Open Access Article
Open Access ArticleChemoselective bioconjugation based on modular click chemistry with 4-halocoumarins and aryl sulfonates†
F. Yushra Thanzeel and
Christian Wolf
and
Christian Wolf *
*
Department of Chemistry, Georgetown University, 37th and O Streets, Washington, DC 20057, USA. E-mail: cw27@georgetown.edu
First published on 25th May 2021
Abstract
We report chemoselective and modular peptide bioconjugation using stoichiometric amounts of 4-halocoumarin and arylsulfonate agents that undergo metal-free C(sp2)-heteroatom bond formation at micromolar concentrations. The underlying ipso-substitution click chemistry is irreversible and generates stable and inherently fluorescent bioconjugates, and the broad selection of coumarin tags offers high labeling flexibility and versatility. Different coumarins and arylsulfonates can be selectively attached to amino and thiol groups in the small peptides glutathione and ornipressin, and both free as well as latent thiols captured in disulfide bridges can be targeted if desired. The broad utility, ease of use, storage, and preparation of 4-halocoumarins and arylsulfonates are very attractive features that extend currently available dual bioconjugation capabilities.
Introduction
Biocompatible chemical modifications of peptides and proteins have received increasing attention in recent years due to the tremendous value in the study of their biomolecular dynamics, trafficking and biological functions. Site-selective bioconjugation with fluorescent tags, affinity probes, polymers such as PEG, or drugs provides an important tool set to investigate and modify protein mobility, distribution, biomolecular interactions and biochemical reaction mechanisms, and it holds considerable promise for the development of bioengineered materials, diagnostics or therapeutics. These exciting prospects have led to considerable interest in chemoselective peptide modification strategies that have emerged as powerful alternatives to genetically engineered proteins exhibiting unnatural amino acids specifically incorporated for chemical derivatization.1–4 Several methods that target an endogenous amino acid in natural peptides and proteins, for example lysine,5–7 histidine,8 methionine,9 tyrosine,10–12 tryptophan,13 serine14 and cysteine,15–23 or the use of chemoenzymatic labeling strategies,24 disulfide bridge modification chemistry25 and lysine–cysteine crosslinking26,27 have been reported.28 Despite the remarkable advance of this field within the last 10 years, chemoselective bioligation remains a challenging task. Persisting shortcomings of currently available methods include the use of potentially toxic organometallic reagents, transition metal complexes29,30 or bioincompatible reaction conditions, instability of the bioconjugate, uncontrolled formation of regio- and stereoisomeric products, or lack of functional group chemoselectivity.31,32 In most cases, modular labeling of different functional groups using the same class of bioconjugation agent is not possible.We envisioned that coumarin click chemistry would provide new effective bioconjugation venues that can address these issues. Our laboratory has recently introduced small molecular optical probes that achieve click chemistry sensing of the enantiomeric composition and concentration of free amino acids in aqueous solutions.33–36 The term ‘click chemistry’ generally refers to a small set of privileged reactions that proceed smoothly with high yields and minimal byproduct formation under mild conditions in environmentally benign solvents. Additional characteristics include operationally simple reaction protocols, the exclusive use of nonhazardous materials and the elimination of cumbersome chromatographic work-up steps, which altogether minimize necessary safety precautions, waste production and cost.37 A wide variety of analytical, synthetic and biomedical applications that display all or at least some of the advantageous features of click chemistry have been reported in recent years.38–43
Results and discussion
We now report chemoselective, modular thiol and amine labeling via ipso-substitution with commercially available or easily prepared, inherently fluorescent 4-halocoumarin and arylsulfonate bioconjugation agents (Fig. 1). Using glutathione (GSH) as a small test peptide we have achieved high-yielding conversion using stoichiometric amounts or minimal excess of a diverse set of bioconjugation agents at room temperature and varying pH. The inexpensive agents are selectively and irreversibly introduced to thiol and amino groups at micromolar concentrations, both free and latent thiols involved in disulfide bridges can be labeled if desired, and the bioconjugates are stable under mass spectrometry conditions and across a wider pH range. The operational simplicity and efficiency of this click chemistry approach allow practical chemoselective bioconjugation with increased labeling flexibility and versatility based on well-defined metal free carbon–heteroatom bond formation which avoids complications that can arise from the formation of regio- and stereoisomeric products when the popular maleimide Michael acceptors are used.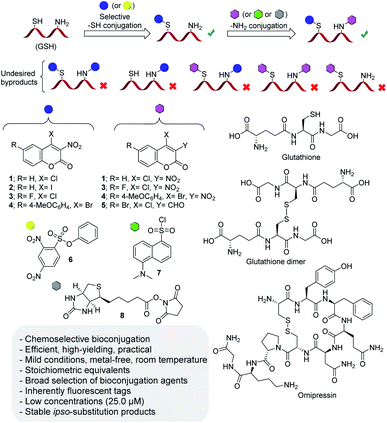 | ||
| Fig. 1 Chemoselective irreversible thiol and amine bioconjugation based on metal-free coumarin and arylation click chemistry. | ||
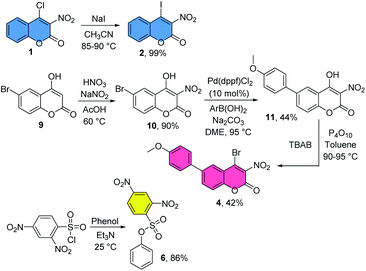
At the onset of this study, we prepared several 4-halocoumarins and phenyl 2,4-dinitrobenzenesulfonate as described in Scheme 1 to first investigate the hydrolytic stability and promise of these agents based on labeling experiments with cysteine and lysine derivatives at room temperature in aqueous solutions. The presence of the nitro group at C-3 in the coumarin scaffold increases the reactivity toward ipso-substitution and was deemed crucial for the envisioned quantitative attachment to thiol and amine nucleophiles. The incorporation of different halides at C-4 is straightforward and allows fine-tuning of the electrophilicity and rate of nucleophilic displacement (Cl > Br > I) if necessary. Treatment of 4-chloro-3-nitrocoumarin, 1, with NaI gave the iodide 2 in quantitative yields.
The remote aryl bromide in commercially available 6-bromo-4-hydroxycoumarin, 9, was appealing to us as this suggested the possibility of coumarin modifications without significantly affecting the bioconjugation chemistry. We therefore developed a protocol for subsequent nitration, Suzuki cross-coupling and halogenation, generating the 4-bromo-3-nitrocoumarin 4 in three steps. As shown below, 4 can be used successfully in chemoselective bioconjugation of GSH and one can imagine that the cross-coupling chemistry provides a convenient entry to the loading of a coumarin with a stapling agent or drug. Alternatively, coumarins are readily synthesized by well-known condensation reactions44,45 which greatly facilitates the incorporation of additional functionalities into the fused benzene ring distant from the carbon-halide bond if necessary. Finally, we prepared phenyl 2,4-dinitrobenzenesulfonate, 6, in 86% yield as described previously.33
Preliminary NMR studies with the 4-chlorocoumarin 1 and the 2,4-dinitrobenzenesulfonate 6 showed fast and quantitative reaction with the thiol group in Cys in aqueous solution at room temperature. We also found that the 4-halo-3-nitrocoumarins react rapidly with amino groups under similar conditions. This ipso-substitution labeling approach is straightforward, does not show side reactions, and displays straightforward click chemistry features unlike bioconjugation methods that rely on transition metal catalyzed arylation.46 We expected that chemoselective tagging of thiol and amino residues with our 4-halocoumarins and the arylsulfonate agent should be possible through pH and buffer optimization. We were aware from previous studies, however, that arylation of free cysteine with 6 is followed by thiol-to-amine migration which would be a problem with a peptide carrying both functionalities in close proximity as in glutathione.33 The complexity of undesired byproducts resulting from incomplete monoconjugation, thiol-to-amine tag-walking, tag replacement and undesired double ligation that altogether need to be suppressed is shown in Fig. 1.
To test the feasibility of controlled dual bioconjugation we began to investigate if thiol-selective tagging of glutathione with chlorocoumarin 1 can be quantitatively achieved without derivatization of the amino group by carefully screening the effects of buffer concentration and pH (Fig. 2). Initial studies using equimolar amounts of GSH and 1 at 5.0 mM and a citrate buffer adjusted to pH 5.0 showed preferential formation of the desired mono-conjugated product 12 but also substantial amounts of the doubly labeled derivative 13 while GSH was mostly consumed after 90 minutes. Although the chemoselectivity toward 12 increased at higher buffer concentrations, we still observed free glutathione while more than 5% of 13 was formed according to ESI-MS analysis (Fig. 2B). Attempts to improve results with the less reactive but presumably more chemoselective 4-iodocoumarin 2 were unsuccessful (see ESI†). We therefore decided to test phosphate, carbonate and TRIZMA buffers at higher pH. Unsatisfactory chemoselectivities were obtained with the inorganic buffers but we were pleased to find that MS analysis indicates that the conversion of GSH to 12 is quantitative while the formation of the undesired byproduct 13 is less than 2% when the bioconjugation is conducted in TRIZMA at pH 9.0 (Fig. 2C and D).
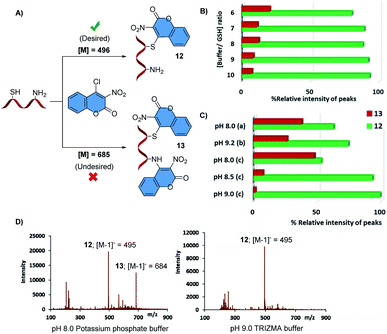 | ||
Fig. 2 Optimization of the thiol-selective bioconjugation of GSH with 1. (A) Desired and undesired reaction outcomes; (B) optimization of buffer/GSH ratio using pH 5.0 citrate phosphate buffer for selective monoconjugation of the thiol moiety in GSH; (C) optimization of buffer type and pH: potassium phosphate buffer (a), sodium carbonate buffer (b), TRIZMA (2-amino-2-(hydroxymethyl)-1,3-propanediol) (c); (D) representative ESI-MS spectra of the reactions between 1 and GSH. Reactions were carried out in acetonitrile–buffer (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution using 5.0 mM concentrations of 1 and GSH, 50.0 mM buffer concentration, at room temperature for 1.5 hours. Relative conversions were calculated using ESI-MS peak intensities of m/z = 495 and 684 corresponding to the desired product and undesired byproduct, respectively, see ESI† for details. 1) solution using 5.0 mM concentrations of 1 and GSH, 50.0 mM buffer concentration, at room temperature for 1.5 hours. Relative conversions were calculated using ESI-MS peak intensities of m/z = 495 and 684 corresponding to the desired product and undesired byproduct, respectively, see ESI† for details. | ||
The optimized protocol for chemoselective monoconjugation of GSH was then applied to 4-chloro-6-fluoro-3-nitrocoumarin, 3, 4-bromo-6-(4-methoxyphenyl)-3-nitro-coumarin, 4, and to the benzenesulfonate 6 (Fig. 3). In accordance with the high-yielding formation of 12, we observed quantitative conversion of the 4-halocoumarins to products 14 and 15 without detectable amounts of the undesired double bioconjugation products. Interestingly, the same high degree of chemoselective transformation of GSH to 16 was observed with 6. In order to confirm that the monoconjugation takes place at the thiol group in GSH, we subjected the corresponding disulfide GSSG, which only carries free amino groups, to equimolar amounts of chlorocoumarin 1 in TRIZMA buffer at pH 8 and 9. In both cases, no sign of C–N bond formation was observed after 24 hours, proving the highly chemoselective thiol ligation outcome with GSH (see ESI†).
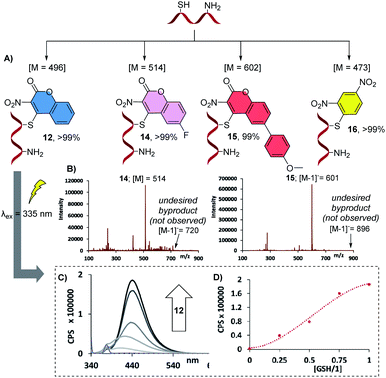 | ||
Fig. 3 Monoconjugated derivatives of GSH obtained with various agents. (A) Structures of bioconjugated GSH products and relative conversions. All reactions were carried out in acetonitrile–buffer (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution, at 5.0 mM concentrations of the bioconjugation agent and GSH in pH 9.0 TRIZMA buffer (50.0 mM), at 25 °C for 2 hours. Relative conversions were calculated using ESI-MS peak intensities of the bioconjugated product and GSH (m/z = 307). (B) Representative MS spectra of products 14 and 15 (see ESI† for details). The undesired byproducts carrying two aryl rings were not detected. (C) Fluorescence emission spectra of increasing concentrations of monoconjugated product 12 (excitation wavelength was 335 nm). (D) The fluorescence intensity at 441 nm plotted against the GSH/1 ratio, see ESI† for details. 1) solution, at 5.0 mM concentrations of the bioconjugation agent and GSH in pH 9.0 TRIZMA buffer (50.0 mM), at 25 °C for 2 hours. Relative conversions were calculated using ESI-MS peak intensities of the bioconjugated product and GSH (m/z = 307). (B) Representative MS spectra of products 14 and 15 (see ESI† for details). The undesired byproducts carrying two aryl rings were not detected. (C) Fluorescence emission spectra of increasing concentrations of monoconjugated product 12 (excitation wavelength was 335 nm). (D) The fluorescence intensity at 441 nm plotted against the GSH/1 ratio, see ESI† for details. | ||
With these results in hand, we continued exploring the possibility of modular chemoselective thiol and amino group bioconjugation (Fig. 4). We first screened the effect of the reaction time, equivalents of the bioconjugation agent and pH to optimize the amine ligation step and found that an increase in reaction time is mostly sufficient (see ESI†). We then attached either a 3-nitrocoumarin or a 2,4-dinitrophenyl ring at the thiol site in GSH, which proceeded according to MS analysis with more than 99% conversion to the desired structures 12 and 16, respectively, thus setting the stage for in situ amine ligation. To these solutions was added another 4-halocoumarin carrying a fluoride, methoxy-phenyl or bromide in the fused benzene ring, dansyl chloride, 7, or the biotin N-succinimidyl ester 8.
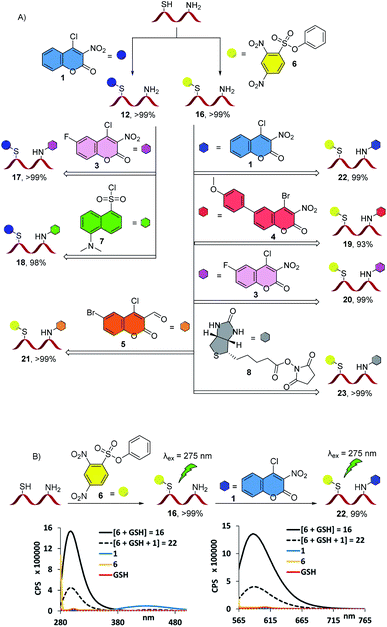 | ||
Fig. 4 (A) Structures of the site-selectively diconjugated GSH derivatives using various reagents. All reactions were carried out in pH 9.0 TRIZMA buffer (50.0 mM)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (1 acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) solutions at 5.0 mM GSH concentrations with 1 to 2 equivalents of the bioconjugation agent. The reaction times were 2 hours for the monoconjugation and 18–24 hours for the second bioconjugation step, respectively. Conversions were determined using the relative ESI-MS intensities of product peaks in comparison to starting materials or monoconjugated intermediates, see ESI† for details. (B) Fluorescence spectra of the bioconjugation products 16 and 22 and of the agents 1 and 6 were collected using an excitation wavelength of 275 nm. Two major emission peaks were observed at 300 nm and 591 nm for the monoconjugated product 16. Fluorescence quenching was observed when 16 was converted to the diconjugated derivative 22. 4) solutions at 5.0 mM GSH concentrations with 1 to 2 equivalents of the bioconjugation agent. The reaction times were 2 hours for the monoconjugation and 18–24 hours for the second bioconjugation step, respectively. Conversions were determined using the relative ESI-MS intensities of product peaks in comparison to starting materials or monoconjugated intermediates, see ESI† for details. (B) Fluorescence spectra of the bioconjugation products 16 and 22 and of the agents 1 and 6 were collected using an excitation wavelength of 275 nm. Two major emission peaks were observed at 300 nm and 591 nm for the monoconjugated product 16. Fluorescence quenching was observed when 16 was converted to the diconjugated derivative 22. | ||
After 12 hours, the desired orthogonally ligated peptide derivatives 17–23 were produced in excellent yields ranging from 93–99%. While we achieved high conversions under mild reaction conditions, we were able to effectively suppress the competing amination with 1 or 6 as well as tag-walking and tag substitution processes. The results show that different coumarins can be chemoselectively introduced to thiol and amino residues or combined with other arylating agents such as phenyl 2,4-dinitrobenzenesulfonate.
The presence of the fluoride in agent 3 provides an opportunity to track the labeled peptide 20 by 19F NMR spectroscopy while the aryl-bromide bond in the double bioconjugation product 21 could be used for late-stage functionalization purposes. The successful use of 4-bromocoumarin 4 shows that modification at C-6 prior to the bioconjugation step is a viable alternative which underscores the versatility of chemoselective amine and thiol bioconjugation with modular coumarin click chemistry. As expected, the placement of two fluorophores in close proximity in 22 results in significant fluorescent quenching47 of the major emission peaks at 300 nm and 591 nm. Importantly, the modular bioconjugation can also be achieved with equal control and efficiency at much lower peptide concentrations. For example, we observed quantitative conversion of GSH to 22 when the reaction was scaled down to 25 μM (see ESI†).
Finally, we applied our method to GSSG and Ornipressin (Fig. 5). As expected, these disulfide peptides do not react with 1 and 6 under the thiol-selective monobioconjugation conditions optimized as described above. However, upon addition of dithiothreitol (DTT), a well-known disulfide reducing agent, the desired thiol labeling occurs with high conversion and selectivity based on MS analysis. The bioconjugates are stable even in the presence of reducing agents and no sign of cleavage of the aryl–sulfur bond in 16 was observed after addition of another equivalent of DTT. These results further prove that the monoconjugation occurs selectively at the free thiol function in the peptide and that the bioconjugation is irreversible. Moreover, addition of 4-chlorocoumarin 1 or the biotin derivative 8 to the solution containing 16 generated in situ from GSSG gave the desired products 22 and 23, respectively, with excellent selectivity and conversion (see ESI† for details).
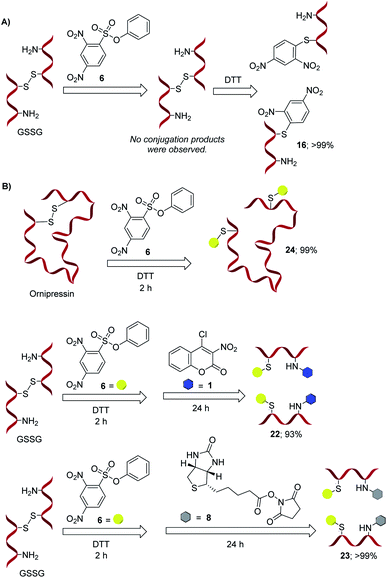 | ||
Fig. 5 Chemoselective thiol ligation upon reductive disulfide cleavage. (A) GSSG (5.0 mM) was exposed to the bioconjugation agent 6 (10.0 mM) in acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TRIZMA pH 9.0 buffer (4 TRIZMA pH 9.0 buffer (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution for 24 hours. The formation of any bioconjugated product was not observed by ESI-MS under these conditions. Instead, a strong signal corresponding to GSSG (m/z = 612) was obtained. An equimolar amount of DTT was added to the above reaction mixture and after 24 hours MS analysis showed formation of the monoconjugated products 16. (B) This protocol was then successfully extended to Ornipressin and for modular GSSG bioconjugation with 6 and either 1 or 8. Conversions were determined using the relative MS intensities of product peaks in comparison to starting materials or monoconjugated intermediates, see ESI.† 1) solution for 24 hours. The formation of any bioconjugated product was not observed by ESI-MS under these conditions. Instead, a strong signal corresponding to GSSG (m/z = 612) was obtained. An equimolar amount of DTT was added to the above reaction mixture and after 24 hours MS analysis showed formation of the monoconjugated products 16. (B) This protocol was then successfully extended to Ornipressin and for modular GSSG bioconjugation with 6 and either 1 or 8. Conversions were determined using the relative MS intensities of product peaks in comparison to starting materials or monoconjugated intermediates, see ESI.† | ||
Conclusions
In conclusion, we have demonstrated chemoselective thiol and amine bioconjugation using stoichiometric amounts of readily available 4-halocoumarin and arylsulfonate agents that undergo C(sp2)-heteroatom bond formation without the common need for transition metal assistance. The peptide modification is high-yielding and occurs at micromolar concentrations at room temperature and varying pH. The underlying ipso-substitution click chemistry is irreversible, generates stable products and the broad selection of coumarin tags offers high labeling flexibility and versatility. Different coumarins can be selectively attached to amino and thiol groups, and both free as well as latent thiols captured in disulfide bridges can be labeled if desired. The 4-halocoumarins and arylsulfonates can also be used in combination with biotin affinity tags or dansyl chloride. Altogether, the broad utility, ease of operation, storage, and preparation of 4-halocoumarins and arylsulfonates make these very attractive bioconjugation agents. We note that the coumarins are inherently fluorescent and can be loaded with drugs or stapling units if desired. The advantageous features embodied in the halocoumarin and arylsulfonate agents are of far-reaching scope and expected to find late-stage functionalization and dual labeling applications in chemical biology, bioengineering, biosensing, and in the biopharmaceutical and materials sciences.Experimental section
General information
All reagents and solvents were commercially available and used without further purification. Flash chromatography was performed on silica gel, particle size 40–63 μm. 1H NMR and 13C NMR spectra were obtained at 400 MHz and 100 MHz, respectively, using deuterated DMSO and chloroform as solvents. Chemical shifts are reported in ppm relative to TMS or to the solvent peak. 4-Chloro-3-nitrocoumarin, 1, 4-chloro-6-fluoro-3-nitrocoumarin, 3, 6-bromo-4-chloro-3-formylcoumarin, 5, dansyl chloride, 7 and NHS-biotin, 8, are commercially available and were used without additional purification. 4-Iodo-3-nitrocoumarin, 2, and phenyl 2,4-dinitrobenzenesulfonate, 6, were synthesized using literature procedures.33,34 4-Bromo-6-(4-methoxyphenyl)-3-nitrocoumarin, 4, was synthesized from 9 by following modified literature protocols, Scheme 1.48,496-Bromo-4-hydroxy-3-nitrocoumarin (10)50
Acetic acid (1.0 mL) was added to a mixture of sodium nitrite (2.8 mg, 0.04 mmol) and 6-bromo-4-hydroxycoumarin, 9 (241.0 mg, 1.00 mmol) in a round bottomed flask and immersed to a pre-heated oil bath of 60 °C. Nitric acid (140.0 μL, 70%) was added to the mixture. After 15 minutes, the reaction was allowed to cool to room temperature. The resultant precipitate was filtered, washed with hexanes (4 × 10 mL) and dried under vacuum to afford 256.0 mg (0.90 mmol, 90%) of a yellow crystalline solid, which was used without further purification. 1H NMR (399 MHz, DMSO-d6) δ 7.92 (d, J = 2.5 Hz, 1H), 7.66 (dd, J = 8.7, 2.6 Hz, 1H), 7.17 (d, J = 8.7 Hz, 1H), 4.33 (bs, 1H). 13C NMR (100 MHz, DMSO-d6) δ 166.4, 157.6, 151.9, 135.1, 128.1, 124.5, 120.8, 119.2, 115.5.4-Hydroxy-6-(4-methoxyphenyl)-3-nitrocoumarin (11)
A mixture of compound 10 (145.0 mg, 0.51 mmol), 4-methoxyphenylboronic acid (152.1 mg, 1.01 mmol), Pd(dppf)Cl2·CH2Cl2 (41.6 mg, 0.05 mmol), aq. Na2CO3 (2.0 mL, 1.4 M) and DME (2.5 mL) were stirred at 90–100 °C. After 72 hours, the reaction was acidified with 1.0 M HCl and extracted with ethyl acetate. The combined organic layers were dried and concentrated under vacuum. Column purification using 0–30% ethyl acetate in hexanes afforded 70.0 mg (0.22 mmol, 44%) of a yellow crystalline solid. 1H NMR: (399 MHz, chloroform-d) δ 8.25 (d, J = 1.7 Hz, 1H), 7.99 (d, J = 8.7 Hz, 1H), 7.55 (d, J = 8.3 Hz, 2H), 7.42 (d, J = 8.7 Hz, 1H), 7.03 (d, J = 8.3 Hz, 2H), 3.88 (s, 3H). 13C NMR (100 MHz, chloroform-d) δ 169.5, 160.0, 152.5, 152.3, 138.6, 136.3, 130.6, 128.1, 123.2, 117.7, 117.5, 114.6, 113.3, 55.4. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C16H11NO6Na: 336.0484; found: 336.0479.4-Bromo-6-(4-methoxyphenyl)-3-nitrocoumarin (4)
A mixture of 11 (70.0 mg, 0.22 mmol), TBAB (386.9 mg, 1.2 mmol) and P4O10 (340.6 mg, 2.4 mmol) in toluene (3 mL) was stirred at 90–95 °C overnight. The mixture was allowed to cool to room temperature and washed with water, sat. NaHCO3 and extracted with dichloromethane. The combined organic layers were dried over MgSO4 and concentrated in vacuo. Purification by flash column chromatography on silica gel (0–50% ethyl acetate in hexanes) afforded 36.1 mg (0.09 mmol, 42%) of a greenish yellow solid. 1H NMR (399 MHz, chloroform-d): δ 8.05 (d, J = 2.2 Hz, 1H), 7.91 (dd, J = 8.7, 2.1 Hz, 1H), 7.55 (d, J = 8.7 Hz, 2H), 7.48 (d, J = 8.7 Hz, 1H), 7.04 (d, J = 8.6, 2H), 3.88 (s, 3H). 13C NMR (100 MHz, chloroform-d): δ 160.1, 151.6, 150.1, 139.6, 133.8, 133.8, 130.7, 128.3, 127.0, 117.8, 117.3, 114.7, 55.4. HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C16H10BrNO5Na: 397.9640; found: 397.9636.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the U. S. National Science Foundation (CHE-1764135).Notes and references
- O. Boutureira and G. J. L. Bernardes, Chem. Rev., 2015, 115, 2174 CrossRef CAS PubMed.
- J. M. J. M. Ravasco, H. Faustino, A. Trindade and P. M. P. Gois, Chem.–Eur. J., 2019, 25, 43 CrossRef CAS PubMed.
- B. Akgun and D. G. Hall, Angew. Chem., Int. Ed., 2018, 57, 13028 CrossRef CAS PubMed.
- C. Zhang, E. V. Vinogradova, A. M. Spokoyny, S. L. Buchwald and B. L. Pentelute, Angew. Chem., Int. Ed., 2019, 58, 4810 CrossRef CAS PubMed.
- F. Liu, H. Wang, S. Li, G. A. L. Bare, X. Chen, C. Wang, J. E. Moses, P. Wu and K. B. Sharpless, Angew. Chem., Int. Ed., 2019, 58, 8029 CrossRef CAS PubMed.
- I. Ivancova, R. Pohl, M. Hubalek and M. Hocek, Angew. Chem., Int. Ed., 2019, 58, 13345 CrossRef CAS PubMed.
- K. L. Diehl, I. V. Lolesnichenko, S. A. Robotham, J. L. Bachman, Y. Zhong, J. S. Brodbelt and E. V. Anslyn, Nat. Chem., 2016, 8, 968 CrossRef CAS.
- S. Jia, D. He and C. J. Chang, J. Am. Chem. Soc., 2019, 141, 7294 CrossRef CAS PubMed.
- A. H. Christian, S. Jia, W. Cao, P. Zhang, A. T. Meza, M. S. Sigman, C. J. Chang and F. D. Toste, J. Am. Chem. Soc., 2019, 141, 12657 CrossRef CAS PubMed.
- E. J. Choi, D. Jung, J.-S. Kim, Y. Lee and B. M. Kim, Chem.–Eur. J., 2018, 24, 10948 CrossRef CAS PubMed.
- D. Alvarez-Dorta, C. Thobie-Gautier, M. Croyal, M. Bouzelha, M. Mevel, D. Deniaud, M. Boujtita and S. G. Gouin, J. Am. Chem. Soc., 2018, 140, 17120 CrossRef CAS PubMed.
- J. Ohata, M. K. Miller, C. M. Mountain, F. Vohidov and Z. T. A. Ball, Angew. Chem., Int. Ed., 2018, 57, 2827 CrossRef CAS PubMed.
- Y. Seki, T. Ishiyama, D. Sasaki, J. Abe, Y. Sohma, K. Oisaki and M. Kanai, J. Am. Chem. Soc., 2016, 138, 10798 CrossRef CAS PubMed.
- J. C. Vantourout, S. R. Adusumalli, K. W. Knouse, D. T. Flood, A. Ramirez, N. M. Padial, A. Istrate, K. Maziarz, J. N. deGruyter, R. R. Merchant, J. X. Qiao, M. A. Schmidt, M. J. Deery, M. D. Eastgate, P. E. Dawson, G. J. L. Bernardes and P. S. Baran, J. Am. Chem. Soc., 2020, 142, 17236 CrossRef CAS PubMed.
- S. Arumugam, J. Guo, N. E. Mbua, F. Friscourt, N. Lin, E. Nekongo, G.-J. Boons and V. V. Popik, Chem. Sci., 2014, 5, 1591 RSC.
- E. V. Vinogradova, C. Zhang, A. M. Spokoyny, B. L. Pentelute and S. L. Buchwald, Nature, 2015, 526, 687–691 CrossRef CAS PubMed.
- D. Kalia, P. V. Malekar and M. Parthasarathy, Angew. Chem., Int. Ed., 2016, 55, 1432 CrossRef CAS PubMed.
- M. S. Messina, J. M. Stauber, M. A. Waddington, A. L. Rheingold, H. D. Maynard and A. M. Spokoyny, J. Am. Chem. Soc., 2018, 140, 7065 CrossRef CAS PubMed.
- D. R. Dempsey, H. Jiang, J. H. Kalin, Z. Chen and P. A. Cole, J. Am. Chem. Soc., 2018, 140, 9374 CrossRef CAS PubMed.
- Y. Chen, K. Tsao, S. L. Acton and J. W. Keillor, Angew. Chem., Int. Ed., 2018, 57, 12390 CrossRef CAS PubMed.
- M.-A. Kasper, M. Glanz, A. Stengl, M. Penkert, S. Klenk, T. Sauer, D. Schumacher, J. Helma, E. Krause, M. C. Cardoso, H. Leonhardt and C. P. R. Hackenberger, Angew. Chem., Int. Ed., 2019, 58, 11625 CrossRef CAS PubMed.
- A. L. Baumann, S. Schwagerus, K. Broi, K. Kemnitz-Hassanin, C. E. Stieger, N. Trieloff, P. Schmieder and C. R. Hackenberger, J. Am. Chem. Soc., 2020, 142, 9544 CrossRef CAS PubMed.
- G.-X. Yin, T.-T. Niu, Y.-B. Gan, T. Yu, P. Yin, H.-M. Chen, Y.-Y. Zhang, H. T. Li and S.-Z. Yao, Angew. Chem., Int. Ed., 2018, 57, 4991 CrossRef CAS PubMed.
- J. E. Glasgow, M. L. Salit and J. R. Cochran, J. Am. Chem. Soc., 2016, 138, 7496 CrossRef CAS PubMed.
- A. Maruani, M. E. B. Smith, E. Miranda, K. A. Chester, V. Chudasama and S. Caddick, Nat. Commun., 2015, 6, 6645 CrossRef CAS PubMed.
- M. Todorovic, K. D. Schwab, J. Zeisler, C. Zhang, F. Benard and D. M. Perrin, Angew. Chem., Int. Ed., 2019, 58, 14120 CrossRef CAS PubMed.
- Y. Zhang, Q. Zhang, C. T. T. Wong and X. Li, J. Am. Chem. Soc., 2019, 141, 12274 CrossRef CAS PubMed.
- T. Tamura and I. Hamachi, J. Am. Chem. Soc., 2018, 141, 2782–2799 CrossRef PubMed.
- J. M. Antos and M. B. Matthew, J. Am. Chem. Soc., 2004, 126, 10256 CrossRef CAS PubMed.
- J.-R. Deng, S.-F. Chung, A. S. Leung, W.-M. Yip, B. Yang, M.-C. Choi, J.-F. Cui, K. K.-Y. Kung, Z. Zhang, K.-W. Lo, Y.-C. Leung and M.-K. Wong, Chem. Commun., 2019, 2, 93 CrossRef.
- C. J. Pickens, S. N. Johnson, M. M. Pressnall, M. A. Leon and C. J. Berkland, Bioconjugate Chem., 2018, 29, 686 CrossRef CAS PubMed.
- N. Krall, F. P. da Cruz, O. Boutureira and G. J. L. Bernardes, Nat. Chem., 2016, 8, 103 CrossRef CAS PubMed.
- F. Y. Thanzeel and C. Wolf, Angew. Chem., Int. Ed., 2017, 56, 7276–7281 CrossRef CAS PubMed.
- F. Y. Thanzeel, K. Balaraman and C. Wolf, Nat. Commun., 2018, 9, 5323 CrossRef CAS PubMed.
- F. Y. Thanzeel, A. Sripada and C. Wolf, J. Am. Chem. Soc., 2019, 141, 16382 CrossRef CAS PubMed.
- S. L. Pilicer, J. M. Dragna, A. Garland, C. J. Welch, E. V. Anslyn and C. Wolf, J. Org. Chem., 2020, 85, 10858 CrossRef CAS PubMed.
- H. C. Kolb, M. G. Finn and B. K. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS PubMed.
- J. E. Moses and A. D. Moorhouse, Chem. Soc. Rev., 2007, 36, 1249 RSC.
- J. M. Baskin, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793 CrossRef CAS PubMed.
- P. V. Chang, J. A. Prescher, E. M. Sletten, J. M. Baskin, I. A. Miller, N. J. Agard, A. Lo and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 1821 CrossRef CAS PubMed.
- S. Li, P. Wu, J. E. Moses and K. B. Sharpless, Angew. Chem., Int. Ed., 2017, 56, 2903 CrossRef CAS PubMed.
- M. Kukwikila, N. Gale, A. H. El-Sagheer, T. Brown and A. Tavassoli, Nat. Chem., 2017, 9, 1089 CrossRef CAS PubMed.
- D. Gahtory, R. Sen, A. R. Kuzmyn, J. Escorihuela and H. Zuilhof, Angew. Chem., Int. Ed., 2018, 57, 10118 CrossRef CAS PubMed.
- M. Lončarić, D. Gašo-Sokač, S. Jokić and M. Molnar, Biomolecules, 2020, 10, 151 CrossRef PubMed.
- A. Stefanachi, F. Leonetti, L. Pisani, M. Catto and A. Carotti, Molecules, 2018, 23, 250 CrossRef PubMed.
- C. P. Toseland, J. Chem. Biol., 2013, 6, 85 CrossRef PubMed.
- C. Munkholm, D. R. Parkinson and D. R. Walt, J. Am. Chem. Soc., 1990, 112, 2608 CrossRef CAS.
- X. Han, J. Luo, F. Wu, X. Hou, G. Yan, M. Zhou, M. Zhang, C. Pu and R. Li, Eur. J. Med. Chem., 2016, 114, 232 CrossRef CAS PubMed.
- M. Sánchez-Peris, E. Falomir, J. Murga, M. Carda and J. A. Marco, Bioorg. Med. Chem., 2016, 24, 3108 CrossRef PubMed.
- D. R. Buckle, B. C. C. Cantello, H. Smith and B. A. Spicer, J. Med. Chem., 1975, 18, 391 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details including coumarin synthesis, bioconjugation optimization studies, ESI-MS analysis. See DOI: 10.1039/d1ra03271b |
| This journal is © The Royal Society of Chemistry 2021 |