Open Access Article
Open Access ArticleA low-cost and high-loading viologen-based organic electrode for rechargeable lithium batteries†
Mao Chen‡
a,
Lei Liu‡ b,
Peiyao Zhanga and
Hongning Chen
b,
Peiyao Zhanga and
Hongning Chen *a
*a
aChemical Hybrid Energy Novel Laboratory, College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen, Guangdong 518055, PR China. E-mail: hnchen@szu.edu.cn
bCollege of Chemistry and Materials Science, Anhui Normal University, Wuhu 241000, China
First published on 13th July 2021
Abstract
Organic active materials are regarded as a very promising choice for lithium batteries because of several outstanding advantages such as low-cost, flexible tunability and pollution-free sources. Viologen compounds are attractive two-electron storage materials with low redox potentials, which are mainly used as anolytes in redox flow batteries (RFBs) considering their high solubility in electrolytes. However, due to their relatively large molecular weight and low density, it is difficult to prepare high-loading and stable-cycling electrodes for lithium battery application. In this research, by adopting 4,4′-bipyridine as the raw material and combining salification with a high-energy ball milling method, a low-solubility and high-stability viologen carbon-coated composite, ethyl viologen dihexafluorophosphate-Ketjen black (EV-KB), is synthesized. Then, by optimizing the electrode preparation process, a high-loading viologen-based electrode is successfully prepared. Salification effectively reduces the solubility of viologen compounds in the electrolyte so that the EV-KB composite can be used in lithium batteries. At the same time, it is pointed out that current collectors and slurry solvents play an important role in achieving the high-loading electrode. By deliberately selecting carbon paper as the current collector and ethanol as the solvent, the EV-KB composite organic electrode with a loading up to 1.5–9 mg cm−2 can achieve a specific capacity of 106–79 mA h g−1 for 400 stable cycles with a coulombic efficiency of 96% as well as a good rate capability. The synthesis method and electrode preparation optimization process introduced in this paper provide a reference for other types of organic active materials to be used in high-loading lithium batteries.
Introduction
With the increasing problem of environmental pollution, the demand for clean energy storage is becoming more and more urgent.1–3 It is known that lithium-ion batteries are the most commercialized electrochemical energy storage device at present; therefore, the environmental protection ability of their electrode active materials has attracted most attention.4–6 In traditional lithium-ion batteries, the active materials contain transition metal elements such as cobalt, titanium, and manganese, so that they are not only expensive but also harmful to the environment due to their toxicity.7–10 Hence, developing a new type of low-cost and environment-friendly active materials has become significant for the better utilization of lithium-ion batteries in the future.Currently, organic active materials are regarded as a very attractive choice for electrodes because of their low-cost, diversity of molecular moieties, wide range of reaction potential and pollution-free sources.11–16 For example, quinone compounds, through appropriate molecular modification, can be synthesized to various derivatives and achieve a stable cycling performance in the potential range of 1.7 V to 3.2 V vs. Li/Li+.17–19 Many sources of quinone compounds can be found in nature. For example, there are many quinone derivatives involved in the electron transport chain of chloroplasts.20,21 In addition, organic active materials such as pteridine,22 nitroxyl radicals23,24 and their derivatives25 have also been used in batteries, showing excellent application prospects. Among these organic active materials, viologen-based active materials are attractive two-electron storage active materials with low redox potentials even if the molecular form of viologen is highly toxic.24,26,27 However, they are now mainly used in RFBs because of their high solubility in electrolytes.28 Viologen-based active materials can be applied in lithium batteries only if their solubility can be inhibited and their stability can be improved in the electrode.29 Viologen-based active materials belong to p-type organics, which the reaction is between the neutral state and the positively charged state.14 This structure leads to a dual-ion configuration relying on both cations and anions. Moreover, the molecular anions are generally used as charge carriers for compensation.30,31 In recent years, salification has been taken as an effective method to suppress the dissolution of organic active materials, and it is able to improve the electrode cycle performance of the materials through the principle of reversing polarity.32–34 Chen et al. used this method to successfully demonstrate two viologen salt compounds which achieved 200 stable cycles.35 This study provides a good strategy for the application of viologen-based active materials in lithium batteries.
Developing high-loading electrodes is crucial for the practical battery application. In classic LIBs, the high-loading electrodes can be achieved by binder enhancement,36 current collector optimization,37 active material modification38 and advanced electrode manufacturing.39 However, the current research of organic electrode is mostly concentrated on developing novel active materials but often overlooks the importance of high-loading. Recent years, novel electrode architectures and electrode fabrication methods have been successfully applied to organic active materials.40,41 For example, the carboxylate compound was designed to achieve high mass loading of 12.0 mg cm−2 with only 0.5 wt% carbon additive.42 Nevertheless, large polarization and poor rate performance issues arise as the mass loading increases. The problems associated with high-loading of organic active materials still need to be addressed in the future. Due to the low density, high molecular weight and poor conductivity of organic active materials, it is difficult to achieve high-loading electrodes. Therefore, compared with the traditional lithium-ion battery electrode, the single cell capacity of organic active material electrode is much smaller, which is not conducive to its further application in more fields. Especially for today's vigorous development of electric vehicles, a higher requirement for the single cell capacity is put forward.43–46 Among the reported work so far, the loading of the organic active material electrode is about 1–5 mg cm−2, and there is still a lot of room for improvement. For the optimization of organic electrodes, many researchers embark on the preparation of composites,47,48 the selection of binders49,50 or solvents,51 which makes the stability and loading of the electrode improved to a certain extent. For example, in the research of Cui and Bao et al., the cycle performance of the organic active material disodium rhodizonate (Na2C6O6) was successfully improved by optimizing the size of the active material and selecting the appropriate electrolyte.52
In this work, 4,4′-bipyridine is used as the raw material to synthesize viologen compounds by salification, and through ion exchange, the almost insoluble ethyl viologen dihexafluorophosphate (EV(PF6)2) is obtained, which can be effectively adopted as the electrode active material for lithium batteries. Then, EV-KB composite is prepared by a simple high-energy ball milling of EV(PF6)2 with conductive carbon Ketjen black (KB) to increase the conductivity and stability of the active material. After optimizing the electrode preparation process, a high-loading viologen-based organic electrode is achieved and applied to lithium batteries. Because the salification method can effectively reduce the solubility of EV in the electrolyte, the active material is no longer dissolved in the electrolyte, thereby improving its stability; at the same time, the EV-KB composite effectively increases the connection between the active materials, so that it can still achieve better cycle stability under high-loading conditions. In our demonstration, the EV-KB composite electrode is able to reach a specific capacity of 106–79 mA h g−1 with a high-loading of 1.5–9 mg cm−2. It can also stably cycle 400 times under certain conditions with the coulombic efficiency of 96%. The synthesis method and electrode preparation optimization process adopted in this paper provide a reference for other types of organic active materials to be used in high-loading lithium batteries.
Experimental
Synthesis of ethyl viologen dihexafluorophosphate (EV(PF6)2)
4,4′-Bipyridine (1.000 g, 6.4 mmol) and bromoethane (1.746 g, 16.0 mmol) were mixed in acetonitrile (ACN, 15 mL) and stirred at 80 °C overnight. After cooling down at room temperature, the suspended solid was collected by filtration and rinsed with acetone for 3 times. Finally, faint yellow solid (1) was obtained after vacuum drying at 50 °C overnight (yield: 2.035 g, 85%).1 (1.000 g, 2.7 mmol) and KPF6 (2.945 g, 16.0 mmol) were mixed and stirred in DI water at room temperature overnight. Then the suspended solid was collected by vacuum filtration and rinsed with DI water. Finally, white solid (EV(PF6)2) was obtained after vacuum drying at 65 °C overnight (yield: 1.225 g, 90%).
Synthesis of EV-KB composite
EV-KB composite was prepared by a simple and scalable high-energy mechanical ball milling method. The synthesized EV(PF6)2 (EV) and Ketjen Black (KB) were mixed in the mass ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in a zirconia vessel. Fritsch Pulverisette-6 was applied at a speed of 350 rpm with the milling duration of 30 min. The collected EV-KB composite was dried in oven at 55 °C overnight.
1 in a zirconia vessel. Fritsch Pulverisette-6 was applied at a speed of 350 rpm with the milling duration of 30 min. The collected EV-KB composite was dried in oven at 55 °C overnight.
Preparation of EV-KB composite electrode
EV-KB composite electrodes were prepared by mixing EV-KB composite with PTFE binder in NMP/ethanol solvent (EV:KB:PTFE = 5.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.6
3.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The mixtures were sonicated in an glass container to get homogeneous slurry and coated on a clean carbon paper/aluminum foil. After drying in a oven at 55 °C for 12 h, the samples were measured with a mass loading of 1.5–9 mg cm−2 for typical electrodes in Φ14 mm discs as shown in Fig. S4a.†
1). The mixtures were sonicated in an glass container to get homogeneous slurry and coated on a clean carbon paper/aluminum foil. After drying in a oven at 55 °C for 12 h, the samples were measured with a mass loading of 1.5–9 mg cm−2 for typical electrodes in Φ14 mm discs as shown in Fig. S4a.†
Assembly of Li-EV based coin cell
Fig. S4b† shows the structure of the Li-EV based coin cell (CR2032). The electrolyte used in the cell assembling was 1 M LiClO4 in propylene carbonate (PC). One piece of lithium foil (Φ16 mm) was attached to a anode plate cell body, which acted as a current collector for the anode side. 50 μL of electrolyte was added on the surface of lithium foil. One glass fiber separator (Φ19 mm) was placed on the surface of the lithium foil followed by adding 50 μL of electrolyte. One piece of EV-KB composite electrode (Φ14 mm) was placed on the separator. Finally, a stainless steel spring and a cathode plate were placed on the cell. The cell assembling process was conducted in an Ar-filled glove box (Etelux, H2O < 1.0 ppm, O2 < 1.0 ppm).Electrochemical characterizations
All the electrochemical characterizations were performed using the Bio-Logic VSP electrochemical workstation. Cyclic voltammogram (CV) tests of active materials (2 mM EV(PF6)2 in 1 M LiClO4 PC or 1 M LiPF6 EC/DEC electrolyte) were conducted between −2.0 and 0 V vs. Ag/Ag+ using a three electrodes configuration with glassy carbon electrode as working electrode, nonaqueous Ag/Ag+ electrode as reference electrode and Pt wire as counter electrode. Galvanostatic discharge–charge tests were performed between 1.5 and 3.0 V vs. Li/Li+ for full cell. Voltage control galvanostatic discharge–charge tests were performed between 2.1 and 3.0 V vs. Li/Li+ for 1st electron and 1.5 and 2.4 V vs. Li/Li+ for 2nd electron. Current density was calculated based on the current collector geometric surface area. The specific capacity was calculated from the mass of active materials (EV(PF6)2).Material characterizations
Scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) characterization were performed on JEOL JSM-7800F. The X-ray photoelectron spectroscopy (XPS) test was conducted on K-Alpha+. Thermogravimetric analysis (TGA) was performed STA409PC (NETZSCH-Gerätebau GmbH). 1H NMR was recorded on VarianVNMRS 400 in solutions of D2O.Results and discussion
In this paper, using 4,4′-bipyridine as the raw material, the viologen-based organic active material EV(PF6)2 is obtained through a two-step synthetic route (Fig. 1a). In this process, we note that Br−1 anions is replaced by PF6−1 through ion exchange, which makes the synthesized viologen active material almost insoluble in the electrolyte. Thus, this material can be effectively used in the electrode of the lithium battery. We observed that the solubility of as-prepared EV(PF6)2 is around 5–10 mM in the 1 M LiClO4 PC electrolyte with obvious precipitate as shown in Fig. S11.† The 1H NMR results in Fig. S1† confirm the chemical structure and purity of the synthesized EV compound, proving the successful synthesis. We conducted the EDX and XPS tests of the as-prepared EV(PF6)2 to examine the structure after ion exchange process as shown in Fig. S13 and S14.† The results indicate that all the Br− ions are replaced by PF6− in our synthesis method. Viologen compounds are attractive organic active materials with two transfer electrons. As shown in Fig. 1c, during the discharge–charge process, the two N atoms on the benzene ring can each gain or lose an electron, thus realizing a reversible double electronic redox reaction, and providing two corresponding discharge–charge plateaus. Based on EV(PF6)2, a high-energy ball milling is then used to mix it with conductive carbon KB to prepare EV-KB composite, thereby increasing the conductivity and stability of EV(PF6)2 (Fig. 1b). The results of thermogravimetric analysis in Fig. S2† show that the yield of this method is very efficient, and the active material in the obtained EV-KB composite accounts for about 61% with almost no loss.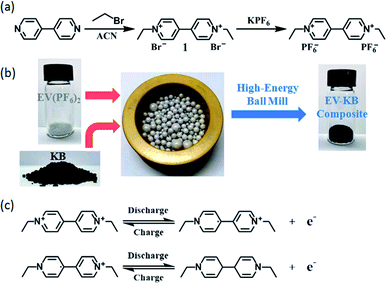 | ||
| Fig. 1 (a) Schematic synthesis process of ethyl viologen dihexafluorophosphate (EV(PF6)2). (b) Schematic synthesis process of EV-KB composite. (c) Redox reaction mechanism of EV(PF6)2. | ||
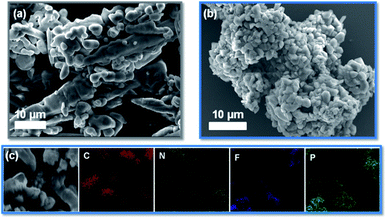
Compared with EV(PF6)2, the EV-KB composite synthesized by high-energy ball milling has more uniform particles and an obvious carbon-coated morphology, which makes it more conductive and more uniformly distributed when preparing electrodes. As shown in the SEM results of Fig. 2, after high-energy ball milling, the morphology of EV(PF6)2 aggregation is broken into smaller and more uniform particles. It can be seen from the EDX results of Fig. 2c that the C atoms and other atoms (N, F, P) in EV are uniformly distributed and overlapped throughout EV-KB composite, which proves the uniformity of EV-KB composite in coating carbon. This simple and efficient composite synthesis method can effectively improve the electrochemical performance of EV(PF6)2. Fig. 3a shows the cyclic voltammetry (CV) results of 2 mM EV(PF6)2 in 1 M LiClO4 in PC at various scanning rates. It can be observed that the redox potentials of EV(PF6)2 are −0.82 V (EV/EV+) and −1.22 V (EV+/EV2+) vs. Ag/Ag+, respectively corresponding to the two-step reaction in Fig. 1c. In addition, the oxidation peaks and reduction peaks are very symmetrical, showing good reversibility. These two reactions come from the gain and loss of electrons of the two N atoms on the benzene ring. However, EV(PF6)2 exhibits poor reversibility in the commonly used 1 M LiPF6 EC/DEC electrolyte as shown in Fig. S12.† The CV results shows asymmetrical configuration for the oxidation and reduction peaks, which implies that the 1 M LiPF6 EC/DEC electrolyte is not suitable to be used in the EV based system. The structure of EV(PF6)2 is then confirmed through X-ray photoelectron spectroscopy (XPS). As shown in Fig. 3b and c, the characteristic peaks of C–C, C–H (284.6 eV) and C–N (285.0 eV) appear in the C 1s spectrum, and the characteristic peaks of N+ (401.6 eV) and C–N (399.8 eV) appear in the N 1s spectrum, all of which are in line with the structural characteristics of EV(PF6)2. Meanwhile, similar results in the XPS of the EV-KB composite (Fig. S3†) are obtained, which suggests that after high-energy ball milling, the structure of the EV are not changed and its electrochemical properties are not affected. Moreover, there is a CF3 (292.2 eV) peak in both EV(PF6)2 and EV-KB composite. This is mainly due to the small amount of impurities introduced in the ion exchange with KPF6 during the synthesis.
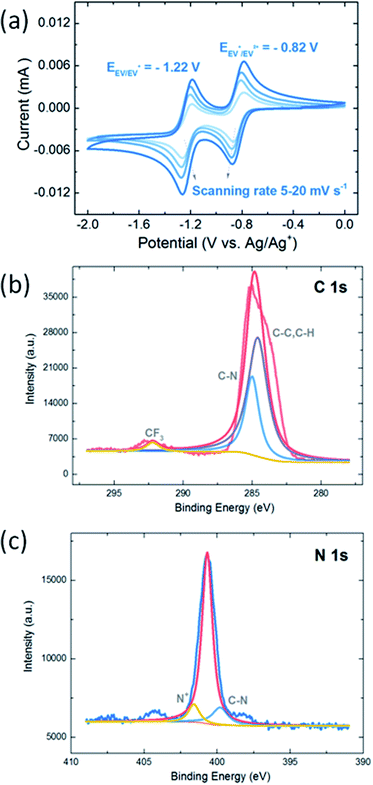 | ||
| Fig. 3 (a) Cyclic voltammetry results of 2 mM EV(PF6)2 in 1 M LiClO4 in PC at various scanning rates. XPS spectra of EV(PF6)2 in (b) C 1s and (c) N 1s regions. | ||
Owing to the large molecular weight and low density of organic active materials, it is difficult to achieve high-loading, or even under high-loading, the electrochemical performance will not be good. When preparing electrodes in this paper, EV-KB composite has been uniformly coated with carbon, so no additional conductive carbon is needed in the electrode slurry (Fig. S4a†); only polytetrafluoroethylene (PTFE) is needed to be mixed with EV-KB composite in the solvent for coating electrodes directly. The aluminum foil is commonly used in lithium batteries as the current collector. However, when it is adopted in the process of preparing EV-KB composite electrodes, it is difficult for slurry to be bonded with aluminum foil, which leads to its obvious separation from aluminum foil. Therefore, carbon paper is used as the current collector in this work to uniformly achieve a high-loading of 1.5–9 mg cm−2. Due to its similar surface properties to active materials, carbon paper is more suitable for connection with EV-KB composite. In addition, the solvents in the slurry also have a great impact on the coating of electrodes. As shown in Fig. S5,† when N-methyl-2-pyrrolidone (NMP) is used, the coated electrodes will become severely cracked after drying, failing to achieve high-loading; however, when ethanol is used as the solvent, the electrodes will be uniformly distributed after drying, and there will be no cracks, achieving high-loading easily. This results demonstrated that it is also possible to optimize the current collectors and slurry solvents for the preparation of uniform organic active material based electrodes. Lithium metal is used as the reference electrodes and counter electrodes in this research to test the electrochemical performance of the EV-KB composite electrodes in CR2032 coin battery (Fig. S4b†).
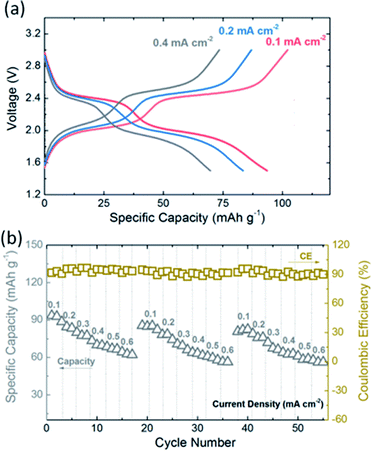
The EV-KB composite electrode presents good rate capability and shows typical two-electron discharge–charge plateaus at various current densities (Fig. 4a). Meanwhile, as shown in Fig. 4b, under the current density range of 0.1–0.6 mA cm−2, the EV-KB composite electrode realizes a specific capacity ranging from 101 to 65 mA h g−1. Furthermore, after discharge–charge at high-rate current, the electrode can remain sound stability when returning to low-rate current with Coulomb efficiency higher than 95%, presenting good rate adaptability. By limiting the discharge–charge voltage, we also study the reversibility of the two-electron of the EV-KB composite electrodes respectively. By setting the discharge–charge voltage at the range of 2.1–3.0 V vs. Li/Li+, it can be seen that the first electron (EV/EV+) achieves 600 stable cycles with Coulomb efficiency about 98% (Fig. S6†). Likewise, when the range of discharge–charge voltage is set as 1.5–2.4 V vs. Li/Li+, the second electron (EV/EV+) can also realize 600 stable cycles with Coulomb efficiency of 98% (Fig. S7†). The two-electron discharge–charge plateau of viologen active materials brings better adaptability, which enables the materials to match with anode and cathode materials with various potentials.
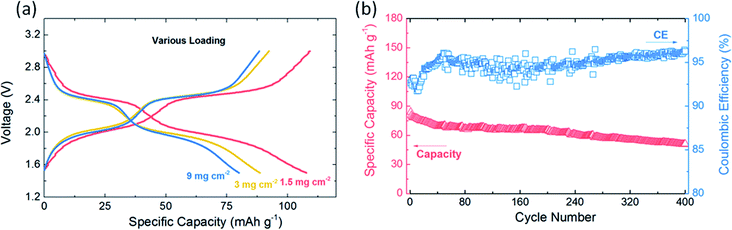
Organic active materials generally have large molecular weight and small density, making it difficult to demonstrate good electrode performance at high-loading. Therefore, a relative large gap exists between the organic active materials and the commonly-used inorganic electrode materials for lithium batteries. However, high-loading is practically significant to the industrial application of organic electrodes. In this work, by optimizing the electrode current collector and slurry solvent, the EV-KB composite electrode successfully achieves stable performance at high-loading. Fig. 5a shows first galvanostatic discharge–charge profiles of EV-KB composite electrode at various loadings. When the electrode loading is between 1.5–9 mg cm−2, the reachable specific capacity range is 106–79 mA h g−1; i.e., when the loading is increased by 6 times, the specific capacity can reach up to 75% of the theoretical value, which represents a relatively high level among the demonstrated organic electrodes at high-loading by far. Meanwhile, we realize 400 stable cycles at loading of 3 mg cm−2 with Coulomb efficiency of 96% (Fig. 5b), with the capacity decayed only 0.049% on each cycle. Moreover, stable cycle performance and rate capability are achieved at other high-loading as well (Fig. S8 & S9†). We also used the EV-KB composite electrode in a self-made pouch cell to further examine its applicability in the larger system. The galvanostatic discharge–charge profiles of EV-KB composite electrode with area of 4 × 2 cm2 were shown in Fig. S10,† which still indicates the reasonable cycling stability and high coulombic efficiency. We believe that the performance of a larger battery system can be further improved by better cell design and manufacturing process.
As illustrated in Table 1, compared with recently-reported organic electrode materials,32,53–65 the EV-KB composite organic electrode demonstrated in this paper reaches relatively high-loading (1.5–9 mg cm−2), which represents a currently leading level. Meanwhile, it shows the reasonable cycle life among all organic electrodes. The demonstration of this stable and high-loading electrode is practically significant to the industrial application of organic active materials. The salification method adopted in this paper, which can effectively reduce the solubility of soluble viologen active materials in electrolyte thus to enable their application in lithium batteries, can also be applied in other kinds of organic active materials. Furthermore, the high-energy ball milling, a simple and effective composite synthesizing method, provides a direction to further optimize the organic electrode performance and loading.
| Active materials | Voltage (vs. Li/Li+) | Specific capacity (mA h g−1) | Cycle life (No.) | Loading (mg cm−2) | Ref. |
|---|---|---|---|---|---|
| EV(PF6)2 | 2.4 & 2.0 V | 106 | 400 | 1.5–9 | This work |
| PTCLi4 | 1.1 V | 110 | 200 | 12 | 53 |
| DMTS | 2.2 V | 720 | 50 | 6.7 | 54 |
| PAQS | 2.2 V | 165 | 100 | 1–2 | 55 |
| PDBS | 2.5 V | 200 | 20 | 1–2 | 56 |
| Li2PDHBQS | 2.0 V | 247 | >1000 | 1–2 | 56 |
| PBQS | 2.7 V | 275 | >1000 | 1–2 | 57 |
| 2HNAQ | 2.4 V | 280 | 1000 | 2–3 | 58 |
| PID | 2.7 V | 207 | 100 | 1.6 | 59 |
| P14AQ | 2.1 V | 263 | 100 | 1–2 | 60 |
| LiDHAQS | 2.5 V | 330 | 400 | 1 | 61 |
| PBDTD | 2.5 V | 200 | 250 | 2–3 | 62 |
| DAAQ-TFP | 2.4 V | 107 | >1000 | 0.45 | 63 |
| Li2C8H4O4 | 0.8 V | 300 | 50 | 8–10 | 32 |
| Li2TDC | 0.65 V | 200 | 50 | 5 | 64 |
| 2,6-Naph(COOLi)2 | 0.8 V | 220 | 100 | 2.5 | 65 |
Conclusions
In summary, by adopting 4,4′-bipyridine as the raw material and combining salification with high-energy ball milling, a low-solubility and high-stability viologen carbon-coated EV-KB composite is synthesized in this research. Then, by optimizing the electrode preparation process, a high-loading viologen-based organic active material electrode is successfully prepared. Salification can effectively reduce the solubility of organic active materials in the electrolytes, thus enable the application of viologen active materials in lithium batteries. The importance of current collectors and slurry solvents is discussed in this research. By selecting carbon paper as the current collector and ethanol as the solvent, the EV-KB composite electrode with a loading up to 1.5–9 mg cm−2 is successfully prepared. This high-loading electrode can stably cycle 400 times at coulombic efficiency of 96%, achieving a specific capacity of 106–79 mA h g−1, and it exhibits a good rate capability. The synthesis method and electrode preparation optimization process introduced in this paper provide a reference for other types of organic active materials to be used in high-loading lithium batteries.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work described in this paper was substantially supported by grants from the National Natural Science Foundation of China (Project No. 21908147 and No. 52076142), Natural Science Foundation of SZU (Grant No. 000002110234), the Shenzhen Science and Technology Fund (RCBS20200714114919158 and JCYJ20170818093905960).Notes and references
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS
.
- K. J. Griffith, K. M. Wiaderek, G. Cibin, L. E. Marbella and C. P. Grey, Nature, 2018, 559, 556–563 CrossRef CAS
.
- B. Scrosati, J. Hassoun and Y. K. Sun, Energy Environ. Sci., 2011, 4, 3287–3295 RSC
.
- B. Scrosati and J. Garche, J. Power Sources, 2010, 195, 2419–2430 CrossRef CAS
.
- Y. Liang, Y. Jing, S. Gheytani, K.-Y. Lee, P. Liu, A. Facchetti and Y. Yao, Nat. Mater., 2017, 16, 841–848 CrossRef CAS
.
- J.-M. Tarascon and M. Armand, Nature, 2011, 414, 171–179 Search PubMed
.
- S. Zhang, S. Ren, D. Han, M. Xiao, S. Wang, L. Sun and Y. Meng, ACS Appl. Mater. Interfaces, 2020, 12, 36237–36246 CrossRef CAS PubMed
.
- A. Manthiram, J. Phys. Chem. Lett., 2011, 2, 176–184 CrossRef CAS
.
- X. Shen, H. Liu, X.-B. Cheng, C. Yan and J.-Q. Huang, Energy Storage Materials, 2018, 12, 161–175 CrossRef
.
- D. Larcher and J.-M. Tarascon, Nat. Chem., 2015, 7, 19–29 CrossRef CAS
.
- Y. Li, Y. Lu, C. Zhao, Y.-S. Hu, M.-M. Titirici, H. Li, X. Huang and L. Chen, Energy Storage Materials, 2017, 7, 130–151 CrossRef
.
- H. Chen, G. Cong and Y. C. Lu, J. Energy Chem., 2018, 27, 1304–1325 CrossRef
.
- X. Wu, S. Jin, Z. Zhang, L. Jiang, L. Mu, Y.-S. Hu, H. Li, X. Chen, M. Armand and L. Chen, Sci. Adv., 2015, 1, e1500330–e1500339 CrossRef
.
- Z. Song and H. Zhou, Energy Environ. Sci., 2013, 6, 2280–2301 RSC
.
- S. Lee, G. Kwon, K. Ku, K. Yoon, S. K. Jung, H. D. Lim and K. Kang, Adv. Mater., 2018, 30, 1704682–1704727 CrossRef
.
- T. B. Schon, B. T. McAllister, P.-F. Li and D. S. Seferos, Chem. Soc. Rev., 2016, 45, 6345–6404 RSC
.
- A. Shimizu, K. Takenaka, N. Handa, T. Nokami, T. Itoh and J.-I. Yoshida, Adv. Mater., 2017, 29, 1606592–1606597 CrossRef PubMed
.
- B. Huskinson, M. P. Marshak, C. Suh, S. Er, M. R. Gerhardt, C. J. Galvin, X. Chen, A. Aspuru-Guzik, R. G. Gordon and M. J. Aziz, Nature, 2014, 505, 195–198 CrossRef CAS PubMed
.
- E. J. Son, J. H. Kim, K. Kim and C. B. Park, J. Mater. Chem. A, 2016, 4, 11179–11202 RSC
.
- H. Chen, M. Armand, G. Demailly, F. Dolhem, P. Poizot and J. M. Tarascon, ChemSusChem, 2008, 1, 348–355 CrossRef CAS PubMed
.
- G. Milczarek and O. Inganäs, Science, 2012, 335, 1468–1471 CrossRef CAS PubMed
.
- J. Hong, M. Lee, B. Lee, D.-H. Seo, C. B. Park and K. Kang, Nat. Commun., 2014, 5, 1–9 Search PubMed
.
- X. Wei, W. Xu, M. Vijayakumar, L. Cosimbescu, T. Liu, V. Sprenkle and W. Wang, Adv. Mater., 2014, 26, 7649–7653 CrossRef CAS PubMed
.
- T. Liu, X. Wei, Z. Nie, V. Sprenkle and W. Wang, Adv. Energy Mater., 2016, 6, 1501449 CrossRef
.
- K. Takechi, Y. Kato and Y. Hase, Adv. Mater., 2015, 27, 2501–2506 CrossRef CAS PubMed
.
- C. DeBruler, B. Hu, J. Moss, X. Liu, J. Luo, Y. Sun and T. L. Liu, Chem, 2017, 3, 961–978 CAS
.
- B. Hu and T. L. Liu, J. Energy Chem., 2018, 27, 1326–1332 CrossRef
.
- L. Liu, Y. Yao, Z. Wang and Y.-C. Lu, Nano Energy, 2021, 84, 105897 CrossRef CAS
.
- K. Amin, Q. Meng, A. Ahmad, M. Cheng, M. Zhang, L. Mao, K. Lu and Z. Wei, Adv. Mater., 2018, 30, 1703868–1703876 CrossRef PubMed
.
- I. A. Rodríguez-Pérez and X. Ji, ACS Energy Lett., 2017, 2, 1762–1770 CrossRef
.
- G. Dai, Y. He, Z. Niu, P. He, C. Zhang, Y. Zhao, X. Zhang and H. Zhou, Angew. Chem., 2019, 131, 10007–10011 CrossRef
.
- M. Armand, S. Grugeon, H. Vezin, S. Laruelle, P. Ribière, P. Poizot and J.-M. Tarascon, Nat. Mater., 2009, 8, 120–125 CrossRef CAS
.
- É. Deunf, P. Moreau, É. Quarez, D. Guyomard, F. Dolhem and P. Poizot, J. Mater. Chem. A, 2016, 4, 6131–6139 RSC
.
- C. Luo, X. Ji, J. Chen, K. J. Gaskell, X. He, Y. Liang, J. Jiang and C. Wang, Angew. Chem., 2018, 130, 8703–8707 CrossRef
.
- T. Ma, L. Liu, J. Wang, Y. Lu and J. Chen, Angew. Chem., 2020, 132, 11630–11636 CrossRef
.
- F. Wu, M. Liu, Y. Li, X. Feng, K. Zhang, Y. Bai, X. Wang and C. Wu, Electrochem. Energy Rev., 2021, 1–65 Search PubMed
.
- Y. Lu, Q. Zhang, L. Li, Z. Niu and J. Chen, Chem, 2018, 4, 2786–2813 CAS
.
- S. H. Chung, P. Han, C. H. Chang and A. Manthiram, Adv. Energy Mater., 2017, 7, 1700537 CrossRef
.
- H. Yang, Z. Feng, X. Teng, L. Guan, H. Hu and M. Wu, InfoMat, 2021, 1–17 Search PubMed
.
- A. Molina, N. Patil, E. Ventosa, M. Liras, J. Palma and R. Marcilla, ACS Energy Lett., 2020, 5, 2945–2953 CrossRef
.
- Y. Hu, W. Chen, T. Lei, Y. Jiao, J. Huang, A. Hu, C. Gong, C. Yan, X. Wang and J. Xiong, Adv. Energy Mater., 2020, 10, 2000082 CrossRef CAS
.
- Y. Lu and J. Chen, Nat. Rev. Chem., 2020, 4, 127–142 CrossRef CAS
.
- J. Liu, J. G. Zhang, Z. G. Yang, J. P. Lemmon, C. Imhoff, G. L. Graff, L. Y. Li, J. Z. Hu, C. M. Wang, J. Xiao, G. Xia, V. V. Viswanathan, S. Baskaran, V. Sprenkle, X. L. Li, Y. Y. Shao and B. Schwenzer, Adv. Funct. Mater., 2013, 23, 929–946 CrossRef CAS
.
- Y. Yang, G. Y. Zheng and Y. Cui, Chem. Soc. Rev., 2013, 42, 3018–3032 RSC
.
- Y. Zhao, Y. Ding, Y. Li, L. Peng, H. R. Byon, J. B. Goodenough and G. Yu, Chem. Soc. Rev., 2015, 44, 7968–7996 RSC
.
- R. Marom, S. F. Amalraj, N. Leifer, D. Jacob and D. Aurbach, J. Mater. Chem., 2011, 21, 9938–9954 RSC
.
- G. Y. Zheng, Y. Yang, J. J. Cha, S. S. Hong and Y. Cui, Nano Lett., 2011, 11, 4462–4467 CrossRef CAS PubMed
.
- H. Chen, Y. Zhou and Y.-C. Lu, ACS Energy Lett., 2018, 3, 1991–1997 CrossRef CAS
.
- X. Zhao, C.-H. Yim, N. Du and Y. Abu-Lebdeh, Ind. Eng. Chem. Res., 2018, 57, 9062–9074 CrossRef CAS
.
- P. Parikh, M. Sina, A. Banerjee, X. Wang, M. S. D'Souza, J.-M. Doux, E. A. Wu, O. Y. Trieu, Y. Gong and Q. Zhou, Chem. Mater., 2019, 31, 2535–2544 CrossRef CAS
.
- L. Zhang, C. Zhang, Y. Ding, K. Ramirez-Meyers and G. Yu, Joule, 2017, 1, 623–633 CrossRef CAS
.
- M. Lee, J. Hong, J. Lopez, Y. Sun, D. Feng, K. Lim, W. C. Chueh, M. F. Toney, Y. Cui and Z. Bao, Nat. Energy, 2017, 2, 861–868 CrossRef CAS
.
- A. Iordache, D. Bresser, S. Solan, M. Retegan, M. Bardet, J. Skrzypski, L. Picard, L. Dubois and T. Gutel, Adv. Sustainable Syst., 2017, 1, 1600032–1600042 CrossRef
.
- M. Wu, Y. Cui, A. Bhargav, Y. Losovyj, A. Siegel, M. Agarwal, Y. Ma and Y. Fu, Angew. Chem., 2016, 128, 10181–10185 CrossRef
.
- Z. Song, T. Xu, M. L. Gordin, Y.-B. Jiang, I.-T. Bae, Q. Xiao, H. Zhan, J. Liu and D. Wang, Nano Lett., 2012, 12, 2205–2211 CrossRef CAS
.
- Z. Song, Y. Qian, X. Liu, T. Zhang, Y. Zhu, H. Yu, M. Otani and H. Zhou, Energy Environ. Sci., 2014, 7, 4077–4086 RSC
.
- Z. Song, Y. Qian, T. Zhang, M. Otani and H. Zhou, Adv. Sci., 2015, 2, 1500124–1500133 CrossRef
.
- J. Lee and M. J. Park, Adv. Energy Mater., 2017, 7, 1602279–1602287 CrossRef
.
- Y. Liang, P. Zhang, S. Yang, Z. Tao and J. Chen, Adv. Energy Mater., 2013, 3, 600–605 CrossRef CAS
.
- Z. Song, Y. Qian, M. L. Gordin, D. Tang, T. Xu, M. Otani, H. Zhan, H. Zhou and D. Wang, Angew. Chem., 2015, 127, 14153–14157 CrossRef
.
- A. Petronico, K. L. Bassett, B. G. Nicolau, A. A. Gewirth and R. G. Nuzzo, Adv. Energy Mater., 2018, 8, 1700960–1700967 CrossRef
.
- Y. Jing, Y. Liang, S. Gheytani and Y. Yao, Nano Energy, 2017, 37, 46–52 CrossRef CAS
.
- S. Wang, Q. Wang, P. Shao, Y. Han, X. Gao, L. Ma, S. Yuan, X. Ma, J. Zhou and X. Feng, J. Am. Chem. Soc., 2017, 139, 4258–4261 CrossRef CAS
.
- W. Walker, S. Grugeon, H. Vezin, S. Laruelle, M. Armand, F. Wudl and J.-M. Tarascon, J. Mater. Chem., 2011, 21, 1615–1620 RSC
.
- T. Yasuda and N. Ogihara, Chem. Commun., 2014, 50, 11565–11567 RSC
.
Footnotes |
| † Electronic supplementary information (ESI) available: 1H NMR of EV in D2O, TGA of EV-KB composite, XPS spectra of EV-KB composite, EV-KB composite electrode preparation comparison and cell configuration, EV-KB composite electrode coating comparison, cycling performance of EV-KB composite electrode for 1st electron, cycling performance of EV-KB composite electrode for 2nd electron, cycling performance of EV-KB composite electrode with mass loading of 9 mg cm−2, cycling performance of EV-KB composite electrode with mass loading of 1.5 mg cm−2. See DOI: 10.1039/d1ra03068j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |