Open Access Article
Open Access ArticleBrønsted acid-promoted hydroamination of unsaturated hydrazones: access to biologically important 5-arylpyrazolines†
Han He‡
,
Ning Xu‡,
Honglin Zhang‡ ,
Bin Chen,
Zhengnan Hu,
Kang Guo,
Jianlin Chun,
Shujun Cao and
Yingguang Zhu
,
Bin Chen,
Zhengnan Hu,
Kang Guo,
Jianlin Chun,
Shujun Cao and
Yingguang Zhu *
*
Jiangsu Key Laboratory of Pesticide Science, Department of Chemistry, College of Sciences, Nanjing Agricultural University, Nanjing 210095, China. E-mail: ygzhu@njau.edu.cn
First published on 11th May 2021
Abstract
A novel and efficient Brønsted acid-promoted hydroamination of hydrazone-tethered olefins has been developed. A variety of pyrazolines have been easily obtained in good to excellent yields with high chemo- and regioselectivity under simple and mild conditions. This method represents a straightforward, facile, and practical approach toward biologically important 5-arylpyrazolines, which are difficult to access by previously reported radical hydroamination of β,γ-unsaturated hydrazones.
Introduction
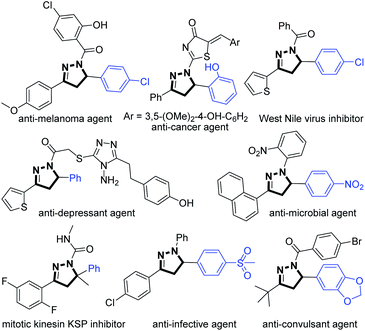
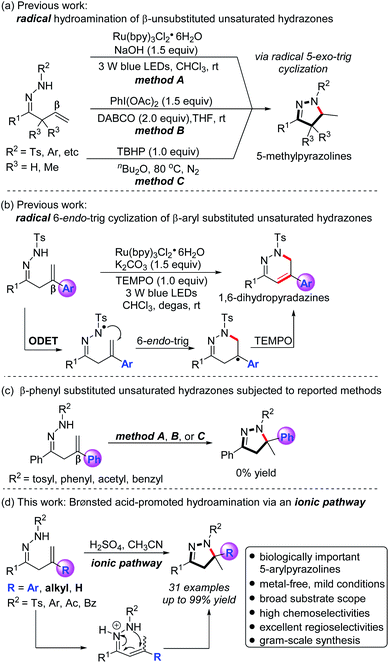
The functionalization of olefins is one of the most efficient and powerful strategies to rapidly construct structurally diverse and valuable molecules.1–3 In this context, the hydroamination of olefins has drawn considerable attention from chemists. Great progress has been made in the transition-metal-catalyzed,4,5 acid-promoted,6,7 and miscellaneous8 hydroaminations of olefins.Pyrazolines are an important class of five-membered nitrogen-containing heterocycles that present in many pharmaceuticals and bioactive molecules.9,10 In this context, 5-arylpyrazolines often exhibit diverse and significant biological properties such as anti-cancer, anti-depressant, anti-infective, and anti-convulsant activities, etc. (Fig. 1).11 Recently, the elegant synthesis of pyrazolines from β,γ-unsaturated hydrazones has been accomplished via a novel C–N bond-forming cyclization strategy by the groups of Loh, Xiao, Han, and others.12–16 Despite recent impressive advances, the examples of the hydroamination of β,γ-unsaturated hydrazones for the construction of pyrazolines are rare.16 In 2014, Xiao and Chen et al. disclosed a novel visible-light-driven photocatalytic hydroamination of β,γ-unsaturated hydrazones, in which the generation of N-centered hydrazonyl radicals has been achieved for the first time by a visible-light photocatalytic oxidation strategy (Scheme 1a, method A).16a Shortly afterwards, the same group developed a mild and efficient radical hydroamination of β,γ-unsaturated hydrazones for the synthesis of pyrazolines with stoichiometric amounts of PhI(OAc)2 as the oxidant and DABCO as the base (Scheme 1a, method B).16b In 2019, the group of Song reported the facile and metal-free access to pyrazolines from β,γ-unsaturated hydrazones via a radical pathway with the use of the oxidant TBHP in n-Bu2O at 80 °C under N2 atmosphere (Scheme 1a, method C).16c The previously reported hydroaminations14 of unsaturated hydrazones represent the rapid, facile, and straightforward approaches to pyrazolines. However, the substrates employed in such hydroamination reactions are only β-unsubstituted unsaturated hydrazones, thus providing the corresponding 5-methylpyrazolines through a radical 5-exo-trig cyclization (Scheme 1a). In 2016, Chen et al. reported an impressive and elegant protocol for the preparation of 1,6-dihydropyradazines from β-aryl substituted β,γ-unsaturated hydrazones, in which a novel visible-light photocatalytic oxidative deprotonation electron transfer (ODET)/6-endo-trig cyclization/TEMPO-mediation strategy was developed (Scheme 1b).17
Given the fact that 5-arylpyrazolines display a broad spectrum of important biological activities, it is highly demanded to develop mild, efficient, practical, and selective methods for the construction of such type of pyrazolines. To test whether 5-arylpyrazolines could be obtained from β-aryl substituted unsaturated hydrazones, we synthesized β-phenyl substituted unsaturated hydrazones (R2 = Ts, Ph, Ac, Bz) and subjected them to the previously reported radical hydroamination methods.16 Surprisingly, no desired 5-phenylpyrazoline products were observed (Scheme 1c). Based on these observations and considering the biological significance of 5-arylpyrazolines, we decided to explore new synthetic methods for the construction of the scaffolds. Inspired by the above breakthrough12–18 and following our continuous interest in the synthesis of N-heterocycles,15f,g we herein report a novel Brønsted acid-promoted hydroamination of β,γ-unsaturated hydrazones to afford a wide range of pyrazolines in generally excellent yields and with high chemo- and regioselectivities through an ionic pathway (Scheme 1d). It is noteworthy that the present method provides a facile, mild, efficient, and practical access to biologically significant 5-arylpyrazolines.
Results and discussion
Our studies commenced with β-phenyl substituted β,γ-unsaturated hydrazone 1a as model substrate to optimize the reaction conditions. To our delight, the desired product 5-arylpyrazoline 2a was obtained in 71% yield by the treatment of 1a with TsOH (1.0 equiv.) in CH3CN at 50 °C for 12 h (Table 1, entry 1). Other Brønsted acids, including TsOH, TfOH, CH3SO3H, CF3COOH, CH3COOH, H3PO4, HCl, HBr, HI, and H2SO4 were also tested (Table 1, entries 2–10). Among the above acids examined, H2SO4 was found to be the best for this transformation, giving product 2a in 94% yield (Table 1, entry 10). The screening of solvents showed that CH3CN was superior to other solvents, such as CH2Cl2, EtOH, DMF, and THF (Table 1, entries 10–14). Decreasing the amount of H2SO4 resulted in the lower yields of 2a (Table 1, entries 15 and 16). Additionally, the significantly decreased yield of 2a was obtained when the reaction was conducted at a lower temperature (Table 1, entry 17). No reaction occurred in the absence of a Brønsted acid, suggesting that the acid plays a crucial role in the transformation (Table 1, entry 18).| Entry | Acid (equiv.) | Solvent | Yieldb (%) |
|---|---|---|---|
| a All reactions were performed with 1a (0.20 mmol) and acid (0.20 mmol) in solvent (2 mL) at 50 °C for 12 h unless otherwise noted.b Isolated yields.c H3PO4 (85 wt% in water).d HCl (36 wt% in water).e HBr (40 wt% in water).f HI (55 wt% in water).g H2SO4 (98 wt% in water).h At 25 °C. Ts = p-toluenesulfonyl, Tf = trifluoromethanesulfonyl, DMF = N,N-dimethyl formamide, THF = tetrahydrofuran. | |||
| 1 | TsOH (1.0) | CH3CN | 71 |
| 2 | TfOH (1.0) | CH3CN | 82 |
| 3 | CH3SO3H (1.0) | CH3CN | 62 |
| 4 | CF3COOH (1.0) | CH3CN | Trace |
| 5 | CH3COOH (1.0) | CH3CN | 0 |
| 6c | H3PO4 (1.0) | CH3CN | 0 |
| 7d | HCl (1.0) | CH3CN | 0 |
| 8e | HBr (1.0) | CH3CN | 62 |
| 9f | HI (1.0) | CH3CN | 81 |
| 10g | H2SO4 (1.0) | CH3CN | 94 |
| 11 | H2SO4 (1.0) | CH2Cl2 | 58 |
| 12 | H2SO4 (1.0) | C2H5OH | 0 |
| 13 | H2SO4 (1.0) | DMF | 0 |
| 14 | H2SO4 (1.0) | THF | 18 |
| 15 | H2SO4 (0.2) | CH3CN | 29 |
| 16 | H2SO4 (0.5) | CH3CN | 65 |
| 17h | H2SO4 (1.0) | CH3CN | 14 |
| 18 | — | CH3CN | 0 |
With the optimized reaction conditions in hand, we subsequently investigated the generality of the Brønsted acid-promoted hydroamination reaction. As exemplified in Scheme 2, the present reaction can be extended to various β,γ-unsaturated hydrazones to give the 5-aryl or 5-alkylpyrazoline products in 60–99% yield. Substrates with either electron-donating or electron-withdrawing groups on the phenyl ring (R1) that is attached to the C–N double bond were smoothly converted into products 2a–2j in 73–99% yield. 2-Thienyl group was also well tolerated to provide the product 2k in 81% yield. Notably, substrates bearing alkyl groups (R1) at the C–N double bond moiety also participated in the reaction to give the corresponding products 2l–2q in 65–98% yield. The effect of the substituents (R) at the alkene moiety on the reaction was next investigated. It was found that electron-rich or electron-poor aryl groups were well tolerated under the reaction conditions, producing the 5-arylpyrazoline products 2r–2w in 85–98% yield. Furthermore, substrates bearing alkyl groups at the alkene moiety could also undergo the reaction to give the 5-alkylpyrazolines 2x and 2y in good yields. Nevertheless, no desired product 2z was obtained when R group in the substrate was changed to H atom. Additionally, the substrate bearing a gem-dimethyl moiety adjacent to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond could not produce the corresponding pyrazoline product 2aa either. It is noteworthy that N-aryl, N-acetyl, and N-benzoyl substituted unsaturated hydrazones also smoothly participated in the transformation, providing the corresponding 5-arylpyrazolines 2ab–2ae in 61–92% yield.
N bond could not produce the corresponding pyrazoline product 2aa either. It is noteworthy that N-aryl, N-acetyl, and N-benzoyl substituted unsaturated hydrazones also smoothly participated in the transformation, providing the corresponding 5-arylpyrazolines 2ab–2ae in 61–92% yield.
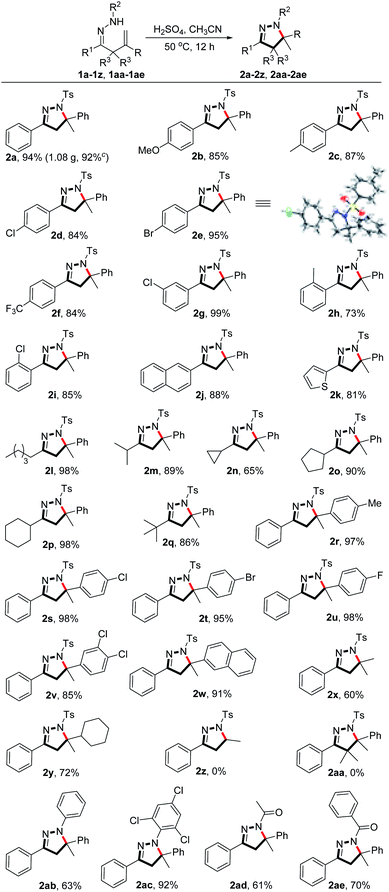 | ||
| Scheme 2 Substrate scope. aAll reactions were performed with 1 (0.20 mmol) and conc. H2SO4 (0.20 mmol) in CH3CN (2 mL) at 50 °C for 12 h unless otherwise noted. b Isolated yields. c 3.0 mmol scale. | ||
The structure of product 2e (CCDC 2018227) was confirmed by single-crystal X-ray diffraction analysis (Scheme 2). To demonstrate the practical application of the method, a gram-scale reaction was carried out under standard conditions and afforded product 2a in 92% yield (1.08 g) (Scheme 2).
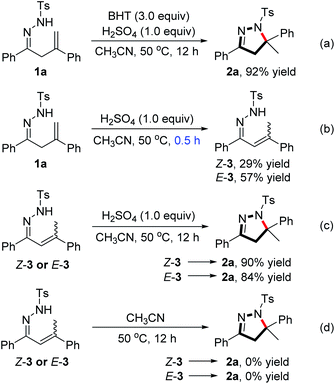
To gain some insight into the reaction pathway, several control experiments were conducted (Scheme 3). Upon the addition of the radical scavenger butylated hydroxytoluene (BHT, 3.0 equiv.) under standard reaction conditions, the reaction still proceeded very well to give the desired product 2a in 92% yield (Scheme 3a), suggesting that a radical pathway could not be involved in this transformation. By the treatment of 1a with conc. H2SO4 (1.0 equiv.) in CH3CN at 50 °C for a much shorter reaction time (0.5 h), two separable isomers Z-3 and E-3, resulting from the isomerization of the terminal olefin moiety of 1a, were obtained in 29% and 57% yields, respectively (Scheme 3b). Subsequent subjection of the isolated Z-3 or E-3 to the standard reaction conditions afforded the product 2a in 90% and 84% yields (Scheme 3c). The results indicate that the isomeric 3 could be the reaction intermediate, and an olefin isomerization process is very likely to be involved in the reaction. No product was observed when isolated Z-3 or E-3 was subjected to the standard reaction conditions in the absence of H2SO4 (Scheme 3d), suggesting that the Brønsted acid plays an important role in the formation of the pyrazoline product from the intermediate.
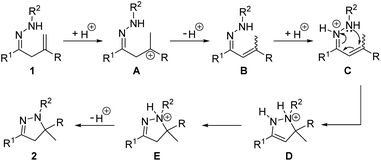
Based on the above experimental results and previous reports,12–19 a plausible reaction mechanism is proposed (Scheme 4). Initial electrophilic attack on the C–C double bond of hydrazone 1 by the proton generates carbocation A, which is deprotonated to afford olefin-isomerized intermediate B.19a–c Subsequent protonation of the hydrazone moiety of B results in cationic species C,18a,b which undergoes a 6π-azaelectrocyclization to provide intermediate D.19d–g Isomerization of D to E,19a,h followed by the final deprotonation to deliver pyrazoline product 2.
Conclusions
In summary, we have developed a novel, facile, efficient, practical, and Brønsted acid-promoted hydroamination protocol that enables the synthesis of various pyrazolines from β,γ-unsaturated hydrazones. Notably, the present method can be applied to construct the biologically significant 5-arylpyrazolines that are difficult to access by the reported radical hydroaminations of β,γ-unsaturated hydrazones. Preliminary mechanistic investigations indicated that an ionic pathway could be involved in this transformation. This reaction is characterized by simple and mild conditions, broad substrate scope, high yields, excellent chemo- and regioselectivities, and amenability to gram-scale synthesis, which makes it particularly attractive and is expected to find potential applications in organic synthesis and drug discovery.Experimental
General information
All commercially available reagents were used without further purification. Column chromatography was performed on silica gel (200–300 mesh). 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a 400 MHz spectrometer. Chemical shifts (δ) were reported in ppm, and coupling constants (J) were given in Hertz (Hz). Data were reported as s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublets, m = multiplet. High-resolution mass spectra (HRMS) were recorded on an AB SCIEX Triple TOF 5600+ mass spectrometer. Melting points were uncorrected. Alkenyl hydrazone substrates 1a–1ac were prepared according to the reported methods.17,20General procedure for the hydroamination reaction
To a reaction tube equipped with a magnetic stir bar were added alkenyl hydrazone 1 (0.20 mmol), conc. H2SO4 (11 μL, 0.20 mmol), and CH3CN (2.0 mL). The reaction mixture was stirred at 50 °C under nitrogen atmosphere for 12 h, cooled to room temperature, and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (eluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate = 10
petroleum ether/ethyl acetate = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give product 2.
1) to give product 2.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (21502096), the Natural Science Foundation of Jiangsu Province (BK20150652), the Student Research Training (SRT) Project of Nanjing Agricultural University (S20190033), the Fundamental Research Funds for the Central Universities (KJQN201629), and the “333 High Level Talent Project” of Jiangsu Province.Notes and references
- For selected reviews, see: (a) A. Minatti and K. Muñiz, Chem. Soc. Rev., 2007, 36, 1142 RSC; (b) S. R. Chemler and M. T. Bovino, ACS Catal., 2013, 3, 1076 CrossRef CAS PubMed; (c) E. Merino and C. Nevado, Chem. Soc. Rev., 2014, 43, 6598 RSC; (d) Y. Zhu, Q. Wang, R. G. Cornwall and Y. Shi, Chem. Rev., 2014, 114, 8199 CrossRef CAS; (e) E. M. Beccalli, G. Broggini, S. Gazzola and A. Mazza, Org. Biomol. Chem., 2014, 12, 6767 RSC; (f) Y. Zhu, R. G. Cornwall, H. Du, B. Zhao and Y. Shi, Acc. Chem. Res., 2014, 47, 3665 CrossRef CAS PubMed; (g) T. Koike and M. Akita, Acc. Chem. Res., 2016, 49, 1937 CrossRef CAS PubMed; (h) G. Yin, X. Mu and G. Liu, Acc. Chem. Res., 2016, 49, 2413 CrossRef CAS PubMed.
- For selected reviews, see: (a) T. Pintauer and K. Matyjaszewski, Chem. Soc. Rev., 2008, 37, 1087 RSC; (b) H. Egami and M. Sodeoka, Angew. Chem., Int. Ed., 2014, 53, 8294 CrossRef CAS PubMed; (c) Z.-M. Chen, X.-M. Zhang and Y.-Q. Tu, Chem. Soc. Rev., 2015, 44, 5220 RSC; (d) A. Studer and D. P. Curran, Angew. Chem., Int. Ed., 2016, 55, 58 CrossRef CAS; (e) X.-W. Lan, N.-X. Wang and Y. Xing, Eur. J. Org. Chem., 2017, 2017, 5821 CrossRef CAS; (f) T. Koike and M. Akita, Chem, 2018, 4, 409 CrossRef CAS.
- For selected reviews, see: (a) J.-R. Chen, X.-Y. Yu and W.-J. Xiao, Synthesis, 2015, 47, 604 CrossRef CAS; (b) R.-J. Song, Y. Liu, Y.-X. Xie and J.-H. Li, Synthesis, 2015, 47, 1195 CrossRef CAS; (c) C.-C. Li and S.-D. Yang, Org. Biomol. Chem., 2016, 14, 4365 RSC; (d) J. Lin, R.-J. Song, M. Hu and J.-H. Li, Chem. Rec., 2019, 19, 440 CrossRef CAS PubMed.
- For selected reviews on intramolecular hydroaminations of olefins, see: (a) K. C. Hultzsch, Adv. Synth. Catal., 2005, 347, 367 CrossRef CAS; (b) T. E. Muller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795 CrossRef PubMed; (c) J. Hannedouche and E. Schulz, Chem.–Eur. J., 2013, 19, 4972 CrossRef CAS PubMed; (d) A. L. Reznichenko, A. J. Nawara-Hultzsch and K. C. Hultzsch, Top. Curr. Chem., 2014, 343, 191 CrossRef CAS PubMed; (e) E. Bernoud, C. Lepori, M. Mellah, E. Schulz and J. Hannedouche, Catal. Sci. Technol., 2015, 5, 2017 RSC; (f) L. Huang, M. Arndt, K. Goossen, H. Heydt and L. J. Goossen, Chem. Rev., 2015, 115, 2596 CrossRef CAS PubMed; (g) J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Acc. Chem. Res., 2016, 49, 1911 CrossRef CAS PubMed; (h) C. Michon, M.-A. Abadie, F. Medina and F. Agbossou-Niedercorn, J. Organomet. Chem., 2017, 847, 13 CrossRef CAS; (i) J. Hannedouche and E. Schulz, Organometallics, 2018, 37, 4313 CrossRef CAS; (j) N. Kaur, P. Grewal, P. Bhardwaj, M. Devi, N. Ahlawat and Y. Verma, Synth. Commun., 2019, 49, 3058 CrossRef CAS; (k) A. Trowbridge, S. M. Walton and M. J. Gaunt, Chem. Rev., 2020, 120, 2613 CrossRef CAS PubMed.
- For selected examples on transition-metal-catalyzed intramolecular hydroaminations, see: (a) S. Hong, S. Tian, M. V. Metz and T. J. Marks, J. Am. Chem. Soc., 2003, 125, 14768 CrossRef CAS PubMed; (b) C. F. Bender and R. A. Widenhoefer, J. Am. Chem. Soc., 2005, 127, 1070 CrossRef CAS PubMed; (c) X. Han and R. A. Widenhoefer, Angew. Chem., Int. Ed., 2006, 45, 1747 CrossRef CAS PubMed; (d) K. Komeyama, T. Morimoto and K. Takaki, Angew. Chem., Int. Ed., 2006, 45, 2938 CrossRef CAS; (e) F. E. Michael and B. M. Cochran, J. Am. Chem. Soc., 2006, 128, 4246 CrossRef CAS; (f) A. Takemiya and J. F. Hartwig, J. Am. Chem. Soc., 2006, 128, 6042 CrossRef CAS PubMed; (g) D. C. Leitch, R. P. Payne, C. R. Dunbar and L. L. Schafer, J. Am. Chem. Soc., 2009, 131, 18246 CrossRef CAS; (h) J. M. Hoover, A. Di Pasquale, J. M. Mayer and F. E. Michael, J. Am. Chem. Soc., 2010, 132, 5043 CrossRef CAS; (i) L. D. Julian and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 13813 CrossRef CAS PubMed; (j) Z. Liu, H. Yamamichi, S. T. Madrahimov and J. F. Hartwig, J. Am. Chem. Soc., 2011, 133, 2772 CrossRef CAS PubMed; (k) K. Manna, S. Xu and A. D. Sadow, Angew. Chem., Int. Ed., 2011, 50, 1865 CrossRef CAS PubMed; (l) E. Bernoud, P. Oulie, R. Guillot, M. Mellah and J. Hannedouche, Angew. Chem., Int. Ed., 2014, 53, 4930 CrossRef CAS PubMed; (m) J. Davies, S. G. Booth, S. Essafi, R. A. W. Dryfe and D. Leonori, Angew. Chem., Int. Ed., 2015, 54, 14017 CrossRef CAS PubMed; (n) K. Manna, N. Eedugurala and A. D. Sadow, J. Am. Chem. Soc., 2015, 137, 425 CrossRef CAS PubMed; (o) S. T. Nguyen, Q. Zhu and R. R. Knowles, ACS Catal., 2019, 9, 4502 CrossRef CAS PubMed; (p) C. B. Roos, J. Demaerel, D. E. Graff and R. R. Knowles, J. Am. Chem. Soc., 2020, 142, 5974 CrossRef CAS PubMed.
- For comprehensive reviews on Brønsted acid catalysis, see: (a) T. Akiyama, Chem. Rev., 2007, 107, 5744–5758 CrossRef CAS PubMed; (b) S.-L. You, Q. Cai and M. Zeng, Chem. Soc. Rev., 2009, 38, 2190 RSC; (c) D. Kampen, C. M. Reisinger and B. List, Top. Curr. Chem., 2010, 291, 395 CrossRef CAS PubMed; (d) M. Terada, Synthesis, 2010, 1929 CrossRef CAS; (e) J. Yu, F. Shi and L.-Z. Gong, Acc. Chem. Res., 2011, 44, 1156 CrossRef CAS PubMed; (f) M. Rueping, A. Kuenkel and I. Atodiresei, Chem. Soc. Rev., 2011, 40, 4539 RSC; (g) M. Mahlau and B. List, Angew. Chem., Int. Ed., 2013, 52, 518 CrossRef CAS PubMed; (h) R. J. Phipps, G. L. Hamilton and F. D. Toste, Nat. Chem., 2012, 4, 603 CrossRef CAS PubMed; (i) D. Parmar, E. Sugiono, S. Raja and M. Rueping, Chem. Rev., 2014, 114, 9047 CrossRef CAS.
- For selected examples of the construction of nitrogen-containing heterocycles through acid-promoted alkene hydroamination, see: (a) C. D. Cox, M. J. Breslin and B. J. Mariano, Tetrahedron Lett., 2004, 45, 1489 CrossRef CAS; (b) D. Enders, A. A. Narine, F. Toulgoat and T. Bisschops, Angew. Chem., Int. Ed., 2008, 47, 5661 CrossRef CAS PubMed; (c) S. Muller and B. List, Angew. Chem., Int. Ed., 2009, 48, 9975 CrossRef PubMed; (d) S. Müller and B. List, Synthesis, 2010, 2010, 2171 CrossRef; (e) I. Dion and A. M. Beauchemin, Angew. Chem., Int. Ed., 2011, 50, 8233 CrossRef CAS PubMed; (f) J.-D. Liu, Y.-C. Chen, G.-B. Zhang, Z.-Q. Li, P. Chen, J.-Y. Du, Y.-Q. Tu and C.-A. Fan, Adv. Synth. Catal., 2011, 353, 2721 CrossRef CAS; (g) N. D. Shapiro, V. Rauniyar, G. L. Hamilton, J. Wu and F. D. Toste, Nature, 2011, 470, 245 CrossRef CAS PubMed; (h) M. Rueping, M. S. Maji, H. B. Küçük and I. Atodiresei, Angew. Chem., Int. Ed., 2012, 51, 12864 CrossRef CAS PubMed; (i) A. Das, C. M. Volla, I. Atodiresei, W. Bettray and M. Rueping, Angew. Chem., Int. Ed., 2013, 52, 8008 CrossRef CAS PubMed; (j) H. Liu, C. Zeng, J. Guo, M. Zhang and S. Yu, RSC Adv., 2013, 3, 1666 RSC; (k) X. Hong, H. B. Kucuk, M. S. Maji, Y. F. Yang, M. Rueping and K. N. Houk, J. Am. Chem. Soc., 2014, 136, 13769 CrossRef CAS PubMed; (l) J.-S. Lin, P. Yu, L. Huang, P. Zhang, B. Tan and X.-Y. Liu, Angew. Chem., Int. Ed., 2015, 54, 7847 CrossRef CAS PubMed; (m) B. Heggen, M. Patil and W. Thiel, J. Comput. Chem., 2016, 37, 280 CrossRef CAS PubMed; (n) J.-S. Lin, T.-T. Li, G.-Y. Jiao, Q.-S. Gu, J.-T. Cheng, L. Lv and X.-Y. Liu, Angew. Chem., Int. Ed., 2019, 58, 7092 CrossRef CAS PubMed.
- For selected examples on miscellaneous hydroaminations, see: (a) J.-G. Roveda, C. Clavette, A. D. Hunt, S. I. Gorelsky, C. J. Whipp and A. M. Beauchemin, J. Am. Chem. Soc., 2009, 131, 8740 CrossRef CAS PubMed; (b) A. R. Brown, C. Uyeda, C. A. Brotherton and E. N. Jacobsen, J. Am. Chem. Soc., 2013, 135, 6747 CrossRef CAS PubMed; (c) T. M. Nguyen and D. A. Nicewicz, J. Am. Chem. Soc., 2013, 135, 9588 CrossRef CAS PubMed; (d) J. Davies, T. D. Svejstrup, D. Fernandez Reina, N. S. Sheikh and D. Leonori, J. Am. Chem. Soc., 2016, 138, 8092 CrossRef CAS PubMed; (e) Z.-Y. Chen, L.-Y. Wu, H.-S. Fang, T. Zhang, Z.-F. Mao, Y. Zou, X.-J. Zhang and M. Yan, Adv. Synth. Catal., 2017, 359, 3894 CrossRef CAS; (f) X. Ma, J. J. Farndon, T. A. Young, N. Fey and J. F. Bower, Angew. Chem., Int. Ed., 2017, 56, 14531 CrossRef CAS PubMed; (g) S. Zou, S. Geng, L. Chen, H. Wang and F. Huang, Org. Biomol. Chem., 2019, 17, 380 RSC.
- For selected reviews on the preparation of nitrogen-containing heterocycles, see: (a) A. Deiters and S. F. Martin, Chem. Rev., 2004, 104, 2199 CrossRef CAS PubMed; (b) B. Han, X.-L. Yang, R. Fang, W. Yu, C. Wang, X.-Y. Duan and S. Liu, Angew. Chem., Int. Ed., 2012, 51, 8816 CrossRef CAS PubMed; (c) R.-H. Liu, D. Wei, B. Han and W. Yu, ACS Catal., 2016, 6, 6525 CrossRef CAS; (d) M. Giustiniano, A. Basso, V. Mercalli, A. Massarotti, E. Novellino, G. C. Tron and J. Zhu, Chem. Soc. Rev., 2017, 46, 1295 RSC; (e) X.-X. Peng, D. Wei, W.-J. Han, F. Chen, W. Yu and B. Han, ACS Catal., 2017, 7, 7830 CrossRef CAS; (f) Y. Xia and J. Wang, Chem. Soc. Rev., 2017, 46, 2306 RSC; (g) J. Fu, G. Zanoni, E. Anderson and X. Bi, Chem. Soc. Rev., 2017, 46, 7208 RSC.
- (a) X.-H. Liu, B.-F. Ruan, J. Li, F.-H. Chen, B.-A. Song, H.-L. Zhu, P. S. Bhadury and J. Zhao, Mini-Rev. Med. Chem., 2011, 11, 771 CrossRef CAS PubMed; (b) A. Marella, R. Ali, T. Alam, R. Saha, O. Tanwar, M. Akhter, M. Shaquiquzzaman and M. Mumtaz Alam, Mini-Rev. Med. Chem., 2013, 13, 921 CrossRef CAS PubMed.
- (a) Q.-S. Li, X.-H. Lv, Y.-B. Zhang, J.-J. Dong, W.-P. Zhou, Y. Yang and H.-L. Zhu, Bioorg. Med. Chem. Lett., 2012, 22, 6596 CrossRef CAS PubMed; (b) D. Havrylyuk, B. Zimenkovsky, O. Vasylenko, L. Zaprutko, A. Gzella and R. Lesyk, Eur. J. Med. Chem., 2009, 44, 1396 CrossRef CAS; (c) J. R. Goodell, F. Puig-Basagoiti, B. M. Forshey, P.-Y. Shi and D. M. Ferguson, J. Med. Chem., 2006, 49, 2127 CrossRef CAS PubMed; (d) Y. R. Prasad, A. L. Rao, L. Prasoona, K. Murali and P. R. Kumar, Bioorg. Med. Chem. Lett., 2005, 15, 5030 CrossRef CAS PubMed; (e) M. Agrawal, P. K. Sonar and S. K. Saraf, Med. Chem. Res., 2011, 21, 3376 CrossRef; (f) C. D. Cox, M. Torrent, M. J. Breslin, B. J. Mariano, D. B. Whitman, P. J. Coleman, C. A. Buser, E. S. Walsh, K. Hamilton, M. D. Schaber, R. B. Lobell, W. Tao, V. J. South, N. E. Kohl, Y. Yan, L. C. Kuo, T. Prueksaritanont, D. E. Slaughter, C. Li, E. Mahan, B. Lu and G. D. Hartman, Bioorg. Med. Chem. Lett., 2006, 16, 3175 CrossRef CAS PubMed; (g) P. M. Sivakumar, S. Prabhu Seenivasan, V. Kumar and M. Doble, Chem. Lett., 2010, 20, 3169 CrossRef CAS PubMed; (h) M. N. Aboul-Enein, A. A. El-Azzouny, M. I. Attia, Y. A. Maklad, K. M. Amin, M. Abdel-Rehim and M. F. El-Behairy, J. Med. Chem., 2012, 47, 360 CrossRef CAS PubMed.
- (a) M.-K. Zhu, Y.-C. Chen and T.-P. Loh, Chem.–Eur. J., 2013, 19, 5250 CrossRef CAS PubMed; (b) X.-Y. Duan, X.-L. Yang, R. Fang, X.-X. Peng, W. Yu and B. Han, J. Org. Chem., 2013, 78, 10692 CrossRef CAS; (c) X.-Y. Duan, N.-N. Zhou, R. Fang, X.-L. Yang, W. Yu and B. Han, Angew. Chem., Int. Ed., 2014, 53, 3158 CrossRef CAS PubMed; (d) X.-Y. Duan, X.-L. Yang, P.-P. Jia, M. Zhang and B. Han, Org. Lett., 2015, 17, 6022 CrossRef CAS; (e) F. Pünner, Y. Sohtome and M. Sodeoka, Chem. Commun., 2016, 52, 14093 RSC; (f) R.-H. Liu, Z.-Q. Wang, B.-Y. Wei, J.-W. Zhang, B. Zhou and B. Han, Org. Lett., 2018, 20, 4183 CrossRef CAS; (g) P. Li, D. Huang, T. Yang, Z. Deng, K. Wang, J. Wang, Y. Su and Y. Hu, Chin. J. Org. Chem., 2019, 39, 2920 CrossRef CAS.
- (a) Q. Wei, J.-R. Chen, X.-Q. Hu, X.-C. Yang, B. Lu and W.-J. Xiao, Org. Lett., 2015, 17, 4464 CrossRef CAS PubMed; (b) X.-Q. Hu, J. Chen, J.-R. Chen, D.-M. Yan and W.-J. Xiao, Chem.–Eur. J., 2016, 22, 14141 CrossRef CAS PubMed; (c) Q.-Q. Zhao, X.-Q. Hu, M.-N. Yang, J.-R. Chen and W.-J. Xiao, Chem. Commun., 2016, 52, 12749 RSC; (d) Q.-Q. Zhao, J. Chen, D.-M. Yan, J.-R. Chen and W.-J. Xiao, Org. Lett., 2017, 19, 3620 CrossRef CAS PubMed.
- (a) M.-N. Yang, D.-M. Yan, Q.-Q. Zhao, J.-R. Chen and W.-J. Xiao, Org. Lett., 2017, 19, 5208 CrossRef CAS PubMed; (b) J. Chen, M.-N. Yang, J.-R. Chen and W.-J. Xiao, Org. Lett., 2018, 20, 3314 CrossRef CAS; (c) X. Kou, Q. Shao, C. Ye, G. Yang and W. Zhang, J. Am. Chem. Soc., 2018, 140, 7587 CrossRef CAS.
- (a) M. Chen, L. Qi, J.-M. Chen, P.-X. Ren, J. Du, L.-J. Wang and W. Li, Org. Biomol. Chem., 2018, 16, 5136 RSC; (b) M. Chen, L.-J. Wang, P.-X. Ren, X.-Y. Hou, Z. Fang, M.-N. Han and W. Li, Org. Lett., 2018, 20, 510 CrossRef CAS PubMed; (c) L.-J. Wang, P.-X. Ren, L. Qi, M. Chen, Y.-L. Lu, J.-Y. Zhao, R. Liu, J.-M. Chen and W. Li, Org. Lett., 2018, 20, 4411 CrossRef CAS PubMed; (d) S. Chen, W. Chen, X. Chen, G. Chen, L. Ackermann and X. Tian, Org. Lett., 2019, 21, 7787 CrossRef CAS PubMed; (e) P. Ren, L. Qi, Z. Fang, T. Wu, Y. Gao, S. Shen, J. Song, L. Wang and W. Li, Chin. J. Org. Chem., 2019, 39, 1776 CrossRef CAS; (f) F. Meng, H. Zhang, H. He, N. Xu, Q. Fang, K. Guo, S. Cao, Y. Shi and Y. Zhu, Adv. Synth. Catal., 2020, 362, 248 CrossRef CAS; (g) F. Meng, Q. Fang, W. Yuan, N. Xu, S. Cao, J. Chun, J. Li, H. Zhang and Y. Zhu, Org. Chem. Front., 2020, 7, 1358 RSC.
- For the synthesis of 5-methylpyrazolines via radical hydroamination of β-unsubstituted β,γ-unsaturated hydrazones, see: (a) X.-Q. Hu, J.-R. Chen, Q. Wei, F.-L. Liu, Q.-H. Deng, A. M. Beauchemin and W.-J. Xiao, Angew. Chem., Int. Ed., 2014, 53, 12163 CrossRef CAS; (b) X.-Q. Hu, G.-Q. Feng, J.-R. Chen, D.-M. Yan, Q.-Q. Zhao, Q. Wei and W.-J. Xiao, Org. Biomol. Chem., 2015, 13, 3457 RSC; (c) X. Liu, Y. Zhou and Q. Song, Chem. Commun., 2019, 55, 8943 RSC.
- X.-Q. Hu, X. Qi, J.-R. Chen, Q.-Q. Zhao, Q. Wei, Y. Lan and W.-J. Xiao, Nat. Commun., 2016, 7, 11188 CrossRef PubMed.
- (a) H. Xie, J. Zhu, Z. Chen, S. Li and Y. Wu, Synthesis, 2011, 2011, 2767 CrossRef; (b) P. Liu, Q.-Q. Xu, C. Dong, X. Lei and G.-q. Lin, Synlett, 2012, 23, 2087 CrossRef CAS.
- (a) Q. Cai, X.-W. Liang, S.-G. Wang, J.-W. Zhang, X. Zhang and S.-L. You, Org. Lett., 2012, 14, 5022 CrossRef CAS PubMed; (b) S. Prevost, N. Dupr, M. Leutzsch, Q. Wang, V. Wakchaure and B. List, Angew. Chem., Int. Ed., 2014, 53, 8770 CrossRef CAS PubMed; (c) Y. Suzuki, Y. Tanaka, S. Nakano, K. Dodo, N. Yoda, K. Shinohara, K. Kita, A. Kaneda, M. Sodeoka, Y. Hamada and T. Nemoto, Chem.–Eur. J., 2016, 22, 4418 CrossRef CAS PubMed; (d) L. M. Bishop, J. E. Barbarow, R. G. Bergman and D. Trauner, Angew. Chem., Int. Ed., 2008, 47, 8100 CrossRef CAS PubMed; (e) D. J. Tantillo, Angew. Chem., Int. Ed., 2009, 48, 31 CrossRef CAS PubMed; (f) X. Liu, N. Zhang, J. Yang, Y. Liang, R. Zhang and D. Dong, J. Org. Chem., 2013, 78, 3323 CrossRef CAS PubMed; (g) Y.-X. Sun, X.-G. Wang, G.-D. Shen, T. Yang, Y.-H. Yang, J. Li, M.-Y. Yang, H.-M. Sun and J.-F. Wei, Adv. Synth. Catal., 2020, 362, 1651 CrossRef CAS; (h) K. Sorimachi and M. Terada, J. Am. Chem. Soc., 2008, 130, 14452 CrossRef CAS PubMed.
- (a) C. B. Tripathi and S. Mukherjee, Org. Lett., 2014, 16, 3368 CrossRef CAS PubMed; (b) T. Imai and S. Nishida, Synthesis, 1993, 1993, 395 CrossRef; (c) S. Rajam, A. V. Jadhav, Q. Li, S. K. Sarkar, P. N. D. Singh, A. Rohr, T. C. S. Pace, R. Li, J. A. Krause, C. Bohne, B. S. Ault and A. D. Gudmundsdottir, J. Org. Chem., 2014, 79, 9325 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2018227 (2e) contains the supplementary crystallographic data for this paper. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra03043d |
| ‡ H. H., N. X., and H. Z. contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |