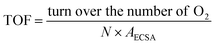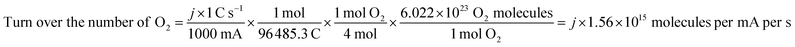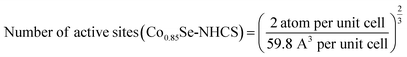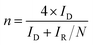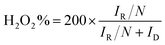Open Access Article
Open Access ArticleA simple method for the preparation of a nickel selenide and cobalt selenide mixed catalyst to enhance bifunctional oxygen activity for Zn–air batteries†
Li-Juan Pengab,
Jie-Ping Huanga,
Qiu-Ren Pana,
Ying Lianga,
Na Yina,
Hang-Chang Xua and
Nan Li *a
*a
aSchool of Chemistry and Chemical Engineering, Guangzhou Key Laboratory for Clean Energy and Materials, Guangzhou University, Guangzhou 510006, China. E-mail: nanli@gzhu.edu.cn
bCollege of Chemistry and Materials Science, Jinan University, Guangzhou 510632, China
First published on 28th May 2021
Abstract
Developing a low-cost, simple, and efficient method to prepare excellent bifunctional electrocatalysts toward the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) is critical in rechargeable zinc–air batteries. Non-stoichiometric M0.85Se (M = Ni or Co) nanoparticles are synthesized and modified on nitrogen-doped hollow carbon sphere (NHCS). The NHCS loaded Ni0.85Se (Ni0.85Se-NHCS) with rich Ni3+ presents higher OER activity, whereas the NHCS-loaded Co0.85Se (Co0.85Se-NHCS) with abundant Co2+ displays better ORR activity, respectively. When Co0.85Se-NHCS is mixed with Ni0.85Se-NHCS in a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the resulting mixture (Ni0.85Se/Co0.85Se-NHCS-2) shows better ORR and OER dual catalytic functions than a single selenide. Moreover, zinc–air batteries equipped with Ni0.85Se/Co0.85Se-NHCS-2 as the oxygen electrode catalyst exhibit excellent charge and discharge performance as well as improved stability over precious metals. This work has developed a simple and effective method to prepare excellent bifunctional electrocatalysts for ORR and OER, which is beneficial for the practical large-scale application of zinc–air batteries.
1, the resulting mixture (Ni0.85Se/Co0.85Se-NHCS-2) shows better ORR and OER dual catalytic functions than a single selenide. Moreover, zinc–air batteries equipped with Ni0.85Se/Co0.85Se-NHCS-2 as the oxygen electrode catalyst exhibit excellent charge and discharge performance as well as improved stability over precious metals. This work has developed a simple and effective method to prepare excellent bifunctional electrocatalysts for ORR and OER, which is beneficial for the practical large-scale application of zinc–air batteries.
Introduction
Due to the exhaustion of fossil fuel energy and environmental deterioration, the development of efficient, clean, and sustainable energy storage technology has attracted worldwide attention.1–3 Zinc–air batteries are considered an ideal candidate for renewable energy technologies due to their high power density, high output capacity, long-term stability, and environmental friendliness.4–6 The performance of rechargeable zinc–air batteries depends mainly on the properties of the oxygen reduction reactions (ORR) and oxygen evolution reactions (OER) that occur during discharging or charging on the cathode, respectively.7–9 Usually, noble metal Pt-based and RuO2 materials are considered as superior catalysts for ORR and OER, respectively, to accelerate the sluggish kinetics of the reaction.10–13 However, due to the price, long-term stability, and limited reserves of noble metal catalysts, the practical application of rechargeable Zn–Air batteries will be severely limited when noble metals are used as cathode catalysts to achieve the dual function of ORR and OER.14–17 Therefore, the development of low-cost, easy-to-prepare, durable, and dual-functional non-precious metal catalysts with high ORR and OER activities has become the key to achieve the practical application of Zn–Air batteries.The transition metal selenide as the earth-abundant and low-cost materials have made great efforts to replace precious metal electrocatalysts to achieve durability and efficiency in electrochemical energy storage.18–20 Compared with transition metal oxides and sulfides, transition metal selenides have greater intrinsic metallic conductivity, which facilitates electron transfer in the electrocatalytic process.21–23 Especially, the non-stoichiometric transition metal selenides such as Co0.85Se and Ni0.85Se exhibit excellent electrocatalysis toward OER and ORR on account of the non-stoichiometric configuration, and corresponding varied metallic valence states and metal vacancies.24,25 Besides, Ni3+ in Ni0.85Se and Co3+ in Co0.85Se are beneficial to the formation of NiOOH or CoOOH,26,27 ascribing to the real active site during the OER process, whereas Ni2+ and Co2+are attributed to the active site for ORR.28
Owing to the poor electronic conduction, the transition metal selenides usually anchor in the high electronic conduction such as carbon-based materials, which effectively are exposed to the more active sites and achieve long-term stability in the electrocatalysis process.29,30 Besides, to further improve the catalytic activity of carbon-based electrocatalysts, the electrocatalytic activity of carbon materials can be enhanced by doping with heteroatoms. For example, Gui et al.28 reported the Co0.85Se nanoparticle encapsulating the 1D nitrogen-doped carbon nanofibers (CNFs), which exhibited excellent bifunctional electrocatalysis activity toward OER and ORR and superior electrochemical stability because of the synergistic effect between Co0.85Se and N-doped CNFs heterostructures and sufficient electrochemically active site. Because of the nitrogen-doped mesoporous carbon (NMC) modifying the CoSe, CoSe@NMC displays excellent performance for OER and ORR.31 However, the transition metal selenides practically gain the bifunctional electrocatalysis by many complicated strategies consisting of the construct of heterointerfaces,32 engineering of the defect,25 and formation of electronic interaction33,34 to optimize the adsorption/desorption of reaction intermediates. Therefore, it is important to develop an excellent, durable, and inexpensive bifunctional electrocatalyst based on transition metal selenides for ORR and OER by a simple and easy method, which really contributes to the practical application of zinc–air batteries.
Because of the high electrocatalytic activity of nitrogen-doped hollow carbon spheres, we synthesized mixed M0.85Se (M = Ni or Co) nanoparticles modified on nitrogen-doped hollow carbon spheres (Ni0.85Se-NHCS mixed with Co0.85Se-NHCS) as OER/OER electrocatalytic materials. The simple physical mixing method simplified the preparation process and reduced the preparation cost. The nitrogen-doped hollow carbon sphere facilitated the mass/charge transfer and reduced aggregation of the nanoparticle during the electrocatalysis process. Due to the suitable ratio of Ni3+/Ni2+ and Co3+/Co2+, Ni0.85Se-NHCS had better catalytic activity for OER, while Co0.85Se-NHCS had higher performance for ORR. The mixture Ni0.85Se/Co0.85Se-NHCS-2 displayed the superior electrocatalytic performance for both ORR and OER than the Ni0.85Se-NHCS or Co0.85Se-NHCS alone because of the synergistic effect between Ni0.85Se-NHCS and Co0.85Se-NHCS. Compared with the zinc–air batteries using Pt/C and RuO2 hybrid catalysts, the batteries using Ni0.85Se/Co0.85Se-NHCS-2 hybrid catalysts showed similar charge and discharge performance and better long-term stability. This work can provide a simple and low-cost method for the design and development of ORR/OER bifunctional electrocatalysts, which is beneficial for the practical application of zinc–air batteries.
Experimental section
Chemicals
NiCl2·6H2O, CoCl2·6H2O, formaldehyde, resorcinol, hexadecyl trimethyl ammonium bromide (CTAB) and tetraethoxysilane (TEOS) were purchased from Aladdin Reagent (Shanghai) Co., Ltd. Ammonia solution (25%) and ethanol were provided from Tianjin Baishi Chemical Co., Ltd. Hydrazine hydrate (N2H4·H2O, 80%) were supplied from Tianjin Damao Chemical Reagent Factory.Synthesis of NHCS
The synthesis of the nitrogen-doped hollow carbon sphere (NHCS) was performed following the hard template method.27 0.5 g of resorcinol was dissolved in 70 mL of ethanol and 10 mL of distilled water. Then 3 mL of ammonia solution added to the above solution under magnetic stirring. Subsequently, 0.72 mL of formaldehyde and 2.8 mL of TEOS were dropped in five minutes. For forming more mesoporous in the carbon sphere, 1.5 mL of TEOS was added to the above-mixed solution after 6 hours. The products were filtered and dried at 50 °C overnight before carbonation at 800 °C for 2 hours. Finally, NHCS was gained after performing 6 M of NaOH solution at 70 °C for 6 hours to etching silica spheres.Synthesis of Ni0.85Se-NHCS and Co0.85Se-NHCS
50 mg of NHCS, 59.4 mg of NiCl2·6H2O, 59.4 mg of NaSeO3, and 50 mg CTAB were dissolved in 60 mL of distilled water under ultrasonic operation for 1 hour. Subsequently, 8 mL of N2H4·H2O was dropped in the above solution and transferred to a Teflon-lined autoclave and kept at 140 °C in an oven for 24 h. After filtered and washed with distilled water, the product was dried at 50 °C for 12 hours. Eventually, the product was performed further calcination in N2 atmosphere at 600 °C for 2 hours. The loading masses of Ni0.85Se and Co0.85Se were calculated to be about 1.29 mg per gram of carbon sphere based on the addition of metal salts and carbon spheres. The synthesis of Co0.85Se-NHCS was following the above process and the difference was that CoCl2·6H2O was used instead of NiCl2·6H2O. 50% Ni-Co0.85Se-NHCS was prepared by a similar co-deposition method as a contrast. The mass of both CoCl2·6H2O and NiCl2·6H2O were 27 mg, other conditions remained the same.Mixing of Ni0.85Se-NHCS and Co0.85Se-NHCS
The above-prepared Ni0.85Se-NHCS and Co0.85Se-NHCS were mixed with the mass radio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was labeled as Ni0.85Se/Co0.85Se-NHCS-1, Ni0.85Se/Co0.85Se-NHCS-2 and Ni0.85Se/Co0.85Se-NHCS-3, respectively.
1, which was labeled as Ni0.85Se/Co0.85Se-NHCS-1, Ni0.85Se/Co0.85Se-NHCS-2 and Ni0.85Se/Co0.85Se-NHCS-3, respectively.
Characterization
The crystal structure's information of the samples was gained via X-ray diffraction (XRD, PANalytical, PW3040/60 diffractometer) with Cu Kα radiation (λ = 0.15418 nm). The surface chemical state of the above samples was tested by X-ray photoelectron spectroscopy (XPS, ESCALab 250) and performed by XPSPEAK software. The surface microstructure and morphology of the above-prepared samples were examined by field-emission scanning electron microscopy (FESEM, JEOL JSM-7001F) and transmission electron microscopy (TEM, JEM2010-HR). The BET specific surface area of the above samples was measured by nitrogen adsorption–desorption isotherm (Micromeritics ASAP 2460) and pore size distribution used the BJH desorption model.Electrochemical measurements
The catalyst ink was contained with 3 mg of catalyst, 500 μL of isopropanol, 500 μL of distilled water, and 20 μL of Nafion®resin solutions (5.0 wt%). And then, 5 μL of catalyst ink was dropped on the glassy carbon rotating disk electrode (RDE, 0.126 cm2) or glassy carbon ring rotating disk electrode (RRDE, 0.196 cm2). Finally, the above catalyst was dried in the air.The ORR and OER catalytic activity was examined on CHI 760e electrochemical workstation (Shanghai, Chenhua) using a three-electrode system, where the RDE (0.126 cm2) with Ni0.85Se-NHCS or Co0.85Se-NHCS film as the working electrode, the Hg/HgO as the reference electrode and a carbon rod of spectral purity as a counter electrode. But the electron transfer numbers and hydrogen peroxide yield was used in an AMETEK Princeton Applied Research PMC 2000A equipped with an RRDE as a working electrode. All the ORR and OER catalytic activity were performed in O2-saturated 0.1 M KOH electrolyte.
The electrochemical surface area (ECSA) was gained by CV measurement in the non-faraday current region by varying the scan rates from 2 mV s−1 to 10 mV s−1. The current is positively related to the scan rate:
| Ic = Cdl × v |
Thereinto, N is the number of active sites
Since the real surface active site was unknown, we evaluated Co and Ni in the surface as the active site. The active sites per real surface area are shown in the following equation:
The number of active sites of Ni0.85Se/Co0.85Se-NHCS-2 is the average of the Ni0.85Se-NHCS and Co0.85Se-NHCS. Besides, the unit cell of Ni0.85Se and Co0.85Se are shown in Fig. S1,† according to the standard crystal card of Ni0.85Se (JCPDS No. 18-0888) and Co0.85Se (JCPDS No. 52-1008), respectively.
The electron transfer number (n) and hydrogen peroxide yield calculate the following equation:
ID and IR represent the disk current and the ring current of RRDE. N means the collection efficiency of the ring (34%).
The Zn–air battery measurement was carried out on CHI 760e electrochemical workstation (Shanghai, Chenhua) at room temperature. Zinc foil was worked as the anode and the catalyst coating in carbon paper was performed as the cathode with a geometric area of 1 cm2. 5 mg of catalyst was dispersed into 20 μL of 5% Nafion solution, 300 μL water, and 700 μL of isopropanol solution. The electrolyte contained 6 M KOH and 0.2 M Zn(Ac)2. As a reference, 2.5 mg of 20% Pt/C and 2.5 mg of RuO2 were performed with the above-same process.
Results and discussion
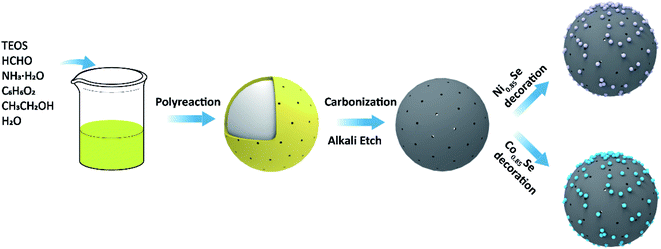
The synthesis process of M0.85Se-NHCS (M = Ni, Co) is shown in Fig. 1. Initially, the phenolic resin was coated on the SiO2 spheres by polyreaction at room temperature. The yellow precursors collecting by filtration were carbonized at 800 °C for 2 hours in the nitrogen atmospheres. After the SiO2 removal, the nitrogen-doped hollow spheres (NHCS) were decorated with M0.85Se (M = Ni, Co) nanoparticles. The M0.85Se-NHCS (M = Ni, Co) was successfully synthesized following the above process.The micromorphology and microstructure of the prepared catalysts Ni0.85Se-NHCS and Co0.85Se-NHCS were carried out by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). As shown in Fig. S2a and b,† the NHCS with an average diameter of 400 nm size exhibited a roughly spherical structure. As shown in the results of SEM (Fig. S2c–f†) and TEM (Fig. 2a and d), the NHCS was uniformly immobilized with tiny nanoparticles (Ni0.85Se or Co0.85Se) abounding on the surface after hydrothermal reaction. For 50% Ni-Co0.85Se, it can be seen the Ni0.85Se and Co0.85Se nanoparticles were co-deposited on the NHCS uniformly (Fig. S2j and k†). Furthermore, high-resolution transmission electron microscopy (HRTEM) was used to analyze the microstructure of the nanoparticles and to confirm the composition of the nanoparticles. As shown in Fig. 2b, the lattice fringes with interplanar width of 2.70 Å and 1.18 Å corresponded to the (101) and (110) planes of Ni0.85Se, respectively. Besides, the crystal interplanar spacings of 2.69 Å corresponding to the (101) planes in Fig. 2e indicated the existence of the Co0.85Se crystal. The EDX mappings of Ni0.85Se-NHCS in Fig. 2c revealed the homogeneous distribution of Ni, Se, N, and C element. Similarly, the element of Co, Se, N, and C were uniformly distributed on the Co0.85Se-NHCS in Fig. 2f. Furthermore, the EDX results implied the successful synthesis of non-stoichiometric Co0.85Se and Ni0.85Se (Fig. S3 and S4†). As we all know, the non-stoichiometric materials provide numerous active sites because of their specific stoichiometry of metal vacancies which makes them abundant unsaturated atoms and boost the ORR and OER activities.24,28 Besides, the presence of the nitrogen component further demonstrated that the successful doping of nitrogen on the hollow carbon spheres, which changed the charge distribution of the carbon material and facilitated the absorption and desorption of oxygen in ORR or OER process.36,37
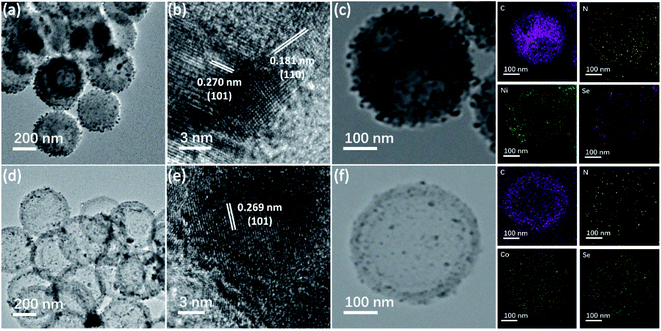 | ||
| Fig. 2 (a and d) The TEM images, (b and e) high-resolution transmission electron microscopy image, and (c and f) EDX mappings of Ni0.85Se-NHCS and Co0.85Se-NHCS, respectively. | ||
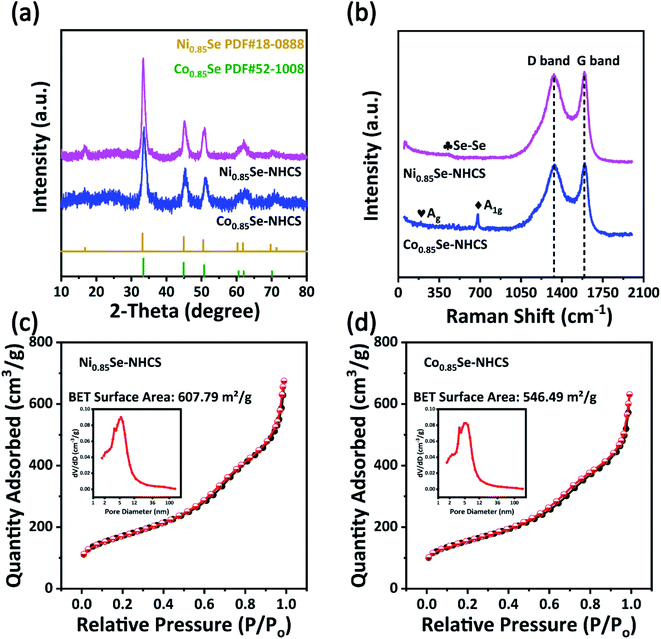
X-ray diffraction (XRD) patterns revealed the crystal structure information of the as-prepared samples. In Fig. 3a, the XRD patterns of Co0.85Se-NHCS and Ni0.85Se-NHCS were in agreement well with the standard crystal card of Ni0.85Se (JCPDS No. 18-0888) and Co0.85Se (JCPDS No. 52-1008), respectively. The diffraction peak positions of 33.15°, 44.95°, and 50.49° were accountable with (101), (102), and (110) crystal planes of hexagonal crystalline Ni0.85Se, correspondingly. Similarly, the diffraction peak positions at 33.26°, 44.74°, and 50.56° were related to (101), (102), and (110) crystal planes of hexagonal crystalline Co0.85Se. The XRD patterns consistent with the standard card further demonstrated the successful synthesis of Co0.85Se and Ni0.85Se. Besides, as shown in Fig. S5a,† when the Co0.85Se-NHCS and Ni0.85Se-NHCS were mixed with the mass radio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the XRD pattern of Co0.85Se/Ni0.85Se-NHCS-2 was unquestionably unchanged. As shown in Fig. 3b, the Raman peaks at 384 cm−1 were ascribed to Se–Se librational and stretching vibrations of the NiSe.38–40 The characteristic peaks at 188 cm−1 and 678 cm−1 were indexed to the Ag and A1g of CoSe, respectively.41,42 Additionally, there were distinctive peaks at 1335 cm−1 (D band) and 1590 cm−1 (G band) attributed to sp3 and sp2 hybridized carbons.43 The intensity ratios of D band and G band of Ni0.85Se-NHCS, Co0.85Se-NHCS, Co0.85Se/Ni0.85Se-NHCS-2 and NHCS (Fig. S5b†) were nearly 0.99, 0.98, 0.99, and 0.88, respectively. Raman results showed that the NHCS possessed a high degree of graphitization, resulting in high electrical conductivity.44 After combined with Co0.85Se or Ni0.85Se, the Ni0.85Se-NHCS, Co0.85Se-NHCS, and Co0.85Se/Ni0.85Se-NHCS-2 had abundant defect because of the existence of the non-stoichiometric M0.85Se (M = Ni or Co) nanoparticle, providing the more active site to boost the ORR and OER activities, which is consistent with previous literature.45–47 The N2 adsorption/desorption isotherms and corresponding pore size distribution of Ni0.85Se-NHCS, Co0.85Se-NHCS, and Co0.85Se/Ni0.85Se-NHCS-2 were revealed in Fig. 3c, d, and S5,† respectively. The specific surface area of the Ni0.85Se-NHCS, Co0.85Se-NHCS, and Co0.85Se/Ni0.85Se-NHCS-2 was 607.79, 546.49, and 554.67 m2 g−1, respectively. Also, the N2 adsorption/desorption isotherms curves of the above samples had a hysteresis loop at a relative pressure between 0.5 and 0.9. These curves belonged to IV profiles and carried out numerous mesoporous structures in these catalysts. Besides, the pore size distribution curves demonstrated that these catalysts retained rich micropores and mesopores. The abundant porous structures of samples accelerated the mass and charge transfer and provided a large amount of specific surface area, which was conducive to the high performance of oxygen reduction and oxygen evolution reactions.48
1, the XRD pattern of Co0.85Se/Ni0.85Se-NHCS-2 was unquestionably unchanged. As shown in Fig. 3b, the Raman peaks at 384 cm−1 were ascribed to Se–Se librational and stretching vibrations of the NiSe.38–40 The characteristic peaks at 188 cm−1 and 678 cm−1 were indexed to the Ag and A1g of CoSe, respectively.41,42 Additionally, there were distinctive peaks at 1335 cm−1 (D band) and 1590 cm−1 (G band) attributed to sp3 and sp2 hybridized carbons.43 The intensity ratios of D band and G band of Ni0.85Se-NHCS, Co0.85Se-NHCS, Co0.85Se/Ni0.85Se-NHCS-2 and NHCS (Fig. S5b†) were nearly 0.99, 0.98, 0.99, and 0.88, respectively. Raman results showed that the NHCS possessed a high degree of graphitization, resulting in high electrical conductivity.44 After combined with Co0.85Se or Ni0.85Se, the Ni0.85Se-NHCS, Co0.85Se-NHCS, and Co0.85Se/Ni0.85Se-NHCS-2 had abundant defect because of the existence of the non-stoichiometric M0.85Se (M = Ni or Co) nanoparticle, providing the more active site to boost the ORR and OER activities, which is consistent with previous literature.45–47 The N2 adsorption/desorption isotherms and corresponding pore size distribution of Ni0.85Se-NHCS, Co0.85Se-NHCS, and Co0.85Se/Ni0.85Se-NHCS-2 were revealed in Fig. 3c, d, and S5,† respectively. The specific surface area of the Ni0.85Se-NHCS, Co0.85Se-NHCS, and Co0.85Se/Ni0.85Se-NHCS-2 was 607.79, 546.49, and 554.67 m2 g−1, respectively. Also, the N2 adsorption/desorption isotherms curves of the above samples had a hysteresis loop at a relative pressure between 0.5 and 0.9. These curves belonged to IV profiles and carried out numerous mesoporous structures in these catalysts. Besides, the pore size distribution curves demonstrated that these catalysts retained rich micropores and mesopores. The abundant porous structures of samples accelerated the mass and charge transfer and provided a large amount of specific surface area, which was conducive to the high performance of oxygen reduction and oxygen evolution reactions.48
The surface chemical state and element composition of the catalyst was carried out by XPS analysis in Fig. 4. The survey spectrum of the Ni0.85Se-NHCS presented the existence of C, N, O, Se and Ni elements, while Co0.85Se-NHCS displayed the coexistence of C, N, O, Se and Co elements (Fig. S6a†). The O element was attributed to the exposure to air, which was consistent with the description in the literature.32 As displayed in Fig. 4a, the Ni 2p spectrum was deconvoluted into two main peaks along with two satellite peaks. The peak located at 853.6 eV of Ni 2p3/2 and 870.9 eV of Ni 2p1/2 were attributed to Ni2+ of Ni0.85Se-NHCS, and the peak located at 856 eV of Ni 2p3/2 and 873.9 eV of Ni 2p1/2 were ascribed to Ni3+ of Ni0.85Se-NHCS.49,50 Similarly, the high-resolution spectrum of Co 3p was split into two main peaks and two satellite peaks in Fig. 4b. The peaks situated at 778.6 eV for Co 2p3/2 and 793.78 eV for Co 2p1/2 demonstrated the existence of Co3+ in Co0.85Se-NHCS, and the peaks situated at 781.1 eV for Co 2p3/2 and 797.1 eV for Co 2p1/2 corresponded to Co2+ in Co0.85Se-NHCS.51–54 As the deconvolution of Ni 2p, the Ni3+/Ni2+ ratio of Ni0.85Se-NHCS was 5.55, implying that the Ni3+ content was much higher than that of Ni2+. It has been reported that Ni3+ was considered the active site for OER by absorbing OH− owing to the formation of NiOOH.12,26 So it can be reasonably inferred that Ni0.85Se-NHCS was favorable to OER. On the other hand, the Co 2p spectrum showed that the Co3+/Co2+ ratio of Co0.85Se-NHCS was 0.06. It indicates a large amount of Co2+, which was considered to favor ORR.28 In the spectrum of Fig. 4c, the N 1s peaks of Ni0.85Se-NHCS and Co0.85Se-NHCS were split into four main peaks ascribed to pyridinic N (398.3 eV), pyrrolic N (399.5 eV), graphitic N (400.9 eV), and oxidic N (402.8 eV), respectively.55 As revealed in the Fig. 4d, the Se 3d spectrums of Ni0.85Se-NHCS and Co0.85Se-NHCS were split into Se 3d5/2 (54.3 eV), Se 3d3/2 (55.75 eV), and SeOx (59.2 eV).56 The existence of SeOx was attributable to exposure to air. So the selenium of transition metal selenides was oxidized to selenites, then to selenates. The adsorption of selenate on the surface of the sample promoted the oxygen evolution reaction, strengthening the OER intermediates bond.57 Interestingly, compared with Ni0.85Se-NHCS, the extra peak located at 61.61 eV in the Se spectrum of Co0.85Se-NHCS was ascribed to Co 3p, which was consistent with the previous literature.55 Furthermore, the C 1 s spectrums of Ni0.85Se-NHCS and Co0.85Se-NHCS were deconvoluted into four main peaks corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (248.8 eV), C-C (285.6 eV), C
C (248.8 eV), C-C (285.6 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (286.9 eV), and C
N (286.9 eV), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (289.4 eV) in Fig. S6b.†
O (289.4 eV) in Fig. S6b.†
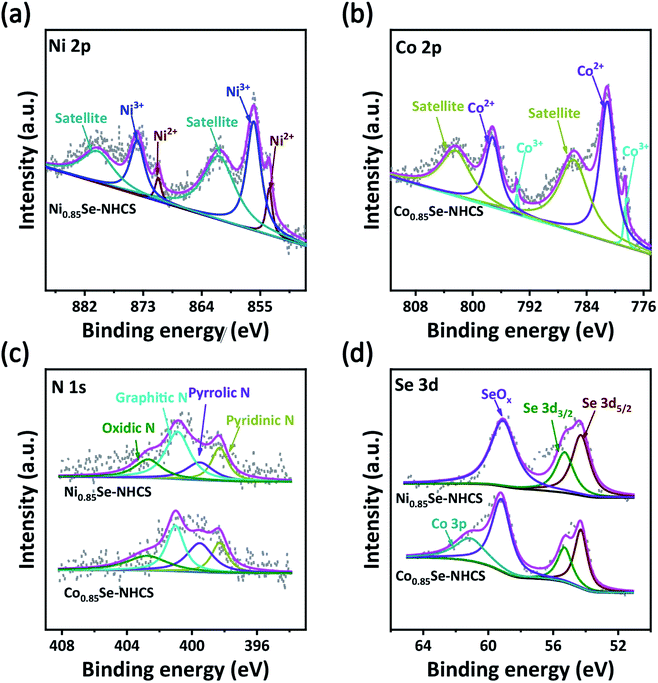 | ||
| Fig. 4 High-resolution XPS of (a) Ni 2p, (b) Co 2p, (c) N 1s and (d) Se 3d of Ni0.85Se-NHCS and Co0.85Se-NHCS. | ||
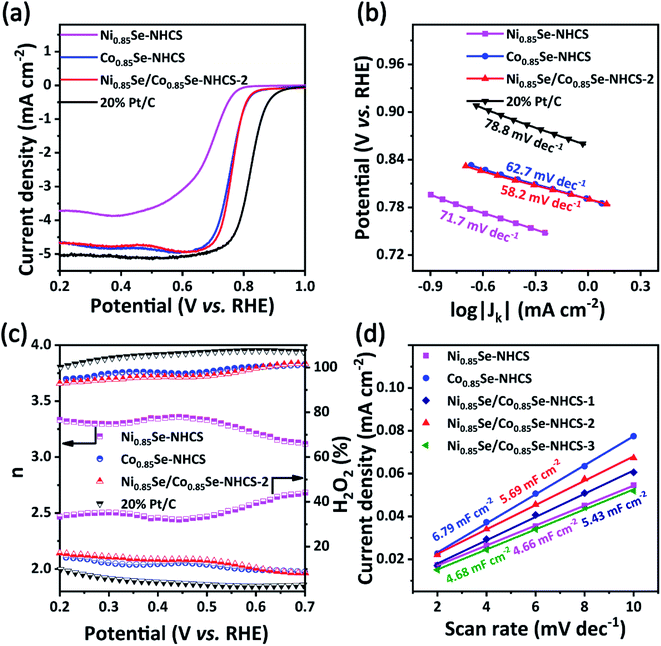
The single NHCS without metal selenide only showed very weak ORR and OER activity (Fig. S7†). After M0.85Se (M = Ni, Co) was decorated on NHCS, the ORR performance of catalysts had been greatly improved due to the intrinsic catalytic quality of Ni0.85Se and Co0.85Se (Fig. 5). As shown in Fig. 5a, the Ni0.85Se/Co0.85Se-NHCS-2 exhibited excellent ORR activities with the onset potential of 0.90 V, a half-wave potential of 0.77 V, and a limiting current density of 4.66 mA cm−2, closing those of the Co0.85Se-NHCS (Eonset = 0.89 V, E1/2 = 0.76 V and JL = 4.67 mA cm−2) and exceeding the Ni0.85Se-NHCS (Eonset = 0.79 V, E1/2 = 0.69 V and JL = 3.71 mA cm−2). Obviously, the ORR performance of the Ni0.85Se/Co0.85Se-NHCS-2 mixture was mainly derived from the high content of Co2+ in Co0.85Se-NHCS. Interestingly, the ORR activities of Ni0.85Se/Co0.85Se-NHCS-2 were better than that of 50% Ni-Co0.85Se-NHCS (Fig. S9a†), which was prepared from CoCl2·6H2O and NiCl2·6H2O by co-precipitation. This result indicated that the catalyst prepared by the simple physical mixing method performed better than that prepared by the complex chemical method. As shown in Fig. 5b, the Tafel slope of Ni0.85Se/Co0.85Se-NHCS-2 was 58.2 mV dec−1, lower than that of Co0.85Se-NHCS (62.7 mV dec−1), Ni0.85Se-NHCS (71.7 mV dec−1), 50% Ni-Co0.85Se-NHCS (60.0 mV dec−1) and 20% Pt/C (78.8 mV dec−1), suggesting a faster kinetics rate of ORR process. Besides, electrochemical impedance spectroscopy (EIS) of these as-prepared catalysts was provided in Fig. S8.† According to the semicircle in the high-frequency region, the charge transfer resistance of Ni0.85Se/Co0.85Se-NHCS-2 was 3.68 Ω, between Co0.85Se-NHCS (3.3 Ω) and Ni0.85Se-NHCS (4.81 Ω), which was consistent with the fact that Ni0.85Se/Co0.85Se-NHCS-2 was a mixture of Ni0.85Se-NHCS and Co0.85Se-NHCS. Furthermore, the detailed data of LSV curves and Tafel slopes of these catalysts were shown in Fig. S9 and Table S1.† The Ni0.85Se/Co0.85Se-NHCS-2 had the highest ORR activity among the mixtures with different mass ratios. Moreover, its activity was even higher than that of some nickel-cobalt-selenides reported in the literature (Table S2†). Remarkably, Ni0.85Se/Co0.85Se-NHCS-2 achieved the same ORR performance as Co0.85Se-NHCS with only half the cobalt content. And with the mass ratio changed, the ORR performance of the mixtures decreased significantly. Therefore, it can be reasonably inferred that there is a synergistic effect between Co0.85Se-NHCS and Ni0.85Se-NHCS, which can affect the ORR activity of the mixture.
The electron transfer number (n) and H2O2 yields in the ORR process were carried out from the rotating ring-disk electrode (RRDE) measurement (Fig. 5c). The average n value evaluated for Ni0.85Se/Co0.85Se-NHCS-2 was 3.75, higher than that of Ni0.85Se-NHCS (3.35), and closed to that of Co0.85Se-NHCS (3.76) and noble metal catalyst 20% Pt/C (3.88), suggesting a four-electron reaction process. Moreover, the H2O2 yield of Ni0.85Se/Co0.85Se-NHCS-2 was less than 12% in the voltage range from 0.2 to 0.7 V, lower than that of the Co0.85Se-NHCS (13%) and commercial catalyst 20% Pt/C (8%). This result of Ni0.85Se/Co0.85Se-NHCS-2 further confirmed its excellent selectivity for ORR activity. For measuring the electrochemical surface area (ECSA), the double-layer capacitance (Cdl) was conducted by cyclic voltammetry at a scan rate from 2 mV s−1 to 10 mV s−1. The CV curves of the as-prepared catalysts were shown in Fig. S10.† As shown in Fig. 5d, the Cdl of the Ni0.85Se/Co0.85Se-NHCS-2 was 5.69 mF cm−2, which was second only to that of Co0.85Se-NHCS and higher than that of other catalysts. This result demonstrates that the Ni0.85Se/Co0.85Se-NHCS-2 has a superior active surface area and active site for electrochemical activity. Besides, the super methanol tolerance was displayed in Fig. S11,† which was carried out the chronoamperometric test at 0.3 V in O2-saturated 0.1 M KOH solution with 1600 rpm. The ORR activity of Ni0.85Se/Co0.85Se-NHCS-2 didn't change obviously after injecting 1 M methanol, while that of 20% Pt/C decreased evidently. It indicates that Ni0.85Se/Co0.85Se-NHCS-2 has outstanding methanol tolerance and a wide practical range. As shown in Fig. S12,† the limiting current density of Ni0.85Se/Co0.85Se-NHCS-2 only declined by 4.05% after 20 hours. By contrast, that of 20% Pt/C dropped off 13.14%. The above measurements result that Ni0.85Se/Co0.85Se-NHCS-2 has excellent ORR performance, good methanol resistance, and remarkable stability, which is derived from the synergistic effect of Ni0.85Se, Co0.85Se, and NHCS.
The excellent cathodic catalysts of the zinc–air battery should possess outstanding bifunctional electrocatalysis for both ORR and OER. The OER performance of the prepared catalysts was analyzed in a three-electrode cell in 0.1 M KOH electrolyte and compared with other catalysts including the noble metal catalyst RuO2. The operating potential at a current density of 10 mA cm−2 was used to evaluate the OER electrocatalytic activity of the material. As shown in Fig. 6a, the operating potential of Ni0.85Se/Co0.85Se-NHCS-2 was 1.63 V. It is a little worse than that of precious metal RuO2 (1.59 V), close to that of Ni0.85Se-NHCS (1.62 V), but better than that of Co0.85Se-NHCS (1.65 V). Fig. 6b showed the Tafel slopes of these catalysts for OER. The Ni0.85Se/Co0.85Se-NHCS-2 exhibited a lower Tafel slope of 118.3 mV dec−1 than that of Co0.85Se-NHCS (141.6 mV dec−1), Ni0.85Se-NHCS (136.0 mV dec−1), and only higher than that of RuO2 (84.1 mV dec−1). Besides, the LSV curves and Tafel slopes of other mixed catalysts were also shown in Fig. S9 and Table S1.† The OER performance of Ni0.85Se/Co0.85Se-NHCS-2 with less Ni content was similar to that of Ni0.85Se-NHCS, and higher than that of the mixtures with other mass ratios. Moreover, to further evaluate the intrinsic performance for OER of the catalysts, the TOF of catalysts was evaluated. Ni0.85Se/Co0.85Se-NHCS-2 was calculated as 0.40 s−1 at the potential of 1.58 V, lower than that of Ni0.85Se-NHCS (0.73 s−1) and higher than that of Co0.85Se-NHCS (0.26 s−1), which demonstrated the OER activity of the mixed catalyst Ni0.85Se/Co0.85Se-NHCS-2 was mainly derived from the Ni0.85Se-NHCS. Like the ORR performance, the Ni0.85Se/Co0.85Se-NHCS-2 with the rich of Ni3+ displayed a better OER performance than that of the other metal selenide materials (Table S2†). Furthermore, the potential difference (ΔE) between half-wave potential (E1/2) for ORR and OER potential at 10 mA cm−2 (Ej=10) was applied to evaluate the performance of the bifunctional catalytic activity. The Ni0.85Se/Co0.85Se-NHCS-2 illustrated a ΔE value of 0.86 V, which was lower than that of Co0.85Se-NHCS (0.89 V), Ni0.85Se-NHCS (0.93 V), indicating that Ni0.85Se/Co0.85Se-NHCS-2 possesses the optimum bifunctional ORR and OER performance among the non-precious metal catalysts (Fig. 6c). From the above results, it is shown that the Ni0.85Se-NHCS-2 as a mixture of Ni3+-rich Ni0.85Se-NHCS and Co2+-rich Co0.85Se-NHCS has good ORR and OER bifunctional electrochemical activity when the same catalyst mass is used.
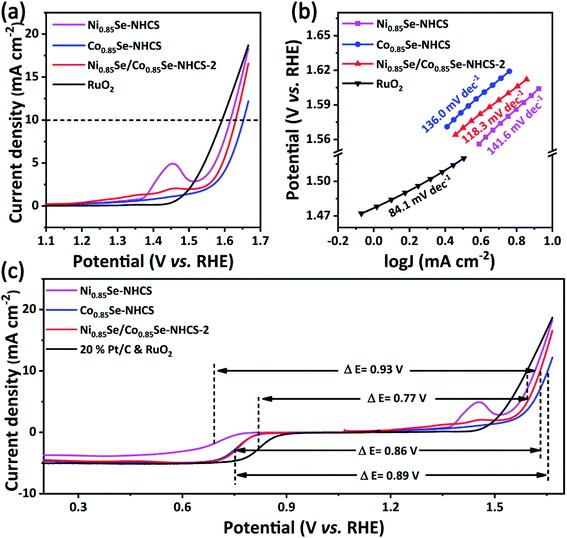 | ||
| Fig. 6 Linear sweep voltammogram curves for (a) OER and corresponding (b) Tafel slope curves, (c) the potential difference (ΔE) between E1/2 for ORR and Ej=10 for OER. | ||
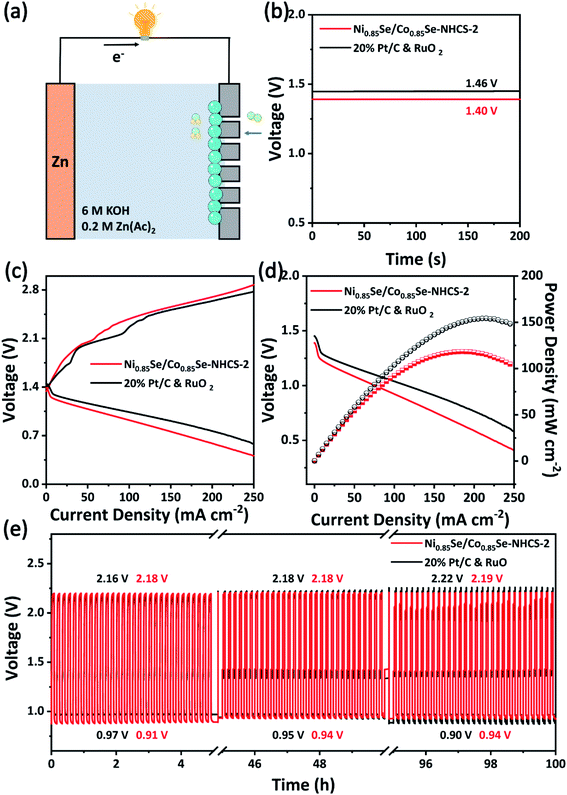
Due to the superior bifunctional activity of Ni0.85Se/Co0.85Se-NHCS-2, the zinc–air battery was assembled with this mixture as cathode catalyst, zinc foil as an anode, and 6 M KOH and 0.2 M Zn(Ac)2 as the electrolyte, respectively (Fig. 7a). The oxygen cathode in the zinc–air battery was made by coating the catalyst on carbon paper with a coating area of 1 cm2. Moreover, the 20% Pt/C and RuO2 noble metal mixture with a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used as a comparison. As shown in Fig. 7b, the zinc–air battery equipped with Ni0.85Se/Co0.85Se-NHCS-2 exhibited the open potential of 1.40 V, coming near that of the battery with Pt/C&RuO2 (1.46 V). Besides, the zinc–air battery based on Ni0.85Se/Co0.85Se-NHCS-2 worked and successfully drived a timer, testifying to the potential application of this catalyst in Fig. S13.† To further assess the performance of zinc–air devices, the charge and discharge curves were carried in Fig. 7c. The Ni0.85Se/Co0.85Se-NHCS-2 based battery exhibited a potential gap in charge and discharge measure approaching that of 20% Pt/C&RuO2, which pointed out the awesome charge and discharge ability of the Ni0.85Se/Co0.85Se-NHCS-2 based battery. Moreover, the Ni0.85Se/Co0.85Se-NHCS-2 based battery was manifested a power density of 118.34 mW cm−2 at a current density of 185.1 mA cm−2, while that of 20% Pt/C&RuO2 achieved 154.13 mW cm−2 at 210.10 mA cm−2 (Fig. 7d). Moreover, the performance of zinc–air batteries with other non-precious catalysts was compared in Table S3.† To further appraise the practical stability application, the long-term charge and discharge activity of the Ni0.85Se/Co0.85Se-NHCS-2 and 20% Pt/C&RuO2 was implemented at a current density of 25 mA cm−2 with 10 min per cycle. As exhibited in Fig. 7e, the zinc–air battery based on Ni0.85Se/Co0.85Se-NHCS-2 displayed better long-term stability through long cycle charging and discharging than that with 20% Pt/C&RuO2. The potential gap of the Ni0.85Se/Co0.85Se-NHCS-2 based battery manifested a subtle change from 1.27 to 1.25 V after 100 hours due to the balanced concentration of dissolved oxygen, which was consistent with previous literature.9,10 In particular, the Ni0.85Se/Co0.85Se-NHCS-2 based battery showed a slight increase in charging potential from 2.18 V to 2.19 V, which confirmed the excellent long-term stability of the OER. For comparison, the Pt/C&RuO2 based battery performed an initial voltage gap of 1.19 V. But after 100 operation hours, the voltage gap changed to 1.32 V, suggesting the performance of the zinc–air battery obviously deteriorated. It can be seen that although the charge and discharge performance of Ni0.85Se/Co0.85Se-NHCS-2 based battery is slightly worse than that of Pt/C&RuO2 based battery, it has better long-term stability. Considering the low cost of the non-precious metal catalyst, the Ni0.85Se/Co0.85Se-NHCS-2 has the potential to replace precious metals for zinc–air batteries.
1 was used as a comparison. As shown in Fig. 7b, the zinc–air battery equipped with Ni0.85Se/Co0.85Se-NHCS-2 exhibited the open potential of 1.40 V, coming near that of the battery with Pt/C&RuO2 (1.46 V). Besides, the zinc–air battery based on Ni0.85Se/Co0.85Se-NHCS-2 worked and successfully drived a timer, testifying to the potential application of this catalyst in Fig. S13.† To further assess the performance of zinc–air devices, the charge and discharge curves were carried in Fig. 7c. The Ni0.85Se/Co0.85Se-NHCS-2 based battery exhibited a potential gap in charge and discharge measure approaching that of 20% Pt/C&RuO2, which pointed out the awesome charge and discharge ability of the Ni0.85Se/Co0.85Se-NHCS-2 based battery. Moreover, the Ni0.85Se/Co0.85Se-NHCS-2 based battery was manifested a power density of 118.34 mW cm−2 at a current density of 185.1 mA cm−2, while that of 20% Pt/C&RuO2 achieved 154.13 mW cm−2 at 210.10 mA cm−2 (Fig. 7d). Moreover, the performance of zinc–air batteries with other non-precious catalysts was compared in Table S3.† To further appraise the practical stability application, the long-term charge and discharge activity of the Ni0.85Se/Co0.85Se-NHCS-2 and 20% Pt/C&RuO2 was implemented at a current density of 25 mA cm−2 with 10 min per cycle. As exhibited in Fig. 7e, the zinc–air battery based on Ni0.85Se/Co0.85Se-NHCS-2 displayed better long-term stability through long cycle charging and discharging than that with 20% Pt/C&RuO2. The potential gap of the Ni0.85Se/Co0.85Se-NHCS-2 based battery manifested a subtle change from 1.27 to 1.25 V after 100 hours due to the balanced concentration of dissolved oxygen, which was consistent with previous literature.9,10 In particular, the Ni0.85Se/Co0.85Se-NHCS-2 based battery showed a slight increase in charging potential from 2.18 V to 2.19 V, which confirmed the excellent long-term stability of the OER. For comparison, the Pt/C&RuO2 based battery performed an initial voltage gap of 1.19 V. But after 100 operation hours, the voltage gap changed to 1.32 V, suggesting the performance of the zinc–air battery obviously deteriorated. It can be seen that although the charge and discharge performance of Ni0.85Se/Co0.85Se-NHCS-2 based battery is slightly worse than that of Pt/C&RuO2 based battery, it has better long-term stability. Considering the low cost of the non-precious metal catalyst, the Ni0.85Se/Co0.85Se-NHCS-2 has the potential to replace precious metals for zinc–air batteries.
Conclusions
In summary, we synthesized Ni0.85Se-NHCS and Co0.85Se-NHCS, respectively, by hydrothermal modification of Ni0.85Se and Co0.85Se nanoparticles on NHCS. The mixture Ni0.85Se/Co0.85Se-NHCS-2, which was prepared by simple mixing of Ni0.85Se-NHCS and Co0.85Se in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio, exhibited better ORR and OER bifunctionality than the single Ni0.85Se-NHCS and Co0.85Se-NHCS. The zinc–air battery equipped with Ni0.85Se/Co0.85Se-NHCS-2 performed more durable than the battery equipped with the 20% Pt/C&RuO2 catalysts. These results identify the rational mixture of nickel selenide and cobalt selenide as a simple, low-cost and efficient method for the preparation of oxygen electrode catalysts, further improving the performance and application prospect of zinc–air batteries.
1 mass ratio, exhibited better ORR and OER bifunctionality than the single Ni0.85Se-NHCS and Co0.85Se-NHCS. The zinc–air battery equipped with Ni0.85Se/Co0.85Se-NHCS-2 performed more durable than the battery equipped with the 20% Pt/C&RuO2 catalysts. These results identify the rational mixture of nickel selenide and cobalt selenide as a simple, low-cost and efficient method for the preparation of oxygen electrode catalysts, further improving the performance and application prospect of zinc–air batteries.
Author contributions
Li-Juan Peng: conceptualization, investigation, writing – original draft. Jie-Ping Huang: investigation, data curation. Qiu-Ren Pan: validation, formal analysis. Ying Liang: investigation. Na Yin: investigation. Hang-Chang Xu: investigation. Nan Li: conceptualization, supervision, writing – original draft, project administration.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by Natural Science Foundation of Guangdong Province (No. 2020A1515011551), Science and Technology Research Project of Guangzhou (No. 202102010484), and Innovative Training Program for University Students (No. 201911078017).References
- C. Wei, R. R. Rao, J. Peng, B. Huang, I. E. L. Stephens, M. Risch, Z. J. Xu and Y. Shao-Horn, Adv. Mater., 2019, 31, 1806296 CrossRef PubMed.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Norskov and T. F. Jaramillo, Science, 2017, 355, 146 CrossRef PubMed.
- X. X. Wang, M. T. Swihart and G. Wu, Nat. Catal., 2019, 2, 578–589 CrossRef CAS.
- J. Pan, Y. Y. Xu, H. Yang, Z. Dong, H. Liu and B. Y. Xia, Adv. Sci., 2018, 5, 1700691 CrossRef PubMed.
- T. Sharifi, E. Gracia-Espino, A. Chen, G. Hu and T. Wågberg, Adv. Energy Mater., 2019, 10, 1902084 CrossRef.
- S. Ren, X. Duan, S. Liang, M. Zhang and H. Zheng, J. Mater. Chem. A, 2020, 8, 6144–6182 RSC.
- A. Kulkarni, S. Siahrostami, A. Patel and J. K. Norskov, Chem. Rev., 2018, 118, 2302–2312 CrossRef CAS PubMed.
- M. Busch, N. B. Halck, U. I. Kramm, S. Siahrostami, P. Krtil and J. Rossmeisl, Nano Energy, 2016, 29, 126–135 CrossRef CAS.
- S. Li, C. Cheng, X. Zhao, J. Schmidt and A. Thomas, Angew. Chem., Int. Ed., 2018, 57, 1856–1862 CrossRef CAS PubMed.
- A. I. Douka, Y. Xu, H. Yang, S. Zaman, Y. Yan, H. Liu, M. A. Salam and B. Y. Xia, Adv. Mater., 2020, 32, 2002170 CrossRef CAS PubMed.
- W. Xie, Z. Li, S. Jiang, J. Li, M. Shao and M. Wei, Chem. Eng. J., 2019, 373, 734–743 CrossRef CAS.
- D. Ding, K. Shen, X. Chen, H. Chen, J. Chen, T. Fan, R. Wu and Y. Li, ACS Catal., 2018, 8, 7879–7888 CrossRef CAS.
- S. Dou, X. Li, L. Tao, J. Huo and S. Wang, Chem. Commun., 2016, 52, 9727–9730 RSC.
- Z.-q. Cao, M.-z. Wu, H.-b. Hu, G.-j. Liang and C.-y. Zhi, NPG Asia Mater., 2018, 10, 670–684 CrossRef CAS.
- Y. Zhang, H. Sun, Y. Qiu, X. Ji, T. Ma, F. Gao, Z. Ma, B. Zhang and P. Hu, Carbon, 2019, 144, 370–381 CrossRef CAS.
- X. Duan, N. Pan, C. Sun, K. Zhang, X. Zhu, M. Zhang, L. Song and H. Zheng, J. Energy Chem., 2021, 56, 290–298 CrossRef.
- X. Duan, S. Ren, N. Pan, M. Zhang and H. Zheng, J. Mater. Chem. A, 2020, 8, 9355–9363 RSC.
- L.-P. Lv, P. Du, P. Liu, X. Li and Y. Wang, ACS Sustainable Chem. Eng., 2020, 8, 8391–8401 CrossRef CAS.
- S. Wang, P. He, L. Jia, M. He, T. Zhang, F. Dong, M. Liu, H. Liu, Y. Zhang, C. Li, J. Gao and L. Bian, Appl. Catal., B, 2019, 243, 463–469 CrossRef CAS.
- S. Liu, Y. Jiang, M. Yang, M. Zhang, Q. Guo, W. Shen, R. He and M. Li, Nanoscale, 2019, 11, 7959–7966 RSC.
- J. Ding, S. Ji, H. Wang, V. Linkov and R. Wang, J. Power Sources, 2019, 423, 1–8 CrossRef CAS.
- J. Dai, D. Zhao, W. Sun, X. Zhu, L.-J. Ma, Z. Wu, C. Yang, Z. Cui, L. Li and S. Chen, ACS Catal., 2019, 9, 10761–10772 CrossRef CAS.
- X. Zheng, X. Han, Y. Cao, Y. Zhang, D. Nordlund, J. Wang, S. Chou, H. Liu, L. Li, C. Zhong, Y. Deng and W. Hu, Adv. Mater., 2020, 32, 2000607 CrossRef CAS PubMed.
- K. Karthick, S. N. Jagadeesan, P. Kumar, S. Patchaiammal and S. Kundu, Inorg. Chem., 2019, 58, 6877–6884 CrossRef CAS PubMed.
- Y. Huang, Z. Wang, Y. Jiang, S. Li, Z. Li, H. Zhang, F. Wu, M. Xie, L. Li and R. Chen, Nano Energy, 2018, 53, 524–535 CrossRef CAS.
- S. Li, S. Peng, L. Huang, X. Cui, A. M. Al-Enizi and G. Zheng, ACS Appl. Mater. Interfaces, 2016, 8, 20534–20539 CrossRef CAS PubMed.
- Q.-R. Pan, S.-J. Li, K. Tong, C. Xie, L. Peng, N. Li, D.-Y. Wang and H. Su, J. Mater. Sci., 2019, 54, 9063–9074 CrossRef CAS.
- L. Gui, Z. Huang, D. Ai, B. He, W. Zhou, J. Sun, J. Xu, Q. Wang and L. Zhao, Chem.–Eur. J., 2020, 26, 4063 CAS.
- T. Meng, J. Qin, S. Wang, D. Zhao, B. Mao and M. Cao, J. Mater. Chem. A, 2017, 5, 7001–7014 RSC.
- P. Xu, J. Zhang, Z. Ye, Y. Liu, T. Cen and D. Yuan, Appl. Surf. Sci., 2019, 494, 749–755 CrossRef CAS.
- X. Hong, Y. Xu, R. Wang, P. Du, Z. Zhao, K. Huang, H. Tang, Y. Liu, M. Lei and H. Wu, Adv. Mater. Interfacesc, 2020, 7, 2000740 CrossRef CAS.
- S. Ibraheem, S. Chen, L. Peng, J. Li, L. Li, Q. Liao, M. Shao and Z. Wei, Appl. Catal., B, 2020, 265, 118569 CrossRef CAS.
- Y. Sun, K. Xu, Z. Wei, H. Li, T. Zhang, X. Li, W. Cai, J. Ma, H. J. Fan and Y. Li, Adv. Mater., 2018, 30, 1802121 CrossRef PubMed.
- X. Zhao, X. Li, Y. Yan, Y. Xing, S. Lu, L. Zhao, S. Zhou, Z. Peng and J. Zeng, Appl. Catal., B, 2018, 236, 569–575 CrossRef CAS.
- C. Huang, T. Ouyang, Y. Zou, N. Li and Z.-Q. Liu, J. Mater. Chem. A, 2018, 6, 7420–7427 RSC.
- D. Guo, R. Shibuya, C. Akiba, S. Saji, T. Kondo and J. Nakamura, Science, 2016, 351, 361–365 CrossRef CAS PubMed.
- L. Yang, J. Shui, L. Du, Y. Shao, J. Liu, L. Dai and Z. Hu, Adv. Mater., 2019, 31, 1804799 CrossRef PubMed.
- S. Kukunuri, M. R. Krishnan and S. Sampath, Phys. Chem. Chem. Phys., 2015, 17, 23448–23459 RSC.
- C. Liu, K. Wang, X. Zheng, X. Liu, Q. Liang and Z. Chen, Carbon, 2018, 139, 1–9 CrossRef CAS.
- W. Shi, X. Zhang and G. Che, Int. J. Hydrogen Energy, 2013, 38, 7037–7045 CrossRef CAS.
- X. Sun, N. Habibul and H. Du, Chin. J. Catal., 2021, 42, 235–243 CrossRef CAS.
- Y. Zhang, Y. Qiu, X. Ji, T. Ma, Z. Ma and P. A. Hu, ChemSusChem, 2019, 12, 3792–3800 CrossRef CAS PubMed.
- W. Zhao, S. Wang, C. Feng, H. Wu, L. Zhang and J. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 40491–40499 CrossRef CAS PubMed.
- D. Lyu, Y. Du, S. Huang, B. Y. Mollamahale, X. Zhang, S. W. Hasan, F. Yu, S. Wang, Z. Q. Tian and P. K. Shen, ACS Appl. Mater. Interfaces, 2019, 11, 39809–39819 CrossRef CAS PubMed.
- N. Zhang, Y. Wang, M. Jia, Y. Liu, J. Xu, L. Jiao and F. Cheng, Chem. Commun., 2018, 54, 1205–1208 RSC.
- C. Li, X. Han, F. Cheng, Y. Hu, C. Chen and J. Chen, Nat. Commun., 2015, 6, 7345 CrossRef CAS PubMed.
- C. Li, W. Yuan, C. Li, H. Wang, L. Wang, Y. Liu and N. Zhang, Chem. Commun., 2021, 57, 4319–4322 RSC.
- S. H. Lee, J. Kim, D. Y. Chung, J. M. Yoo, H. S. Lee, M. J. Kim, B. S. Mun, S. G. Kwon, Y. E. Sung and T. Hyeon, J. Am. Chem. Soc., 2019, 141, 2035–2045 CrossRef CAS PubMed.
- M. Zhu, Y. Yan, Q. Yan, J. Yin, K. Cheng, K. Ye, K. Zhu, J. Yan, D. Cao and G. Wang, Int. J. Hydrogen Energy, 2020, 45, 10486–10493 CrossRef CAS.
- B. Yu, Y. Hu, F. Qi, X. Wang, B. Zheng, K. Liu, W. Zhang, Y. Li and Y. Chen, Electrochim. Acta, 2017, 242, 25–30 CrossRef CAS.
- Z. Jin, M. Zhang, M. Wang, C. Feng and Z.-S. Wang, J. Power Sources, 2018, 378, 475–482 CrossRef CAS.
- Z. Lin, C. Wang, Z. Wang, Q. Liu, C. Le, B. Lin and S. Chen, Electrochim. Acta, 2019, 294, 142–147 CrossRef CAS.
- S. Wu, Q. Hu, T. Cui, H. Zhang, Q. Su, Y. Wang, Y. Xue, L. Wu and Q. Yang, Synth. Met., 2020, 268, 116499 CrossRef CAS.
- Y. Zhu, X. Yun, J. Li, K. Xiang, L. Xiao, H. Chen and X. Chen, J. Electrochem. Soc., 2018, 165, 3723–3731 CrossRef.
- B. Yu, F. Qi, Y. Chen, X. Wang, B. Zheng, W. Zhang, Y. Li and L. C. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 30703–30710 CrossRef CAS PubMed.
- X. Zheng, X. Han, H. Liu, J. Chen, D. Fu, J. Wang, C. Zhong, Y. Deng and W. Hu, ACS Appl. Mater. Interfaces, 2018, 10, 13675–13684 CrossRef CAS PubMed.
- Y. Shi, W. Du, W. Zhou, C. Wang, S. Lu, S. Lu and B. Zhang, Angew. Chem., Int. Ed., 2020, 59, 22470–22474 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02861h |
| This journal is © The Royal Society of Chemistry 2021 |