Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tandem imine formation via auto-hydrogen transfer from alcohols to nitro compounds catalyzed by a nanomagnetically recyclable copper catalyst under solvent-free conditions†
Sara Sobhani *a,
Hadis Hosseini Moghadama,
Seyed Ruhollah Derakhshan
*a,
Hadis Hosseini Moghadama,
Seyed Ruhollah Derakhshan a and
José Miguel Sansano
a and
José Miguel Sansano b
b
aDepartment of Chemistry, College of Sciences, University of Birjand, Birjand, Iran. E-mail: ssobhani@birjand.ac.ir; sobhanisara@yahoo.com
bDepartamento de Química Orgánica, Facultad de Ciencias, Centro de Innovación en Química Avanzada (ORFEO-CINQA), Instituto de Síntesis Orgánica (ISO), Universidad de Alicante, Apdo. 99, 03080-Alicante, Spain
First published on 26th May 2021
Abstract
A direct imination reaction was developed by tandem reaction of alcohols and nitro compounds in the presence of Cu-isatin Schiff base-γ-Fe2O3 as a nanomagnetically recyclable catalyst under solvent-free conditions. By this method, various imines were prepared in good to high yields from one-pot reaction of various alcohols (primary aromatic and aliphatic) and nitro compounds (aromatic and aliphatic) via an auto-hydrogen transfer reaction. Use of an inexpensive and easily reusable catalyst, without requiring any additives or excess amounts of benzyl alcohol as the reaction solvent are the other advantages of this method. This catalytic system has the merits of cost effectiveness, environmental benignity, excellent recyclability and good reproducibility.
Introduction
Imines are crucial intermediates in the synthesis of biologically active nitrogen compounds, such as β-lactams, dyes, fragrances, pharmaceuticals, fungicides, and agricultural chemicals.1 The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond in imines has electrophilic properties and is widely used in organic transformations such as reduction, addition, cyclization, and aziridination reactions.2 Traditionally, imines are produced from the condensation of primary amines with carbonyl compounds.3 In recent years, much attention has been paid to tandem processes as an attractive synthetic concept for improving overall process efficiency and reducing waste production by converting simple starting materials into more complex products in a single reaction vessel.4 In this regard, tandem synthesis of imines from alcohols and nitro compounds is a more advantageous method than traditional procedures, because alcohols are much more stable and readily available starting materials.5 The process involves selective oxidation of benzyl alcohol to benzaldehyde followed by the coupling reaction of amines with benzaldehyde.6 From the standpoint of sustainable development, an atom-economic, “green” and operationally convenient method, the synthesis of imines from nitro compounds is highly desirable.7 In this regard, the borrowing-hydrogen methodology (auto-hydrogen transfer), which transfers hydrogen from readily available alcohols to nitro compounds to produce amines as well as valuable aldehydes, provides a promising alternative to the existing method for the synthesis of imines.8 Some complexes and compounds derived from transition metals of the second and third rows, such as ruthenium,9 palladium,10 silver11 iridium12 and gold13 have been introduced as promoters of this strategy of the imine synthesis. However, the toxicities, pricing, and stability of these metals prohibit their general use for industrial purposes.14 Surprisingly, there is only one report on the use of copper for imines formation via auto-hydrogen transfer,15 although copper is a low cost, easily available and relatively environmentally benign metal. This reported method suffers from severe problems related to the use of a toxic organic solvent, excess amount of the base, high temperature, and requiring prolonged reaction time (3 days).
N bond in imines has electrophilic properties and is widely used in organic transformations such as reduction, addition, cyclization, and aziridination reactions.2 Traditionally, imines are produced from the condensation of primary amines with carbonyl compounds.3 In recent years, much attention has been paid to tandem processes as an attractive synthetic concept for improving overall process efficiency and reducing waste production by converting simple starting materials into more complex products in a single reaction vessel.4 In this regard, tandem synthesis of imines from alcohols and nitro compounds is a more advantageous method than traditional procedures, because alcohols are much more stable and readily available starting materials.5 The process involves selective oxidation of benzyl alcohol to benzaldehyde followed by the coupling reaction of amines with benzaldehyde.6 From the standpoint of sustainable development, an atom-economic, “green” and operationally convenient method, the synthesis of imines from nitro compounds is highly desirable.7 In this regard, the borrowing-hydrogen methodology (auto-hydrogen transfer), which transfers hydrogen from readily available alcohols to nitro compounds to produce amines as well as valuable aldehydes, provides a promising alternative to the existing method for the synthesis of imines.8 Some complexes and compounds derived from transition metals of the second and third rows, such as ruthenium,9 palladium,10 silver11 iridium12 and gold13 have been introduced as promoters of this strategy of the imine synthesis. However, the toxicities, pricing, and stability of these metals prohibit their general use for industrial purposes.14 Surprisingly, there is only one report on the use of copper for imines formation via auto-hydrogen transfer,15 although copper is a low cost, easily available and relatively environmentally benign metal. This reported method suffers from severe problems related to the use of a toxic organic solvent, excess amount of the base, high temperature, and requiring prolonged reaction time (3 days).
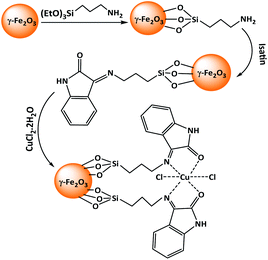
In continuation of our efforts on the introduction of new heterogeneous catalysts for organic transformations,16 we have recently synthesized Cu-isatin Schiff base-γ-Fe2O3 (Scheme 1) and used it as a new heterogeneous catalyst for the synthesis of bis(indolyl)methanes and bis(pyrazolyl)methanes.17 Herein, in this paper, we have expanded the scope of application of this catalyst for the tandem imine formation by auto-hydrogen transfer from alcohols to nitro compounds under solvent-free conditions.
Result and discussion
Cu-isatin Schiff base-γ-Fe2O3 as a nanomagnetically heterogeneous catalyst was synthesized according to our previously reported method.16 Cu-isatin Schiff base-γ-Fe2O3 as a nanomagnetically heterogeneous catalyst, was synthesized by functionalization of γ-Fe2O3 with 3-aminopropyltriethoxysilane and then the reaction with isatin to produce isatin Schiff base-γ-Fe2O3 followed by the reaction with dissolving CuCl2·2H2O in methanol (Scheme 1).The catalyst was characterized by a series of techniques such as FT-IR, XRD, TGA, TEM, SEM, VSM, ICP and elemental analysis.
The FT-IR spectra of (a) amino-functionalized γ-Fe2O3, (b) isatin Schiff base-γ-Fe2O3 and Cu-isatin Schiff base-γ-Fe2O3 are exhibited in Fig. 1. The FT-IR spectrum of amino-functionalized γ-Fe2O3 exhibited a broad band at around 550–670 cm−1 due to the stretching vibrations of Fe–O. The appeared peaks at 1085, 3438, 3480 and around 2869–2906 cm−1 in the FT-IR spectrum of amino-functionalized γ-Fe2O3 are related to C–N, N–H and C–H stretching modes of the alkyl chain, respectively. N–H bending was observed at 1631 cm−1 (Fig. 1a). New bands at 1455, 1608 and 1718 cm−1 in the FT-IR spectrum of isatin Schiff base-γ-Fe2O3 (Fig. 1b) refer to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations, respectively. These bands proved that isatin has been successfully reacted with amino-functionalized γ-Fe2O3. N–H stretching band of the amide group in isatin overlapped with the broad O–H band, which was found at 3390 cm−1. In the FT-IR spectra of Cu-isatin Schiff base-γ-Fe2O3 (Fig. 1c), the C
O stretching vibrations, respectively. These bands proved that isatin has been successfully reacted with amino-functionalized γ-Fe2O3. N–H stretching band of the amide group in isatin overlapped with the broad O–H band, which was found at 3390 cm−1. In the FT-IR spectra of Cu-isatin Schiff base-γ-Fe2O3 (Fig. 1c), the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequencies were shifted to the lower wave numbers (1600, 1704 cm−1), which showed the successful coordination of nitrogen and oxygen to the metal center.
O stretching frequencies were shifted to the lower wave numbers (1600, 1704 cm−1), which showed the successful coordination of nitrogen and oxygen to the metal center.
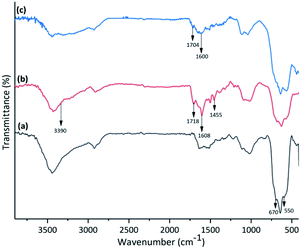 | ||
| Fig. 1 FT-IR spectra of (a) amino-functionalized γ-Fe2O3, (b) isatin Schiff base-γ-Fe2O3 and (c) Cu-isatin Schiff base-γ-Fe2O3. | ||
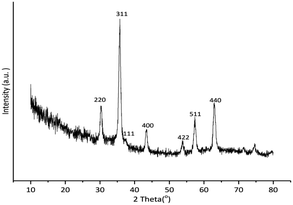
As presented in Fig. 2, the reflection planes of (2 2 0), (3 1 1), (1 1 1), (4 0 0), (4 2 2), (5 1 1) and (4 4 0) at 2θ = 30.3°, 35.7°, 39.9°, 43.4°, 53.8°, 57.4° and 63.0° were readily recognized in the XRD pattern of Cu-isatin Schiff base-γ-Fe2O3. These characteristic peaks matched with those of standard γ-Fe2O3 (JCPDS file no. 04-0755). The observed diffraction peaks was indicated that γ-Fe2O3 mostly exists in face-centered cubic structure. In addition, the diffraction plane of (111) at 2θ = 37.4° in the XRD pattern of Cu-isatin Schiff base-γ-Fe2O3 is ascribed to Cu.
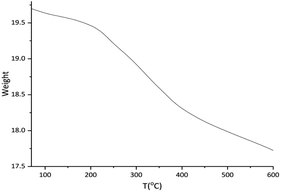
The thermogravimetric analysis (TGA) of Cu-isatin Schiff base-γ-Fe2O3 was used to determine the thermal stability and content of organic functional groups on the surface of magnetic nano particles (Fig. 3). TG curve of the catalyst showed the weight loss at around 182 °C, which was related to the adsorbed water molecules on the support. The organic parts were decomposed completely in the temperature range of 200–600 °C.
The metal content of the complex was determined by ICP-OES, and showed a value of 0.11 mmol g−1. Elemental analysis showed that the loading of isatin-Schiff base on γ-Fe2O3, was 0.31 mmol g−1 based on the nitrogen and carbon amounts (0.86% and 3.95%, respectively).
The size and morphology of the synthesized catalyst were characterized using TEM and SEM (Fig. 4). The TEM image is clearly showed that Cu-isatin Schiff base-γ-Fe2O3 exhibits spherical morphology with relatively good monodispersity (Fig. 4a). The particle size distribution of Cu-isatin Schiff base-γ-Fe2O3 was evaluated using TEM and showed that the average diameter of the particles was 13 nm (Fig. 4b). The SEM image analysis indicates that the-γ-Fe2O3 nanoparticles are spherical in shape (Fig. 4c).
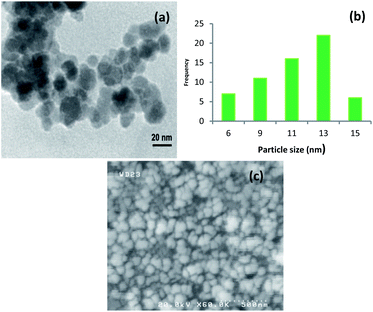 | ||
| Fig. 4 (a) TEM image of Cu-isatin Schiff base-γ-Fe2O3 and (b) particle size distribution histogram of Cu-isatin Schiff base-γ-Fe2O3. (c) SEM image of Cu-isatin Schiff base-γ-Fe2O3. | ||
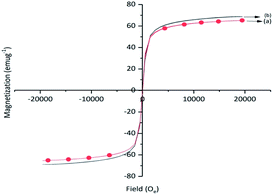
The saturation magnetization values for γ-Fe2O3 and Cu-isatin Schiff base-γ-Fe2O3 were 68.9 and 65.2 emu g−1, respectively (Fig. 5). A slight decrease of the saturation magnetization of Cu-isatin Schiff base-γ-Fe2O3 was due to the immobilization of Cu complex on the surface of γ-Fe2O3.
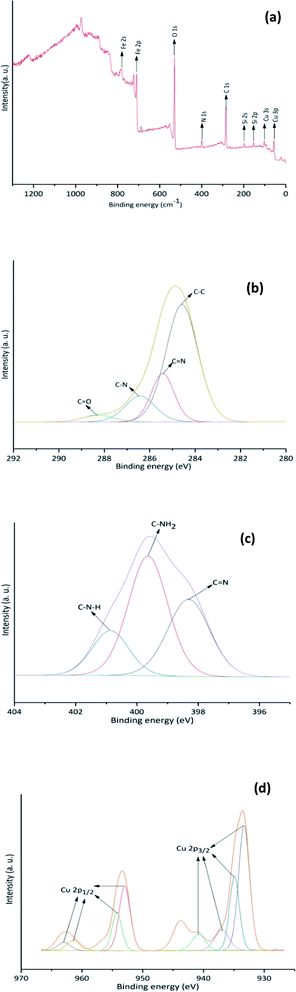
Cu-isatin Schiff base-γ-Fe2O3 was also analyzed by XPS spectroscopy (Fig. 6). The observed characteristic peaks in the XPS elemental survey are attributed to carbon (C 1s), nitrogen (N 1s), oxygen (O 1s), iron, silicon and copper (Fig. 6a).18 The C 1s spectrum (Fig. 6b) showed binding energies of 284.5 (C–C), 285.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 286.4 (C–N) and 288.3 eV (C
N), 286.4 (C–N) and 288.3 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).19 Furthermore, high resolution XPS of N 1s region confirmed the presence of N–H, imine and –NH2 by revealing three peaks at 400.8, 399.6 and 398.3 eV, respectively (Fig. 6c).20 In the XPS spectrum of Cu (Fig. 6d), the presence of binding energies at 933.3 and 940.8 eV (Cu 2p3/2) along with the binding energies at 952.9 and 963.1 eV (Cu 2p1/2) could be ascribed to Cu with (I) oxidation state. The peaks centered at 934.9, 936.9 (Cu 2p3/2) and 954.2, 961.3 eV (Cu 2p1/2) are attributed to the existence of Cu(II) in the catalyst.21
O).19 Furthermore, high resolution XPS of N 1s region confirmed the presence of N–H, imine and –NH2 by revealing three peaks at 400.8, 399.6 and 398.3 eV, respectively (Fig. 6c).20 In the XPS spectrum of Cu (Fig. 6d), the presence of binding energies at 933.3 and 940.8 eV (Cu 2p3/2) along with the binding energies at 952.9 and 963.1 eV (Cu 2p1/2) could be ascribed to Cu with (I) oxidation state. The peaks centered at 934.9, 936.9 (Cu 2p3/2) and 954.2, 961.3 eV (Cu 2p1/2) are attributed to the existence of Cu(II) in the catalyst.21
After catalyst characterization, its catalytic activity was studied in the tandem imines formation by auto-hydrogen transfer from alcohols to nitro compounds. At first, to find out the best reaction conditions, a set of factors including base, solvent, temperature and the amount of the catalyst were screened in the reaction of benzyl alcohol and nitrobenzene as the model reaction. The study of the influence of the base, as well as its absence, showed that KOH rendered the best results (Table 1, entry 4). The solvent dependency of the model reaction was investigated (Table 1, entries 7–13) and found that the reaction proceeded efficiently under solvent-free conditions as green and sustainable reaction conditions. In an effort to optimize the reaction temperature, temperatures below 100 °C were found to minimize the imines formation (Table 1, entries 14 and 15). Screening the amount of the catalyst showed that decreasing the catalyst amount from 2 to 1 mol% led to a negligible decrease in the yield of the product (Table 1, entry 16). Poorer activity was obtained by further lowering the catalyst amount (Table 1, entry 17). To show the role of the catalyst, the reaction was performed in the absence of the catalyst and also in the presence of CuCl2 and isatin Schiff base-γ-Fe2O3 as a copper less analogue of the catalyst (Table 1, entries 18–20). It was found that the desired product was obtained in low yield after 24 h. These findings indicated that a desirable activation of copper was occurred by complexation in the catalyst. The reaction of benzyl alcohol was studied in the presence of the catalyst under optimized reaction conditions (Table 1, entry 21). The results showed that benzyl alcohol was oxidized and produced benzaldehyde in 60% yield after 10 h. The reaction of nitrobenzene in the presence of the catalyst was also studied and any product was not obtained (Table 1, entry 22). This should be attributed to the lack of benzyl alcohol as a driving force for subsequent reduction of nitro groups. Performing the model reaction under O2 and Ar atmosphere proceeded with no change in the product yields (Table 1, entries 22 and 23) compared with the optimal conditions (Table 1, entry 16), which showed that alcohol dehydrogenized without requiring specific atmospheric conditions.
| Entrya | Catalyst (mol%) | Solvent | Base | Temperature (°C) | Time (h) | Isolated yield (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: nitrobenzene (0.5 mmol, except for entry 21), benzyl alcohol (0.5 mmol, except for entry 22), base (0.5 mmol, except for entry 6), solvent (3 mL, entries 7–13).b CuCl2·2H2O was used as a catalyst.c Isatin Schiff base-γ-Fe2O3 as a catalyst.d Without nitrobenzene.e Benzaldehyde was obtained as the only product in 60% yield.f Without benzyl alcohol.g Under O2.h Under Ar. | ||||||
| 1 | 2 | — | K2CO3 | 100 | 5 | 42 |
| 2 | 2 | — | Et3N | 100 | 24 | Trace |
| 3 | 2 | — | Na2CO3 | 100 | 24 | 35 |
| 4 | 2 | — | KOH | 100 | 9 | 95 |
| 5 | 2 | — | NaOH | 100 | 9 | 86 |
| 6 | 2 | — | — | 100 | 6 | 30 |
| 7 | 2 | EtOH | KOH | 65 | 3 | 22 |
| 8 | 2 | H2O | KOH | 80 | 24 | 0 |
| 9 | 2 | Acetonitrile | KOH | 68 | 24 | Trace |
| 10 | 2 | Benzyl alcohol | KOH | 100 | 7 | 81 |
| 11 | 2 | n-Hexane | KOH | 50 | 5 | 35 |
| 12 | 2 | Toluene | KOH | 90 | 3 | 32 |
| 13 | 2 | Ethyl acetate | KOH | 60 | 24 | Trace |
| 14 | 2 | — | KOH | r.t. | 24 | Trace |
| 15 | 2 | — | KOH | 65 | 7 | 56 |
| 16 | 1 | — | KOH | 100 | 10 | 92 |
| 17 | 0.5 | — | KOH | 100 | 6 | 63 |
| 18 | 0 | — | KOH | 100 | 24 | 21 |
| 19b | 1 | — | KOH | 100 | 24 | 43 |
| 20c | 1 | — | KOH | 100 | 24 | 30 |
| 21d | 1 | — | KOH | 100 | 10 | 0e |
| 22f | 1 | — | KOH | 100 | 10 | 0 |
| 22g | 1 | — | KOH | 100 | 10 | 90 |
| 23h | 1 | — | KOH | 100 | 12 | 87 |
With an optimized catalytic system in hand (Table 1), we set out to probe versatility of the present method in the direct imine synthesis of various alcohols and nitro compounds by auto-hydrogen transfer strategy. As depicted in Table 2, the reaction of various nitrobenzenes and benzyl alcohols catalyzed by Cu-isatin Schiff base-γ-Fe2O3 gave expected imines (a–m) in good to high yields (Table 2, entries 1–13). The reaction of benzyl alcohol with nitroethane as an aliphatic compound and also the reaction of nitrobenzene with 1-butanol as an aliphatic alcohol proceeded well under optimized reaction conditions (Table 2, entries 14 and 15). The imine products in the current study have widespread usages in the synthesis of biologically active compounds with antibacterial and antifungal activities.22
| Entrya | R1 | R2 | Product | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: nitro compound (1 mmol), alcohol (1 mmol), KOH (1 mmol), solvent-free, catalyst (1 mol%), 100 °C. Melting points of the solid products were compared with the reported ones in the ESI (Table S1). | |||||
| 1 | C6H5 | C6H5 | a | 10 | 92 |
| 2 | C6H5 | 4-OH–C6H4 | b | 8 | 90 |
| 3 | C6H5 | 4-OMe–C6H4 | c | 8 | 84 |
| 4 | C6H5 | 4-Me–C6H4 | d | 9 | 87 |
| 5 | C6H5 | 4-Cl–C6H4 | e | 12 | 71 |
| 6 | 4-Me–C6H4 | C6H5 | f | 13 | 91 |
| 7 | 4-Me–C6H4 | 4-Cl–C6H4 | g | 12 | 68 |
| 8 | 4-Me–C6H4 | 4-OMe–C6H4 | h | 10 | 85 |
| 9 | 4-Me–C6H4 | 4-Me–C6H4 | i | 12 | 83 |
| 10 | 4-OMe–C6H4 | C6H5 | j | 12 | 75 |
| 11 | 4-OMe–C6H4 | 4-Cl–C6H4 | k | 13 | 72 |
| 12 | 4-OMe–C6H4 | 4-OMe–C6H4 | l | 11 | 84 |
| 13 | 4-OMe–C6H4 | 4-Me–C6H4 | m | 14 | 70 |
| 14 | C6H5 | CH3–CH2– | n | 10 | 72 |
| 15 | CH3–CH3–CH2– | C6H5 | o | 12 | 85 |
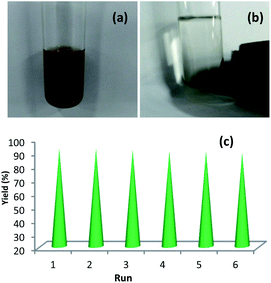
The recyclability of Cu-isatin Schiff base-γ-Fe2O3 was investigated in a model reaction of benzyl alcohol and nitrobenzene in the auto-hydrogen transfer reaction, under optimized reaction conditions. EtOAc was added to the reaction mixture after 10 h (Fig. 7a). The catalyst was isolated by an external magnet (Fig. 7b), washed with EtOAc and EtOH (2 × 10 mL) and dried under vacuum. The catalyst was successfully recycled for six times. Loss of the catalytic activity was not considerably observed for Cu-isatin Schiff base-γ-Fe2O3 in these reactions (Fig. 7c).
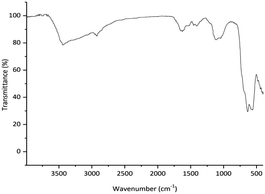
Comparison of FT-IR of the reused catalyst (Fig. 8) with the freshly prepared one (Fig. 1c) indicated that any significant changes in the chemical structure of the catalyst was not observed.
Moreover, TEM and FE-SEM images illustrated that the nanoparticles were still spherical in shape even after six times reuse (Fig. 9a and b) and the mean diameter size of the recycled catalyst was 14 nm (Fig. 9c).
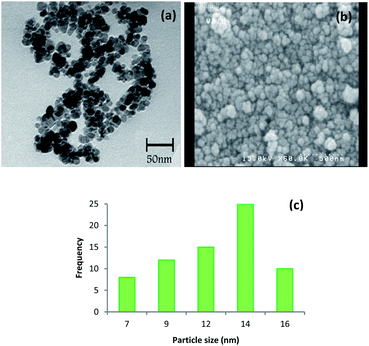 | ||
| Fig. 9 (a) TEM image, (b) FE-SEM image and (c) size distribution of the Cu-isatin Schiff base-γ-Fe2O3 after six times reuse. | ||
To study the synthetic applications and practical activity of this protocol, the reaction of benzyl alcohol (50 mmol) and nitrobenzene (50 mmol) was assessed under the optimized reaction conditions and the desired product was isolated in 90% yield after 13 h.
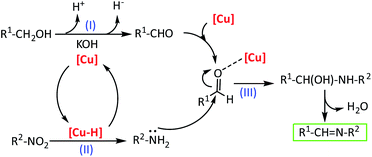
Based on our experiment results and previous literature reports,10b,12,13,14,26 a reaction mechanism to rationalize the direct imines formation from the reaction of nitroarenes with alcohols was depicted in Scheme 2. First, the alcohol is dehydrogenated to aldehyde in the presence of Cu-isatin Schiff base-γ-Fe2O3 as a catalyst promoted by base, releasing proton and hydride (step I). Simultaneously, the hydride is transferred to the copper complex to give a copper–hydride complex. This copper–hydride complex reduces the nitro group to amine via N-phenylhydroxylamine as a well-known intermediate (step II).23 In the next step, the nucleophilic attack of the amine to the activated aldehyde by the copper catalyst followed by water elimination gives the imine product (step III). In this mechanism the copper catalyst promoted both the hydrogen transfer from the alcohol to the nitro compounds and also the imine formation from the in situ produced aldehyde and amine. To investigate the mechanism in detail, the reaction of N-phenylhydroxylamine and benzyl alcohol under optimized reaction conditions was employed. The imine was obtained in 80% yield after 8 h. The reaction of benzaldehyde with aniline was also studied and the desired imine was produced in 93% yield after 6 h.
The applicability of this method was also examined for the one-pot reaction of 2-nitroaniline with benzyl alcohol under the optimized reaction conditions at the last part of our studies. Benzimidazole (p) was produced in 82% yields by auto-hydrogen transfer reaction in the presence of Cu-isatin Schiff base-γ-Fe2O3 (Scheme 3).
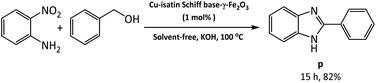 | ||
| Scheme 3 Tandem reaction of 2-nitroaniline with benzyl alcohol under the optimized reaction conditions. | ||
To show the merits of the present protocol for the synthesis of imines, we have compared the catalytic activity of Cu-isatin Schiff base-γ-Fe2O3 with those of some reported catalysts in the auto-hydrogen transfer reactions (Table 3). As depicted in Table 3, Cu-isatin Schiff base-γ-Fe2O3 is the most effective catalyst for the reaction of benzyl alcohols and nitrobenzenes. The reported synthetic routes have certain limitations such as requiring high temperature, long reaction times, expensive catalysts, additives, large amount of the catalyst, bimetallic catalysts, especial atmospheric conditions, toxic organic solvents and most importantly use of excess amounts of benzyl alcohol as the reaction solvent. More importantly, our catalyst is a magnetic heterogeneous catalyst and can be easily separated from the reaction mixture using an external magnet.
| Entry (ref.) | Catalyst (mol%) | Reaction conditions | Time (h) | Yield (%) |
|---|---|---|---|---|
| a Hydrotalcite.b Polyacrylic acid.c Co, N and C composite encapsulated carbon nanotube grown in situ on the surface of activated carbon.d Cobalt nanoparticles modified with N-doped hierarchical porous carbon derived from biomass.e Au/Ag–Mo nano-rods. | ||||
| 1 (ref. 10a) | Pd/HTa (2) | Toluene, 130 °C | 24 | 35–93 |
| 2 (ref. 11) | Ag-MCP-1b (25 mg) | Toluene, K2CO3, glycerol, 120 °C | 12 | 70–99 |
| 3 (ref. 24) | Rh/Au (0.5) | Benzyl alcohol (solvent), Cs2CO3, 100 °C | 22 | 57 |
| 4 (ref. 25) | Co–N–C/CNT@ACc (0.4 g) | Solvent free, N2 atmosphere, 160 °C | 18–42 | 12–100 |
| 5 (ref. 26) | IrIII–AuI heterodimetallic complex (1) | Benzyl alcohol (solvent), Cs2CO3, 100 °C, aerobic condition | 15–22 | 4–99 |
| 6 (ref. 14) | CoOX@NC-800d (10) | Toluene, tBuOK, 120 °C | 15 | 57–87 |
| 7 (ref. 12) | Ir–Pd heterodimetallic catalyst (2) | Benzyl alcohol (solvent), Cs2CO3, 110 °C | 3–20 | 16–92 |
| 8 (ref. 15) | CuO–Fe3O4 (1.3) | Toluene, NaOH, 130 °C | 3 d | 58–84 |
| 9 (ref. 10b) | Pd/DNA (2.9) | Water, LiOH·H2O, 50 °C, N2 balloon | 24 | 51–95 |
| 10 (ref. 13) | Au/Ag–Mo-NRe (40 mg) | Toluene, K2CO3, glycerol, Ar, 120 °C | 24 | 60–98 |
| 11 (ref. 9) | RuCl3 (3) | K2CO3, glycerol, N2 atmosphere | 24 | 72–99 |
| 12 (This work) | Cu-isatin Schiff base-γ-Fe2O3 (1) | Solvent-free, KOH, 100 °C | 8–14 | 65–92 |
Experimental
General procedure for the tandem synthesis of imine from alcohols and nitro compounds
Cu-isatin Schiff base-γ-Fe2O3 (1 mol%) was added to a mixture of alcohol (1 mmol), nitro compound (1 mmol) and KOH (1 mmol) and the resulting mixture was stirred at 100 °C for an appropriate time (Table 2). After cooling the reaction mixture to room temperature, EtOAc (5 mL) was added to the reaction mixture. The catalyst was separated by an external magnet, washed with EtOAc (2 × 10 mL) and EtOH (2 × 10 mL), dried in vacuum and reused. The solvent of the combined organic layer was evaporated under vacuum. Pure products were obtained by recrystallization in EtOH or by column chromatography eluted with n-hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (5
EtOAc (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 2
1 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR spectra of the products (a, c, d, e, g, k and p) have been presented in the ESI.†
1). 1H NMR spectra of the products (a, c, d, e, g, k and p) have been presented in the ESI.†
Conclusion
In summary, in this paper, an efficient method for the synthesis of imines by tandem reaction of various alcohols (primary aromatic and aliphatic) and nitro compounds (aromatic and aliphatic) was developed via auto-hydrogen transfer reaction, catalyzed by Cu-isatin Schiff base-γ-Fe2O3 as a nanomagnetically reusable and inexpensive catalyst under solvent-free conditions. Good to high yields of imines were achieved under mild reaction conditions without requiring any additive. After EtOAc was added to the reaction mixture, the catalyst was easily isolated by using an external magnet and reused successfully for six runs without any significant changes on the chemical structure of the catalyst. This catalytic system had the merits of cost effectiveness, environmental benignity, excellent recyclability and good reproducibility for the direct synthesis of imines from alcohols and nitro compounds without requiring high temperature, long reaction times, expensive catalysts, additives, large amounts of the catalyst, bimetallic catalysts, especial atmospheric conditions, hydrogen gas, toxic organic solvents and most importantly use of excess amounts of benzyl alcohol as the reaction solvent.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support of this project by University of Birjand Research Council is acknowledged. Access to the XPS facilities of University of Alicante is appreciated.References
- (a) A. Jarrahpour, P. Shirvani, V. Sinou, Ch. Latour and J. M. Brunel, Med. Chem. Res., 2016, 25, 149 CrossRef CAS; (b) M. S. Kiakhani and S. Safapour, Clean Technol. Environ. Policy, 2015, 17, 1019 CrossRef; (c) W. A. Zoubi, S. G. Mohamed, A. A. S. Al-Hamdani, A. P. Mahendradhany and Y. G. Ko, RSC Adv., 2018, 8, 23294 RSC; (d) M. Jafari, M. Hasanzadeh, R. Karimian and N. Shadjou, Microchem. J., 2019, 147, 741 CrossRef CAS.
- (a) Y. Feng, J. Yin, Sh. Liu, Y. Wang, B. Li and T. Jiao, ACS Omega, 2020, 7, 3725 CrossRef PubMed; (b) Y. Yamashita, H. Suzuki, I. Sato, T. Hirata and S. Kobayashi, Angew. Chem., Int. Ed., 2018, 57, 6896 CrossRef CAS PubMed; (c) M. Bai, B. Cui, J. Zuo, J. Zhao, Y. You, Y. Chen, X. Xu, X. Zhang and W. Yuan, Tetrahedron, 2015, 71, 949 CrossRef CAS; (d) H. Luo, K. Chen, H. Jiang and S. Zhu, Org. Lett., 2016, 20, 5208 CrossRef PubMed.
- (a) W. Qin, S. Long, M. Panunzio and S. Biondi, Molecules, 2013, 18, 12264 CrossRef CAS PubMed; (b) R. D. Patil and S. Adimurthy, Asian J. Org. Chem., 2013, 2, 726 CrossRef CAS.
- G. J. Chen, H. C. Ma, W. L. Xin, X. B. Li, F. Z. Jin, J. S. Wang, M. Y. Liu and Y. B. Dong, Inorg. Chem., 2017, 56, 654 CrossRef CAS PubMed.
- G. M. Zhao, H. L. Liu, X. R. Huang, D. D. Zhang and X. Yang, RSC Adv., 2015, 5, 22996 RSC.
- S. Mandal, S. Maity, S. Saha and B. Banerjee, RSC Adv., 2016, 6, 73906 RSC.
- K. Wang, W. Gao, P. Jiang, K. Lan, M. Yang, X. Huang, L. Ma, F. Niu and R. Li, Mol. Catal., 2019, 456, 43 CrossRef.
- H. Liu, G. K. Chuah and S. Jaenicke, Phys. Chem. Chem. Phys., 2015, 17, 15012 RSC.
- D. Tan, H. Li, D. J. Young and J. Long, Tetrahedron, 2016, 72, 4169 CrossRef CAS.
- (a) J. Chen, S. Huang, J. Lin and W. Su, Appl. Catal., A, 2014, 470, 1–7 CrossRef CAS; (b) L. Tang, H. Sun, Y. Li, Z. Zha and Z. Wang, Green Chem., 2012, 14, 3423 RSC.
- U. Mandi, A. S. Roy, S. K. Kundu, S. Roy, A. Bhaumik and S. M. Islam, J. Colloid Interface Sci., 2016, 472, 202 CrossRef CAS PubMed.
- A. Zanardi, J. A. Mata and E. Peris, Chem.–Eur. J., 2010, 16, 10502 CrossRef CAS PubMed.
- X. Cui, C. Zhang, F. Shi and Y. Deng, Chem. Commun., 2012, 48, 9391 RSC.
- T. Song, Y. Duan, X. Chen and Y. Yang, Catalysts, 2019, 9, 116 CrossRef.
- J. M. Perez, R. Cano, M. Yus and D. J. Ramon, Eur. J. Org. Chem., 2012, 24, 4548 CrossRef.
- (a) S. Sobhani, A. Habibollahi and Z. Zeraatkar, Org. Process Res. Dev., 2019, 23, 1321 CrossRef CAS; (b) H. H. Moghadam, S. Sobhani and J. M. Sansano, ACS Omega, 2020, 5, 18619 CrossRef CAS PubMed; (c) A. Khazaee, R. Jahanshahi, S. Sobhani, J. Skibsted and J. M. Sansano, Green Chem., 2020, 22, 4604 RSC; (d) The method for the preparation of the catalyst is explained in the ESI.†.
- S. Sobhani, S. Asadi, M. Salimi and F. Zarifi, J. Organomet. Chem., 2016, 822, 154 CrossRef CAS.
- M. Niakan, Z. Asadi and M. M. Farahani, Appl. Surf. Sci., 2019, 481, 394 CrossRef CAS.
- B. Huang, J. Chen, S. Zhan and J. Ye, J. Electrochem. Soc., 2016, 163, G26 CrossRef CAS.
- X. Zhang, X. Hu, P. Guan, N. Zhang, J. Li and C. Du, J. Mater. Sci., 2017, 52, 4713 CrossRef CAS.
- Y. Liu, L. Ma, D. Zhang, G. Han and Y. Chang, RSC Adv., 2017, 7, 12027 RSC.
- L. Ravishankar, S. A. Patwe, N. Gosarani and A. Roy, Synth. Commun., 2010, 40, 3177 CrossRef CAS.
- X. Cui, Y. Zhang, F. Shi and Y. Deng, Chem.–Eur. J., 2011, 17, 2587 CrossRef CAS PubMed.
- M. Böhmer, F. Kampert, T. T. Y. Tan, G. G. Barrios, E. Peris and F. E. Hahn, Organometallics, 2018, 37, 4092 CrossRef.
- D. Liu, P. Yang, H. Zhang, M. Liu, W. Zhang, D. Xu and J. Gao, Green Chem., 2019, 21, 2129 RSC.
- S. Sabater, J. A. Mata and E. Peris, Chem.–Eur. J., 2012, 18, 6380 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The synthesis and analysis of the catalyst. See DOI: 10.1039/d1ra02347k |
| This journal is © The Royal Society of Chemistry 2021 |