 Open Access Article
Open Access ArticleZirconia/phenylsiloxane nano-composite for LED encapsulation with high and stable light extraction efficiency†
Ying Lu‡
ab,
Zhihang Zhao‡a,
Xianpeng Fana,
Xinyu Cao *a,
Mingtan Haib,
Zhou Yang*b,
Kun Zheng
*a,
Mingtan Haib,
Zhou Yang*b,
Kun Zheng a,
Jiaxin Lua,
Jingnan Zhang
a,
Jiaxin Lua,
Jingnan Zhang a,
Yongmei Ma
a,
Yongmei Ma *a,
Rongben Zhanga and
Shibi Fanga
*a,
Rongben Zhanga and
Shibi Fanga
aKey Laboratory of Green Printing, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: xinyucao@iccas.ac.cn; yangz@ust.edu.cn; maym@iccas.ac.cn
bDepartment of Materials Physics and Chemistry, School of Materials Science and Engineering, University of Science and Technology Beijing, Beijing, 100083, China
First published on 21st May 2021
Abstract
To obtain a rapid processible LED encapsulant that leads to high and stable light extraction efficiency (LEE), UV curable ZrO2/phenyl-siloxane nano-composite (ZSC) double-layer encapsulants were prepared and optimized. The highly crystalline ZrO2 nanoparticles with a diameter of ∼14 nm were synthesized through a modified hydrothermal method at mild conditions, and a UV curable methacryl-diphenyl-polysiloxane (MDPS) with a refractive index (RI) of 1.54 (at 633 nm) was synthesized from self-condensation of diphenylsilanediol and an end-capping reaction. High refractive indexes (RIs) from 1.54–1.61 have been obtained for ZSC composites by adding 0–20 wt% ZrO2. Before and after sulfur vapor erosion, the double-layer encapsulated sample (M-10/M) showed 11.2% and 64.8% higher LEE respectively than that of Dow Corning OE-7662. Meanwhile, the variation of LED light color temperature (Tc) was less than 1%. The effect of the ZrO2 nanoparticle content on LEE of double-layer and single-layer encapsulation were compared and discussed based on Fresnel loss and Rayleigh scattering theories. The double-layered UV curing processing took only 1/6 of the time needed for common thermal curing.
1 Introduction
Light-emitting diodes (LEDs) have been widely accepted as light sources in various circumstances owing to their low power consumption and long lifetime. The encapsulation materials that directly contact the blue-chip provide close protection to the package, and also seriously affect its output efficiency and stability.1 Silicone-based materials have become the most studied encapsulants for LEDs because of their high transparency and resistance to ultraviolet (UV) radiation and heat.2 Nevertheless, silicone materials are classical with a refractive index (RI) of ∼1.4–1.5, which is much lower than that of the LED substrate (∼2.6 for GaN or 3.5 for GaP). The mismatch of RI can dramatically reduce the light extraction efficiency (LEE) due to light loss at the interface. On the other hand, because of the extreme flexibility of silicone chains, their gas or vapor permeation is quite high and is harmful to the inner metal components and leads to quick decline or even extinction of the light. A lot of efforts have been made to increase the RI of silicone materials3–6 and their gas barrier properties7–11 to enhance the light extraction efficiency (LEE) value and stability.The RI of material is contributed by the sum of each chemical unit and composite, so there are generally two ways to increase the RI of silicone materials. One is to introduce organic groups with high polarizability and small molecular volume to the molecular chain or substituents by a chemical bond, such as halogen atoms, sulfur atoms, and π-conjugated groups. In considering thermal stability and cost, phenyl siloxanes12–18 are the most commonly used. However, with the highest phenyl substitution, the siloxanes may still subject to a RI ceiling of ∼1.6.19–22
The other way to increase the RI of siloxane is by introducing inorganic nanoparticles with high RI, such as ZrO2, TiO2, and ZnO into the matrix. The RI of the composite can be varied in a wide range according to the method of preparation and nanoparticle content. To maintain the transparency of the composite, the particle size, fraction, and dispersion in the matrix are critical. Studies have shown that when the size of the inorganic particles is less than one-tenth of the wavelength of visible light, especially 20 nm, the light scattering of the nanocomposite can be greatly reduced.13,23
The copolymerization of Si and A (Zr,2,24–26 Ti,27–29 Zn,30 etc.) base precursors by the sol–gel process can lead to high transparent hybrid material, in which the hybrid nano-domains are uniformly dispersed and form Si–O–A bond with silicone.2 In some stepwise sol–gel methods, ZrO2,23,31 TiO2 (ref. 22 and 32) particles are formed in situ and then further composite with siloxanes. The RI was reportedly reached 1.59 (ref. 24) to 1.60.22
The introduction of crystalline ZrO2,1,33–43 TiO2 (ref. 44–49) nanoparticles into siloxane are more effective to increase the RI because the RI of the nanoparticle increases dramatically with crystallinity. ZrO2 has excellent optical properties, such as Abbe number and transparency on a broad spectral range,50 as well as chemical inertness and thermal stability. ZrO2/siloxane nano-composites with RIs of 1.7–1.9 were reported when the mass fraction of the nanoparticles raised to 50% (ref. 1 and 38) to 80%.50 It is well aware that the nano-particles tend to aggregate in high loading. The optical transparency and mechanical property of the material could be much compromised at such high ZrO2 content.23,50 A few reports did demonstrate 10–13% LEE enhancement by using ZrO2/epoxy or siloxane composite encapsulation with ZrO2 loading of ∼50 wt%. However, the LEE stabilities were reportedly decreased compare with the matrixes.1,41,42 It could be caused by a large amount of un-condensed Zr–OH in the system.
Here, we report a UV curable transparent ZrO2/siloxane nano-composite (ZSC) with an RI of 1.55–1.61 at 5–20 wt% ZrO2 loading. The ∼14 nm ZrO2 was highly crystalline and the surface Zr–OHs were sealed by a mono-OH silane coupling agent. The high RI silicone resin was a methacrylate-end-capped polydiphenylsiloxane (MDPS resin) with an RI of 1.54. Furthermore, double-layer LED encapsulation was designed and optimized based on the Fresnel loss theory, and experimental data. The double-layered encapsulant demonstrated an effective and efficient utilization of such the composite that leads to 11% and 65% higher LEE and anti-sulfuric corrosion respectively compared to commercial high RI product Dow Corning OE-7662, and the LEE is also higher than the ZSC single-layer encapsulation. Meanwhile, the double-layer encapsulation showed good adhesive at the interfaces characterized by SEM and red ink tests. The double-layer encapsulation can be accomplished in 40 min that shows promising potential for further gradient content layer encapsulation and scale-up application.
2 Results and discussion
2.1 Zirconia/siloxane composite
Fig. 1 illustrates the preparation method for the transparent high RI ZSC. To achieve high RI of the ZSC, the matrix was designed as a high phenyl content siloxane. It was synthesized by methacryloxy end-capped of diphenylsiloxane oligomers (MDPS) (Fig. 2). The highly crystalline ZrO2 nanoparticles were synthesized from a modified hydrothermal method at comparatively mild conditions.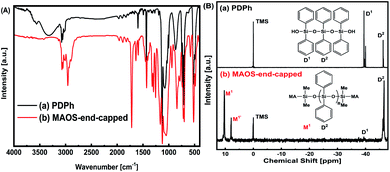 | ||
| Fig. 2 The product of MDPS was characterized by (A) FT-IR spectra and (B) 29Si-NMR spectra. In each digraph, spectra before (a) and after (b) MAOS end-cap are compared. MA represents methacryloyloxy. | ||
The phenyl-siloxane products were monitored by FTIR and 29Si-NMR (Fig. 2). In the FTIR spectrum (Fig. 2A), the strong absorption around 3070 cm−1, 1728 cm−1, and 1558 cm−1 are assigned to the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H, C
C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C
O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. After end-capped, the absorptions at 3290 cm−1 that assigned to Si–OH for polydiphenylsiloxane oligomer are nearly disappeared.
C. After end-capped, the absorptions at 3290 cm−1 that assigned to Si–OH for polydiphenylsiloxane oligomer are nearly disappeared.
In the 29Si-NMR spectrum of the oligo-polydiphenylsiloxane, the peaks at ca. −46 ppm corresponds to the fully condensed Si (D2) from –O2SiPh2, and the peaks at −40 ppm belongs to the Si (D1) in –OSiPh–OH. After the end-cap reaction, the D1 peak almost disappeared, and the peak corresponds to the Si (M1) from MASiMe2O– appears at ∼10 ppm. From the corresponding peak area ratio, the polymerization degree of oligo-polydiphenylsiloxane (PDPh) is estimated as 2.8. After end-cap reaction, it is 4.1 (including end-capping groups), and the end-capping ratio is 96% (see ESI and Fig. S1†). The MDPS resin is highly transparent with an RI of 1.541 at 633 nm.
TEM and XRD analysis (Fig. 3, S2 and S3 in ESI†) show that the as-prepared ZrO2 particles are highly crystalline tetragonal phases with an average size of ∼14 nm. The HR-TEM image in Fig. 3A shows that the spacing of the lattice fringe is about 0.252 nm that belongs to the tetragonal phase (110) crystal plane that agrees with the XRD result. Simulation from the XRD pattern of the modified nanoparticles (Fig. 3D) indicates that the ZrO2 nanoparticles are tetragonal with a crystallinity of ca. 80% (Fig. S3†).
In the FTIR spectrum of the as-prepared ZrO2 nanoparticle, the broad peak at ∼3330 cm−1 is assigned to the Zr–OH stretching. After surface modification, the Zr–OH absorption decreased, and new peaks at 1025 cm−1 assigned to the Si–O–Zr appear (Fig. 3E). The appearance of absorptions at 3070 cm−1, 1728 cm−1, and 1558 cm−1 assigned to the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H, C
C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C
O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C respectively indicating the successful graft of the methacryloxylsiloxyl. After surface modification, the average diameter of the collected M-ZrO2 nanoparticle increased a little bit to 17 nm (Fig. S2 ESI†). The M-ZrO2 nanoparticle can then be well dispersed in THF and MDPS resin (Fig. 3A–C).
C respectively indicating the successful graft of the methacryloxylsiloxyl. After surface modification, the average diameter of the collected M-ZrO2 nanoparticle increased a little bit to 17 nm (Fig. S2 ESI†). The M-ZrO2 nanoparticle can then be well dispersed in THF and MDPS resin (Fig. 3A–C).
Transparent films were obtained from the ZSC with ZrO2 content of 5–20 wt% (Fig. 4A). Although the transmittance decreases with the content of the ZrO2 nano-particle, it is still 92.2% at 450 nm for the 20 wt% ZrO2 content film. The RI of pure MDPS (M) resin, M-5, M-10, M-15, and M-20 at 633 nm was 1.540, 1.551, 1.573, 1.584, and 1.611, respectively, measured by Abbe refractometer (Fig. 4B). It has to be mentioned that when the RI of ZSC films were measured by ellipsometric method, the values were much higher. It probably because of the gradient distribution of ZrO2 in the film, which need to be studied in detail in the future (Fig. S4†). Table 1 lists the optical and thermal properties of ZSCs with different ZrO2 content (also see Fig. S5†). The RIs measured with different ZrO2 content were generally agree with those calculated by Lorentz–Lorenz equation. The thermal stability of the nano-composites increases with the content of ZrO2 wt% in term of both starting degradation temperature and residue at 800 °C.
| Sample name | ZrO2 [wt%] | ZrO2 [v/v%] | Td-10% [°C] | RIa [633 nm] | RIb [633 nm] | T% [633 nm] |
|---|---|---|---|---|---|---|
a Measured by Abbe refractometer at 633 nm.b Calculate from Lorentz–Lorenz equation:  , where nc is RI of ZSC; np is RI of ZrO2; nm is RI of MDPS resin. , where nc is RI of ZSC; np is RI of ZrO2; nm is RI of MDPS resin. |
||||||
| M | 0 | 0 | 315.2 | 1.54 | — | 98.5 |
| M-5 | 5% | 1.72% | 317.7 | 1.55 | 1.55 | 98.0 |
| M-10 | 10% | 3.56% | 330.2 | 1.57 | 1.56 | 97.5 |
| M-15 | 15% | 5.54% | 340.6 | 1.58 | 1.57 | 96.6 |
| M-20 | 20% | 7.68% | 353.7 | 1.61 | 1.58 | 96.5 |
2.2 LED encapsulation
It has been reported that for a single-layer encapsulant of LED (GaN) there is a maximum transmission coefficient of 0.8931 when the RI of the encapsulant reaches 1.612 (at 633 nm) according to Fresnel loss theory.36,54 Once the RI exceeds the maximum value, the transmission coefficient begins to decline. When applying this theory to the above ZSC with ZrO2 content from 0 to 20%, the best transmittance is expected from the sample M-5 (RI = 1.551 at 633 nm), and the highest LEE is also expected. However, the experiments show that the transmittance obtained monotonically decreases with the ZrO2 nano-particle content from M to M-20 (Fig. 5A), and the LEE of the single-layer encapsulated was also monotonically decreased with ZrO2 content (Fig. 5B).In the above Fresnel loss simulation, it was assumed that only the RIs affect the result. For silicone nano-composites, the situation is more complicated, and the experimental data suggest that the light loss from nanoparticle scattering and the film thickness cannot be ignored even within nano-size below 20 nm. Fig. 5A shows that when the film thickness of ZSC increased from 80 μm to 280 μm, the transmittance of M-10 decreased from 94% to 85%, and the transmittance decreases more quickly when ZrO2 content goes up (also seeing Fig. S5B ESI†).
If a double-layered encapsulant of MDPS (up layer) and ZSC (bottom layer) combination is used, it not only is more effective in decreasing the mismatch of RI between the substrate and air but also reduces the thickness of the composite film compare to single-layer encapsulation strategy that can greatly reduce the light scattering loss according to Rayleigh scattering theory. The effect of double-layer encapsulation can be estimated by the Fresnel transmission coefficient (FTC) expressed as eqn (1) and (2) that proposed by Mont et al.54
Fresnel loss:
 | (1) |
Fresnel transmission coefficient:
 | (2) |
The simulation for double-layer encapsulation demonstrates that with the continuous change of refractive index combination (n1 and n2), the FTC results fall on a curved surface (Fig. 6A). The highest FTC of 0.9288 can be achieved when n1 equals 1.89 and n2 equals 1.37. When applied for the ZSC/MDPS samples, the calculated FTC values of double-layer encapsulation are all higher than that of the single-layer encapsulation, and it increases with the ZrO2 nanoparticle content in ZSC (Fig. 6B). The increase in FTC is more evident when the ZrO2 content was from 0 to 10%.
 | ||
| Fig. 6 (A) Schematic diagram of the refractive index combination and interfaces of (a) LED chip/air, (b) LED chip/singer layer encapsulant/air, and (c) LED chip/double-layer encapsulant/air. (B) The simulation of FTC is derived from eqn (2). (C) The calculated FTCs of doubled-layer encapsulation of M and ZSC/M with different ZrO2 content. | ||
The two-layer combination of ZSC (M-5, M-10, M-15, M-20) and MDPS (M) was also optimized by experimental characterization (Fig. 7). The thickness of the whole encapsulant is ca. 500 μm. A typical cross-section SEM photo of two-layer encapsulation is shown in Fig. S7.† It shows that all the double-layer encapsulation did lead to higher LEEs than those of single-layer encapsulation. The LEE increased obviously at the beginning when ZrO2 nano-particle content increases from 0 to 10% in the ZSC layer, but then the LEE decreases with the ZrO2 nano-particle content. The best LEE value was obtained from M-10/M (Fig. 7B) which is 11.2% higher than that of OE-7662 and 7.6% higher than that of M (MDPS single-layer encapsulation). The Tc of ZSC/M system encapsulated samples are in the range of 5000–6000 K, which is preferred for many commercial usages, while the Tc of OE-7662 is much higher in this case.
For LED, the water resistance and gas/vapor barrier property of the encapsulants are very important for the life time. In this work, it is demonstrated by the anti-sulfur vapor test and red ink test. The M-10/M double-layer encapsulated sample kept the highest LEE value after 2–4 hours of sulfur vapor corrosion. It is worth noticing that the novel polydiphenylsiloxane MDPS encapsulant has a much better anti-sulfur property itself, and the composition of the ZrO2 nanoparticle make the LEE value even higher. The LEE of OE-7662 was reduced by 8.4% and 36.5% respectively after 2 hours and 4 hours sulfur vapor corrosion, while the reduction of LEE of M-10/M double-layer encapsulated sample is 3.3% and 5.9% respectively upon 2 hours and 4 hours test (Fig. 7C). It means after 4 hours of sulfur vapor erosion, the LEE of M-10/M is 64.8% higher than that of the OE-7662. The Tc changes of M-10/M and M encapsulation kept within 1%, while that of OE-7662 drifted up by 40% (Fig. 7D and E).
All the double-layer encapsulated samples passed the red ink test showing that they have good adhesion with the chip substrate and anti-humidity property. There is no trace of red ink permeation and cracks can be observed by optical and SEM observation (Fig. S6 and S7†).
The SEM observation (Fig. 8A) also shows that the ZSC layer and the MDPS layer are well compatible and bonded. Fig. 8B shows that the SEM observation of the encapsulation of LED chip (M-10/M) after the red ink test. After the red ink test, the encapsulation resin has good adhesion to the substrate, and this also provides a basis for its anti-sulfur and stable LEE output.
 | ||
| Fig. 8 (A) The SEM observation of the cross section of the two-layer film on silicon wafer (M-10/M). (B) The SEM observation of the encapsulation of LED chip (M-10/M) after the red ink test. | ||
3 Experimental section
3.1 Materials
Zirconyl chloride octahydrate (ZrOCl2·8H2O) was purchased from Aladdin. 2-Hydroxy-2-methylpropiophenone (97%) (photoinitiator-1173, 98%) was purchased from Sigma-Aldrich LLC. The phosphor YGG-530 was from China Minmetals Corporation. Irganox-1076 (>98%) and Irgafos-168 (>98%) were purchased from TCI. Trimethylolpropane trimethacrylate was purchased from Energy Chemical. Diphenylsilanediol (DPSD) (98%), 3-methacryloxypropyltrimethoxysilane (MPTMS), and methacryloyloxydimethylmethoxysilane (MAOS) were purchased from Innochem (Beijing) Technology Co., Ltd. The reference silicone encapsulants OE-7662 were purchased from Dow Corning. Triethanolamine (TEA), tetrahydrofuran (THF) and toluene were purchase from Aladdin. All chemicals were used without further purification.3.2 Synthesis and preparation
For surface modification, ZrO2, MPTMS with the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 was mixed with 30 ml of THF. The mixture was first treated by ultrasound for 30 min and then stirred at 60 °C for 12 h. The product was precipitated by n-hexane and washed with ethanol to remove extra MPTMS and other residuals. The precipitation of MPTMS-ZrO2 nanoparticles (M-ZrO2) was re-dispersed in THF under ultrasonic.1 The ZrO2 content in M-ZrO2 was estimated to be ∼88 wt% by compare the RI of composite resin with ∼5.7 wt% M-ZrO2 (M-5 in Table 1) with that calculated from Lorentz–Lorenz equation.
1.9 was mixed with 30 ml of THF. The mixture was first treated by ultrasound for 30 min and then stirred at 60 °C for 12 h. The product was precipitated by n-hexane and washed with ethanol to remove extra MPTMS and other residuals. The precipitation of MPTMS-ZrO2 nanoparticles (M-ZrO2) was re-dispersed in THF under ultrasonic.1 The ZrO2 content in M-ZrO2 was estimated to be ∼88 wt% by compare the RI of composite resin with ∼5.7 wt% M-ZrO2 (M-5 in Table 1) with that calculated from Lorentz–Lorenz equation.
3.3 Characterization
Fourier transform infrared spectrometry (FTIR) spectra were recorded by a Bruker EQUINOX55 spectrophotometer. 29Si-NMR spectrum was performed on Bruker 300 MHz instruments (DMX300) in THF solution with TMS and relaxation reagent (chromium acetylacetonate). X-ray diffraction (XRD) analysis was recorded on Empyrean. The transmission electron microscope (TEM) images were captured by TEM, JEM-2200FS, JEOL, Japan. The thermal gravimetric analysis (TGA) was performed on a PerkinElmer/Pyris 1 TGA by the heating rate of 20 °C min−1 in a nitrogen atmosphere. The optical transmittance was measured by an ultraviolet-visible spectrophotometer (UV2600, SHIMADZU). RI of the L-MPS resin was measured by an Abbe refractometer (2WAJ, Shanghai Optical Instrument Factory No. 1). The LEE was detected by spectrophotocolorimeter with the LED spec analysis system (STC 4000, Everfine Photo-E-Info Co., Ltd.). The gas barrier property was demonstrated by the LEE and Tc change in the anti-sulfur test, in which samples and 1.34 g sulfur powder were kept at 105 °C for 4 h in an 800 ml sealed container. The red ink test was processed as follows. The 2% of red ink solution 2 ml was added to 100 ml DI water then heated to 100 °C. Then put the encapsulated LED chip into the solution and continued heating for 2 h. When the chip was cooled down to room temperature and dried in the air, it was observed under a microscope to find whether leakage happed, and the sample was also tested for the ability to be lit.4 Conclusions
Transparent UV curable nano-composite ZSC with high RI was prepared from a highly crystalline ∼14 nm ZrO2 nano-particle and a polydiphenylsiloxane MDPS. When the ZrO2 nano-particle content was increases from 0 to 20%, the RI of the composite increase from 1.54–1.61, and the transmittance was all above 92% with a film thickness of ∼80 μm. The double-layer encapsulation M-10/M obtained 11.2% higher LEE compares to that of OE-7662, and higher LEE than those of the single-layer encapsulated samples. Furthermore, after 4 h of sulfur vapor erosion, the LEE of the M-10/M sample is 64.8% higher than that of OE-7662. At the same time, the Tc changes of M-10/M and M encapsulation kept within 1%, while that of OE-7662 drifted by 40%. The UV curing of the double layer encapsulation can be accomplished in 40 min which is still much time and energy saving compare to traditional thermal curing. The combination of highly crystalline zirconia nano-particles and UV curable polydiphenylsiloxane shows promising potential for further multi-layer LED encapsulation with enhanced quality.Author contributions
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the support from NSFC (51373179, 51673023, 51773017, 51973017, 51803218, 51373184), the National Key R&D Program of China (2018YFB0703703, 2016YFB0303000 and 2016YFB1100800), the State Key Laboratory for Advanced Metals and Materials (2018Z-06) and the Fundamental Research Funds for the Central Universities (FRF-DF-19-001). The authors also thank Mr Rui Wang and Beijing Kehua Advanced Materials Technology Co., Ltd for helpful discussion and sample test.References
- P.-T. Chung, S.-H. Chiou, C.-Y. Tseng and A. S. T. Chiang, ACS Appl. Mater. Interfaces, 2016, 8(15), 9986–9993 CrossRef CAS PubMed.
- Y. H. Kim, J.-Y. Bae, J. Jin and B.-S. Bae, ACS Appl. Mater. Interfaces, 2014, 6(5), 3115–3121 CrossRef CAS PubMed.
- H. J. Duan, W. J. Dong, X. Wang, X. X. Tao and H. R. Ma, J. Appl. Polym. Sci., 2019, 136(43), 9 CrossRef.
- P.-H. Carlos, L. J. Guo and P.-F. Fu, ACS Nano, 2010, 4(8), 4776–4784 CrossRef PubMed.
- T. T. Zhao, R. Yu, S. Li, X. P. Li, Y. Zhang, X. Yang, X. J. Zhao, C. Wang, Z. C. Liu, R. Dou and W. Huang, ACS Appl. Mater. Interfaces, 2019, 11(15), 14391–14398 CrossRef CAS PubMed.
- J.-Y. Bae, Y. H. Kim, H. Y. Kim, Y. B. Kim, J. h. Jin and B.-S. Bae, ACS Appl. Mater. Interfaces, 2015, 7(2), 1035–1039 CrossRef CAS PubMed.
- M. Velderrain, Designing low permeability, optical-grade silicone systems: guidelines for choosing a silicone based on transmission rates for barrier applications, in Advances in Display Technologies II, International Society for Optics and Photonics, 2012 Search PubMed.
- S. Lee, J.-Y. Hong and J. Jang, ACS Nano, 2013, 7(7), 5784–5790 CrossRef CAS PubMed.
- Y. Tokudome, T. Hara, R. Abe and M. Takahashi, ACS Appl. Mater. Interfaces, 2014, 6(21), 19355–19359 CrossRef CAS PubMed.
- C. Zhang, C. Zhang, R. M. Ding, X. M. Cui, J. Wang, Q. H. Zhang and Y. Xu, ACS Appl. Mater. Interfaces, 2016, 8(23), 14766–14775 CrossRef PubMed.
- J. Gao, F. Bao, Q. X. Wu, R. Ma, X. B. Han, D. P. Jin, K. Y. Chena, J. Y. He, Z. F. Guo and C. J. Yan, Mater. Today Commun., 2016, 7, 149–154 CrossRef CAS.
- Y. H. Kim, Y.-W. Lim, D. Lee, Y. H. Kim and B.-S. Bae, J. Mater. Chem. C, 2016, 4(46), 10791–10796 RSC.
- J. Jin, S. Yang and B.-S. Bae, J. Sol-Gel Sci. Technol., 2012, 61(2), 321–327 CrossRef CAS.
- M. Zhao, Y. K. Feng, Y. Li, G. Li, Y. Wang, Y. Han, X. J. Sun and X. H. Tan, J. Macromol. Sci., Part A: Pure Appl. Chem., 2014, 51(8), 653–658 CrossRef CAS.
- J.-S. Kim, S. C. Yang, S.-Y. Kwak, Y. W. Choi, K.-W. Paik and B.-S. Bae, J. Mater. Chem., 2012, 22(16), 7954–7960 RSC.
- T. C. Loh, C. M. Ng, R. N. Kumar, H. Ismail and Z. Ahmad, J. Appl. Polym. Sci., 2017, 134(37), 45285 CrossRef.
- J.-Y. Bae, Y. H. Kim, H.-Y. Kim, Y.-W. Lim and B. S. Bae, RSC Adv., 2013, 3(23), 8871–8877 RSC.
- J.-S. Kim, S. C. Yang and B.-S. Bae, Chem. Mater., 2010, 22(11), 3549–3555 CrossRef CAS.
- Y. H. Kim, Y.-W. Lim, D. Lee, Y. H. Kima and B.-S. Bae, J. Mater. Chem. C, 2016, 4(46), 10791–10796 RSC.
- S. C. Yang, S.-Y. Kwak, J. H. Jin, J.-S. Kim, Y. W. Choi, K.-W. Paik and B.-S. Bae, J. Mater. Chem., 2012, 22(18), 8874–8880 RSC.
- X. X. Shang, S. Duan, M. Zhang, X. Y. Cao, K. Zheng, J. N. Zhang, Y. M. Ma and R. B. Zhang, RSC Adv., 2018, 8(17), 9049–9056 RSC.
- P. Huang, H.-Q. Shi, H.-M. Xiao, Y.-Q. Li, N. Hu and S.-Y. Fu, Sci. Rep., 2017, 7(1), 5951 CrossRef.
- C.-L. Tsai and G.-S. Liou, Chem. Commun., 2015, 51(70), 13523–13526 RSC.
- T. Z. Guo, X. D. Lin, X. S. Hu, S. W. Cui and X. L. Chen, Int. J. Polym. Anal. Charact., 2018, 23(2), 120–127 CrossRef CAS.
- J.-Y. Bae, S. C. Yang, J. H. Jin, J.-S. Kim and B.-S. Bae, J. Sol-Gel Sci. Technol., 2011, 58(1), 114–120 CrossRef CAS.
- M. Ochi, D. Nii, Y. Suzuki and M. Harada, J. Mater. Sci., 2010, 45(10), 2655–2661 CrossRef CAS.
- Q. Lu and M. E. Mullins, MRS Online Proc. Libr., 2012, 1400, 23–28 Search PubMed.
- C.-C. Chang, L.-P. Cheng, F.-H. Huang, C.-Y. Lin, C.-F. Hsieh and W.-H. Wang, J. Sol-Gel Sci. Technol., 2010, 55(2), 199–206 CrossRef CAS.
- A. R. M. Dalod, O. G. Grendal, A. B. Blichfeld, V. Furtula, J. Pérez, L. Henriksen, T. Grande and M. Einarsrud, Nanomaterials, 2017, 7(12), 460 CrossRef PubMed.
- X. Zhao, L. Li, Z. Li and C. P. Wong, Stretchable and transparent silicone/zinc oxide nanocomposite for advanced LED packaging, in 2014 IEEE 64th Electronic Components and Technology Conference (ECTC), IEEE, 2014 Search PubMed.
- T. Otsuka and Y. Chujo, Polym. J., 2008, 40(12), 1157 CrossRef CAS.
- Y. W. Lai, L. J. Jin, J. Z. Hang, X. Y. Sun and L. Y. Shi, J. Coat. Technol. Res., 2015, 12(6), 1185–1192 CrossRef CAS.
- Y. Li, L. Wang, B. Natarajan, P. Tao, B. C. Benicewicz, C. Ullala and L. S. Schadler, RSC Adv., 2015, 5(19), 14788–14795 RSC.
- K. Xu, S. Zhou and L. Wu, Prog. Org. Coat., 2010, 67(3), 302–310 CrossRef CAS.
- C. Liu, T. Hajagos, D. Y. Chen, Y. Chen, D. Kishpaugh and Q. B. Pei, ACS Appl. Mater. Interfaces, 2016, 8(7), 4795–4802 CrossRef CAS PubMed.
- Y.-T. Lin, Y.-H. Li, I.-A. Lei, C.-Y. Kuo, C.-F. Lee, W.-Y. Chiu and T.-M. Don, Mater. Chem. Phys., 2018, 206, 136–143 CrossRef CAS.
- X. L. He, Z. Wang, Y. Pu, D. Wang, R. J. Tang, S. Cui, J.-X. Wang and J.-F. Chen, Chem. Eng. Sci., 2019, 195, 1–10 CrossRef CAS.
- Y. Li, P. Tao, R. W. Siegel and L. S. Schadler, MRS Online Proc. Libr., 2013, 1547, 161–166 CrossRef.
- T. A. Cheema and G. Garnweitner, CrystEngComm, 2014, 16(16), 3366–3375 RSC.
- J. M. Park, H. S. Lee and H. M. Lim, Preparation and Characterization of Nano-size ZrO2 Particle for Highly Refractive Index Nanocomposite Depending on Zirconium Precursor and Concentration, in Materials Science Forum, Trans Tech Publ., 2018 Search PubMed.
- P. T. Chung, C. T. Yang, S. H. Wang, C. W. Chen, A. S. T. Chiang and C.-Y. Liu, Mater. Chem. Phys., 2012, 136(2–3), 868–876 CrossRef CAS.
- P. Tao, Y. Li, R. W. Siegel and L. S. Schadler, J. Appl. Polym. Sci., 2013, 130(5), 3785–3793 CrossRef CAS.
- X. l. He, R. J. Tang, Y. Pu, J.-X. Wang, Z. Wang, D. Wang and J.-F. Chen, Nano Energy, 2019, 62, 1–10 CrossRef CAS.
- W.-H. Liao, S.-T. Hsiao, Y.-F. Wu, S.-J. Zeng, S.-M. Li, Y.-S. Wang and C.-C. M. Ma, RSC Adv., 2014, 4(73), 38614–38622 RSC.
- Y. Liu, Z. Y. Lin, X. Y. Zhao, C.-C. Tuan, K.-S. Moon, S. Yoo, M.-G. Jang and C.-P. Wong, IEEE Trans. Compon., Packag., Manuf. Technol., 2014, 4(7), 1125–1130 CAS.
- J.-H. Huang, C.-P. Li, C.-W. C. Jian, K.-C. Lee and J.-H. Huang, J. Taiwan Inst. Chem. Eng., 2015, 46, 168–175 CrossRef CAS.
- N. Nakayama and T. Hayashi, J. Appl. Polym. Sci., 2007, 105(6), 3662–3672 CrossRef CAS.
- Y. Shen, L. Wang, H. Zhang, T. Wu and H. Y. Pan, Materials Science and Engineering, IOP Publishing, 2015 Search PubMed.
- X. L. He, Y. Pu, J.-X. Wang, D. Wang and J.-F. Chen, Langmuir, 2021, 37(8), 2707–2713 CrossRef CAS PubMed.
- Y. Xia, C. Zhang, J.-X. Wang, D. Wang, X.-F. Zeng and J.-F. Chen, Langmuir, 2018, 34(23), 6806–6813 CrossRef CAS PubMed.
- C.-W. Chen, X.-S. Yang and A. S. Chiang, J. Taiwan Inst. Chem. Eng., 2009, 40(3), 296–301 CrossRef CAS.
- X. Y. Cao, Y. Lu, X. P. Fan, Y. M. Ma, C. X. Yang, Z. H. Zhao, J. N. Zhang, K. Zheng, G. Ye, R. B. Zhang and S. B. Fang, CN2019111045869, 2019.
- X. Y. Cao, X. P. Fan, Y. Lu, Y. M. Ma, C. X. Yang, Z. H. Zhao, J. N. Zhang, K. Zheng, G. Ye, R. B. Zhang, S. B. Fang and X. P. Fan, CN2019110991817, 2019.
- F. W. Mont, J. K. Kim, M. F. Schubert, H. Luo, E. F. Schubert and R. W. Siegel, High refractive index nanoparticle- loaded encapsulants for light-emitting diodes, Proc. SPIE 6486, Light-Emitting Diodes: Research, Manufacturing, and Applications XI, Integrated Optoelectronic Devices, San Jose, CA, 2007 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02230j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |





