Open Access Article
Open Access ArticleFactor analysis of the influence of environmental conditions on VOC emissions from medium density fibreboard and the correlation of the factors with fitting parameters†
Huiqi Shaoa,
Yifan Rena,
Yan Zhangb,
Chuandong Wua,
Wenhui Lia and
Jiemin Liu *a
*a
aSchool of Chemistry and Biological Engineering, University of Science and Technology Beijing, Beijing 100083, China. E-mail: liujm@ustb.edu.cn
bSchool of Science, Beijing University of Civil Engineering and Architecture, Beijing 100044, China
First published on 30th July 2021
Abstract
Volatile organic compounds (VOCs) emitting from building materials are one of the main sources of indoor pollution. Environmental factors have obvious effects on VOC emissions from building materials. However, no unified conclusions have been achieved on the influence of relative humidity (RH) and air change rate (ACR), and there is little research on the correlations of RH and ACR with parameters in VOCs emission fitting models. Therefore, factor analysis was applied in this paper to study the influence of RH and ACR on VOCs emissions. Medium density fibreboard pannels with the coating of oil-based paint were applied at four ACR (0.5 h−1, 1.0 h−1, 2.0 h−1, 3.0 h−1) and four RH (20%, 30%, 50%, 70%) conditions in 60 L environmental chambers. Tenax TA tubes were used to collect VOCs and thermal desorption-gas chromatography mass spectrometry was applied to determine the concentrations. The results show that RH influences the initial stage of VOCs emission and has a positive correlation with the emission concentrations. In the later emission stage, RH has no obvious influence on VOCs emissions, while the concentrations of VOCs are inversely proportional to ACR. The parameters in the single exponential model a1 and b1 have power-law or polynomial relationships with ACR and RH. ACR has negative correlations with a1 and positive correlations with b1, resulting in a negative influence on VOCs emissions, while RH has a complex influence on VOCs emissions. This study elucidated how RH and ACR impact VOCs emissions from oil-based paint coating medium density fibreboard and further influence human health exposure risks, which can then be used to improve indoor air quality.
1 Introduction
Indoor pollution will seriously reduce indoor air quality (IAQ), and further affect humans' comfort, health, and work efficiency.1–3 Volatile organic compounds (VOCs) emitting from wood-based materials and furniture are one of the main sources of indoor air pollution, and have attracted extensive attention.4–6Previous studies show that environmental factors have significant impacts on VOC emissions from building materials.7,8 Xiong et al.9,10 studied the combined influence of temperature and relative humidity (RH) on the emission of VOCs from building materials. They found that the emission rates of the pollutants rose with the increase of temperature and RH. Markowicz et al.11 illustrated that the influence of RH on the emission of pollutants should not be ignored by studying the release of VOCs in damp and normal rooms under high and low RH conditions respectively. However, Liang et al. demonstrated that formaldehyde emission was positively correlated with absolute humidity but not with RH through a 29 months' emission from a medium density fibreboard in a full-scale environmental room.7 Caron et al.12 studied formaldehyde and VOCs emissions from a particleboard with a waterproof coating in an environmental chamber under different air change rates (ACR) and found that the emission rate of formaldehyde was significantly affected by ACR, while VOCs were not.
Numerous studies have shown that temperature has a positive correlation with VOCs emissions, while there are no uniform conclusions on how RH and ACR affect the behavior of VOCs emissions.13 As many studies were conducted in practical dwelling environments, the environmental conditions couldn't be controlled precisely, and temperature, RH, and ACR might have a mixed influence on pollutant emissions. Besides, the emission characteristics of wood-based panels that have been stored for a long period should be different from that of newly manufactured panels, which made the conclusions drawn by different researchers different. Therefore, it is of significance to separately study the influence of RH and ACR on VOCs emissions in an experimental environment.
Moreover, most studies were concentrated on the relationships between environmental factors and key physical parameters (diffusion coefficient Dm, initial emittable concentration C0, and partition coefficient K14) in the mass transfer process.15–18 The study of Xu et al. indicated that with the increase of temperature, Dm and C0 increased, while K decreased. Liang et al.19–21 studied the influence of RH on the values of three parameters (C0, Dm, K) and found that there was a positive relation between C0 and RH. While RH had a negligible impact on Dm and K. However, C0, Dm, and K only devoted to investigating VOCs emission mechanisms, not for predicting VOCs concentrations releasing from building materials. Generally speaking, the single exponential model has been widely applied to fit VOCs emissions and predict VOCs concentrations in environmental chambers in previous studies.22–24 This model can be derived from the diffusion and mass transfer process of VOCs emissions from the material to the ambient air.25 Its form is simple, which is beneficial to the application in practical engineering. The two parameters in the model are key parameters to predict VOCs emissions. As we know, VOCs emissions vary with RH and ACR. Then there should be relationships of RH and ACR with the parameters in the model. However, studies related to these factors are limited.
Therefore, this study mainly focused on the influence of RH and ACR on VOCs emissions from a medium density fibreboard with the coating of oil-based paint (a representative of wood-based panels) based on environmental chamber experiments. The relationships between the parameters of the single exponential model and the environmental factors were researched, which will be of great significance to predict VOCs emissions and will further improve indoor air quality.
2 Materials and methods
2.1 Materials
A medium density fibreboard was purchased in the market and treated with oil-based paint on the surfaces in a furniture company. The emissions of pollutants were evaluated before the experiment. The concentrations of formaldehyde were lower than the detection limit, while the VOCs emission concentrations were very high, which made it easier to study VOCs emission characteristics. Eight pieces of panels with the same size of 10 cm × 30 cm were cut from the same oil-based paint coating medium density fibreboard panel. Four panels were used for VOCs emissions at different RH conditions, and the other four were used for VOCs emissions at different ACR conditions. The edge areas were sealed with aluminum foil to make sure that the pollutants only emitted from the upper and lower sides of the materials during the experiments.26 Then the emission area of each panel was 0.06 m2 and the loading rate was 1 m2 m−3. After that, the panels were immediately wrapped with plastic films and stored at room temperature until the start of the experiments.27 Four panels for RH influence studies were stored for half a month while the other four for ACR influence studies were stored for three months respectively.2.2 Environmental chamber
The experiments to test the emissions of VOCs from wood-based panels were carried out in 60 L (0.3 m × 0.3 m × 0.67 m) environmental chambers coated with Teflon on the inner walls. The chambers were cleaned under a high temperature of 250 °C to reduce the background concentrations of TVOC to below 0.02 mg m−3.28 After cleaning, the temperature in the chamber was maintained at 23 ± 0.5 °C.29,30 Two groups of the experiment based on factor analysis were conducted to study the influence of RH and ACR on VOCs emissions from the oil-based paint coating medium density fibreboard. The conditions are presented in Table 1.2.3 Sampling method
The panels were placed in the middle of the chamber, with the surfaces parallel to the airflow. VOCs emitting from the materials were sampled with Tenax TA tubes using a constant flow pump. At the condition of ACR 0.5 h−1, the sampling rate was 200 mL min−1, and the sampling time was 25 min. At other conditions, the sampling flow rate was 500 mL min−1 and the sampling time was 10 min, resulting in the total sampling volume of 5 L.31 Sampling was conducted every several hours until the balanced state of emissions.32 The sampling schedule is shown in Table 2.22 The ambient temperature and atmospheric pressure during sampling were recorded.| Days | Sampling intervals |
|---|---|
| 1st day | 2 h |
| 2nd day | 3 h |
| 3rd day | 4 h |
| 4th day | 6 h |
| 5th day | 12 h |
| 6th day to the balanced state | 24 h |
2.4 Testing method
VOCs sampled by Tenax TA tubes were analyzed by thermal desorption-gas chromatography mass spectrometry (TD-GCMS) (TD: TD 100-xr, Markes International; GC: 7890B, MS: 5977B, Agilent Technology). The determination conditions of TD-GCMS are shown in Table S1.† All the VOCs with the retention time between n-hexane and n-hexadecane were identified automatically using NIST library.33 The concentrations were quantified using peak areas with the response coefficient of toluene,34 as seen in eqn (1) and (2). Six consecutive samples were collected at the condition of RH 50% and ACR 1.0 h−1, and the relative standard deviation (RSD) of the VOCs concentrations was calculated to evaluate the precision of the measurement. The main components were selected to analyze the relationships of emission characteristics with RH and ACR.| ma = A/k | (1) |
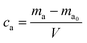 | (2) |
2.5 Simulation of VOCs emissions
The single exponential model was applied to simulate VOCs emission concentrations in environmental chambers, as shown in eqn (3).| Ca = a1 × e−b1t | (3) |
The values of a1 and b1 can be obtained through the simulating results. Then the relationships of a1 and b1 with RH and ACR were examined. Five kinds of fitting approaches, which are exponential fitting, linear fitting, logarithmic fitting, polynomial fitting, and power fitting, were applied to fit the values of a1 and b1 with RH and ACR.
3 Results and discussion
3.1 Precision evaluation
VOCs emitting from an oil-based paint coating medium density fibreboard in the environmental chamber were collected consecutively with six Tenax TA tubes and analyzed by TD-GCMS. The main pollutants contained seven VOCs, which were acetic acid butyl ester, ethylbenzene, propylene glycol monomethyl ether acetate (PGMEA), p/m-xylene, o-xylene, isopropyl benzene, and 1, 2, 4-trimethylbenzene. Concentrations of TVOC and the main pollutants were calculated respectively and the precision of the measurement was evaluated. The results are shown in Table 3.| No. | Concentrations/mg m−3 | |||||||
|---|---|---|---|---|---|---|---|---|
| TVOC | Acetic acid butyl ester | Ethylbenzene | PGMEA | p/m-Xylene | o-Xylene | Isopropyl benzene | 1,2,4-Trimethylbenzene | |
| 1 | 0.772 | 6.10 × 10−2 | 8.00 × 10−3 | 0.363 | 6.36 × 10−2 | 0.123 | 3.23 × 10−2 | 2.61 × 10−2 |
| 2 | 0.770 | 6.06 × 10−2 | 8.14 × 10−3 | 0.362 | 6.16 × 10−2 | 0.122 | 3.19 × 10−2 | 2.60 × 10−2 |
| 3 | 0.745 | 5.97 × 10−2 | 7.69 × 10−3 | 0.356 | 6.14 × 10−2 | 0.117 | 3.11 × 10−2 | 2.57 × 10−2 |
| 4 | 0.757 | 5.99 × 10−2 | 7.89 × 10−3 | 0.358 | 6.33 × 10−2 | 0.120 | 3.14 × 10−2 | 2.59 × 10−2 |
| 5 | 0.756 | 5.94 × 10−2 | 7.84 × 10−3 | 0.355 | 6.26 × 10−2 | 0.121 | 3.19 × 10−2 | 2.56 × 10−2 |
| 6 | 0.749 | 5.92 × 10−2 | 8.20 × 10−3 | 0.354 | 6.19 × 10−2 | 0.121 | 3.02 × 10−2 | 2.56 × 10−2 |
| Average/mg m−3 | 0.758 | 6.00 × 10−2 | 7.96 × 10−3 | 0.358 | 6.24 × 10−2 | 0.121 | 3.15 × 10−2 | 2.58 × 10−2 |
| SD/mg m−3 | 1.11 × 10−2 | 6.85 × 10−4 | 1.93 × 10−4 | 3.76 × 10−3 | 9.22 × 10−4 | 2.00 × 10−3 | 7.29 × 10−4 | 2.49 × 10−4 |
| RSD/% | 1.46 | 1.14 | 2.42 | 1.05 | 1.48 | 1.66 | 2.32 | 0.97 |
The RSD values of the concentration of TVOC and individual VOCs are all lower than 5%, which means that the precision of VOCs measurement is more than 95% and the test method is reliable. Therefore, collecting one sample at a specific time is enough to represent the VOCs emissions and there is no need to conduct a duplicate experiment in the test of VOCs emissions.
3.2 The influence of RH on VOCs emissions
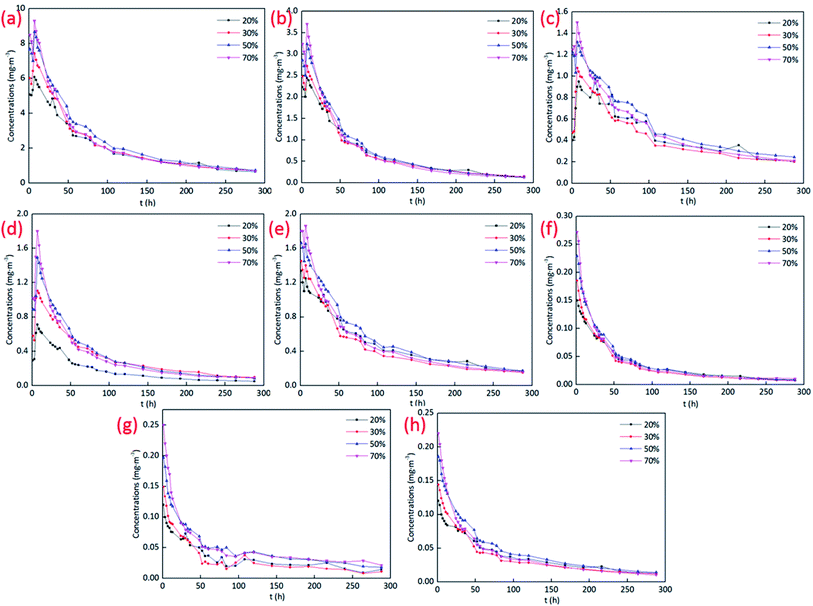
Four panels were put into the environmental chambers at different RH conditions to test VOCs emissions. The chromatograms of VOCs emissions at 50% RH and different RH conditions are shown in Fig. 1(a) and (b) respectively, with the emission at 24 h as the representative.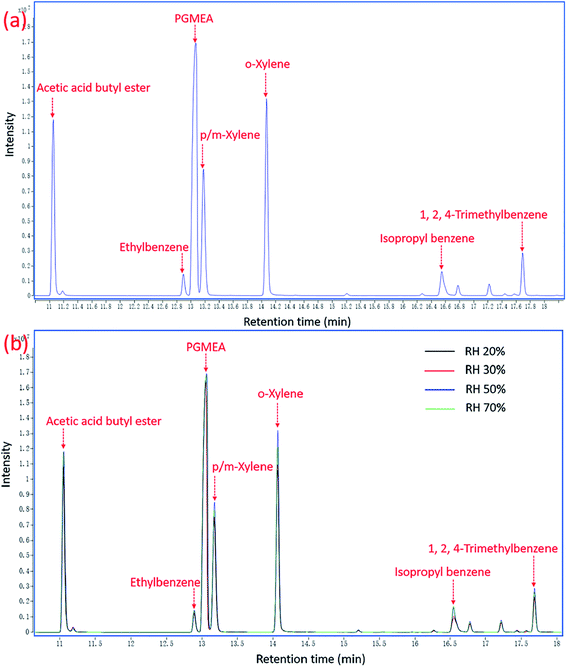 | ||
| Fig. 1 Chromatograms of VOCs emissions at 24 h from the medium density fibreboard coated with oil-based paint: (a) at 50% RH; (b) at different RH conditions. | ||
The results in Fig. 1 depict that the difference between the peaks of VOCs at different conditions is not too much, which means that there is no significant difference in VOCs emissions under different RH conditions. Then peak areas were integrated and the concentrations were calculated based on eqn (1) and (2) to quantify VOCs emissions at different conditions. The experiment went on for a total of 288 h for 12 days. The concentrations over time of TVOC and the major pollutants at different RH conditions are displayed in Fig. 2.
The results in Fig. 2 depict that the concentrations of acetic acid butyl ester, m/p-xylene, PGMEA, and o-xylene increase first and decay over time after the peak values. While the concentrations of ethylbenzene, isopropyl benzene, 1,2,4-trimethylbenzene decay with the increase of time. There are no peak values for the concentrations of these three compounds, which is most probably due to that the C0 of the pollutants is relatively low. The pollutants at free state quickly escaped from the material and reached the maximum values within 1 h. Then the rate of VOCs emitting from the materials to the chamber was slower than that escaped to the outlet, resulting in the decay of VOCs concentrations after 1 h.
RH mainly influences the early stage of VOCs emissions, while in the later period, especially in the equilibrium stage, the concentrations are almost the same, which means that the effect of RH on VOCs emissions at the equilibrium state can be ignored. In the early emission stage, VOCs concentrations are proportional to RH. The larger the RH, the higher the VOCs emission concentrations in the early emission stages.
The influence mechanism of RH on VOCs emissions is complex. RH has an impact on the Dm of VOCs. The increase of the RH will increase the Dm of the pollutants, resulting in the increase of VOCs emission concentrations.20 Moreover, most of the pollutants emitting from building materials are not water-soluble. As VOCs can't dissolve in water, there are competitive relationships between water and VOCs molecules to occupy the adsorption sites in the materials. The increase of RH will increase the content of water molecules in materials and occupy more adsorption sites, which will increase the competitiveness between water and VOCs molecules. The adsorption of pollutants decreases and then decreases the value of K of VOCs. Then more VOCs will emit from the materials and the concentrations will increase.19,35,36 Meanwhile, the existence of water molecules has a more significant impact on hydrophilic target compounds. In the seven main pollutants emitting from the materials, only PGMEA is water-soluble. As seen from Fig. 2, the influence of RH on PGMEA lasts longer than other compounds, even at the equilibrium state.
Moreover, in the initial emission period, there are numerous dissociative VOCs molecules in the material. Diffusion and convective mass transfer in the boundary layer should be the main controlling step of VOCs emissions. However, in the later stage, most of the dissociative VOCs molecules have released into the chamber. Then VOCs emissions are limited by the mass transfer process in the material.12,37 The water molecules will occupy the adsorption sites on the surface of the material first and then invade into the interior of the material gradually, which results that RH affects VOCs more in the initial emission period.
3.3 The influence of ACR on VOCs emissions
Four panels were put in the environmental chambers to test VOCs emissions. The chromatograms of VOCs obtained from GCMS at ACR 1.0 h−1 and different ACR conditions are shown in Fig. 3(a) and (b) respectively, with the emission at 24 h as the representative.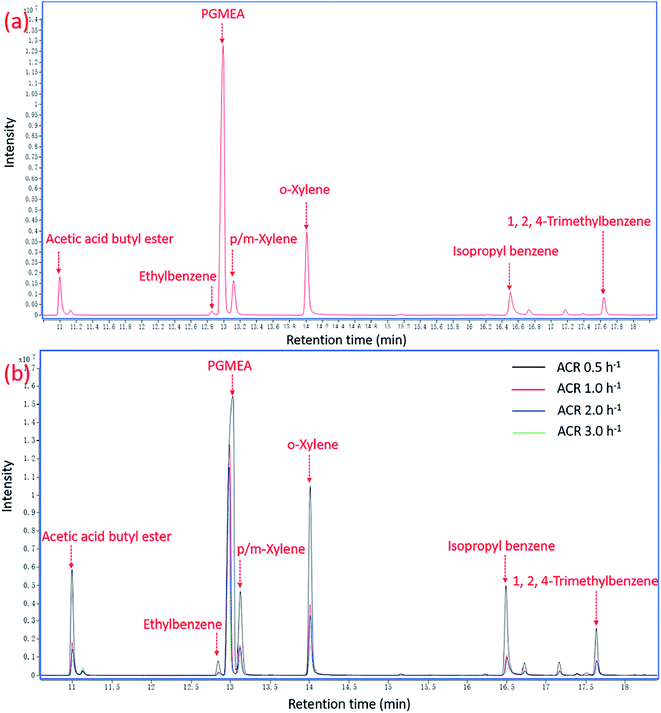 | ||
| Fig. 3 Chromatograms of VOCs emissions at 24 h from the medium density fibreboard coated with oil-based paint: (a) at ACR 1.0 h−1; (b) at different ACR conditions. | ||
The results in Fig. 3 show that the larger the ACR, the smaller the peak areas and intensity of the pollutants. The main pollutants are the same as that obtained in the study of the influence of RH on VOCs emissions in Section 3.2. The concentrations of VOCs were calculated to compare the difference at different conditions quantitatively. The experiment ran for a total of 384 h for 16 days. The variation curves of the concentrations of the major pollutants in the chambers over time at different conditions are displayed in Fig. 4.
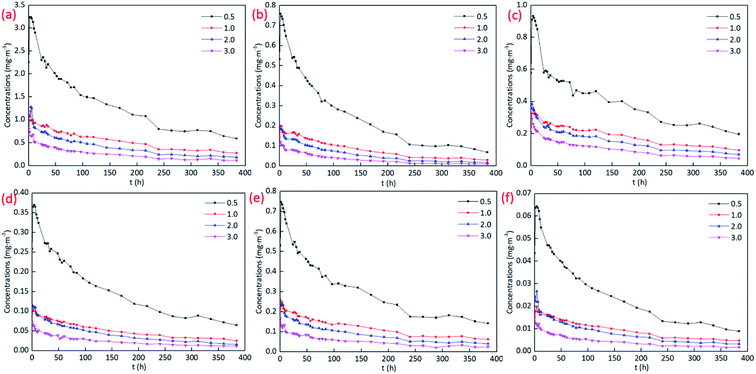 | ||
| Fig. 4 Concentrations of VOCs in the chambers at different ACR conditions: (a) TVOC; (b) acetic acid butyl ester; (c) PGMEA; (d) p/m-xylene; (e) o-xylene; (f) 1,2,4-trimethylbenzene. | ||
The results in Fig. 4 show that in the initial stage of VOCs emission, the concentrations increase first and then decay with time. The peak values are achieved in the first 10 hours. The higher the ACR is, the earlier it is to achieve the peak value of concentrations. Meanwhile, the concentrations of VOCs decrease with the increase of ACR. The influence is significant and gradually increases in the increasing stage, but diminishes at the decaying stage. While at the equilibrium stage, the difference of VOCs concentrations between different conditions is stable and not significant.
Meanwhile, ACR influences VOCs emissions in different degrees. The concentration difference between the ACR of 0.5 h−1 and 1.0 h−1 is significantly higher than that between 1.0 h−1 and 2.0 h−1, as well as between 2.0 h−1 and 3.0 h−1. According to the mass balance equation and convective mass transfer theory, the concentration gradient of VOCs at the boundary layer between the material and air will increase with the increase of ACR. Therefore, the convective mass transfer of the pollutants from the materials to the air increases, which will accelerate the diffusion of VOCs from the material to the air.38 While the increase of ACR will bring more fresh air into the chamber, which will dilute VOCs concentrations in the chamber. As a result, the amount emitting from the materials to the chamber is much lower than that is taken away from the chamber to the ambient air,12 which makes the concentrations of VOCs at the ACR of 1.0 h−1 much lower than that of 0.5 h−1. At the ACR conditions of 1.0 h−1, 2.0 h−1, and 3.0 h−1, the enormous concentration gradient makes the emission of VOCs from the material to the air much faster. Therefore, the difference of the emission amount from the materials to the chamber and from the chamber to the ambient air is much less than that between the ACR conditions of 0.5 h−1 and 1.0 h−1, resulting in the concentration difference of VOCs in the chamber much lower.
3.4 Simulation of VOCs emissions at different conditions
The concentrations of VOCs emissions at different conditions over time were simulated with the single exponential model according to the methods in Section 2.5. The results of 1,2,4-trimethyl benzene at the conditions of 50% RH and ACR of 0.5 h−1, 1.0 h−1, 2.0 h−1, 3.0 h−1 are shown in Fig. 5 as the representative. The simulating results of other compounds at different RH and ACR conditions are shown in Fig. S1 to S13 in the ESI.†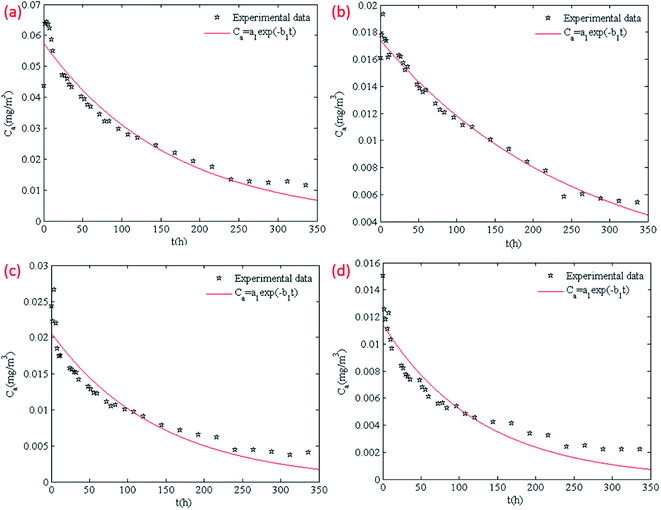 | ||
| Fig. 5 Simulation of 1,2,4-trimethylbenzene concentrations at RH 50% and different ACR with single exponential model: (a) 0.5 h−1; (b) 1.0 h−1; (c) 2.0 h−1; (d) 3.0 h−1. | ||
In Fig. 5, we can notice that the single exponential model can fit the emission rates well. The fitting formulas at four different conditions are Ca = 0.0576 × e−0.061t, Ca = 0.0174 × e−0.039t, Ca = 0.0206 × e−0.071t, Ca = 0.0114 × e−0.079t respectively. The fitting results indicate that the values of a1 and b1 of the same compound at different conditions are different, which means RH and ACR influence the values of a1 and b1.
From eqn (1), we know that Ca has a positive correlation with a1 and a negative correlation with b1. The results in Section 3.2 and Section 3.3 shows that RH influences initial VOCs emissions from building materials, and the higher the RH, the higher the VOCs concentrations in the initial stage. While the concentrations of VOCs in the chamber decrease with the increase of ACR. Then the values of a1 and b1 should be related to RH and ACR. The relationships of a1 and b1 with RH and ACR were examined according to the method in Section 2.5. The fitting results and corresponding R-squared are summarized in Tables S2 to S5.†
In Table S2,† R-squared values of VOCs fitted by different models are over 0.9 except for the exponential fitting of p/m-xylene. The result indicates that a1 has a significantly strong correlation with RH and the value of a1 doesn't have an exponential relationship with RH. Nevertheless, most of the values of R-squared obtained by polynomial fitting are the highest. Therefore, it can be inferred that there is a polynomial relationship between a1 and RH. According to the results by different types fitting of a1 with ACR in Table S3,† the R-squared obtained by power fitting is the largest and this model fits well. Therefore, there is a power-law relationship between a1 and ACR. While the fitting results of b1 with RH and ACR in Tables S4 and S5† show that b1 has a polynomial relationship both with RH and with ACR.
The fitting results in Tables S2–S5† indicate that RH has positive correlations with a1 and b1, while ACR has a negative correlation with a1 and a positive correlation with b1. As shown in eqn (3), large a1 and small b1 can lead to large VOCs concentrations. It means that factors that have positive correlations with a1 and negative correlations with b1 will have a positive influence on VOCs emissions. While factors that have negative correlations with a1 and positive correlations with b1 will have a negative influence on VOCs emissions. Combining the analyses above, it can be concluded that ACR is a factor that can promote VOCs emissions, while RH can either promote or prevent VOCs emissions from building material. It may also explain why RH mainly influences VOCs emissions in the initial stage. As there are quantitative relationships between these factors and parameters in eqn (3), VOCs concentrations can be calculated according to the environmental conditions and the time, which is meaningful for the prediction of VOCs emission concentrations.
Furthermore, the panels used to explore the influence of RH and ACR are cut from the same oil-based paint coating medium density fibreboard. There is the same condition applied during these two groups' studies, which is RH 50% and ACR 1.0 h−1. However, the values of a1 and b1 obtained from these two experiments and the numbers of the main pollutants are different. This is probably due to that the C0 of the compounds in these two groups of experiments are different because of the different storage periods. The panels applied for VOCs emission at different ACR conditions were stored for a longer period, which led to the release of some VOCs from materials into ambient air before the start of the experiment.2 Therefore, the obtained VOCs concentrations and species are less than those obtained at different RH conditions. Meanwhile, the values of a1 and b1 of different compounds at the same condition are also different from each other, which is related to the molecular weight, boiling points, as well as C0.36,39
3.5 The influence of the parameters on human health
The exposure of VOCs will affect humans' health and cause health risks. From the results in the study above, RH mainly influences VOCs concentrations in the initial emission stage. The larger RH will lead to larger VOCs concentrations, which will increase the exposure risks for humans. While in the latter emission stage, especially at the balanced state, RH has little influence on VOCs concentrations, which will have little influence on human exposure risks. Meanwhile, ACR has a negative influence on VOCs emission concentrations and high ACR will bring forward the time to achieve the balanced states. Therefore, higher ACR will decrease the exposure concentrations as well as the exposure time at high concentrations, which will reduce the exposure risks for humans. Moreover, high a1 and low b1 will increase VOCs concentrations, which will increase human exposure risks. Overall, decreasing RH and a1 and increasing ACR and b1 will contribute to the decrease of human exposure risks of VOCs emitting from building materials.4. Conclusions
VOCs emitting from oil-based paint coating medium density fibreboard at different RH and ACR conditions were studied in environmental chambers based on the factor analysis. The relationships between the parameters in the single exponential model (a1 and b1) with RH and ACR were studied. The following conclusions are drawn:(1) The concentrations of VOCs emitting from the oil-based paint coating medium density fibreboard have a negative correlation with ACR and a positive correlation with RH in the initial emission stage. While at the later period of emission, RH has no obvious influence on VOCs releasing.
(2) The parameters of the single exponential model a1 and b1 are related to ACR and RH. The value of a1 has a power relationship with ACR and a polynomial relationship with RH. b1 has a polynomial relationship with both ACR and RH, which is meaningful for the prediction of VOCs emissions.
(3) ACR has negative correlations with a1 and positive correlations with b1, while RH has positive correlations with both a1 and b1, which has complex effects on VOCs emissions. Meanwhile, decreasing RH and a1 as well as increasing ACR and b1 will contribute to the decrease of human exposure risks of VOCs emitting from building materials.
The results in this study give an explicit conclusion about how RH and ACR influence VOCs emissions from medium density fibreboard, which is beneficial for the promotion of indoor air quality.
Author contributions
Huiqi Shao: conceptualization, investigation, methodology, formal analysis, writing – original draft. Yifan Ren: data curation. Yan Zhang: formal analysis. Chuandong Wu, Wenhui Li, and Jiemin Liu: supervision, Writing–review & editing.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China [Nos. 2016YFC0700601, 2016YFC0700603]; and the National Natural Science Foundation of China [No. 21878018].References
- WHO R. O. of Europe, WHO Guidelines For Indoor Air Quality: Selected Pollutants, World Health Organization, Regional Office for Europe, Copenhagen, 2010 Search PubMed.
- V. Gallon, P. Le Cann, M. Sanchez, C. Dematteo and B. Le Bot, Indoor Air, 2020, 30, 691–710 CrossRef CAS PubMed.
- H. S. Park, C. Ji and T. Hong, Build. Environ., 2016, 95, 133–144 CrossRef.
- P. Harb, N. Locoge and F. Thevenet, Chem. Eng. J., 2018, 354, 641–652 CrossRef CAS.
- S. W. Tang, E. Chen, Z. J. Li and H. Y. Shao, Build. Environ., 2015, 84, 221–227 CrossRef.
- Y. Z. Wang, T. Yang, Z. C. He, L. H. Sun, X. F. Yu, J. Zhao, Y. J. Hu, S. H. Zhang and J. Y. Xiong, Atmos. Environ., 2020, 231 CAS.
- W. Liang, S. Yang and X. Yang, Environ. Sci. Technol., 2015, 49, 10349–10356 CrossRef CAS PubMed.
- P. A. Clausen, Z. Liu, V. Kofoed-Sorensen, J. Little and P. Wolkoff, Environ. Sci. Technol., 2012, 46, 909–915 CrossRef CAS PubMed.
- J. Xiong, P. Zhang, S. Huang and Y. Zhang, Environ. Res., 2016, 151, 734–741 CrossRef CAS PubMed.
- C. Zhou, Y. Zhan, S. Chen, M. Xia, C. Ronda, M. Sun, H. Chen and X. Shen, Build. Environ., 2017, 121, 26–34 CrossRef.
- P. Markowicz and L. Larsson, Environ. Sci. Pollut. Res., 2015, 22, 5772–5779 CrossRef CAS PubMed.
- F. Caron, R. Guichard, L. Robert, M. Verriele and F. Thevenet, Atmos. Environ., 2020, 243, 117713 CrossRef CAS.
- J. Xiong, W. Wei, S. Huang and Y. Zhang, Environ. Sci. Technol., 2013, 47, 8540–8547 CrossRef CAS PubMed.
- B. Xu and Z. Q. Chen, Int. Commun. Heat Mass Transfer, 2017, 87, 105–111 CrossRef CAS.
- W. Wei, C. Howard-Reed, A. Persily and Y. Zhang, Environ. Sci. Technol., 2013, 47, 7848–7854 CrossRef CAS PubMed.
- Y. Zhang, X. Luo, X. Wang, K. Qian and R. Zhao, Atmos. Environ., 2007, 41, 3203–3216 CrossRef CAS.
- S. Huang, J. Xiong and Y. Zhang, Environ. Sci. Technol., 2015, 49, 1537–1544 CrossRef CAS PubMed.
- Q. Deng, X. Yang and J. Zhang, Atmos. Environ., 2009, 43, 2080–2083 CrossRef CAS.
- W. Liang, M. Lv and X. Yang, Build. Environ., 2016, 101, 110–115 CrossRef.
- T. Yang, P. Zhang, B. Xu and J. Xiong, Int. J. Heat Mass Transfer, 2017, 110, 671–679 CrossRef CAS.
- Z. Liu, C. Howard-Reed, S. S. Cox, W. Ye and J. C. Little, Indoor Air, 2014, 24, 283–291 CrossRef CAS PubMed.
- X. Liu, M. A. Mason, Z. Guo, K. A. Krebs and N. F. Roache, Atmos. Environ., 2015, 122, 561–568 CrossRef CAS.
- J. P. Zhu, J. S. Zhang and C. Y. Shaw, Chemosphere, 2001, 44, 1253–1257 CrossRef CAS PubMed.
- P. A. Clausen, Indoor Air, 1993, 3, 269–275 CrossRef CAS.
- B. Deng and C. N. Kim, Atmos. Environ., 2004, 38, 1173–1180 CrossRef CAS.
- C. Jiang, D. Li, P. Zhang, J. Li, J. Wang and J. Yu, Build. Environ., 2017, 117, 118–126 CrossRef.
- C. Howard-Reed, Z. Liu, J. Benning, S. Cox, D. Samarov, D. Leber, A. T. Hodgson, S. Mason, D. Won and J. C. Little, Build. Environ., 2011, 46, 1504–1511 CrossRef.
- S. Wi, M. G. Kim, S. W. Myung, Y. K. Baik, K. B. Lee, H. S. Song, M. J. Kwak and S. Kim, J. Hazard. Mater., 2020, 393, 122381 CrossRef CAS PubMed.
- M. Even, C. Hutzler, O. Wilke and A. Luch, Indoor Air, 2020, 30, 40–48 CrossRef CAS PubMed.
- Y. Yao, J. Xiong, W. Liu, J. Mo and Y. Zhang, Atmos. Environ., 2011, 45, 5602–5611 CrossRef CAS.
- P. Markowicz and L. Larsson, Atmos. Environ., 2015, 106, 376–381 CrossRef CAS.
- J. Xiong, Y. Yao and Y. Zhang, Environ. Sci. Technol., 2011, 45, 3584–3590 CrossRef CAS PubMed.
- B. E. Boor, H. Jarnstrom, A. Novoselac and Y. Xu, Environ. Sci. Technol., 2014, 48, 3541–3549 CrossRef CAS PubMed.
- The International Organization for Standardization, ISO 16000-6-2011, 2011.
- J. Xu and J. S. Zhang, Build. Environ., 2011, 46, 1785–1796 CrossRef.
- C.-C. Lin, K.-P. Yu, P. Zhao and G. W.-M. Lee, Build. Environ., 2009, 44, 525–533 CrossRef.
- P. Wolkoff, Atmos. Environ., 1998, 32, 2659–2668 CrossRef CAS.
- D. E. Hun, R. L. Corsi, M. T. Morandi and J. A. Siegel, Indoor Air, 2010, 20, 196–203 CrossRef CAS PubMed.
- Z. L. Liu, A. Nicolai, M. Abadie, M. H. Qin, J. Grunewald and J. S. Zhang, Build. Simul., 2020, 14(2), 269–282 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02164h |
| This journal is © The Royal Society of Chemistry 2021 |