Open Access Article
Open Access ArticleRe(I)-nitroxide complexes†
Kseniya Maryunina *a,
Gleb Letyagin
*a,
Gleb Letyagin ab,
Artem Bogomyakov
ab,
Artem Bogomyakov abc,
Vitaly Morozovab,
Sergey Tumanov
abc,
Vitaly Morozovab,
Sergey Tumanov ab,
Sergey Veber
ab,
Sergey Veber ab,
Matvey Fedin
ab,
Matvey Fedin ab,
Evgeniya Saverina
ab,
Evgeniya Saverina c,
Mikhail Syroeshkin
c,
Mikhail Syroeshkin c,
Mikhail Egorovc,
Galina Romanenko
c,
Mikhail Egorovc,
Galina Romanenko a and
Victor Ovcharenko
a and
Victor Ovcharenko ac
ac
aInternational Tomography Center SB RAS, Institutskaya Str. 3a, 630090 Novosibirsk, Russia. E-mail: mks@tomo.nsc.ru
bNovosibirsk State University, Pirogova Str. 1, 630090 Novosibirsk, Russia
cN. D. Zelinsky Institute of Organic Chemistry RAS, Leninsky Prospect, 47, Moscow, 119991, Russia
First published on 2nd June 2021
Abstract
Spin-labeled cyrhetrenes [(NNCp)Re(CO)3] and [(INCp)Re(CO)3], where NNCp is nitronyl nitroxide 2-(η5-cyclopentadienyl)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole-3-oxide-1-oxyl and INCp is the corresponding imino nitroxide, were synthesized and characterized by EPR, CV, XRD, magnetochemistry and quantum chemistry methods. The correlations between different arrangements of paramagnetic centers and the magnetic exchange interactions for three polymorphs of [(NNCp)Re(CO)3] were studied. It was concluded that high kinetic stability of nitroxide-substituted cyrhetrenes is a promising feature of compounds for the creation of multifunctional contrast agents.
Introduction
In recent years, the design of cyrhetrene derivatives [(RCp)Re(CO)3] exhibiting pharmacological activity has been of significant interest.1–3 The robust coordination of the η5-cyclopentadienyl ligand and the low-spin d6 electronic configuration of Re(I) provide high kinetic stability of this class of compounds under ambient conditions.1–4 This makes cyrhetrenes suitable prototypes for developing the synthesis of their technetium and manganese analogs, which are much less radio-, photo-, and electrochemically stable.1–5The possibility of introducing various functional substituents into the cyclopentadienyl ring, small size and the ability to mimic the benzene ring of the [(Cp)Re(CO)3] fragment open the broad prospects for the creation of theranostics drugs providing both a targeted therapy and monitoring their accumulation and removal in vivo.1,3,4 Despite the high potential of cyrhetrens for use in chemotherapy, photodynamic therapy, and radiological treatment by 186/188Re isotopes, only single-photon emission computed tomography based on 99mTc agents has become widespread for the noninvasive diagnostic localization of pathological processes.1,3,4 Alternative methods for visualization of carbonyl complexes Re(I)/Tc(I) in tissues and organs based on fluorescence or UV/FTIR/Raman microscopy have a number of limitations3,4 and require the use of complex facility, often equipped with specific sources of laser or synchrotron radiation.3
Another promising approach to the creation of rhenium(I) compounds for theranostics in our view is the combination in one molecule of cyrhetrene moiety and paramagnetic organic fragments for their use as contrast agents in magnetic resonance imaging. Kinetic stability, low toxicity and effective contrast properties of 2-imidazoline nitroxide radicals6–9 are promising for their introduction as spin-labeled substituents R in [(RCp)Re(CO)3] derivatives. However, the synthesis and physicochemical properties of such compounds are a scarce studied area of organometallic chemistry. Previously, a ferrocene and ruthenocene/osmocene species substituted with various nitroxide fragments have been extensively explored, while other nitroxide-bound derivatives of metallocenes,10–15 including half-sandwich ones, are still unknown. We succeeded in synthesizing organometallic complexes [(RCp)Re(CO)3] bearing nitroxide substituents R that could be used as a convenient approach in the design of paramagnetic targeted drugs for theranostics.
Results and discussion
Cyrhetren derivatives substituted with 2-imidazoline nitroxides were synthesized by the Ullman's method.16,17 The nitronyl nitroxide [(NNCp)Re(CO)3], imino nitroxide [(INCp)Re(CO)3] and their diamagnetic precursor [(BHACp)Re(CO)3] (Scheme 1) are kinetically stable both in the solid state and in solutions, and do not require special precautions when handling them. It could be suggested that an electrochemical impact or other physical stimuli are capable to induce electron transfer between the nitroxide fragment and the Re1+ ion.18,19 However, as the results of electrochemical measurements, magnetochemical studies and quantum chemical calculations show below, intramolecular redox processes are not inherent of this type of compounds.EPR spectroscopy study of [(NNCp)Re(CO)3] and [(INCp)Re(CO)3] solutions has shown paramagnetic signals with hyperfine coupling on 14N and 185Re/187Re nuclei (Fig. 1). EPR spectra modeling used spin Hamiltonian in form 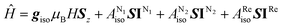 (S = 1/2, IN1 = IN2 = 1, and IRe = 5/2). Simulation of EPR spectra gave giso = 2.009 for both compounds and hyperfine coupling constants
(S = 1/2, IN1 = IN2 = 1, and IRe = 5/2). Simulation of EPR spectra gave giso = 2.009 for both compounds and hyperfine coupling constants  AReiso = 0.462 mT for [(NNCp)Re(CO)3], and
AReiso = 0.462 mT for [(NNCp)Re(CO)3], and  = 0.905 mT,
= 0.905 mT,  = 0.445 mT, AReiso = 0.373 mT for [(INCp)Re(CO)3]. Calculated parameters are in good agreement with typical giso, AReiso and ANiiso values of nitronyl/imino nitroxides6,12 and reduced derivatives of rhenium(I) tricarbonyl diimine complexes fac-[(a-diimine−˙)ReI(CO)3(L)].20–23 The high natural isotropic hyperfine splitting constant of Re nuclei24 makes it possible to clearly observe their coupling with the unpaired electron of nitroxide fragments. According to the results of molecular DFT calculations (ORCA 4.2 quantum chemistry package25 with range-separated LC-BLYP functional and def2-TZVPP basis set), the spin density is found to be mainly localized on {O←N
= 0.445 mT, AReiso = 0.373 mT for [(INCp)Re(CO)3]. Calculated parameters are in good agreement with typical giso, AReiso and ANiiso values of nitronyl/imino nitroxides6,12 and reduced derivatives of rhenium(I) tricarbonyl diimine complexes fac-[(a-diimine−˙)ReI(CO)3(L)].20–23 The high natural isotropic hyperfine splitting constant of Re nuclei24 makes it possible to clearly observe their coupling with the unpaired electron of nitroxide fragments. According to the results of molecular DFT calculations (ORCA 4.2 quantum chemistry package25 with range-separated LC-BLYP functional and def2-TZVPP basis set), the spin density is found to be mainly localized on {O←N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N–˙O}/{N
C–N–˙O}/{N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N–˙O} fragments and has a very small value at the rhenium(I) center (Fig. S1†). It is noteworthy that the Mulliken spin density values for [(NNCp)Re(CO)3] (−0.017) are higher than for [(INCp)Re(CO)3] (−0.005), which is consistent with higher values of AReiso for nitronyl nitroxide compared to that of imino nitroxide.
C–N–˙O} fragments and has a very small value at the rhenium(I) center (Fig. S1†). It is noteworthy that the Mulliken spin density values for [(NNCp)Re(CO)3] (−0.017) are higher than for [(INCp)Re(CO)3] (−0.005), which is consistent with higher values of AReiso for nitronyl nitroxide compared to that of imino nitroxide.
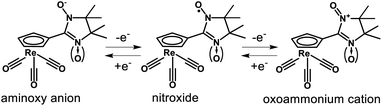
According to the cyclic voltammetry (CV) data [(NNCp)Re(CO)3] undergoes one-electron reversible oxidation (Fig. 2 and S2, ESI†). The value of the half-wave potential E0′ = 681 mV of the oxoammonium cation formation (Scheme 2) is comparable with the data for other reversibly oxidized nitronyl nitroxides.12,26 The imino nitroxide [(INCp)Re(CO)3] is oxidized irreversibly (Fig. 1); its oxidation potential is 557 mV higher than that of [(NNCp)Re(CO)3], which could be associated with a much higher electron transfer energy and lower stability of the primary oxidation product (Scheme 2). At the higher anodic areas of 1400–2500 mV signals of subsequent irreversible oxidation processes for both [(NNCp)Re(CO)3] and [(INCp)Re(CO)3] were recorded (Fig. S3a and b, ESI†). [(NNCp)Re(CO)3] and [(INCp)Re(CO)3] are irreversibly reduced in the potential range of −950 to −1100 mV (Fig. 2), which is typical for most compounds containing a nitroxide fragment12,27,28 due to the high basicity of the formed aminoxy anions (Scheme 2).29 These results are in line with the data on the electrochemical behavior of the dihydroxyimidazolidine precursor – [(BHACp)Re(CO)3] (Fig. S3c, ESI†), which can be considered as a product of complete reduction of [(NNCp)Re(CO)3]. [(BHACp)Re(CO)3] could not be reduced in the experimentally available potential range, but it is irreversibly oxidized at Epox = 886 mV with a number of subsequent electrochemical transformations in higher anodic areas. Thus, external electrochemical impact on [(NNCp)Re(CO)3] and [(INCp)Re(CO)3] induces electron transfer only for the nitroxide fragment of the molecules, while their cyrhetrene moiety remains unchanged.
X-ray diffraction study of [(NNCp)Re(CO)3], [(INCp)Re(CO)3] (Fig. 3) and their diamagnetic precursor [(BHACp)Re(CO)3] (Fig. S4, ESI†) showed that the bond lengths in coordination sphere of Re and ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–˙O groups are in good agreement with the previously reported ones for other cyrhetrenes and nitroxides species30 (Re–CCO – 1.86–1.92 Å, Re–CCp – 2.27–2.35 Å, N–O – 1.25–1.30 Å, Table S2, ESI†).
N–˙O groups are in good agreement with the previously reported ones for other cyrhetrenes and nitroxides species30 (Re–CCO – 1.86–1.92 Å, Re–CCp – 2.27–2.35 Å, N–O – 1.25–1.30 Å, Table S2, ESI†).
 | ||
| Fig. 3 Molecular structure and the shortest intermolecular distances (Å) of I (a), II (b), III (c) and [(INCp)Re(CO)3] (d). The H atoms and the geminal CH3 groups are omitted for clarity. | ||
The formation of polymorphic modifications seems to be inherent in the nature of [(NNCp)Re(CO)3]. We did obtain crystals of three polymorphs (here and further appointed as I, II and III). The crystal structures of II and III are formed by two crystallographically independent molecules – IIa, IIb and IIIa, IIIb, respectively (Fig. 3 and Table 1). The {ONCN(O)} nitroxide fragment and the Cp ring are practically coplanar for the most solids under study, which could be favorable for the spread of the spin density to the conjugated aromatic {ONCN(O)–Cp} system.6,7
| Compound | OCO⋯ONO | CCp⋯CCp centroids | ∠Cp⋯Cp | Cp⋯ONO | ONO⋯ONO | N⋯ONO | |
|---|---|---|---|---|---|---|---|
| [(INCp)Re(CO)3] | 3.95(1) | 3.33(2) | 0.0 | 3.12(1)–3.17(1) | 3.69(1) | 4.55(1) | |
| [(NNCp)Re(CO)3] | I | 3.89(1)–3.81(1) | — | — | 3.54(1) | — | — |
| IIa–IIa | — | — | — | 3.45(2) | 4.16(1) | 4.66(1) | |
| IIa–IIb | 3.82(1) | 3.38(2) | 3.4 | 3.47(2) | 3.82(1) | 3.69(1)–3.72(1) | |
| IIb–IIb | 3.98(1)–3.52(1) | — | — | 3.29(2)–3.42(2) | 4.44(2) | 5.22(2) | |
| IIIa–IIIa | 4.22(1)–4.06(1) | — | — | 3.20(3) | — | — | |
| IIIa–IIIb | — | 3.24(1) | 20.4 | 3.60(1) | 4.07(1) | 3.93(1)–3.94(1) | |
| IIIb–IIIb | 4.14(1) | — | — | — | — | — | |
Polymorphs of [(NNCp)Re(CO)3] differ in the type and parameters of intermolecular contacts. Of particular interest is the environment of ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–˙O groups, since almost all of the spin density is concentrated on their π-antibonding orbitals (Fig. S1†). The efficiency of overlapping of these so-called magnetic orbitals,31 is very important for the formation of channels of the strongest magnetic exchange interactions (Fig. 3, Tables 1 and 2).
N–˙O groups, since almost all of the spin density is concentrated on their π-antibonding orbitals (Fig. S1†). The efficiency of overlapping of these so-called magnetic orbitals,31 is very important for the formation of channels of the strongest magnetic exchange interactions (Fig. 3, Tables 1 and 2).
Molecules a and b are combined into π-associated dimers with close location of ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–˙O groups in modifications II and III (II: CCp⋯CCp 3.38(2) Å and 3.4°, N⋯ONO 3.69(1)/3.72(1) and ONO⋯ONO 3.82(1) Å; III: CCp⋯CCp 3.20(1) Å and 20.4°, N⋯ONO 3.93(1)/3.94(1) and ONO⋯ONO and 4.07(1) Å; Fig. 3b and c). In addition, there are two pairs of short distances ONO⋯CCp = 3.29(2) Å between IIb molecules. In polymorph III molecules IIIa are connected in a chain by a H-bond CCp–H⋯ONO (C⋯O 3.20(3), H⋯O 2.33 Å). In the crystal structure of I, the distances both between neighboring
N–˙O groups in modifications II and III (II: CCp⋯CCp 3.38(2) Å and 3.4°, N⋯ONO 3.69(1)/3.72(1) and ONO⋯ONO 3.82(1) Å; III: CCp⋯CCp 3.20(1) Å and 20.4°, N⋯ONO 3.93(1)/3.94(1) and ONO⋯ONO and 4.07(1) Å; Fig. 3b and c). In addition, there are two pairs of short distances ONO⋯CCp = 3.29(2) Å between IIb molecules. In polymorph III molecules IIIa are connected in a chain by a H-bond CCp–H⋯ONO (C⋯O 3.20(3), H⋯O 2.33 Å). In the crystal structure of I, the distances both between neighboring ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–˙O groups and between the cyclopentadienyl ring and the nitroxide group are too large (N⋯ONO and ONO⋯ONO > 4.5 Å, ONO⋯CCp = 3.54(1) Å; Fig. 3a) to expect any significant magnetic exchange interactions for this polymorph. π-associated dimers in [(INCp)Re(CO)3] are connected into a ribbon by short contacts between O of nitroxide groups and C of cyclopentadienyl ring (CCp⋯CCp 3.33(2) Å and 0°, ONO⋯CCp = 3.12(1) Å; Fig. 3d). Distances ONO⋯ONO and N⋯ONO between
N–˙O groups and between the cyclopentadienyl ring and the nitroxide group are too large (N⋯ONO and ONO⋯ONO > 4.5 Å, ONO⋯CCp = 3.54(1) Å; Fig. 3a) to expect any significant magnetic exchange interactions for this polymorph. π-associated dimers in [(INCp)Re(CO)3] are connected into a ribbon by short contacts between O of nitroxide groups and C of cyclopentadienyl ring (CCp⋯CCp 3.33(2) Å and 0°, ONO⋯CCp = 3.12(1) Å; Fig. 3d). Distances ONO⋯ONO and N⋯ONO between ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–˙O groups of adjacent π-dimers are 3.69(1) and 4.55(1) Å, respectively.
N–˙O groups of adjacent π-dimers are 3.69(1) and 4.55(1) Å, respectively.
The magnetochemical study confirmed that solids of the obtained nitroxides [(NNCp)Re(CO)3] and [(INCp)Re(CO)3] are paramagnetic (Fig. 4 and Table 2). For all studied spin-labeled derivatives of cyrhetrene the values of the effective magnetic moment μeff at 100–300 K are close to the theoretical spin-only value 1.73 μB for the monoradical with S = 1/2 and g = 2. There are no magnetic anomalies at the μeff(T) curves associated with intramolecular charge transfer or a change in the spin state of the nitroxide fragments and/or the Re+ ion. Molecular DFT calculations (LC-BLYP/def2-TZVPP)25 showed that the nearest in energy spin state isomers are above the ground state of the initial nitroxides by about 70 and 90 kcal M−1 for [(NNCp)Re(CO)3] and [(INCp)Re(CO)3], respectively. This manifests that the thermally induced spin state change-over or the intramolecular redox processes are energetically unfavorable.
 | ||
| Fig. 4 Experimental dependences μeff(T) for the polymorphs of nitronyl nitroxide [(NNCp)Re(CO)3] (a: I – dark cyan triangles; II – dark blue squares; III – blue cycles) and imino nitroxide [(INCp)Re(CO)3] (b: red squares). Solid thick lines – fitted data based on optimized parameters (Table 2). | ||
| Compound | Fitted | Calculated | |||||
|---|---|---|---|---|---|---|---|
| J | g | Spin Hamiltonian33 | J | Contact type | Spin Hamiltonian33 | ||
| a The molecular DFT calculation25 (LC-BLYP functional/def2-TZVPP) gave a similar evaluation – JcalcIN-NO⋯ON = −0.75 cm−1. | |||||||
| [(INCp)Re(CO)3] | −1.0 | 2.02 | H = −2JSR1SR2, SR1 = SR2 = ½ | −2.6a | NO…ON | H = −2JSR1SR2, SR1 = SR2 = ½ | |
| [(NNCp)Re(CO)3] | I | −2.0 | 2.03 | −1.4 | Cp⋯ON | ||
| II | −20.6 | 1.93 | −15.6 | NO…ON | |||
| −5.3 | NO…ON | ||||||
| III | −12.3 | 2.03 | −1.8 | Cp⋯ON |  SRi = ½ SRi = ½ |
||
A monotonic decrease of μeff with lowering temperature from 100 to 2 K indicates weak antiferromagnetic exchange interactions. Periodic DFT+U calculations32 using the crystallographic geometry at room temperature of [(NNCp)Re(CO)3] and [(INCp)Re(CO)3] nitroxides revealed the most important passways of intermolecular magnetic exchange interactions (Table 2). In this respect, the most effective is the close mutual arrangement of ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–˙O groups in the π-associated dimers of polymorphs II and III. The calculated values of exchange interactions are JcalcII-NO⋯ON = −15.6 cm−1 and JcalcIII-NO⋯ON = −5.3 cm−1. These differences in the values of intermolecular magnetic exchange interactions are in good agreement with the best fit parameters within the same exchange-coupled dimer model: JII = −20.6 cm−1 (gII = 1.93) and JIII = −12.3 cm−1 (gIII = 2.03).
N–˙O groups in the π-associated dimers of polymorphs II and III. The calculated values of exchange interactions are JcalcII-NO⋯ON = −15.6 cm−1 and JcalcIII-NO⋯ON = −5.3 cm−1. These differences in the values of intermolecular magnetic exchange interactions are in good agreement with the best fit parameters within the same exchange-coupled dimer model: JII = −20.6 cm−1 (gII = 1.93) and JIII = −12.3 cm−1 (gIII = 2.03).
The magnitude of intermolecular exchange interactions, formed by the overlapping of the magnetic orbitals of the ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–˙O group and the Cp fragment of neighboring molecules, was estimated too. For modification I, where there are no other contacts between the spin density bearing fragments (Fig. 3a), the calculated value JcalcI-NO⋯Cp = −1.4 cm−1 agrees with the value of the exchange parameter JI = −2.0 cm−1 (gI = 2.03) obtained by fitting the experimental data. Among all the studied nitroxides, the shortest ONO⋯CCp contacts were found for III; they bind IIIa molecules in the chain. The calculated JcalcIII-NO⋯Cp values are also very small (−1.8 cm−1). Comparison of the calculated values of the exchange integrals JcalcIII-NO⋯ON and JcalcIII-NO⋯Cp for III confirms that the direct overlap of the magnetic orbitals of the
N–˙O group and the Cp fragment of neighboring molecules, was estimated too. For modification I, where there are no other contacts between the spin density bearing fragments (Fig. 3a), the calculated value JcalcI-NO⋯Cp = −1.4 cm−1 agrees with the value of the exchange parameter JI = −2.0 cm−1 (gI = 2.03) obtained by fitting the experimental data. Among all the studied nitroxides, the shortest ONO⋯CCp contacts were found for III; they bind IIIa molecules in the chain. The calculated JcalcIII-NO⋯Cp values are also very small (−1.8 cm−1). Comparison of the calculated values of the exchange integrals JcalcIII-NO⋯ON and JcalcIII-NO⋯Cp for III confirms that the direct overlap of the magnetic orbitals of the ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–˙O groups of adjacent molecules is most effective for the onset of the strongest magnetic exchange interactions. The contribution of the exchange channels passing through the Cp fragments should be insignificant due to the poor transmitting of the spin density via the α-carbon atom to substituents of 2-imidazoline nitroxides.6
N–˙O groups of adjacent molecules is most effective for the onset of the strongest magnetic exchange interactions. The contribution of the exchange channels passing through the Cp fragments should be insignificant due to the poor transmitting of the spin density via the α-carbon atom to substituents of 2-imidazoline nitroxides.6
Periodic DFT calculations for [(INCp)Re(CO)3] revealed only weak antiferromagnetic exchange interactions JcalcIN-NO⋯ON = −2.6 cm−1 for dimers with the most closely located ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–˙O groups (Fig. 3d, Tables 1 and 2). These data are consistent with the values obtained by fitting the experimental dependence μeff(T) for exchange-coupled dimers: JIN = −1.0 cm−1 and gIN = 2.02 (Fig. 4b). This type of mutual arrangement of
N–˙O groups (Fig. 3d, Tables 1 and 2). These data are consistent with the values obtained by fitting the experimental dependence μeff(T) for exchange-coupled dimers: JIN = −1.0 cm−1 and gIN = 2.02 (Fig. 4b). This type of mutual arrangement of ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–˙O groups does not provide reliable overlap of their magnetic orbitals that determines the low efficiency of this channel of intermolecular magnetic exchange interactions.
N–˙O groups does not provide reliable overlap of their magnetic orbitals that determines the low efficiency of this channel of intermolecular magnetic exchange interactions.
Conclusions
Thus, the paramagnetic tricarbonyl half-sandwich rhenium(I) complexes [(NNCp)Re(CO)3] and [(INCp)Re(CO)3] with cyclopentadienyl-substituted nitronyl nitroxide NNCp and imino nitroxide INCp ligands were obtained as a result of this study. The correlation between the mutual arrangement of paramagnetic molecules in the solid phases of polymorphs [(NNCp)Re(CO)3] and the energy of their magnetic exchange interactions was established. High kinetic stability of [(NNCp)Re(CO)3] and [(INCp)Re(CO)3] is of particular interest for the design of new multifunctional targeted drugs on their basis.Author contributions
The manuscript was written through contributions of all authors. V. O. and K. M. developed the main conception and conceived the synthesis and physicochemical study of Re(I)-nitroxide complexes; V. O. and M. E. served as scientific advisors. K. M. synthesized of the Re(I)-nitroxide complexes under study. E. S., M. S. and M. E. performed the CV measurements and analysis; S. T., S. V. and M. F. studied Re(I)-nitroxide complexes by the EPR spectroscopy. G. R. and G. L. carried out X-ray experiment, refined the X-ray data and solved structures; A. B. performed the magnetochemistry measurements and analysis; V. M. carried out the quantum chemical calculations. All authors contributed in analysis and interpretation of obtained data and co-wrote the paper. K. M., G. R., and M. F. drafted the manuscript. All authors gave approvals to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Russian Foundation for Basic Research (Grant No. 19-29-08005, synthesis, electrochemical measurements and magnetochemistry studies) and the Russian Science Foundation (Grant No. 17-13-01022, XRD study).References
- E. B. Bauer, A. A. Haase, R. M. Reich, D. C. Crans and F. E. Kühn, Coord. Chem. Rev., 2019, 393, 79–117 CrossRef CAS.
- Bioorganometallic Chemistry: Applications in Dug Discovery, Biocatalysis, and Imaging, ed. G. Jaouen and M. Salmain, Wiley-VCH Verlag GmbH & Co, Weinheim, Germany, 2015, p. 398 Search PubMed.
- S. Hostachy, C. Policar and N. Delsuc, Coord. Chem. Rev., 2017, 351, 172–188 CrossRef CAS.
- A. Leonidova and G. Gasser, ACS Chem. Biol., 2014, 9, 2180–2193 CrossRef CAS PubMed.
- N. Lepareur, F. Lacoeuille, C. Bouvry, F. Hindré, E. Garcion, M. Chérel, N. Noiret, E. Garin and F. F. R. Knapp, Front. Med., 2019, 6, 00132 CrossRef PubMed.
- Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds, ed. R. G. Hicks, John Wiley & Sons, Ltd., 2010 Search PubMed.
- E. V. Tretyakov and V. I. Ovcharenko, Russ. Chem. Rev., 2009, 78, 971–1012 CrossRef CAS.
- V. I. Ovcharenko, E. Yu. Fursova, T. G. Tolstikova, K. N. Sorokina, A. Y. Letyagin and A. A. Savelov, Dokl. Chem., 2005, 404, 171–173 CrossRef CAS.
- N. A. Artyukhova, G. V. Romanenko, E. Y. Fursova and V. I. Ovcharenko, RF Pat., RU2642468C2, 2015 Search PubMed.
- W. Owtscharenko, W. Huber and K. E. Schwarzhans, Z. Naturforsch., B: J. Chem. Sci., 1986, 41, 1587–1588 Search PubMed.
- Y. Nakamura, N. Koga and H. Iwamura, Chem. Lett., 1991, 20, 69–72 CrossRef.
- O. Jürgens, J. Vidal-Gancedo, C. Rovira, K. Wurst, C. Sporer, B. Bildstein, H. Schottenberger, P. Jaitner and J. Veciana, Inorg. Chem., 1998, 37, 4547–4558 CrossRef PubMed.
- C. Sporer, D. Ruiz-Molina, K. Wurst, H. Kopacka, J. Veciana and P. Jaitner, J. Organomet. Chem., 2001, 637–639, 507–513 CrossRef CAS.
- A. R. Forrester, S. P. Hepburn, R. S. Dunlop and H. H. Mills, J. Chem. Soc. D, 1969, 698–699 RSC.
- S. Nakatsuji, K. Fujiwara, H. Akutsu, J. Yamada and M. Satoh, New J. Chem., 2013, 37, 2468–2472 RSC.
- E. F. Ullman, J. H. Osiecki, D. G. B. Boocock and R. Darcy, J. Am. Chem. Soc., 1972, 94, 7049–7059 CrossRef CAS.
- J. Becher and E. F. Ullman, US Pat., 3927019, 1975 Search PubMed.
- V. W.-W. Yam, K. K.-W. Lo and K. M.-C. Wong, J. Organomet. Chem., 1999, 578, 3–30 CrossRef CAS.
- A. Lannes, Y. Suffren, J. B. Tommasino, R. Chiriac, F. Toche, L. Khrouz, F. Molton, C. Duboc, I. Kieffer, J.-L. Hazemann, C. Reber, A. Hauser and D. Luneau, J. Am. Chem. Soc., 2016, 138, 16493–16501 CrossRef CAS PubMed.
- T. Scheiring, A. Klein and W. Kaim, J. Chem. Soc., Perkin Trans. 2, 1997, 2569–2571 RSC.
- A. Klein, C. Volger and W. Kaim, Organometallics, 1996, 15, 236–244 CrossRef CAS.
- I. Löw, M. Bubrin, A. Paretzki, J. Fiedler, S. Záliš and W. Kaim, Inorg. Chim. Acta, 2017, 455, 540–548 CrossRef.
- P. A. Abramov, A. A. Dmitriev, K. V. Kholin, N. P. Gritsan, M. K. Kadirov, A. L. Gushchin and M. N. Sokolov, Electrochim. Acta, 2018, 270, 526–534 CrossRef CAS.
- B. A. Goodman and J. B. Raynor, Adv. Inorg. Chem. Radiochem., 1970, 13, 135–362 CrossRef CAS.
- F. Neese, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS.
- Y. G. Budnikova, T. V. Gryaznova, M. K. Kadirov, E. V. Tret'yakov, K. V. Kholin, V. I. Ovcharenko, R. Z. Sagdeev and O. G. Sinyashin, Russ. J. Phys. Chem. A, 2009, 83, 1976–1980 CrossRef CAS.
- A. S. Mendkovich, V. B. Luzhkov, M. A. Syroeshkin, V. D. Sen, D. I. Khartsii and A. I. Rusakov, Russ. Chem. Bull., 2017, 66, 683–689 CrossRef CAS.
- V. D. Sen', I. V. Tikhonov, L. I. Borodin, E. M. Pliss, V. A. Golubev, M. A. Syroeshkin and A. I. Rusakov, J. Phys. Org. Chem., 2015, 28, 17–24 CrossRef.
- F. G. Bordwell and W.-Z. Liu, J. Am. Chem. Soc., 1996, 118, 8777–8781 CrossRef CAS.
- Cambridge Structural Database, Ver. 5.42, University of Cambridge, UK, 2020 Search PubMed.
- O. Kahn, Molecular Magnetism, Wiley-VCH, Weinheim, 1993 Search PubMed.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni, I. Dabo, A. Dal Corso, S. de Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A. P. Seitsonen, A. Smogunov, P. Umari and R. M. Wentzcovitch, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef PubMed.
- R. Boča, A Handbook of Magnetochemical Formulae, Elsevier Inc., 2012, p. 1010 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details relating to the synthesis, EPR spectroscopy and electrochemical studies, single crystals XRD experiments and crystallographic data, magnetochemistry measurements, fitting and analysis, and procedures and results of quantum-chemical calculations. CCDC 2064831–2064835. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra02159a |
| This journal is © The Royal Society of Chemistry 2021 |