Open Access Article
Open Access ArticleElectron beam lithography for direct patterning of MoS2 on PDMS substrates†
Gil Jumbertab,
Marcel Placidicd,
Francesc Alzinaa,
Clivia M. Sotomayor Torresae and
Marianna Sledzinska *a
*a
aCatalan Institute of Nanoscience and Nanotechnology (ICN2), CSIC, BIST, Campus UAB, Bellaterra, 08193 Barcelona, Spain. E-mail: marianna.sledzinska@icn2.cat
bDepartament de Fisica, Universitat Autònoma de Barcelona, Bellaterra, E-08193 Barcelona, Spain
cCatalonia Institute for Energy Research (IREC), Jardins de les Dones de Negre 1, Sant Adrià de Besòs, Spain
dPhotovoltaic Group, Electronic Engineering Department, Polytechnic University of Catalonia (UPC), Barcelona, Spain
eICREA, Pg. Lluís Companys 23, 08010 Barcelona, Spain
First published on 9th June 2021
Abstract
Precise patterning of 2D materials into micro- and nanostructures presents a considerable challenge and many efforts are dedicated to the development of processes alternative to the standard lithography. In this work we show a fabrication technique based on direct electron beam lithography (EBL) on MoS2 on polydimethylsiloxane (PDMS) substrates. This easy and fast method takes advantage of the interaction of the electron beam with the PDMS, which at high enough doses leads to cross-linking and shrinking of the polymer. At the same time, the adhesion of MoS2 to PDMS is enhanced in the exposed regions. The EBL acceleration voltages and doses are optimized in order to fabricate well-defined microstructures, which can be subsequently transferred to either a flexible or a rigid substrate, to obtain the negative of the exposed image. The reported procedure greatly simplifies the fabrication process and reduces the number of steps compared to standard lithography and etching. As no additional polymer, such as polymethyl methacrylate (PMMA) or photoresists, are used during the whole process the resulting samples are free of residues.
Introduction
2D materials, such as transition metal dichalcogenides (TMDs), offer unprecedented possibilities in applications such as electronics, optics or sensing.1,2 However, the precise control of the size and shape of 2D materials is critical for the fabrication of functional devices. As for now, their integration in electronic circuits requires a large number of fabrication steps, such as lithography, etching, and transfer.3 In the future the number of these steps will increase with the increasing complexity of the devices. At the same time 2D materials are particularly sensitive to contamination and residues, which can introduce defects, doping and seriously degrade their optical and electronic properties.4–7 Moreover, many cleaning techniques commonly used in silicon industry, such as strong acids or oxygen plasma, can no longer be used for 2D materials processing.Therefore, many efforts have been dedicated to the development of cleaner alternative fabrication methods. Instead of the electron beam or optical lithography8,9 one of the ideas has been to directly pattern the substrate and achieve the selective growth in the desired areas.10,11 In the case of MoS2 on SiO2/Si substrates, photolithography, O2 plasma and a specific chemical surface treatment was applied before chemical vapour deposition (CVD) growth.12 This process resulted in a single layer MoS2 with desired shapes and a minimal feature lateral size of 2 μm. Similarly chalcogenide crystals of Bi2Se3 and In2Se3 were grown by a method which combines solvent nanoimprinting with van der Waals epitaxy on mica substrates.13
Other alternative nanofabrication approaches have been developed to directly pattern the 2D materials on different substrates. In order to avoid the use of a photoresist or PMMA it has been proposed to pattern graphene films through a stencil mask by magnetic-assisted ultraviolet ozone treatment.14 However, high resolution patterns would be difficult to achieve using such a method. Furthermore, laser ablation has been reported as a relatively fast and precise method in order to thin a multilayer MoS2 down to a monolayer,15 bilayer and trilayer16 with both vertical and lateral control and maintaining good electronic properties. Similarly an optothermoplasmonic nanolithography is possible for 2D films placed on top of substrates made of gold nanoparticles.17 It takes advantage of the localized surface plasmon resonances, which enhance thermal oxidation and sublimation of the films when the substrate is exposed to the laser irradiation. However, the main drawback of this method is the need of a metallic substrate.
In this work, we report an easy patterning method for MoS2 on PDMS, which is the most common material employed for 2D materials exfoliation and transfer. The process is based on direct electron beam lithography (EBL), however, instead of using a standard PMMA mask and dry etching pattern transfer, we take advantage of the interaction between the electron beam and the MoS2 on the PDMS. The electron beam exposure strongly enhances the adhesion of MoS2 to the PDMS and afterwards the film can be dry-transferred either to a rigid substrate, such as Si/SiO2, or to another flexible substrate, such as PET, in order to obtain the negative of the exposed image. The main advantage of this method is the significant reduction of the number of fabrication steps to three (transfer to PDMS, EBL and transfer to the final substrate). The use of EBL allows for exposition of arbitrary patterns without a physical mask and with the resolution comparable to the above-discussed processes.
Nanofabrication process
MoS2 was grown on glass substrates, by direct sulfurization of a Mo thin film layer. It is a kinetic synthesis method, which has also been used to produce structures such as nanotubes or fullerene-like nanoparticles.18,19 In this way, both horizontally and vertically aligned layers can be formed, depending on the sample thickness and process conditions.20–22 In this work a continuous MoS2 polycrystalline film with thickness of 2 nm is used. Detailed sample characterisation can be found in the ESI (ESI, Fig. S1 to S4†).Polymer-free, wet transfer was performed in order to transfer the MoS2 from the original glass substrate to PDMS films (GelPak).21 The direct transfer procedure consisted in bringing the PDMS film into contact with MoS2 surface. A drop of DI water was placed with a pipette at the interface of the PDMS and the MoS2/glass. Due to the surface tension difference, the MoS2 layer is lifted off from the glass substrate. The PDMS is then peeled off together with the attached MoS2 during approx. 5–10 s (ESI, Fig. S5†). The process was performed in the ambient environment (20 °C, 40% relative humidity).
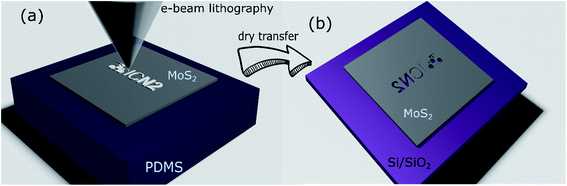
The EBL was performed to directly pattern the MoS2 on PDMS samples (Fig. 1(a)). Parameters such as the exposition dose and acceleration voltage are discussed in the section below.
Finally, after the exposure the MoS2 film was dry-transferred to the final substrate, where we obtained the negative of the exposed pattern (Fig. 1(b)). For the dry transfer process, the patterned PDMS was brought in contact with the final substrate (Si/SiO2 or PET). A finger-pressure was applied for a few minutes. Then the PDMS was gently peeled off during approx. 5–10 s. In general, we found that the transfer process quality does not strongly depend on the pressure and time. The process was performed in the ambient environment (20 °C, 40% relative humidity).
Results and discussion
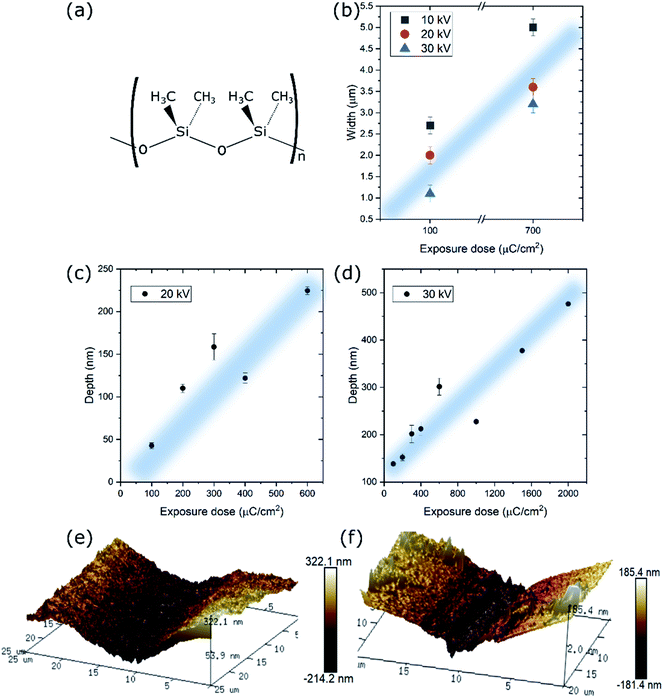
While PDMS has been extensively used in nanoimprint lithography not many works have been dedicated to its use in EBL and, more specifically, to its interaction with an electron beam. Such a study can give rise to a new nanofabrication tool for 2D materials patterning. It has been reported that during e-beam exposure methyl groups of the PDMS are removed allowing the backbones of different polymer chains to become cross-linked (Fig. 2(a)). However, at large doses, the electrons also can cause chain scission and the formation of other chemical species.23,24 At the same time, the PDMS hardness increases with the increase in the exposure dose, which has been attributed to the increase in cross-link density and decrease in molecular mobility. Higher degree of cross-linking results in a tighter network, which is responsible for the increase e in hardness.25 Moreover, a monotonic increase in the Young's modulus of PDMS from ∼50 to ∼350 MPa as a function of the e-beam dose was reported.26In a previous work, we studied the interactions at the interface between MoS2 and PDMS induced by an e-beam27 unveiling that well-defined micro-patterns can be obtained in the MoS2 regions. Due to the insulating nature of the PDMS the majority of the dose is accumulated on the surface and the MoS2 film-PDMS interface undergoes strong cross-linking. We have observed stronger adhesion of the MoS2 to the PDMS substrate, enhanced by the short-bonded PDMS chains.
First of all, a set of 200 μm long rectangles with nominal width of1 μm have been exposed at three acceleration voltages of 10, 20 and 30 kV and currents of 0.12, 0.14 and 0.15 nA, respectively. In Fig. 2(b) we compare the real width of a rectangle designed to be of 1 μm exposed at two doses, 100 and 700 μC cm−2, for each of the acceleration voltages. Higher acceleration voltages and smaller doses yield thinner patterns. Clearly, the closest to the original design is the rectangle exposed at 30 kV and the dose of 100 μC cm−2 (1.1 ± 0.2 μm). Therefore, we can conclude that the easily achievable minimum size of the features exposed on the MoS2/PDMS is between 1 and 2 μm, comparable to the techniques reported previously.
Secondly, a set of 1 mm long rectangles with nominal widths between 1 and 25 μm have been exposed at the same three acceleration voltages. At the voltages of 20 and 30 kV the exposed patterns were clearly visible under the optical microscope because of the strong cross-linking of the PDMS. We performed AFM on the set of rectangles exposed with doses from 100 to 2000 μC cm−2 in order to determine the level of contraction of the PDMS due to the e-beam interaction. The results are compared in Fig. 2(c) and (d). For both voltages we observe a linear increase in depth of the features with the increasing dose. As expected, higher acceleration voltages lead to deeper penetration of the electrons in the PDMS which in consequence leads to larger depth of exposed features. Examples of the AFM profiles are shown in Fig. 2(e) and (f). Patterns exposed using 10 kV acceleration voltage yield small contrast under the optical microscope, therefore it was not possible to locate them for the AFM measurements (Fig. S6†). However, after the dry transfer to Si/SiO2 the negatives of the patterns were clearly visible under the optical microscope. This further confirms that the penetration depth and the resulting PDMS contraction are acceleration dependent. In this work we have opted for exposition using 20 kV acceleration voltage, which combines good visibility under optical microscope and smaller contraction of the PDMS and, accordingly, shallow and narrow patterns.
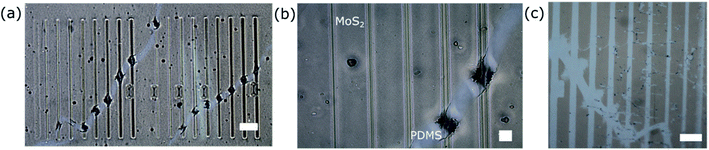
All exposure doses between 100 and 1000 μC cm−2 generate well defined rectangles, as seen in the optical microscope (Fig. 3(a) and (b)). The MoS2 can be transferred to the PET film using dry transfer in order to obtain a well-defined negative of the exposed patterns (Fig. 3(c) and S7†). The thinnest lines which can be successfully transferred are of about 2 μm at the exposure dose of 100 μC cm−2.
However, the patterns are only well exposed in the regions where MoS2 is present on the PDMS. In the regions where the MoS2 is not present (Fig. 3(b) and S8†) we observe that PDMS colour changed to almost black and the pattern is not well defined. We believe this is due to the fact that MoS2 is conductive and helps eliminate extra charge, in a similar way in which a conductive polymer or thin metal layer is used for EBL on insulating substrates.
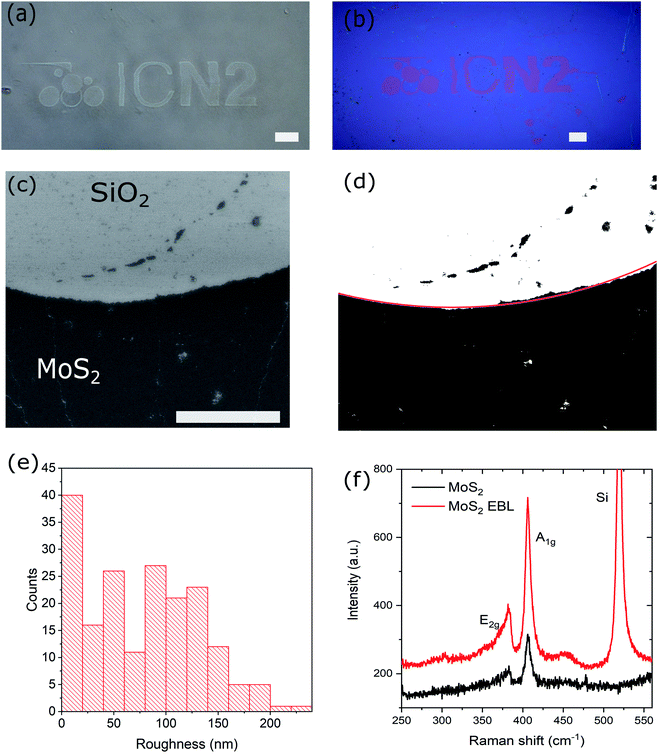
To assess the potential of the present method, we patterned and transferred a more complex design than the simple rectangles, i.e., the ICN2 logo. The design was exposed with EBL using 20 kV and various doses between 100 and 1000 μC cm−2. Optical images of the exposed sample and the transferred MoS2 pattern to Si/SiO2 are shown in Fig. 4(a) and (b), respectively. All of the exposure doses led to a good pattern definition and a successful transfer. A SEM image of a detail of the fabricated sample is shown in Fig. 4(c).
Using Matlab the SEM image was converted to black and white (Fig. 4(d)). In the resulting image, the pixels corresponding to MoS2 pattern were black while the pixels of the background were white. The MoS2 pattern was segmented by an arch of a circle fitted on the boundary by an iterative process. In the process the white area inside the arch and black area outside were maximized. Fig. 4(e) represents the roughness distribution, calculated as the distances between the pattern edge and the fitted arch. From the latter, we can extract an average (root mean square) roughness of the transferred patterns of about 100 nm, which proves that our technique is suitable for fabricating microstructures.
Raman spectra taken on both as-grown and transferred layers show no structural changes occurred in the material during the processing (Fig. 4(f)). Since no carrier polymer is used during the entire process the presence of organic contamination or residues is minimized. However, in some of the samples we have observed wrinkles and fractures of the MoS2 film after the transfer from PDMS to the Si/SiO2 (Fig. S9†). This effect is not caused directly by the EBL, but by the fact that large area transfer of ultrathin 2D materials is difficult to optimize. The fractures can occur both during the wet transfer to PDMS as well as during the dry transfer to the final substrate. Also polycrystalline MoS2 has a relatively low fracture strength, in order of 5%, making it prone to breaking.27 However, we believe it should be possible to perform the same process on the CVD grown single crystalline MoS2 or exfoliated flakes, which will have more possibilities for a successful transfer and device applications. The crystalline MoS2 has much higher Young modulus and its critical strain increases significantly when placed on an underlying substrate.28,29
One possible application of the proposed process could be in the field of micro- and nanoelectronics. 2D materials offer higher mobility than that of silicon and better scalability, which can lead to producing ultra-low noise field effect transistors and radiofrequency components with low cross talk and radiative losses and thus improved performance. Properly fabricated 2D transistor arrays are crucial for scale-up applications.30,31
Another application which can potentially benefit from our fabrication technique is the hydrogen evolution reaction (HER). MoS2 has become one of the most promising electrocatalysts for both the HER as well as for hydro-desulfurization (HDS) of sulfur-rich hydrocarbon fuels.32,33 Specifically, the edges of MoS2 nanostructures are known to be far more active as compared to its basal planes. Bulk MoS2 has relatively low electrical conductivity and small number of catalytically active edges, which seriously compromises its HER and HDS catalytic performance. On the other hand, patterning of MoS2 thin films increases the number of active edges as well as allows for spatial tuning of the edge density for local catalytic reactions. The possibility for transfer to arbitrary substrates can further increase the process efficiency.
Conclusions
In this work we have studied the interaction between an electron beam and the PDMS/MoS2 interface. We observed that exposure to the e-beam leads to cross-linking and contraction of PDMS, where the feature depth depends on the acceleration voltage and is roughly linearly proportional to the exposure dose. This effect was used to develop a new fabrication technique based on direct EBL on MoS2 on PDMS substrates. This easy and fast method allows fabricating well-defined microstructures, which can be subsequently transferred to either a rigid or flexible substrate, to obtain a negative of the exposed image. As no additional polymer, such as PMMA, is used during the EBL the resulting samples are free of residues. While this technique has been proven on MoS2 we believe it can be used for a variety of conducting and semiconducting 2D materials, as the mechanism behind the technique relies on the interaction between the electron beam and the PDMS.Experimental methods
MoS2 characterisation
Atomic force microscope topographical images were obtained using a Nanoscope IV controller and a Dimension 3100 head (Veeco). SEM images were taken in the FEI Quanta 650FEG ESEM and the FEI MAGELLAN 400L. Raman scattering measurements were performed using the Horiba T64000 Raman spectrometer and a 532 nm laser (cobolt). The Raman spectra at each point was obtained at the same incident laser power (0.3 mW) and integrated for the same amount of time (180 s). Elphy Quantum (Inspect F50 FEI SEM based system) was used for EBL. High-resolution imaging of the structure and morphology of the samples was obtained using the FEI Tecnai F20 in TEM and scanning TEM (STEM) modes.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
ICN2 is funded by the CERCA program/Generalitat de Catalunya, and is supported by the Severo Ochoa program from Spanish MINECO (Grant No. SEV-2017-0706). We acknowledge support from EU Project NANOPOLY (GA 289061) and the Spanish MICINN project SIP (PGC2018-101743-B-I00). MP acknowledges the financial support from Spanish MICINN within the Ramón y Cajal Program (RYC-2017-23758).References
- D. Jariwala, V. K. Sangwan, L. J. Lauhon, T. J. Marks and M. C. Hersam, ACS Nano, 2014, 8, 1102 CrossRef CAS PubMed.
- W. Choi, N. Choudhary, G. H. Han, J. Park, D. Akinwande and Y. H. Lee, Mater. Today, 2017, 20, 116 CrossRef CAS.
- Y. Chen, X.-L. Gong and J.-G. Gai, Adv. Sci., 2016, 3, 1500343 CrossRef PubMed.
- T. Hallam, N. C. Berner, C. Yim and G. S. Duesberg, Adv. Mater. Interfaces, 2014, 1, 1400115 CrossRef.
- L. Gammelgaard, J. M. Caridad, A. Cagliani, D. M. A. Mackenzie, D. H. Petersen, T. J. Booth and P. Bøggild, 2D Mater, 2014, 1, 035005 CrossRef CAS.
- W. Choi, M. A. Shehzad, S. Park and Y. Seo, RSC Adv., 2017, 7, 6943 RSC.
- A. Quellmalz, X. Wang, S. Sawallich, B. Uzlu, M. Otto, S. Wagner, Z. Wang, M. Prechtl, O. Hartwig, S. Luo, G. S. Duesberg, M. C. Lemme, K. B. Gylfason, N. Roxhed, G. Stemme and F. Niklaus, Nat. Commun., 2021, 12, 917 CrossRef CAS PubMed.
- L. Ju, B. Geng, J. Horng, C. Girit, M. Martin, Z. Hao, H. A. Bechtel, X. Liang, A. Zettl, Y. R. Shen and F. Wang, Nat. Nanotechnol., 2011, 6, 630 CrossRef CAS PubMed.
- Y. Chen, N. Zhang, Y.-F. Li, Y.-G. Bi, Y.-Y. Yue, J. Feng and H.-B. Sun, Adv. Opt. Mater., 2018, 6, 1701348 CrossRef.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi and B. H. Hong, Nature, 2009, 457, 706 CrossRef CAS PubMed.
- Z. Liu, L. Ma, G. Shi, W. Zhou, Y. Gong, S. Lei, X. Yang, J. Zhang, J. Yu, K. P. Hackenberg, A. Babakhani, J.-C. Idrobo, R. Vajtai, J. Lou and P. M. Ajayan, Nat. Nanotechnol., 2013, 8, 119 CrossRef CAS PubMed.
- Y. Guo, P.-C. Shen, C. Su, A.-Y. Lu, M. Hempel, Y. Han, Q. Ji, Y. Lin, E. Shi, E. McVay, L. Dou, D. A. Muller, T. Palacios, J. Li, X. Ling and J. Kong, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 3437 CrossRef CAS PubMed.
- W. Zheng, T. Xie, Y. Zhou, Y. L. Chen, W. Jiang, S. Zhao, J. Wu, Y. Jing, Y. Wu, G. Chen, Y. Guo, J. Yin, S. Huang, H. Q. Xu, Z. Liu and H. Peng, Nat. Commun., 2015, 6, 6972 CrossRef CAS PubMed.
- Y. Wu, H. Tao, S. Su, H. Yue, H. Li, Z. Zhang, Z. Ni and X. Chen, Sci. Rep., 2017, 7, 1 CrossRef PubMed.
- A. Castellanos-Gomez, M. Barkelid, A. M. Goossens, V. E. Calado, H. S. J. van der Zant and G. A. Steele, Nano Lett., 2012, 12, 3187 CrossRef CAS PubMed.
- L. Hu, X. Shan, Y. Wu, J. Zhao and X. Lu, Sci. Rep., 2017, 7, 15538 CrossRef PubMed.
- L. Lin, J. Li, W. Li, M. N. Yogeesh, J. Shi, X. Peng, Y. Liu, B. B. Rajeeva, M. F. Becker, Y. Liu, D. Akinwande and Y. Zheng, Adv. Funct. Mater., 2018, 28, 1803990 CrossRef.
- R. Tenne, L. Margulis, M. Genut and G. Hodes, Nature, 1992, 360, 444 CrossRef CAS.
- Y. Feldman, E. Wasserman, D. J. Srolovitz and R. Tenne, Science, 1995, 267, 222 CrossRef CAS PubMed.
- D. Kong, H. Wang, J. J. Cha, M. Pasta, K. J. Koski, J. Yao and Y. Cui, Nano Lett., 2013, 13, 1341 CrossRef CAS PubMed.
- M. Sledzinska, B. Graczykowski, M. Placidi, D. S. Reig, A. E. Sachat, J. S. Reparaz, F. Alzina, B. Mortazavi, R. Quey, L. Colombo, S. Roche and C. M. S. Torres, 2D Mater, 2016, 3, 035016 CrossRef.
- M. Sledzinska, R. Quey, B. Mortazavi, B. Graczykowski, M. Placidi, D. Saleta Reig, D. Navarro-Urrios, F. Alzina, L. Colombo, S. Roche and C. M. Sotomayor Torres, ACS Appl. Mater. Interfaces, 2017, 9, 37905 CrossRef CAS PubMed.
- J. Bowen, D. Cheneler and A. P. G. Robinson, Microelectron. Eng., 2012, 97, 34 CrossRef CAS.
- M. T. Russell, L. S. C. Pingree, M. C. Hersam and T. J. Marks, Langmuir, 2006, 22, 6712 CrossRef CAS PubMed.
- R. Giri, M. S. Sureshkumar, K. Naskar, Y. K. Bharadwaj, K. S. S. Sarma, S. Sabharwal and G. B. Nando, Adv. Polym. Technol., 2009, 27, 98 CrossRef.
- M. J. P. Biggs, M. Fernandez, D. Thomas, R. Cooper, M. Palma, J. Liao, T. Fazio, C. Dahlberg, H. Wheadon, A. Pallipurath, A. Pandit, J. Kysar and S. J. Wind, Adv. Mater., 2017, 29, 1702119 CrossRef PubMed.
- M. Sledzinska, G. Jumbert, M. Placidi, A. Arrighi, P. Xiao, F. Alzina and C. M. Sotomayor Torres, ACS Appl. Electron. Mater., 2020, 2, 1169 CrossRef CAS.
- Y. Li, P. Chen, H. Liu, J. Peng, F. Gao and N. Luo, Phys. Chem. Chem. Phys., 2019, 21, 19115 RSC.
- Y. Li, P. Chen, H. Liu, J. Peng and N. Luo, J. Appl. Phys., 2021, 129, 014302 CrossRef CAS.
- H. Nam, S. Wi, H. Rokni, M. Chen, G. Priessnitz, W. Lu and X. Liang, ACS Nano, 2013, 7, 5870 CrossRef CAS PubMed.
- M. G. Stanford, P. D. Rack and D. Jariwala, npj 2D Mater. Appl., 2018, 2, 1 CrossRef CAS.
- A. Parija, Y.-H. Choi, Z. Liu, J. L. Andrews, L. R. De Jesus, S. C. Fakra, M. Al-Hashimi, J. D. Batteas, D. Prendergast and S. Banerjee, ACS Cent. Sci., 2018, 4, 493 CrossRef CAS PubMed.
- X. Kong, X. Shen, C. Zhang, S. Norooz Oliaee and Z. Peng, Inorg. Chem. Front., 2016, 3, 1376 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra00885d |
| This journal is © The Royal Society of Chemistry 2021 |