Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper as an antimicrobial agent: recent advances
Intisar Salah a,
Ivan P. Parkin
a,
Ivan P. Parkin a and
Elaine Allan*b
a and
Elaine Allan*b
aMaterials Chemistry Research Centre, Department of Chemistry, University College London, 20 Gordon Street, London, UK
bDepartment of Microbial Diseases, Eastman Dental Institute, University College London, Royal Free Campus, Rowland Hill Street, London, UK. E-mail: e.allan@ucl.ac.uk
First published on 19th May 2021
Abstract
From its uses in ancient civilisations, copper has an established history as an antimicrobial agent. Extensive research has determined the efficacy and mechanism of copper's antimicrobial activity against microorganisms. The process is multifaceted with the main mechanism of bactericidal activity being the generation of reactive oxygen species (ROS), which irreversibly damages membranes. Copper ions released from surfaces lead to RNA degradation and membrane disruption of enveloped viruses. For fungi, the mechanism involves the physical deterioration of the membrane and copper ion influx. Due to variations in the experimental parameters, it is difficult to compare studies directly. In this review article, we outline the importance of the experimental conditions currently employed and how they bear little resemblance to real-world conditions. We endorse previous recommendations calling for an update to industrial standard tests.
Introduction
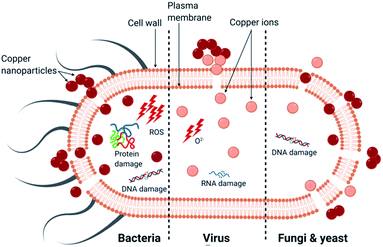
The ability of transition metals and metalloids to non-specifically target bacteria, viruses and fungi make them attractive antimicrobials. Specifically, copper is an esteemed antimicrobial agent and was utilised in ancient civilisation for its properties in medicinal products and water vessels before an understanding of the role of microbes in biofouling were acquired.1 Improvements in nanotechnology in recent years has led to a focus on developing copper nanoparticles (CuNPs) for antimicrobial performance. They have a substantially larger surface-area-to-volume ratio resulting in increased toxicity compared to the metal, alongside improved optical properties making them a desirable replacement.2–5Pathogenic microbes may be transmitted directly from person-to-person or indirectly via contamination of surfaces leading to both healthcare-acquired infections (HAIs) and community-acquired infections (CAIs).6–8 The exact mechanism of microbial death from copper is controversial and the significance and relative contribution of each proposed mechanism are unclear. One method is the physical interaction of the CuNPs with the cell, or virus, plasma membrane leading to its destruction, making the microbe susceptible to damage from copper ions.9–15 The smaller nanoparticles (NPs), between 1–10 nm, favour this mechanism as they can attach to the membrane and infiltrate the cell.16,17 Another method of copper's action is the generation of reactive oxygen species (ROS) by reduction of copper through a Fenton-like reaction, leading to enzyme and non-enzyme mediated oxidative damage involving lipid peroxidation, protein oxidation and DNA damage.18–20 The final mechanism is the release of copper ions, Cu+ and Cu2+, which damage the membrane and infiltrate the cell, inducing an oxidative stress response involving endogenous ROS.21–23 The consensus view of the cause of microbial cell death due to copper is a combination of these processes with the relative importance of each dependent on the microorganism; this will be explored in this paper.
The relative contributions of copper ions and ROS to efficacy can be ascertained by performing experiments in the presence and absence of chelating agents and quenching agents, respectively. Ethylenediaminetetraacetic acid (EDTA) is a chelating agent that forms complexes with Cu2+ released from the metal, and bathocuproine disulfonic acid complexes with Cu+. A commonly used agent to quench hydroxyl radicals is D-mannitol and for superoxide anions, 4,5-dihydroxy-1,3-benzene disulfonic acid. DNA integrity can be assessed by gel electrophoresis to separate DNA fragments. Membrane integrity can be visualised using a fluorescent live/dead stain which displays the viable and non-viable cells as green and red, respectively, using a fluorescence microscope.24,25 However, since permeability can be transient, fluorescent dyes alone cannot determine the extent of the damage and a quantitative assessment of the ability of the microorganism (bacteria or yeast) to form colonies is often employed using stainless steel as a negative control.26–29
Activity against bacteria
There is a distinction between the Gram-negative and Gram-positive bacterial response to copper potentially due to structural differences as seen in Fig. 1. Gram-negative bacteria have an outer membrane which makes them less susceptible to antibacterial agents.17,30,31 The normal mode of bacterial growth is in the form of a biofilm where the bacterial cells are adherent to a surface (or each other) and surrounded by a self-produced exopolymeric matrix comprising of polysaccharides, proteins and nucleic acids. Bacteria growing in a biofilm are challenging to remove, display increased resistance to antimicrobial agents compared to planktonic bacteria and are associated with medical device-related and tissue-related infections including urinary tract infections (UTIs), pneumonia and chronic wound infections.32–36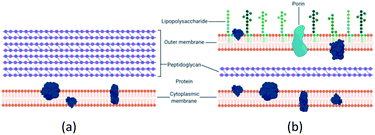 | ||
| Fig. 1 (a) Gram-positive bacteria possess a characteristic thick peptidoglycan layer. (b) Gram-negative bacteria exhibit an outer membrane and a thinner peptidoglycan layer. | ||
Activity against Escherichia coli
Pathogenic Escherichia coli is a common cause of HAIs and CAIs, including UTIs, and gastrointestinal disease, amongst other illnesses.37,38 E. coli is often used as the model Gram-negative bacterium to test bactericidal efficacy. E. coli is sub-divided into pathotypes which differ in their complement of accessory genes (i.e., virulence factors) but share core genomes. Different pathotypes are associated with different diseases. For instance, O157:H7 is a foodborne pathogen and responsible for diarrhoea and haemorrhagic colitis, as well as the life-threatening haemolytic-uremic syndrome.39 E. coli K12 is a domesticated strain known for being one of the first isolated microbes but is also notorious for being ‘far from [the] model’ microbe found in the clinical setting.40 This is because it is non-pathogenic and is therefore commonly employed in research as a model bacterium.Copper surface
The behaviour of three strains of E. coli, a pathogenic O157:H7 strain, and two K12 strains, on a copper surface were studied.41 Using a dry inoculum, the pathogenic strain survived for longer than the other two strains but all were non-viable after 10–20 minutes of exposure to the copper surface in comparison to stainless steel which still had live bacteria after 30 minutes. Staining with rhodamine 123 (where cells with intact membranes fluoresce) showed that fluorescence was rapidly lost on exposure to the copper surface indicating membrane disruption. Protection was detected by copper ion chelators alongside D-mannitol, which offered more protection than the superoxide anion quencher, suggesting most of the ROS present were hydroxyl radicals. This confirms the generation of ROS by a mechanism independent of the Fenton reaction, as proposed by the authors.Copper nanoparticles
In a study where E. coli ATCC 15224 was exposed to increasing concentrations of 12 nm CuNPs, the authors reported a progressive inhibition of bacterial growth with increasing concentration.42 Cu2+ ion release was measured and increased proportionally with the increased concentration of CuNPs suggesting that ion release is important for activity. The small size of the nanoparticles makes them easy to penetrate the cells and, through scanning electron microscopy (SEM), the authors demonstrated a shift in the morphology of the rod-shaped E. coli to irregular shapes in the presence of copper. This suggests that the CuNPs interact with the cell wall and damage the plasma membrane affecting its integrity as proposed by previous authors.43–45 However, the role of ROS was not investigated.In another study, evidence was presented for the role of Cu2+ ions in the degradation of E. coli as the size of CuNPs were reduced from 62.5 nm to 23.4 nm in the presence of EDTA for 30 minutes.46 Presumably, this was a result of gradual leaching (and subsequent chelation) of copper ions from the NP surface as the CuNPs left in suspension for 1 hour revealed no decrease in size. The generation of ROS inside bacterial cells were studied using the 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), a reduced form of fluorescein that is oxidised and highly fluorescent in the presence of ROS. E. coli K12 was exposed to CuNPs sized 62.5 nm for 1 hour at the minimum inhibitory concentration and the minimum bactericidal concentration which resulted in 1.8 and 2.5 times, respectively, overproduction of cellular reactive singlet oxygen and hydroxyl radicals in comparison with untreated cells. This study used E. coli K12 and studies with different E. coli strains have confirmed that even at low concentrations, CuNPs show good activity by invoking a strong ROS response.46–48
Activity against Staphylococcus aureus
Staphylococcus aureus is a major cause of skin and soft tissue infections.49,50 After the introduction of meticillin, resistant strains rapidly evolved51 and meticillin-resistant S. aureus (MRSA) has become a major problem in hospitals and increasingly in the community. Hence, research has focused on finding ways to reduce its transmission between patients.52Copper surface
In a study exposing MRSA to copper metal surfaces, complete kill was observed after 20 μL drops of bacterial suspension (containing 107 CFU) were exposed for 90 minutes at 22 °C in air, for three different strains including representatives of the epidemic clones, EMRSA-1 (NCTC 11939) and 16 (NCTC 13143).27 In comparison, stainless steel had viable cells after 72 hours of exposure, as anticipated. A notable finding was that there was no significant difference in the numbers of surviving bacteria between samples recovered with or without a Cu2+ chelator. This suggests that another mechanism independent of the presence of Cu2+ ions is responsible for the bactericidal activity of the copper surfaces.| Test organism & strain | Inoculum | Antimicrobial agent & UNS name | NP concentration & size | Inactivation time | Primary/significant MoA | Ref. |
|---|---|---|---|---|---|---|
| a UNS: unified numbering system for metals and alloys; MoA: mechanism of action; ref: reference. | ||||||
| E. coli O157:H7 | 1 × 107 CFU | Cu surface (C11000) | — | 10 min | ROS not via Fenton | 41 |
| E. coli K12 | 1 × 107 CFU | Cu surface (C11000) | — | 10 min | ||
| E. coli-ATCC 15224 | <1.0 × 108 CFU | CuNP suspension | 20–100 μg mL−1, 12 nm | Not specified | Physical interaction | 42 |
| E. coli K12 | 1 × 108 CFU | CuNP suspension | 3.0 & 7.5 μg mL−1 62.5 nm | 1 h | ROS via Fenton | 46 |
| MRSA-NCTC 10442 | 1 × 107 CFU | Cu surface (C19700) | — | 45 min | ROS not via Fenton | 27 |
| EMRSA-1 NCTC 11939 | 1.9 × 107 CFU | Cu surface (C19700) | — | 60 min | ||
| EMRSA-16 NCTC 13143 | 1.5 × 107 CFU | Cu surface (C19700) | — | 90 min | ||
| EMRSA-16 NCTC 13143 | 1 × 107 CFU | Cu surface | — | 20 min | 53 | |
| MSSA-ATCC 49230 | 1 × 107 CFU | Cu surface | — | 15 min | ||
| S. aureus-ATCC 6538 | 1 × 105 CFU | CuNP suspension | 200–3200 μg mL−1, 9 nm | 16 h | Physical interaction | 54 |
| SARS-CoV-2 – USA-WA1/2020 | 5 μm × 105.25 TCID50/mL | Cu surface | — | 4 h | RNA degradation | 29 |
| HuCoV-229E | 1 × 103 PFU | Cu surface (C11000) | — | 60 min | 62 | |
| Influenza A H1N1 | 2 × 106 PFU | Cu surface (C11000) | — | 6 h | Cu ions cause RNA degradation | 28 |
| Norovirus-murine norovirus | 5 × 104 PFU | Cu surface (C11000) | — | 30 min | Cu ions, particularly, Cu+, cause RNA degradation | 65 |
| Aspergillus spp. | 1 × 106 spores | CuNP suspension | 100 μg mL−1, 25.5 nm | 5 days | Physical interaction, copper ions | 71 |
| C. albicans SC5314, CAF3-1, CaCUP1, CaCRP1 | 12.5 × 106 CFU | Cu surface (C11000) | — | 60 min (mutants 5–10 min) | 9 | |
| Candida spp., C. Albicans ATCC MYA-2876, C. glabrata ATCC 2001, C. tropicalis ATCC 750 | 1–5 × 105 CFU | CuONP suspension | 25–200 mM, 35 nm | Not specified | 73 | |
Another study applied smaller droplets (1 μL containing 107 CFU) of the same EMRSA-16 strain (NCTC 13143) to examine the activity of a copper surface compared to a meticillin-sensitive S. aureus (MSSA).53 The authors reported complete kill of EMRSA in 20 minutes and MSSA in 15 minutes. The addition of a chelating agent and a superoxide anion quencher protected the bacteria; however, the addition of a hydroxyl quencher and a hydrogen peroxide-decomposing agent offered no protection. This indicated there was ROS generation, however not via Fenton chemistry.
Copper nanoparticles
Betancourt-Galindo and co-workers assessed the effect of exposure to increasing concentrations of 9 nm CuNPs on S. aureus ATCC 6538.54 As expected, increasing CuNP concentration resulted in a progressive increase in growth inhibition and the highest test concentration (3200 μg mL−1) was required for complete inhibition of growth. In contrast, 1600 μg mL−1 was sufficient for complete inhibition of the Gram-negative bacterium, Pseudomonas aeruginosa. Although there was no attempt to elucidate the mechanism, we can speculate that the presence of a thicker peptidoglycan layer in S. aureus compared to P. aeruginosa may be responsible for reduced cellular penetration by the CuNPs.Activity against viruses
Viruses have either DNA or RNA genomes and the nucleic acid is either double-stranded or single-stranded, respectively. RNA viruses are more likely to cause outbreaks of infections as they have a higher mutation and evolutionary rates making them more virulent.55 In this article, we review the literature on the effect of copper surfaces on the RNA viruses, SARS-CoV-2, influenza A and norovirus. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the highly infectious virus responsible for the COVID-19 pandemic. It is spread through droplets exhaled from infected people and through aerosols propelled by coughs and sneezes.56,57 Influenza A causes the common flu and can lead to pneumonia and high fever.58 It has a high morbidity and mortality rate amongst high-risk groups, particularly the elderly. Norovirus, also known as the ‘winter vomiting bug’ is also common, responsible for epidemic gastroenteritis and annually costs the NHS £100 million.59,60 Therefore, solutions to reduce the spread of this highly contagious virus is in high demand. Because of the risk of handling highly infectious viruses like SARS-CoV-2, less infectious surrogates are often used and potential biological differences between the actual pathogen and its surrogates should be borne in mind when the results are interpreted.Activity against SARS-CoV-2
Recent studies have demonstrated that SARS-Cov-2 is stable on copper surfaces for up to 4 hours after exposure.29,61 This is in comparison to stainless steel and plastic surfaces where the virus survives for up to 48 and 72 h, respectively.29 The strain used in this study serves as the reference for SARS-CoV-2.62 Another study demonstrated the degradation of genomic RNA of another human coronavirus (HuCoV-229E) by copper.63 These authors also examined copper ion release by using copper ion chelators which protected the virus for up to 2 hours suggesting both ions are involved in the virucidal mechanism. They also assessed the generation of ROS by using D-mannitol and a superoxide anion quencher. They found that the latter protected the virus for a considerably longer period of time compared to exposure in the presence of D-mannitol, suggesting superoxide radicals are significant in the inactivation of this coronavirus. As a negative control, the authors used the same chelating and quenching agents on stainless steel and reported no significant effect.Activity against influenza A
Research comparing the rate of inactivation of influenza A on a copper surface with stainless steel showed promising results.28 This study demonstrated a nearly 4-log reduction of infectious particles on the copper surface after just 6 hours of incubation at room temperature compared to a 1-log reduction after 24 hours on stainless steel. The authors suggest the degradation of the genomic material may be responsible for the activity although no supporting evidence was presented.Activity against norovirus
The first study to look at the antiviral properties of a copper surface against a surrogate murine norovirus reported zero-counts of virus particles after only 30 minutes of exposure.64 The murine surrogate is phylogenetically closest to the human norovirus and is regarded as an appropriate model.65 Analysis of genomic RNA by gel electrophoresis showed complete genome degradation following exposure to copper. To study the mechanism, quenchers and chelators were utilised. The results demonstrated that the quenchers did not affect virus inactivation whereas the chelators offered protection. Due to the lack of action of the quenching agents, it can be inferred that ROS generation was not the primary mechanism and instead copper ions contributed significantly to virucidal activity.Activity against fungi and yeast
Fungi are a diverse group of microorganisms including both yeast and filamentous fungi and are a cause of subcutaneous, cutaneous and systemic disease and if not treated, can result in chronic infections.66 Aspergillus is a filamentous fungus that produces spores which are ubiquitous in the environment. Inhalation of spores can lead to fatal systemic infections in immunocompromised hospital patients.67,68 Several studies have concentrated on Candida sp., the genus of yeast responsible for candidiasis, a common fungal infection.67,69 Nearly a million cases of invasive and potentially deadly candidiasis are diagnosed annually and cases are on the rise, particularly in immunocompromised individuals, including patients undergoing cancer chemotherapy, transplants and haemodialysis.67,70 C. albicans, C. glabrata, and C. tropicalis are the three species collectively responsible for 83.8% of candidiasis cases.71Activity against Aspergillus species
In one study, numerous species, including opportunistic A. flavus and A. terreus, were presented with 25.5 nm CuNPs and incubated for 5 days at 28 °C in air.71 Relatively long periods of exposure are required because of the slow growth rate of the fungi.72 The parameters set in this experiment were sufficient to achieve 58–73% inhibition of fungal growth although whether this reflects inhibition of spore germination or growth of vegetative filaments is not clear. Agarose gel electrophoresis of genomic DNA isolated after exposure to NPs indicated fragmentation of the genome suggesting NP penetration of the cell wall.Activity against Candida species
One study exposed C. albicans to a copper surface and found the yeast was inactivated in 5 minutes at 23 °C.9 A fluorescent dye, dihydroethidium, was employed to detect cytoplasmic ROS within 1 minute of exposure alongside a copper-sensitive dye that exhibited an uptake of copper ions. A mutant lacking the copper ion efflux system showed increased susceptibility to copper compared to the wild-type strain, demonstrating the importance of copper ions in the fungicidal activity. Live/dead staining showed the membranes were damaged instantly on exposure to the copper surface, suggesting the physical interaction of CuNPs with the yeast cell is vital in the mechanism of yeast cell death.Another study prepared 35 nm CuONPs and demonstrated a 53% inhibition of growth for C. albicans and C. glabrata, and 59% for C. tropicalis.73 An alteration of the cellular morphology was evident by SEM. Although this is a promising demonstration of the potential of CuONPs in preventing yeast infections, further studies are required to elucidate the precise mechanism of growth inhibition.
Summary of copper's activity against microorganisms
In summary, copper has an intrinsic antimicrobial effect on bacteria, viruses and fungi. The activity of CuNP appears to depend more on their size rather than their concentration: the smaller the nanoparticles, the greater the efficacy.46–48 The primary causes of death for each microorganism is presented in Fig. 2. The main mechanism of bactericidal activity is the generation of ROS, both dependent and independent from Fenton chemistry, and results in membrane damage. The principal mechanism of activity of copper surface against viruses was ion release leading to RNA degradation and membrane disruption in the case of enveloped viruses. For fungi, the uptake of copper ions and the physical deterioration of the membrane leading to copper influx are considered to be the primary mechanisms (Table 1).Antimicrobial properties of copper alloys
Commonly used copper alloys are brass and bronze, the former consisting of copper and zinc and the latter, copper and other metals, typically tin, aluminium, nickel and metalloids such as silicon.26 The copper alloys also have antimicrobial activity and studies have shown that there is a direct correlation between the copper content and antimicrobial efficacy.26,74–76 Alongside improving the antimicrobial activity, changing the composition of metals can affect other properties; for instance, the ratio of copper-to-zinc in brass can be altered to ensure the surface is sufficiently hard for applications such as hospital furniture and doorknobs.77Brass
Due to zinc's inherent antimicrobial activity, when combined with copper, there is a synergy between the two metals, therefore, even at low copper concentrations, there is notable antiviral activity.63,78 Nickel, on the other hand, has no intrinsic antimicrobial properties, thus, the increase in copper content has a proportional impact on activity. A comparison of brass and CuNi, both with 70% copper, revealed that brass inactivates human coronavirus (HuCoV-229E) faster.63 After 30 minutes, the authors reported a 4-log reduction in virus particles compared to only 1-log reduction after 2 hours on CuNi. In another study, brass with 86% and 63% copper also demonstrated bactericidal activity against E. coli at higher copper concentrations; it took 15 minutes for the 86% copper and 30 minutes for 63% copper to achieve full inactivation of bacteria.26 The synergy between copper and zinc has demonstrated that brass can be a cost-effective option for large surfaces such as those required in hospitals.26 Another study looked at the antibacterial performance of brass (80% copper) against MRSA and reported a 4-log reduction in bacterial numbers within 3 hours compared to no significant reduction on stainless steel after 6 hours.27In a 10 week study in a busy British hospital, plastic, chrome-plated and aluminium surfaces were replaced with brass, of 60% or 70% copper.79 In comparison to traditional surfaces, brass resulted in 90–100% inactivation of MSSA, vancomycin-resistant enterococcus (VRE) and E. coli. Another study similarly replaced hospital surfaces with copper alloys and showed that the reduced surface colonisation by bacteria and yeast was associated with a 58% decrease in the incidence of HAIs.80 Although the exact alloys were not disclosed in this study, the results are promising and demonstrate the potential of copper alloys potential as antimicrobial agents for surface disinfection in hospitals.
Bronze
Three bronze surfaces, C65500 (97% copper, 3% silicon), C61500 (90% copper, 8% aluminium, 2% nickel) and C51000 (95% copper, 5% tin) were exposed to E. coli O157:H7 (ref. 81) and a reduction in bacterial numbers to below the detection limit in 65, 180 and 105 minutes, respectively, was reported. Silicon has proven to have antibacterial and antifungal properties and tin is a powerful antimicrobial agent which means when these elements are combined with copper to form bronze, they reduce the inactivation time as seen in C65500 and C51000.82–85 Although aluminium has intrinsic antimicrobial properties, C61500 took the longest to reduce bacterial numbers to below the detection limit suggesting that a higher copper content is required for maximum activity.86,87Summary of copper alloys' activity against microorganisms
Brass and bronze present excellent antimicrobial efficacy and the activity of the alloys increases proportionally with the concentration of copper. The suggested antimicrobial mechanism is copper ion release supporting the Fenton reaction leading to the production of hydroxyl radicals.63 This was established by using copper ion chelators, with brass and copper exposed to a human coronavirus (HuCoV-229E). The chelators protected the virus by increasing the time taken for inactivation, indicating that the release of copper ions is important in the inactivation mechanism. There is also evidence to determine DNA degradation is not necessarily a cause of bacterial cell death.12 The relative contributions of membrane damage, penetration into the cytoplasm of bacteria and fungi, and the generation of ROS by copper alloys need to be elucidated (Table 2).| Test organism & strain | Inoculum | Antimicrobial surface & UNS name | % Copper | Extent of reduction | Inactivation time | Ref. |
|---|---|---|---|---|---|---|
| a UNS: unified numbering system for metals and alloys; %copper: percentage of copper in alloy; ref.: references. | ||||||
| Murine norovirus-HuCoV-229E | 5 × 105 PFU | CuZn (C26000) | 70 | 4-log | 30 min | 62 |
| CuNi (C71500) | 70 | 1-log | 2 h | |||
| E. coli-ATCC 25922 | 1 × 107 CFU | CuZn (C23000 & C24700) | 86 | 100% | 15 min | 26 |
| 63 | 100% | 30 min | ||||
| MRSA-NCTC 10442 | 1 × 107 CFU | CuZn (C24000) | 80 | 4-log | 3 h | 27 |
| MSSA, VRE, E. coli | Not specified | CuZn | 60 & 70 | 90–100% | 10 weeks | 79 |
| E. coli-O157:H7 | 1 × 107 CFU | CuSi (C65500) | 97 | 100% | 65 min | 81 |
| CuAlNi (C61500) | 90 | 100% | 180 min | |||
| CuSn (C51000) | 95 | 100% | 105 min | |||
Critical remarks and future perspectives
In our review of the literature, it is apparent that the specific experimental conditions play a major role in the results obtained and differences between studies make comparison difficult. We have noted differences between studies in the type of microorganism selected including genus, species and strain, and few papers provide justification for their choice. Microbial cultures are often obtained from collections such as the UK's National Collection of Typed Cultures (NCTC) or the American Typed Culture Collection (ATCC) in the US. While the use of these authenticated strains is essential to enable intra-laboratory comparison, there are two important issues to bear in mind: (i) in many cases, the extent of laboratory culture (and hence the extent of genomic evolution as a result of domestication) is undocumented and (ii) the same strains maintained in different laboratories for many years may exhibit genetic differences.88–90 This has implications for reproducibility within, and comparison between, studies. It also must be borne in mind that strains within a species are often heterogeneous, and therefore, a single strain is unlikely to be representative of the species as a whole.91 Thus, it is advisable to use multiple strains and including freshly isolated (and minimally sub-cultured) clinical isolates as well as standard strains from culture collections.Studies also differ in the phase of growth microorganisms are harvested for testing, with some using stationary phase cultures where growth is slowed and others using exponential phase cultures where replication rates are maximal. The differences in the physiological state of the microbial population will undoubtedly affect their susceptibility to antibacterials; indeed some studies have documented the differences.36,78,92 Other experimental conditions which vary between studies and are known to affect microbial susceptibility to copper include factors that affect inoculum-drying time, like temperature, airflow and humidity.21,88,93–95 Issues with the lack of ‘real world’ conditions in the industrial standard tests (e.g., ISO 22196, ISO 21702, ISO 846) and the vast collection of modified protocols that now exist as a result have been previously acknowledged and adequately discussed.21,93,96,97 In addition, the need to take into account the effect of cleaning protocols, surface soiling and wear on material efficacy has been documented.93,97 We reiterate previous recommendations calling for updated industrial standard tests which apply environmental parameters more reflective of actual conditions within the healthcare setting.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to thank A. O. Smith from NSG Group for financial support and for useful discussion. The authors would also like to acklowledge the EPSRC Centre for Doctoral Training in Molecular Modelling and Material Science at University College London (EP/L015862/1) for financial support.References
- V. B. Sudha, K. O. Singh, S. R. Prasad and P. Venkatasubramanian, Trans. R. Soc. Trop. Med. Hyg., 2009, 103, 819–822 CrossRef CAS PubMed.
- A. S. Karakoti, L. L. Hench and S. Seal, JOM, 2006, 58, 77–82 CrossRef CAS.
- S. Shahzadi, N. Zafar and R. Sharif, Antibacterial Activity of Metallic Nanoparticles, Bacterial Pathogenesis and Antibacterial Control, IntechOpen, 2018 Search PubMed.
- G. Sánchez-Sanhueza, D. Fuentes-Rodrígue and H. Bello-Toledo, Int. J. Odontostomat., 2016, 10, 547–554 CrossRef.
- B. A. Camacho-Flores, O. Martínez-Álvarez, M. C. Arenas-Arrocena, R. Garcia-Contreras, L. Argueta-Figueroa, J. Fuente-Hernández and L. S. Acosta-Torres, J. Nanomater., 2015, 415238 Search PubMed.
- P. Gastmeier, S. Stamm-Balderjahn, S. Hansen, F. Nitzschke-Tiemann, I. Zuschneid, K. Groneberg and H. Rüden, Infect. Control Hosp. Epidemiol., 2005, 26, 357–361 CrossRef PubMed.
- G. U. Lopez, C. P. Gerba, A. H. Tamimi, M. Kitajima, S. L. Maxwell and J. B. Rose, Appl. Environ. Microbiol., 2013, 79, 5728–5734 CrossRef CAS PubMed.
- N. L. Turnage and K. E. Gibson, J. Virol. Methods, 2017, 248, 31–38 CrossRef CAS PubMed.
- D. Quaranta, T. Krans, C. Espírito Santo, C. G. Elowsky, D. W. Domaille, C. J. Chang and G. Grass, Appl. Environ. Microbiol., 2011, 77, 416–426 CrossRef CAS PubMed.
- C. Espírito Santo, E. W. Lam, C. G. Elowsky, D. Quaranta, D. W. Domaille, C. J. Chang and G. Grass, Appl. Environ. Microbiol., 2011, 77, 794–802 CrossRef PubMed.
- C. E. Santo, D. Quaranta and G. Grass, MicrobiologyOpen, 2012, 1, 46–52 CrossRef CAS PubMed.
- R. Hong, T. Y. Kang, C. A. Michels and N. Gadura, Appl. Environ. Microbiol., 2012, 78, 1776–1784 CrossRef CAS PubMed.
- W. X. Tian, S. Yu, M. Ibrahim, A. W. Almonaofy, L. He, Q. Hui, Z. Bo, B. Li and G. L. Xie, J. Microbiol., 2012, 50, 586–593 CrossRef CAS PubMed.
- S. Mathews, M. Hans, F. Mücklich and M. Solioz, Appl. Environ. Microbiol., 2013, 79, 2605–2611 CrossRef CAS PubMed.
- H. Gutierrez, T. Portman, V. Pershin and M. Ringuette, J. Appl. Microbiol., 2013, 114, 680–687 CrossRef CAS PubMed.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramírez and M. J. Yacaman, Nanotechnology, 2005, 16, 2346–2353 CrossRef CAS PubMed.
- K. Giannousi, A. Pantazaki and C. Dendrinou-Samara, Nanostructures for Antimicrobial Therapy, 2017, pp. 515–529 Search PubMed.
- A. N. Pham, G. Xing, C. J. Miller and D. T. Waite, J. Catal., 2013, 301, 54–64 CrossRef CAS.
- C. Yang, L. Jiang, H. Zhang, L. A. Shimoda, R. J. DeBerardinis and G. L. Semenza, Methods Enzymol., 2014, 542, 425–455 CAS.
- P. D. Ray, B. W. Huang and Y. Tsuji, Cell. Signalling, 2012, 24, 981–990 CrossRef CAS PubMed.
- G. Grass, C. Rensing and M. Solioz, Appl. Environ. Microbiol., 2011, 77, 1541–1547 CrossRef CAS PubMed.
- B. M. Prabhu, S. F. Ali, R. C. Murdock, S. M. Hussain and M. Srivatsan, Nanotoxicology, 2010, 4, 150–160 CrossRef CAS PubMed.
- I. C. Lee, J. W. Ko, S. H. Park, J. O. Lim, I. S. Shin, C. Moon, S. H. Kim, J. D. Heo and J. C. Kim, Int. J. Nanomed., 2016, 11, 2883–2900 CAS.
- P. Breeuwer and T. Abee, Mol. Microb. Ecol. Man., 2004, 8, 1563–1580 Search PubMed.
- D. Voytas, Current Protocols, 1992 Search PubMed.
- A. Różańska, A. Chmielarczyk, D. Romaniszyn, A. Sroka-Oleksiak, M. Bulanda, M. Walkowicz, P. Osuch and T. Knych, Int. J. Environ. Res. Public Health, 2017, 14, 813 CrossRef PubMed.
- J. O. Noyce, H. Michels and C. W. Keevil, J. Hosp. Infect., 2006, 63, 289–297 CrossRef CAS PubMed.
- J. O. Noyce, H. Michels and C. W. Keevil, Appl. Environ. Microbiol., 2007, 73, 2748–2750 CrossRef CAS PubMed.
- N. van Doremalen, T. Bushmaker, D. H. Morris, M. G. Holbrook, A. Gamble, B. N. Williamson, A. Tamin, J. L. Harcourt, N. J. Thornburg, S. I. Gerber, J. O. Lloyd-Smith, E. de Wit and V. J. Munster, N. Engl. J. Med., 2020, 1564–1567 CrossRef PubMed.
- K. San, J. Long, C. A. Michels and N. Gadura, MicrobiologyOpen, 2015, 4, 753–763 CrossRef CAS PubMed.
- A. L. Koch, Am. Sci., 1990, 78, 15 Search PubMed.
- H. Koo, R. N. Allan, R. P. Howlin, P. Stoodley and L. Hall-Stoodley, Nat. Rev. Microbiol., 2017, 15, 740–755 CrossRef CAS PubMed.
- D. Lebeaux, J. M. Ghigo and C. Beloin, Microbiol. Mol. Biol. Rev., 2014, 78, 510–543 CrossRef PubMed.
- T. Bjarnsholt, APMIS, Suppl., 2013, 1–51 Search PubMed.
- M. Jamal, W. Ahmad, S. Andleeb, F. Jalil, M. Imran, M. A. Nawaz, T. Hussain, M. Ali, M. Rafiq and M. A. Kamil, J. Chin. Med. Assoc., 2018, 81, 7–11 CrossRef PubMed.
- G. M. Teitzel and M. R. Parsek, Appl. Environ. Microbiol., 2003, 69, 2313–2320 CrossRef CAS PubMed.
- A. Clements, J. C. Young, N. Constantinou and G. Frankel, Gut Microbes, 2012, 3, 71–87 CrossRef PubMed.
- N. Allocati, M. Masulli, M. F. Alexeyev and C. Di Ilio, Int. J. Environ. Res. Public Health, 2013, 10, 6235–6254 CrossRef PubMed.
- J. Y. Lim, J. Yoon and C. J. Hovde, J. Microbiol. Biotechnol., 2010, 20, 5–14 CrossRef CAS PubMed.
- J. L. Hobman, C. W. Penn and M. J. Pallen, Mol. Microbiol., 2007, 64, 881–885 CrossRef CAS PubMed.
- S. L. Warnes, V. Caves and C. W. Keevil, Environ. Microbiol., 2012, 14, 1730–1743 CrossRef CAS PubMed.
- M. Raffi, S. Mehrwan, T. M. Bhatti, J. I. Akhter, A. Hameed, W. Yawar and M. M. Ul Hassan, Ann. Microbiol., 2010, 60, 75–80 CrossRef CAS.
- A. Thill, O. Zeyons, O. Spalla, F. Chauvat, J. Rose, M. Auffan and A. M. Flank, Environ. Sci. Technol., 2006, 40, 6151–6156 CrossRef CAS PubMed.
- C. Ning, X. Wang, L. Li, Y. Zhu, M. Li, P. Yu, L. Zhou, Z. Zhou, J. Chen, G. Tan, Y. Zhang, Y. Wang and C. Mao, Chem. Res. Toxicol., 2015, 28, 1815–1822 Search PubMed.
- R. Gyawali, S. A. Ibrahim, S. H. Abu Hasfa, S. Q. Smqadri and Y. Haik, J. Pathog., 2011, 2011, 650968 Search PubMed.
- A. K. Chatterjee, R. Chakraborty and T. Basu, Nanotechnology, 2014, 25, 135101 CrossRef PubMed.
- C. Kaweeteerawat, P. Na Ubol, S. Sangmuang, S. Aueviriyavit and R. Maniratanachote, J. Toxicol. Environ. Health, Part A, 2017, 80, 1276–1289 CrossRef CAS PubMed.
- K. Y. Yoon, J. Hoon Byeon, J. H. Park and J. Hwang, Sci. Total Environ., 2007, 373, 572–575 CrossRef CAS PubMed.
- P. M. Schlievert, K. N. Shands, B. B. Dan, G. P. Schmid and R. D. Nishimura, J. Infect. Dis., 1981, 143, 509–516 CrossRef CAS PubMed.
- A. Russo, E. Concia, F. Cristini, F. G. De Rosa, S. Esposito, F. Menichetti, N. Petrosillo, M. Tumbarello, M. Venditti, P. Viale, C. Viscoli and M. Bassetti, Clin. Microbiol. Infect., 2016, 22, 27–36 CrossRef.
- P. D. Stapleton and P. W. Taylor, Sci. Prog., 2002, 85, 57–72 CrossRef CAS PubMed.
- B. P. Howden, P. B. Ward, P. G. Charles, T. M. Korman, A. Fuller, P. du Cros, E. A. Grabsch, S. A. Roberts, J. Robson, K. Read, N. Bak, J. Hurley, P. D. Johnson, A. J. Morris, B. C. Mayall and M. L. Grayson, Clin. Infect. Dis., 2004, 38, 521–528 CrossRef CAS PubMed.
- S. L. Warnes and C. W. Keevil, Appl. Environ. Microbiol., 2016, 82, 2132–2136 CrossRef CAS PubMed.
- R. Betancourt-Galindo, P. Y. Reyes-Rodriguez, B. A. Puente-Urbina, C. A. Avila-Orta, O. S. Rodríguez-Fernández, G. Cadenas-Pliego, R. H. Lira-Saldivar and L. A. García-Cerda, J. Nanomater., 2014, 2014, 5 Search PubMed.
- E. C. Holmes, Annual Review of Ecology, Evolution, and Systematics, 2009, 40, 353–372 CrossRef.
- S. Tang, Y. Mao, R. M. Jones, Q. Tan, J. S. Ji, N. Li, J. Shen, Y. Lv, L. Pan, P. Ding, X. Wang, Y. Wang, C. R. MacIntyre and X. Shi, Environ. Int., 2020, 144, 106039 CrossRef CAS PubMed.
- C. Poggio, M. Colombo, C. R. Arciola, T. Greggi, A. Scribante and A. Dagna, Materials, 2020, 13, 3244 CrossRef CAS PubMed.
- J. K. Taubenberger and D. M. Morens, Annu. Rev. Phytopathol., 2008, 3, 499–522 CrossRef CAS PubMed.
- F. G. Sandmann, L. Shallcross, N. Adams, D. J. Allen, P. G. Coen, A. Jeanes, Z. Kozlakidis, L. Larkin, F. Wurie, J. V. Robotham, M. Jit and S. R. Deeny, Clin. Infect. Dis., 2018, 67, 693–700 CrossRef PubMed.
- E. Robilotti, S. Deresinski and B. A. Pinsky, Clin. Microbiol. Rev., 2015, 28, 134–164 CrossRef CAS PubMed.
- N. Hutasoit, B. Kennedy, S. Hamilton, A. Luttick, R. A. Rahman Rashid and S. Palanisamy, Manuf. Lett., 2020, 25, 93–97 CrossRef.
- J. Harcourt, A. Tamin, X. Lu, S. Kamili, S. K. Sakthivel, J. Murray, K. Queen, Y. Tao, C. R. Paden, J. Zhang, Y. Li, A. Uehara, H. Wang, C. Goldsmith, H. A. Bullock, L. Wang, B. Whitaker, B. Lynch, R. Gautam, C. Schindewolf, K. G. Lokugamage, D. Scharton, J. A. Plante, D. Mirchandani, S. G. Widen, K. Narayanan, S. Makino, T. G. Ksiazek, K. S. Plante, S. C. Weaver, S. Lindstrom, S. Tong, V. D. Menachery and N. J. Thornburg, Emerging Infect. Dis., 2020, 26, 1266–1273 CrossRef CAS PubMed.
- S. L. Warnes, Z. R. Little and C. W. Keevil, mBio, 2015, 6, 01697-15 CrossRef PubMed.
- S. L. Warnes and C. W. Keevil, PLoS One, 2013, 8, e75017 CrossRef CAS PubMed.
- C. E. Wobus, L. B. Thackray and H. W. Virgin, J. Virol., 2006, 80, 5104–5112 CrossRef CAS PubMed.
- H. J. Yoon, H. Y. Choi, Y. K. Kim, Y. J. Song and M. Ki, Epidemiol. Health, 2014, 36, e2014017 CrossRef PubMed.
- F. Bongomin, S. Gago, R. O. Oladele and D. W. Denning, J. Fungi, 2017, 3, 1–29 CrossRef PubMed.
- M. V. Bandres and S. Sharma, Aspergillus Fumigatus, StatsPearls Publishing, Treasure Island, Florida, 2020 Search PubMed.
- B. E. de Pauw, Mediterr. J. Hematol. Infect. Dis., 2011, 3, e2011001 CrossRef PubMed.
- S. Randolph, RN, 2002, 65, 41–44 Search PubMed.
- E. Z. Gomaa, M. M. Housseiny and A. A. A.-K. Omran, J. Cluster Sci., 2019, 30, 181–196 CrossRef CAS.
- J. E. Smith, Aspergillus, Plenum Press, New York, 1994 Search PubMed.
- A. Muñoz-Escobar and S. Y. Reyes-López, PLoS One, 2020, 15, e0228864 CrossRef PubMed.
- H. T. Michels, J. P. Noyce, S. A. Wilks and C. W. Keevil, Adv. Mater. Processes, 2005, 1–12 Search PubMed.
- E. Zhang, J. Ren, S. Li, L. Yang and G. Qin, Biomed. Mater., 2016, 11, 065001 CrossRef.
- L. Fowler, O. Janson, H. Engqvist, S. Norgren and C. Öhman-Mägi, Mater. Sci. Eng., C, 2019, 97, 707–714 CrossRef CAS PubMed.
- V. M. Villapún, L. G. Dover, A. Cross and S. González, Materials, 2016, 9, 1–23 CrossRef PubMed.
- S. L. Warnes, E. N. Summersgill and C. W. Keevil, Appl. Environ. Microbiol., 2015, 81, 1085–1091 CrossRef PubMed.
- A. L. Casey, D. Adams, T. J. Karpanen, P. A. Lambert, B. D. Cookson, P. Nightingale, L. Miruszenko, R. Shillam, P. Christian and T. S. Elliott, J. Hosp. Infect., 2010, 74, 72–77 CrossRef CAS PubMed.
- C. D. Salgado, K. A. Sepkowitz, J. F. John, J. R. Cantey, H. H. Attaway, K. D. Freeman, P. A. Sharpe, H. T. Michels and M. G. Schmidt, Infect. Control Hosp. Epidemiol., 2013, 34, 479–486 CrossRef PubMed.
- S. A. Wilks, H. Michels and C. W. Keevil, Int. J. Food Microbiol., 2005, 105, 445–454 CrossRef CAS PubMed.
- M. Yasuyuki, K. Kunihiro, S. Kurissery, N. Kanavillil, Y. Sato and Y. Kikuchi, Biofouling, 2010, 26, 851–858 CrossRef CAS PubMed.
- P. H. Carey, F. Ren, Z. Jia, C. D. Batich, S. E. A. Camargo, A. E. Clark, V. Craciun, D. W. Neal and J. F. Esquivel-Upshaw, ChemistrySelect, 2019, 4, 9185–9189 CrossRef CAS PubMed.
- A. Ozdamar, C. Aras, R. Ozturk, E. Akin, M. Karacorlu and C. Ercikan, Retina, 1999, 19, 122–126 CrossRef CAS.
- C. J. Boyer, J. Ambrose, S. Das, A. Humayun, D. Chappidi, R. Giorno and D. K. Mills, Med. Devices, 2018, 11, 123–137 CrossRef CAS PubMed.
- A. Valiei, M. Okshevsky, N. Lin and N. Tufenkji, ACS Appl. Mater. Interfaces, 2018, 10, 41207–41214 CrossRef CAS PubMed.
- S. C. Londono, H. E. Hartnett and L. B. Williams, Environ. Sci. Technol., 2017, 51, 2401–2408 CrossRef CAS PubMed.
- J. Klockgether, A. Munder, J. Neugebauer, C. F. Davenport, F. Stanke, K. D. Larbig, S. Heeb, U. Schöck, T. M. Pohl, L. Wiehlmann and B. Tümmler, J. Bacteriol., 2010, 192, 1113–1121 CrossRef CAS PubMed.
- B. Pascoe, L. K. Williams, J. K. Calland, G. Meric, M. D. Hitchings, M. Dyer, J. Ryder, S. Shaw, B. S. Lopes, C. Chintoan-Uta, E. Allan, A. Vidal, C. Fearnley, P. Everest, J. A. Pachebat, T. A. Cogan, M. P. Stevens, T. J. Humphrey, T. S. Wilkinson, A. J. Cody, F. M. Colles, K. A. Jolley, M. C. J. Maiden, N. Strachan, B. M. Pearson, D. Linton, B. W. Wren, J. Parkhill, D. J. Kelly, A. H. M. van Vliet, K. J. Forbes and S. K. Sheppard, Microb. Genomes, 2019, 5, 1–12 CAS.
- A. L. McLoon, S. B. Guttenplan, D. B. Kearns, R. Kolter and R. Losick, J. Bacteriol., 2011, 193, 2027–2034 CrossRef CAS PubMed.
- B. Ryall, G. Eydallin and T. Ferenci, Microbiol. Mol. Biol. Rev., 2012, 76, 597–625 CrossRef CAS PubMed.
- J. M. Stokes, A. J. Lopatkin, M. A. Lobritz and C. J. James, Cell Metab., 2019, 30, 251–259 CrossRef CAS PubMed.
- J. Redfern, J. Tucker, L. M. Simmons, P. Askew, I. Stephan and J. Verran, Methods Protoc., 2018, 1, 1–10 Search PubMed.
- H. T. Michels, J. O. Noyce and C. W. Keevil, Lett. Appl. Microbiol., 2009, 49, 191–195 CrossRef CAS PubMed.
- J. Elguindi, J. Wagner and C. Rensing, J. Appl. Microbiol., 2009, 106, 1448–1455 CrossRef CAS PubMed.
- J. Sjollema, S. A. J. Zaat, V. Fontaine, M. Ramstedt, R. Luginbuehl, K. Thevissen, J. Li, H. C. van der Mei and H. J. Busscher, Acta Biomater., 2018, 70, 12–24 CrossRef CAS PubMed.
- S. S. Dunne, M. Ahonen, M. Modic, F. R. L. Crijns, M. M. Keinänen-Toivola, R. Meinke, C. W. Keevil, J. Gray, N. H. O'Connell and C. P. Dunne, J. Hosp. Infect., 2018, 99, 250–255 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |