Open Access Article
Open Access ArticleHigh Tm linear poly(L-lactide)s prepared via alcohol-initiated ROPs of L-lactide†
Hans R. Kricheldorf*a,
Steffen M. Weidner b and
Andreas Meyerc
b and
Andreas Meyerc
aInstitut für Technische und Makromolekulare Chemie der Universität Hamburg, Bundesstr. 45, D-20146 Hamburg, Germany. E-mail: kricheld@chemie.uni-hamburg.de
bBAM, Bundesanstalt für Materialforschung und -prüfung, Richard Willstätter Straße 11, 12489 Berlin, Germany
cInstitut für Physikalische Chemie der Universität Hamburg, Grindelallee 117, D-20147 Hamburg, Germany
First published on 14th April 2021
Abstract
Alcohol-initiated ROPs of L-lactide were performed in bulk at 160 °C for 72 h with variation of the catalyst or with variation of the initiator (aliphatic alcohols). Spontaneous crystallization was only observed when cyclic Sn(II) compounds were used as a catalyst. Regardless of initiator, high melting crystallites with melting temperatures (Tm) of 189–193 °C were obtained in almost all experiments with Sn(II) 2,2′-dioxybiphenyl (SnBiph) as catalyst, even when the time was shortened to 24 h. These HTm poly(lactide)s represent the thermodynamically most stable form of poly(L-lactide). Regardless of the reaction conditions, such high melting crystallites were never obtained when Sn(II) 2-ethylhexanoate (SnOct2) was used as catalyst. SAXS measurements evidenced that formation of HTm poly(L-lactide) involves growth of the crystallite thickness, but chemical modification of the crystallite surface (smoothing) seems to be of greater importance. A hypothesis, why the “surface smoothing” is more effective for crystallites of linear chains than for crystallites composed of cycles is discussed.
Introduction
Poly(L-lactide) is a technically produced biodegradable and biosourced engineering plastic, which is semicrystalline when optically pure.1,2 Poly(L-lactide) is known to have a melting temperature (Tm) in the range of 175–177 °C and after annealing in the absence of a catalyst or in the presence of a poisoned catalyst the Tm may rise to 178–180 °C.1–7 Quite recently the authors have shown8 that cyclic poly(L-lactide), when prepared at 150–160 °C with a highly reactive catalyst, may form a high melting type of crystallite, so that the Tm rises to 190–196 °C. However, only polymerizations obeying a ring-expansion polymerization mechanism or a ROPPOC mechanism (simultaneous ROP and polycondensation) were successful. In contrast, the standard catalyst used for the technical production of linear poly(L-lactide)s, namely tin(II) 2-ethylhexanoate (SnOct2) combined with an alcohol as initiator (cocatalyst), proved to be ineffective.8 Furthermore, it was found that the most reactive cyclic catalysts SnBiph (Scheme 1) and Sn-salicylate are capable of transforming the “low melting” (LTm) crystallites (Tm < 180 °C) into the high melting (HTm) counterparts. In a series of eight polymerization experiments involving alcohols as initiators not only SnOct2 failed to yield HTm crystallites but also three cyclic tin catalysts were unsuccessful.8 Hence, despite numerous successful experiments with cyclic poly(L-lactide)s, direct syntheses of high Tm linear poly(L-lactide)s are still unknown. Poly(L-lactide) having a Tm around 193 °C was first reported by Pennings and co-workers who studied the isothermal crystallization of linear poly(L-lactide) at various temperatures.9,10 Those authors synthesized their poly(L-lactide) themselves using neat SnOct2 as catalyst and did not add a catalyst poison (rapid and homogeneous distribution of a catalyst poison is difficult to achieve in small scale experiments).Quite recently, the authors have reported that neat SnOct2 produces cyclic polylactides and not as believed for several decades' linear ones.11 Hence, it is highly likely that the Pennings group had cyclic polylactide in hand and observed properties influenced by transesterification reactions catalyzed by SnOct2.
In this context, it was the purpose of this work to find conditions allowing for the preparation of high Tm linear pol(L-lactide)s initiated by alcohols (or primary amines), and to find an explanation why alcohol-initiated ROPs and polymerization mechanisms yielding preferentially cyclic polylactides behave quite differently with regard to formation of HTm crystallites. Structures and labels of the catalysts used and/or discussed in this work were compiled in Scheme 1.
Experimental
Materials
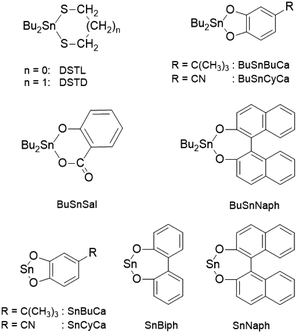
L-Lactide, a product of PURAC, kindly supplied by Thyssen-Uhde AG (Berlin, Germany) was recrystallized from “toluene 99.89% extra dry” purchased from ACROS Chem. (Geel, Belgium). L-Lactylamide, ethyl L-lactate (EtLac), n-butanol, 10-undecenol (10-UND), benzyl alcohol (BnOH), benzylamine (BnNH2), 2-hydroxymethyl naphthalene (2-HMN), 1,12-dodecanediol (DOD) and SnOct2 were all purchased from Alfa Aesar (Karlsruhe, Germany) and used as received. The catalysts DSTL,12 BuSnBuca,13 BuSnBiph,13 BuSnSal,14 SnSal,14 BuSnNaph,13 SnBiph15 and SnNaph15 were those already used in the cited previous publications.Polymerizations
Syntheses of linear poly(L-lactide)s
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; Mw = 43
000; Mw = 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw = 49
000, Mw = 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.Annealing experiments with preformed poly(L-lactide) ethyl esters (Table 6).
A portion of 5.76 g (80 mmol of lactyl units) was dissolved in dry dichloromethane (50 mL) and a catalyst (0.04 mmol of SnOct2, DSTL or BuSnNaph) was added. The resulting solution was evaporated under normal pressure with slow heating up to 120 °C whereupon the polylactide crystallized.
Measurements
The MALDI TOF mass spectra were measured with Bruker AutoflexMax mass spectrometer. All spectra were recorded in the positive ion linear mode. The MALDI stainless steel sample targets were prepared from chloroform solutions of poly(L-lactide) or solutions of the stereocomplex in hexafluoroisopropanol (3–5 mg mL−1) doped with potassium trifluoroacetate (2 mg mL−1). Typically, 20 μL of sample the solution, 2 μL of the potassium salt solution and 50 μL of a solution of trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidene]malononitrile (DCTB, 20 mg mL−1 in CHCl3) serving as matrix were mixed in an Eppendorf vial. 1 μL of the corresponding solution was deposited on the MALDI target.The GPC measurements were performed in a modular system kept at 40 °C consisting of an isocratic pump, 1 mL min−1 and a refractive index detector (RI-501-Shodex). Samples were manually injected (100 μL, 2–4 mg mL−1). For instrument control and data calculation Clarity software (GPC extension, DataApex) was used. The calibration was performed using polystyrene standard sets (Polymer Standards Service – PSS, Mainz). All molecular weights listed in Tables 1–3 and 5 are uncorrected. They overestimate the real molecular weights by approximately 50% corresponding to a correction factor of 0.67 (±0.01).
The DSC heating traces were recorded on a (freshly with indium and zinc calibrated) Mettler-Toledo DSC-1 equipped with Stare Software-11 using a heating rate of 10 K min−1. Only the first heating traces were considered. The crystallinities were calculated with a maximum melting enthalpy of −106 J g−1
The SAXS measurements were performed using our in-house SAXS/WAXS apparatus equipped with an Incoatec™ X-ray source IμS and Quazar Montel optics. The wavelength of the X-ray beam was 0.154 nm and the focal spot size at the sample position was 0.6 mm2. The samples were measured in transmission geometry. The sample-detector distance for SAXS was 1.6 m. For WAXS, a distance of 0.1 m was used, allowing us to detect an angular range of 2Θ = 5°–33°. The patterns were recorded with a Rayonix™ SX165 CCD-Detector and the accumulation time per SAXS measurement was 1200 s and for WAXS 300 s.
DPDAK, a customizable software for reduction and analysis of X-ray scattering data sets was used for gathering 1D scattering curves.16 For the evaluation of the crystallinity of the samples the data were imported in Origin™ and analyzed with the curve fitting module. After subtracting of the instrumental background, the integral intensity of the crystalline reflections was divided by the overall integral intensity to determine the crystallinity xc.
Results and discussion
Ethyl L-lactate-initiated polymerizations with variation of cyclic tin catalysts
A first series of polymerizations was conducted at 160 °C in bulk with EtLac as initiator and variation of the catalyst (Table 1) to elucidate the role of the catalyst. A first trend which was discovered, is the rather low tendency of dibutyltin(IV) catalysts to yield crystalline polylactides. Only the most reactive member of this series, BuSnNaph, gave a low percentage of spherulites. The Sn(II)-based catalyst showed a better performance, but nearly complete crystallization of the reaction product was only observed for SnBiph-catalyzed polymerizations. Therefore, SnBiph was used for all further experiments with a cyclic tin catalyst. Nonetheless, all crystallites isolated from the polymerizations listed in Table 1 had Tm's around 190 °C and thus, demonstrated that it is possible to obtain HTm polylactides via alcohol initiated ROPs. The reason why SnBiph turned out to be the most successful catalyst of this study seems to be its extraordinarily high activity as transesterification catalyst. This is also supported be the results of hundreds of ROPs of L-lactide performed during the past five years, where SnBiph was found to be the most active polymerization catalyst.| Exp. no. | Catalyst | Spherulitesa (%) | Mn | Mw | Tm (°C) | ΔHm (J g−1) |
|---|---|---|---|---|---|---|
| a Estimated volume fraction of the reaction mixture.b Almost completely crystallized.c With another batch of L-lactide only 50–60 vol% of the reaction mixture had crystallized. | ||||||
| 1 | DSTL | 0 | — | — | — | — |
| 2 | DSTD | ∼5–10 | — | — | — | — |
| 3 | BuSnBuca | 0 | — | — | — | — |
| 4 | BuSnCyca | 0 | — | — | — | — |
| 5 | BuSnBiph | 0 | — | — | — | — |
| 6 | BuSnNaph | ∼20–25 | — | — | — | — |
| 8 | SnBuca | ∼60–80 | — | — | — | — |
| 10 | SnBiph | 80–95 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
193.0 | 87 |
| 11 | SnNaphb,c | 80–95 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
178.0 | 65 |
On the basis of these results (Table 1), a series of ROPs was performed with SnBiph as catalyst and variation of the initiator (Table 2). This second series of polymerizations had the purpose to find out if and to what extent the initiator influences the formation of HTm crystallites. The steric demands of the initiator were varied over a broad range, but crystallization was observed for all initiators (Table 2) and all experiments yielded polylactides having Tm's > 189 °C. However, the first experiment with EtLac at a Lac/In ratio of 100/1 did not crystallize although the parallel experiment with a Lac/In ratio of 300/1 had crystallized (No. 2). For experiment 1A the EtLac was added in the form of a 2 M solution in toluene and when the experiment was repeated (No. 1B) with addition of neat EtLac complete crystallization was achieved. A similar observation was made for initiation with n-butanol. The first experiment failed to crystallize, whereas the second polymerization yielded a completely crystallized polylactide. These observations suggest that a lack of crystallization may in individual cases result from slow or hindered nucleation. Therefore, several experiments of Table 1 were repeated to check, if the failure of crystallization was reproducible and it was found to be reproducible. At this point, it should also be mentioned that the SEC data listed in Table 2 evidence a rough dependence of the molecular weights on the Lac/In ratio. A more precise control of the molecular weights may not be expected because of transesterification reactions including formation of cycles. In summary, the results obtained from the experiments of Table 2 underline the extraordinary usefulness of SnBiph and confirm that with an optimum catalyst linear HTm polylactides can be prepared via alcohol initiated ROPs. After this success, those experiments based on a Lac/In ratio of 200/1 were repeated with a time of 1 d for the following reasons:
| Exp. no. | Initiator | Lac/In | Mn | Mw | Tm (°C) | ΔHm (J g−1) | Cryst. (%) | L (nm) | lc (nm) |
|---|---|---|---|---|---|---|---|---|---|
| a Ethyl lactate was added as 2 M solution in toluene.b Neat ethyl lactate was added.c 10-Undecenol.d 2-hydroxymethyl naphthalene.e 1,12-Dodecane diol. | |||||||||
| 1Aa | EtLac | 100/1 | No cryst. | — | — | — | — | — | — |
| 1Bb | EtLac | 100/1 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
192.5 | 84.0 | 80 | 28 | 23 |
| 2 | EtLac | 300/1 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
195.5 | 83.5 | 79 | 30 | 24 |
| 3 | n-Butanol | 200/1 | No cryst. | — | — | — | — | — | — |
| 4 | 10-Undc | 100/1 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
190.5 | 84.5 | 80 | 25 | 20 |
| 5 | 10-Undc | 200/1 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
192.3 | 83.5 | 79 | 28 | 23 |
| 6 | 1-Hmnd | 100/1 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
188.5 | 87.0 | 82 | 26 | 21 |
| 7 | 1-Hmnc | 200/1 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
190.0 | 89.0 | 83 | 27 | 23 |
| 8 | 1,12-Dode | 200/1 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
190.2 | 84.0 | 77 | 30 | 23 |
| 9 | 1,12-Dode | 400/1 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
193.5 | 92.0 | 86 | 26 | 23 |
(1) A shorter time is more attractive from a preparative point of view and reduces the risk of partial racemization and other side reaction.
(2) It should be found out, if the longer time of three days has a significant influence on Tm and ΔHm.
(3) The results should be compared with those obtained from polymerizations catalyzed by SnOct2 (see Table 4 below).
All polymerizations stopped after 1 d yielded crystalline polylactides but only in three experiments a Tm of 190 °C or higher was achieved, whereas in the case of n-butanol and 1,12-dodecanediol the Tm's were as low as 184.0 and 183.5 °C (Table 3). Hence, these experiments demonstrate that at the shorter time the initiator has indeed an influence on the perfection of the resulting crystallites. However, a straightforward explanation based on the steric demands of the initiator proved impossible, because the volume of 10-undecenol, which gave a high Tm, is more than twice as high than that of n-butanol. These unexpected results like those of Table 1 justified, why it made sense to conduct numerous experiments with broad variation of all experimental parameters.
| Exp. no. | Alcohol | Mn | Mw | Tm | ΔHm (J g−1) | Crystal (%) |
|---|---|---|---|---|---|---|
| 1 | EtLac | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
190.0 | 83.5 | 79 |
| 2 | n-BuOHl | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
184.0 | 75.0 | 71 |
| 3 | 10-Und | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
192.0 | 80.5 | 76 |
| 4 | 1-Hmn | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
190.5 | 79.0 | 75 |
| 5 | Dod | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
183.5 | 71.0 | 67 |
Polymerizations catalyzed by SnOct2
SnOct2 is an extraordinarily important catalyst, because it is used for all technical productions of poly(L-lactide), and thus, almost all studies of properties and applications of poly(L-lactide) are based on this catalyst.1–3,5–7,9,17–20 Therefore, it was of high interest to find out, how this catalyst compares with the afore mentioned cyclic tin compounds under the given reaction conditions. In a previous publication8 three polymerizations using a monofunctional alcohol were performed in bulk at 160 °C with EtLac, n-butanol and 10-undecenol as initiators. Regardless of the initiator, HTm crystallites were not formed, although in the case of EtLac crystallization of the reaction mixture took place. This was a first demonstration that SnOct2 is a relatively unfavourable catalyst for the formation of HTm polylactide. In this work twelve more experiments were conducted (Table 4). The variation of the initiator included two cases that yielded amide end groups (No. 3 and 13). Furthermore, the concentration of SnOct2 was doubled in three experiments to enhance its effectiveness (Lac/Cat ratios = 100/1, No. 1, 3 and 5).| Exp. no. | Initiator | Lac/Cat | Lac/In | Spherulitesa (%) | Tm (°C) | ΔHm (J g−1) |
|---|---|---|---|---|---|---|
| a Estimated vol% of the reaction mixture.b These experiments were repeated and the failure to crystallize was reproduced. | ||||||
| 1 | EtLac | 100/1 | 1000/1 | 0 | — | — |
| 2b | EtLac | 200/1 | 1000/1 | 0 | — | — |
| 3 | Lac. amide | 200/1 | 1000/1 | 20–30 | — | — |
| 4 | n-Butanol | 100/1 | 1000/1 | 0 | — | — |
| 5b | n-Butanol | 200/1 | 1000/1 | 0 | — | — |
| 6 | 10-UND | 100/1 | 1000/1 | 0 | — | — |
| 7b | 10-UND | 200/1 | 1000/1 | c. 5 | — | — |
| 8 | 2-HMN | 100/1 | 500/1 | c. 50 | 184.0 | 69.0 |
| 9 | 2-HMN | 100/1 | 1000/1 | c. 60–70 | 187.5 | 79.5 |
| 10 | 2-HMN | 200/1 | 500/1 | c. 15 | — | — |
| 11 | 2-HMN | 200/1 | 1000/1 | c. 5 | — | — |
| 12 | BnOH | 100/1 | 1000/1 | 0 | — | — |
| 13 | BnNH2 | 100/1 | 1000/1 | 0 | — | — |
A first noteworthy result is the crystallization of the polylactides prepared with EtLac as initiator (No. 1 and 2, Table 3). Since Tm's < 180 °C were found, these results demonstrate the reproducibility of an experiment presented in ref. 8, which was conducted under exactly the same conditions as experiment No. 2 in Table 3 of this work. This point needs to be emphasized for two reasons. Firstly, it illustrates the conspicuous difference between the catalysts SnBiph and SnOct2. Secondly, it demonstrates together with the other results compiled in Table 4, that in the case of SnOct2 the initiator has a considerable influence on formation and perfection of the crystallites. With butanol and 10-undecenol no crystallization occurred, and this failure was reproducible. However, partial crystallization was again observable, when 2-hydroxymethyl naphthalene served as initiator and it was also found that a low Lac/In ratio was advantageous for the extent of crystallization. The most surprising result came up when the DSC measurements showed that Tm's of the polylactides from the 100/1 experiments (No. 6 and 7, Table 3) were as high as 184.0 and 187.5 °C. These Tm's were somewhere in between those of typical LTm and HTm crystallites and demonstrated for the first time that SnOct2 may produce crystallites with higher perfection than the normal LTm species, but the results of experiments No. 6 and 7 were still far from the optimum which was achieved with SnBiph under the same conditions. Nonetheless, these unexpected results prompted the authors to study the effect of two other aromatic initiators and since the highest Tm was obtained with 2-HMN at a Lac/In ratio of 100/1, the additional experiments with benzyl alcohol and benzyl amine (No. 12 and 13) were performed with this Lac/In ratio, but no crystallization was observed.
To complete the comparison of SnOct2 with SnBiph, two small series of polymerizations were performed with variation of the temperature (Table 5). All SnBiph-catalyzed polymerizations crystallized, but HTm crystallites with a Tm > 190 °C were only obtained at the highest temperature (160 °C). This result contrasts with SnBiph-catalyzed polymerization performed in the absence of an alcohol, because neat SnBiph yielded HTm polylactide even at 120 °C.8 This comparison demonstrates again that alcohol initiated ROPs are unfavourable for the formation of HTm crystallites. With SnOct2 no crystallization occurred at 160 °C and at lower polymerization temperatures the Tm's were all below 184 °C. In other words, the combination of SnOct2 with alcohol proved again to be particularly unfavourable for the formation of HTm crystallites.
| Exp. no. | Catalyst | Temp. (°C) | Spherulites (vol%) | Mn | Mw | Tm (°C) | ΔHm (J g−1) | Cryst. | L (nm) | lc |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SnBiph | 160 | 90–100 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
191.5 | 84.0 | 79 | 28 | 22 |
| 2 | SnBiph | 150 | 90–100 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
188.5 | 88.0 | 83 | 21 | 18 |
| 3 | SnBiph | 140 | 90–100 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
187.0 | 87.0 | 82 | 20 | 16 |
| 4 | SnBiph | 130 | 90–100 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
182.5 | 87.5 | 83 | 19 | 15/16 |
| 5 | SnOct2 | 160 | — | — | — | — | — | — | — | — |
| 6 | SnOct2 | 150 | 30–40 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
183.5 | 80.5 | 76 | 18 | 14 |
| 7 | SnOct2 | 140 | 90–100 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
183.5 | 79.0 | 75 | 19 | 14 |
| 8 | SnOct2 | 130 | 90–100 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
179.5 | 78.5 | 74 | 17 | 12/13 |
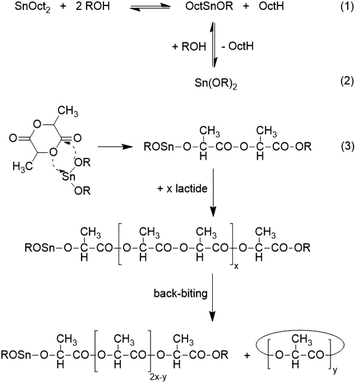
All the results reported above suggested that a larger number of OH end groups on the surface of the lamellar crystal is unfavourable for the formation of HTm poly(L-lactide). As a hypothetical explanation of these results, it may be concluded that the OH groups reduce the mobility of the catalyst either by donor–acceptor interaction (coordination) with the tin atoms or by temporarily (reversible) formation of covalent bonds. This assumption is supported by the well-known mechanism of SnOct2/alcohol catalyzed polymerizations which includes as first step the reversible formation of Sn-alkoxide groups (Scheme 2, eqn (1) and (2)). This hypothesis also explains why crystallites consisting of cyclic polylactides are more prone to modification by transesterification on their surface. Its surface is exclusively covered with loops and thus, their ester groups are easily accessible to the catalysts. Furthermore, the ester groups in the loops are slightly more reactive than those in linear chain segments, due to energetically less favourable conformations (cisoid versus transoid). In order to shed more light on this phenomenon, the annealing experiments summarized in Table 6 were conducted.
| Exp. no. | Substrate | Cat. used for annealing | T (°C) | t (h) | Tm (°C) | ΔHm (J g−1) | Cryst. (%) |
|---|---|---|---|---|---|---|---|
| a The EtLac-initiated, SnOct2-catalyzed product of exp. no. 8, Table 5, was used as substrate.b AA preformed poly(L-lactide) prepared with EtLac as initiator (Lac/In = 200/1) and SnOct2 as catalyst (Lac/Cat = 1000/1) was acetylated with acetic anhydride plus pyridine and used as substrate. | |||||||
| 1 | HO-PLA-Eta | SnOct2 | 160 | 48 | 180.5 | 92 | 86 |
| 2 | HO-PLA-Eta | SnOct2 | 170 | 24 | 184.0 | 77 | 72 |
| 3 | AcO-PLA-Etb | — | 160 | 48 | 180.0 | 84 | 79 |
| 4 | AcO-PLA-Etb | — | 170 | 24 | 180.5 | 80 | 75 |
| 5 | AcO-PLA-Etb | SnOct2 | 160 | 48 | 183.5 | 91 | 86 |
| 6 | AcO-PLA-Etb | SnOct2 | 170 | 24 | 184.5 | 87.5 | 82 |
| 7 | AcO-PLA-Etb | DSTL | 170 | 24 | 182.5 | 90.5 | 86 |
| 8 | AcO-PLA-Etb | BuSnNaph | 179 | 24 | 184.5 | 89.0 | 85 |
Annealing experiments
These experiments were conducted in such a way that a linear poly(L-lactide) was prepared with ethyl L-lactate as initiator and a Lac/In ratio of 100/1, whereupon SnOct2 (Lac/Cat = 1000/1) served as catalyst (acronym: HO-PLA-Et, see Table 6). Part of this polylactide was modified by acetylation of the HO–CH end group with acetic anhydride. The resulting polylactide was named AcO-PLA-Et in Table 6. When HO-PLA-Et was heated with SnOct2 to 160 or 170 °C. Tm's of 180.5 and 184.5 °C were reached (No. 1 and 2, Table 6). Analogous experiments with Ac-PLA-Et gave similar temperatures (No. 5 and 6). Almost the same temperatures were achieved with two cyclic catalysts (No. 7 and 8). In summary, these model experiments evidenced that higher Tm's may be obtained in the presence of a catalyst, but they did not prove that OH end groups on the surface of thy crystallites act as specific hindrance for the catalytic activity of SnOct2.DSC measurements
In this section characteristic features of the DSC measurements should be discussed. When the crystallization of the reaction mixture was almost complete as it was typical for SnBiph-catalyzed ROPs, the only feature observable in the DSC heating traces was a monomodal melting endotherm and a glass-transition was not detectable. A characteristic example of such DSC traces is presented in Fig. 1A, which represents most of the experiments performed with SnBiph (Tables 1, 2 and 5). A couple of exceptions are illustrated in Figure 1B, 2 and 3. Firstly, the small fraction of crystallites obtained with DSTD as catalyst (No. 2, Table 1) is worth mentioning. The crystalline phase was isolated after removal of the amorphous phase by careful washing with cold dichloromethane. In this case the DSC trace revealed two melting endotherms indicating simultaneous presence of LTm and HTm crystallites. A glass-transition was again not detectable (Fig. 1A).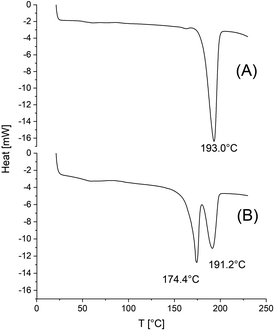 | ||
| Fig. 1 DSC heating traces of polylactides prepared by ethyl L-lactate-initiated ROPs in bulk at 160 °C/3 d: (A) with SnBiph (No. 10, Table 1), (B) with DSTD (No. 2, Table 1). | ||
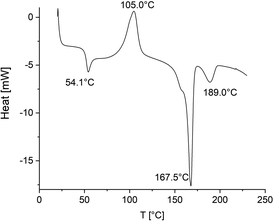 | ||
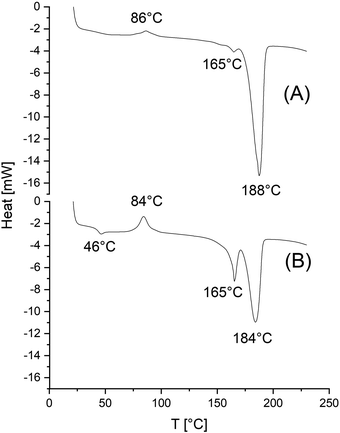
| Fig. 2 DSC heating traces of a polyLA prepared with SnNaph as catalyst and ethyl L-lactate as initiator at 160 °C/3 d (No. 8, Table 1, see footnote c). | ||
 | ||
| Fig. 3 DSC heating traces of polyLA prepared with SnOct2 as catalyst and 1-HMN as initiator (LA/In = 100/1) at 160 °C, 3 d: (A) after 1 h (No. 9, Table 4), (B) after 0.5 h (No. 8, Table 4). | ||
When the sample picked up for the DSC measurements contained a large amount of amorphous phase, the glass-transition (around 50 °C) was clearly observable and followed by crystallization endotherm with a maximum around 105 °C (Fig. 2). The crystallites formed during this crystallization process had a low perfection and melted around 167 °C. The fourth feature was finally the melting endotherm of the HTm crystallites formed during the polymerization. DSC heating traces with a similar structure were also obtained from polylactides prepared with SnOct2 in the experiments of Table 5 (Fig. 3). Again, crystallization of the amorphous phase followed by a melting endotherm at low temperature (around 167 °C) can be seen. What is different from the DSC traces discussed before, is a strong endotherm at a temperature between 180 and 190 °C indicating a partial transformation of LTm into HTm crystallites.
MALDI TOF mass spectrometry
Alcohol-initiated catalyzed by SnOct2 or cyclic tin catalysts such as DSTL12 and BuSnBuca13 typically yield linear chains with considerable predominance of even-numbered species when performed at 130 °C or below and polymerization times limited to a few hours. Furthermore, cyclics are not formed. Yet, at temperatures of 160 °C and higher in combination with a reaction time of 3 d considerable influence of transesterifications may be expected. To elucidate this aspect MALDI-TOF mass spectra of the crystalline sample listed in Tables 2 and 6 were recorded. These mass spectra indeed confirmed this expectation. A first important result are the equal intensities of even and odd chains, regardless if cyclics were present or not. This finding demonstrates that intensive interchain transesterification occurred at all temperatures studied in this work and that this equilibration does not depend on the existence of ring-chain equilibration via “back-biting”. This result is in perfect agreement with a previous study dealing with transesterification in SnOct2-catalyzed and alcohol-initiated ROP of lactide.21 The intermolecular random transesterification between linear chains has a slightly higher rate than formation of cycles by back-biting. Mass spectra free of cyclics were typically obtained at temperatures below 160 °C as illustrated by Fig. 4A and B.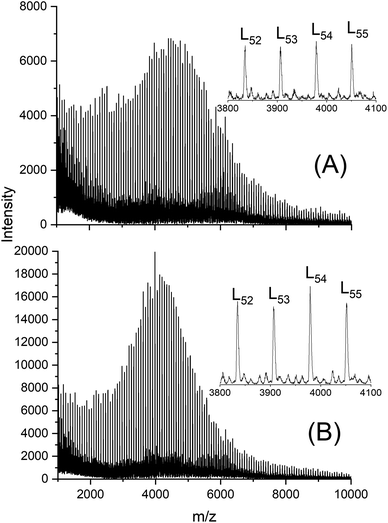 | ||
| Fig. 4 MALDI TOF mass spectra of ethyl lactate-initiated polylactides polymerized with (A) SnBiph at 150 °C/1 d (No. 2, Table 5), (B) SnOct2 at 140 °C/1 d (No. 7, Table 5). L = linear chains having ethyl ester end groups. | ||
However, when UND was used as initiator with a Lac/In ratio of 100/1 cycles were also absent after 3 d at 160 °C (Fig. 5A). Yet, at Lac/In ratio of 200/1 small amounts of cycles were formed as demonstrated in Fig. 5B. This difference agrees with results of a previous study which showed that back-biting is favors by lower In/Cat ratios corresponding to higher Lac/In ratios at constant catalyst concentration. However, with other alcohols cycles were also formed at Lac/Cat ratios of 100/1 as demonstrated in Fig. S1A (see ESI).†
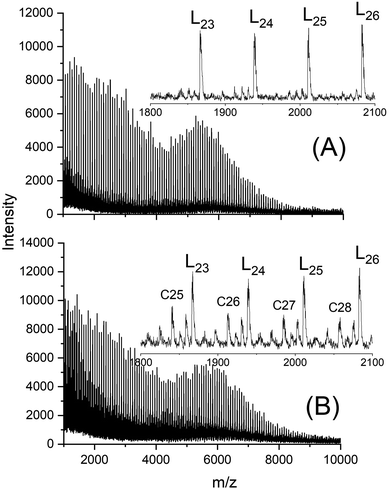 | ||
| Fig. 5 MALDI TOF mass spectra of polylactides initiated with 11-undecenol and catalyzed with SnBiph at 160 °C: (A) LA/In = 100/1, No. 4 Table 2, (B) LA/In = 200/1, No. 5, Table 2. L = linear chains having undecenyl ester end groups. | ||
The most interesting and somewhat surprising result was the existence of a maximum in the molecular weight distribution (MWD) between m/z 5000 and 6000, whenever semi-crystalline polylactides were measured (Fig. 4, 5, S1 and S2†). In principle, one may expect that a fully equilibrated polymer possesses a “most probable” distribution according to eqn (1) as first demonstrated by Flory for irreversible and reversible polycondensations.22
| nx = p(x−1)(1 − p) | (1) |
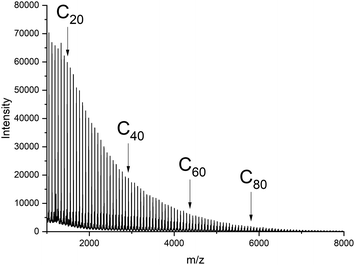 | ||
| Fig. 6 MALDI TOF mass spectrum of a polylactides prepared with Ph2SnCl2 as catalyst in bulk at 160 °C/1 h (reproduced from ref. 23). | ||
The most probable MWD results from an optimization of the entropy. Hence, it is obvious that the maxima observed in mass spectra of the semi-crystalline poly(L-lactide)s result from a gain in enthalpy, and the only source of a negative enthalpy change is in this system formation of more and more perfect crystallites. In other words, these maxima of the MWD suggest that a certain chain length is for thermodynamic reasons particularly prone to crystallize. Their “extraction” from the amorphous phase into the crystallites has the consequence that such chains are regenerated from the pool of shorter and longer chains by transesterification. Such a scenario was also observed, when cyclic polylactides were annealed at 120 °C for 15 d with a large amount of DSTL (Lac/Cat = 200/1).25 It was concluded that enthalpy-driven formation of new maxima in the MWD is characteristic for cyclic polylactides. The results presented in this study clearly prove that not the topology, but the chain length is decisive. A more detailed study and explanation of this phenomenon was outside the scope of this work.
WAXS and SAXS measurements
WAXS measurements of samples No. 1, 2, 7 and 8 of Table 5 were conducted, and in all four cases the typical WAXS patterns of the orthorhombic α-modification, first described by DeSantis and Kovacs,26 were found (Fig. S3;† numerous examples of such WAXS patterns were also presented in previous publications11,27). This result was predictable, because the α-modification is thermodynamically most stable modification of poly(L-lactide) and because the same result has been obtained in previous studies. In other words, the WAXS powder patterns do not contribute anything to a differentiation between LTm and HTm crystallites, but they reflect, as usual, variation of the crystallinity as illustrated by the two examples presented in Fig. S3.†In the previous study27 dealing with HTm crystallites of predominantly cyclic poly(L-lactide)s, it was found by means of SAXS measurements that the HTm polylactides possess lamellar crystallites with a greater thickness (lc values), than the standard LTm crystallites. Whereas lc-values in the range of 25–35 nm were typical for HTm crystallites, lc values in the range of 8–11 nm were found for the LTm counterparts. Hence, it was of interest, if this trend could be confirmed by the alcohol-initiated polylactides prepared in this work. For the first SAXS measurements, samples listed in Table 2 were selected, because for these samples the highest degree of perfection was expected. The lc-values listed in the last column of that table corresponded indeed to distances in the range of 21–25 nm (Fig. S4†), corresponding to a thickness growth by a factor of 2.0–2.2 relative to the usual thickness of LTm crystallites. However, a factor of 2.5–3.5 was found for most polymerization and annealing experiments in previous studies based on cyclic polyLAs.27
Another series of experiments which was particular interesting for SAXS measurements was the variation of the polymerization temperature illustrated in Table 5. As expected, form the relatively high Tm, the lc-value of 22 nm found for sample No. 1 (Fig. 7B) was in agreement with the formation of HTm crystallites. Furthermore, the other extreme, namely the LTm sample No. 8, prepared with SnOct2 at 130 °C was again in agreement with the expected trend: low Tm combined with a rather low lc-value (12–13 nm; Fig. 7A). The other measurements listed in Table 5 gave Tm and lc values in between these extremes.
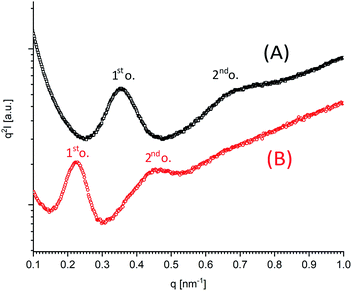 | ||
| Fig. 7 SAXS Kratky plots of polylactides prepared with ethyl lactate as initiator and with: (A) SnOct2 as catalyst at 130 °C (No. 8, Table 5), (B) SnBiph as catalyst at 160 °C (No. 1, Table 5), 1st o. = reflection of first order of lamellar long period L. | ||
In this connection, the most interesting result of this work is the finding that Tm's > 191 °C were achieved with a rather moderate increase of the lamellar thickness when the crystallites are formed by linear chains compared with crystallites composed of cyclic polyLA. This means that another factor played an important role, namely a considerable reduction of the surface free energy σe. According to the Gibbs–Thomson – eqn (2), a high Tm may be a consequence of a low σe or a high lc value. At identical Tm a lower crystal thickness as observed for the linear polyLAs of this work is therefore, correlated with a rather low surface free energy.
| Tm= Tm0[1 − (2σe/r × ΔHm0 × lc)] | (2) |
Hence, the results of this work indicate that the alcohol-initiated linear chains are less favorable for the thickness growth of the crystallites than predominantly cyclic polylactides, but more favorable for a “smoothing” of the surface via transesterification. The transesterification reactions responsible for such a “smoothing” have been discussed in two previous publications, and thus should not be repeated here again. The demonstration that chemical “smoothing” of the crystallite surface plays an important role for the rise of Tm is remarkable for two reasons. First, to the best knowledge of the authors this phenomenon has never been documented before. Second, an explanation is required why the chemical smoothing is more effective when crystallites of linear chains are involved relative to crystallites composed of cyclic polyLAs. Illustrated in Fig. 8A, the surface of crystallites composed of cycles is exclusively covered by loops, which include energetically unfavorable conformations of the repeat units. Due to the steric demands of these loops, directly neighboring loops cannot have the same size. However, in the case of crystallites based on linear chains, loops may be neighbored by sterically less demanding and more flexible linear chain ends (Fig. 8B). Hence, smoothing by transesterification may create a more homogeneous surface with a smaller thickness of the rigid amorphous fraction (RAF), whereas the surface of crystals formed by cycles remains rougher and includes a higher level of conformational energy.
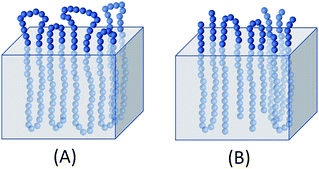 | ||
| Fig. 8 Hypothetical illustration of the smoothed surface of crystallites consisting of cyclic polyLA (A) or of linear polyLA (B). | ||
Finally, it should be mentioned that the SAXS patterns of all samples having Tm's around 184 °C or higher show a second order refection (Fig. 7 and S4†), which indicates a rather high 3-dimensional order of the crystallites in the spherulites. Fig. 9 presents a schematic summary and illustration of the consequences that transesterification reactions in the mobile and immobile disordered phase have on shape and 3-dimensional ordering of the crystallites.
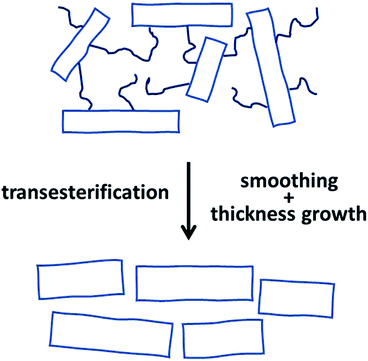 | ||
| Fig. 9 Schematic illustration of the morphological change caused by transesterification reactions across the rigid amorphous phase on the large surfaces of the lamellar crystallites. | ||
Conclusions
The results of this work allow first of all for the conclusion that HTm polylactides can be prepared via alcohol-initiated polymerizations. Yet, surprisingly, only cyclic tin(II) catalysts, such as SnBiph, gave satisfactory results regardless which alcohol served as initiator. Particularly remarkable is also the finding that none of the numerous experiments performed here and previously with SnOct2 as catalyst and various alcohols as initiators yielded a HTm polylactide having a Tm > 190 °C. Since the technical production of poly(L-lactide) and almost all studies of properties of poly(L-lactide)s published over the past 50 years are based on ROPs catalyzed with SnOct2 + alcohol, the present results explain why the HTm crystallites were not detected in experiments performed with this catalyst system. A third important result is the finding that the chemical modification of the crystallite surfaces by transesterification (smoothing) plays a key role for the formation of HTm crystallites based on linear chains. The smaller number of energetically unfavourable loops on their surface seems to be responsible for the greater effectiveness of chemical smoothing.Author contributions
HK – conceptualization, supervision, syntheses, writing-original draft; SW – investigation, visualization, writing – review & editing; AM – SAXS measurements – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank S. Bleck (Institut für TMC Hamburg) for the DSC measurements and A. Myxa (BAM, Berlin) for the GPC measurements. Furthermore, we thank Dr U. Mühlbauer (Thyssen-Uhde AG, Berlin) for kindly supplying all the L-lactide.Notes and references
- R. A. Auras, L.-T. Lim, S. E. Selke and H. Tsuji, Poly(lactic acid): synthesis, structures, properties, processing, and applications, John Wiley & Sons, 2011 Search PubMed.
- M. L. Di Lorenzo and R. Androsch, Synthesis, Structure and Properties of Poly(lactic acid), Springer International Publishing, Cham, 2018 Search PubMed.
- D. Bigg, Technical papers of the annual technical conference-society of plastics engineers incorporated, 1996, pp. 2028–2039 Search PubMed.
- M. L. Di Lorenzo, J. Appl. Polym. Sci., 2006, 100, 3145–3151 CrossRef CAS.
- J. R. Dorgan, J. Janzen, M. P. Clayton, S. B. Hait and D. M. Knauss, J. Rheol., 2005, 49, 607–619 CrossRef CAS.
- K. Jamshidi, S.-H. Hyon and Y. Ikada, Polymer, 1988, 29, 2229–2234 CrossRef CAS.
- J. J. Kolstad, J. Appl. Polym. Sci., 1996, 62, 1079–1091 CrossRef CAS.
- H. R. Kricheldorf, S. M. Weidner and A. Meyer, Polym. Chem., 2020, 11, 2182–2193 RSC.
- B. Kalb and A. J. Pennings, Polymer, 1980, 21, 607–612 CrossRef CAS.
- K. Kishore, R. Vasanthakumari and A. J. Pennings, J. Polym. Sci., Polym. Phys. Ed., 1984, 22, 537–542 CrossRef CAS.
- H. R. Kricheldorf and S. M. Weidner, Polym. Chem., 2020, 11, 5249–5260 RSC.
- H. R. Kricheldorf, S. M. Weidner and F. Scheliga, Polym. Chem., 2017, 8, 1589–1596 RSC.
- H. R. Kricheldorf, S. M. Weidner and F. Scheliga, Macromol. Chem. Phys., 2017, 218, 1700274 CrossRef.
- H. R. Kricheldorf and S. M. Weidner, Eur. Polym. J., 2018, 105, 158–166 CrossRef CAS.
- H. R. Kricheldorf, S. M. Weidner and F. Scheliga, Eur. Polym. J., 2019, 116, 256–264 CrossRef CAS.
- G. Benecke, W. Wagermaier, C. Li, M. Schwartzkopf, G. Flucke, R. Hoerth, I. Zizak, M. Burghammer, E. Metwalli and P. Müller-Buschbaum, J. Appl. Crystallogr., 2014, 47, 1797–1803 CrossRef CAS PubMed.
- G. Schwach, J. Coudane, R. Engel and M. Vert, Polym. Int., 1998, 46, 177–182 CrossRef CAS.
- G. Kharas, F. Sanchez-Riera and D. Severson, Polymers of Lactic Acid in Plastics from Microbes, ed. D. P. Mobley, Hanser Gardner Publications, Inc., 1994 Search PubMed.
- A. Södergård and M. Stolt, Industrial production of high molecular weight poly(lactic acid), Wiley & Sons, Hoboken, 2010 Search PubMed.
- J. A. Byers, A. B. Biernesser, K. R. Delle Chiaie, A. Kaur and J. A. Kehl, in Synthesis, Structure and Properties of Poly(lactic acid), ed. R. Di Lorenzo and R. Androsch, Springer, Cham, 2017, pp. 67–118 Search PubMed.
- S. M. Weidner and H. R. Kricheldorf, Macromol. Chem. Phys., 2017, 218, 1600331 CrossRef.
- P. J. Flory, Principles of polymer chemistry, Cornell University Press, 1953 Search PubMed.
- H. R. Kricheldorf, S. M. Weidner and F. Scheliga, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 952–960 CrossRef CAS.
- H. R. Kricheldorf and S. M. Weidner, Macromol. Rapid Commun., 2020, 2000152 CrossRef CAS PubMed.
- S. M. Weidner and H. R. Kricheldorf, Macromol. Chem. Phys., 2017, 218, 1700114 CrossRef.
- P. DeSantis and A. J. Kovacs, Biopolymers, 1968, 6, 299–306 CrossRef CAS PubMed.
- H. Kricheldorf, S. Chatti, A. Meyer and S. Weidner, RSC Adv., 2021, 11, 2872–2883 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01990b |
| This journal is © The Royal Society of Chemistry 2021 |