Open Access Article
Open Access ArticleQuality assessment of large yellow croaker (Larimichthys crocea) roe oil before and after refining
Lingyun Zhang†
,
Wei Wei†,
Luyao Huang,
Tingting Zheng,
Rongbin Zhong,
Jie Pang,
Lijiao Chen,
Wenjian Cheng and
Peng Liang *
*
College of Food Science, Fujian Agriculture and Forestry University, No. 15, Shangxiadian Road, Cangshan District, Fuzhou 350002, Fujian Province, P. R. China. E-mail: liangpeng137@sina.com; Tel: +86 591-83789348
First published on 15th April 2021
Abstract
This research aimed to assess the quality of the large yellow croaker (Larimichthys crocea) roe oil before and after refining. The crude and refined L. crocea roe oils were compared based on their peroxide value (PV), acid value (AV), iodine value (IV), saponification value (SV), and fatty acid composition. Furthermore, the volatile compounds were identified and analyzed via gas chromatography-mass spectroscopy (GC-MS) and electronic nose (E-nose) analysis. Meanwhile, the flavor fingerprint was established via headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS). The results showed that the PV, AV, IV, and SV of the refined oil were 4.44 ± 0.04 mmol kg−1, 2.86 ± 0.01 mgKOH g−1, 163.1 ± 0.8 g/100 g, and 222.9 ± 0.7 mg g−1, respectively. The docosahexaenoic acids (DHAs) content in the total polyunsaturated fatty acids (PUFAs) was increased. Moreover, 55 volatile compounds were identified in the refined oil; among these compounds, the contents of carboxylic acids, aldehydes, alcohols, ketones, and esters were reasonably increased, while the hydrocarbon and heterocyclic compound contents were decreased. The flavor fingerprints of the crude and refined L. crocea roe oils were established by HS-GC-IMS. The results demonstrated that the refining improved the quality of L. crocea roe oil.
1. Introduction
Large yellow croaker (Larimichthys crocea) is an important economic and marine fish resource in China loved by many consumers because of its delicious taste and high nutritional value.1,2 In 2019, the L. crocea production from aquatic breeding was almost 225![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 549 tons.3 Larimichthys crocea roe is a major byproduct in the fish industry and accounts for 20–30% of the fresh weight of L. crocea. However, the utilization of L. crocea roe remains problematic because of its poor taste and strong fishy smell, and thus, the roe is often considered as waste from fish processing, leading to a great waste of resources.4 It has been reported that fish roe can yield high-value-added fish oils, which are sources of polyunsaturated fatty acids (PUFAs). Fish oils are essential to the human diet as they are sources of PUFAs, and they are considered capable of reducing the occurrence of coronary heart diseases and autoimmune and inflammatory disorders.5,6 Our previous studies confirmed the properties and classes of the phospholipids in L. crocea roe, and the results showed that the L. crocea roe is rich in PUFAs.2,4,7 There is no relevant report that explores the potential of L. crocea roe as a good biological resource for fish oil extraction.
549 tons.3 Larimichthys crocea roe is a major byproduct in the fish industry and accounts for 20–30% of the fresh weight of L. crocea. However, the utilization of L. crocea roe remains problematic because of its poor taste and strong fishy smell, and thus, the roe is often considered as waste from fish processing, leading to a great waste of resources.4 It has been reported that fish roe can yield high-value-added fish oils, which are sources of polyunsaturated fatty acids (PUFAs). Fish oils are essential to the human diet as they are sources of PUFAs, and they are considered capable of reducing the occurrence of coronary heart diseases and autoimmune and inflammatory disorders.5,6 Our previous studies confirmed the properties and classes of the phospholipids in L. crocea roe, and the results showed that the L. crocea roe is rich in PUFAs.2,4,7 There is no relevant report that explores the potential of L. crocea roe as a good biological resource for fish oil extraction.
Generally, crude fish oils contain impurities and other undesirable compounds such as pigment, moisture, free fatty acids (FFAs), phospholipids, and volatile compounds, and this affects the stability, overall quality, and consumers' acceptability of fish oils. Chemical refining is performed to remove the undesirable compounds and improve the characteristics of fish oils; the refining processes may include degumming, neutralization, washing, bleaching, and deodorization. Chakraborty et al. successfully obtained refined fish oils from the Indian sardine (Sardinella longiceps) through a chemical refining process.8 Crexi also obtained refined oil from carp (Cyprinus carpio) viscera through chemical refinement.9 In the current study, crude L. crocea roe oil was processed by degumming, deacidification, decolorization, and deodorization treatments according to the method by Chakraborty.8
The traditional quality criteria for evaluating the crude and refined fish oil include the physicochemical property, the saturability and variation of fatty acids, and the quantitative and qualitative analyses of volatile compounds by headspace solid-phase microextraction combined with gas chromatography-mass spectrometry (HS-SPME-GC-MS). Flavor usually determines the overall unique sensory characteristics of food and is also an important parameter for evaluating the nutritional value and freshness of food.10 However, only few studies are associated with the non-target-based volatiles fingerprints of fish roe oil during refining.
Ion mobility spectrometry (IMS) is an analytical technique for detecting trace gases and characterizing chemical ionic substances based on the difference in the migration rate of gas-phase ions in an electric field; this technique is characterized by ultra-high sensitivity and ultra-high analytical speed.11 In recent years, IMS has been widely applied in the quality control of food processing, quality appraisal and optimization, food additives analysis, and toxic chemical detection; moreover, it is effective for analyzing and characterizing the volatile compounds of different properties.12,13 In this study, gas chromatography (GC) coupled with IMS is used for establishing the flavor fingerprints of crude and refined L. crocea roe oils, so that compounds that cannot be completely separated in the GC column can be separated via IMS after secondary separation.14
The objectives of this work are to evaluate the effect of the refining process on the L. crocea roe oil quality. The peroxide value (PV), acid value (AV), iodine value (IV), saponification value (SV), and fatty acid composition of L. crocea roe oil were compared. Electronic-nose (E-nose) analysis, HS-GC-IMS, and HS-SPME-GC-MS were utilized to comprehensively compare the differences in volatile compounds. In addition, the flavor fingerprints were established. This study can not only improve the high value utilization of L. crocea roe, but also reduce the environmental pollution caused by these wastes.
2. Materials and methods
2.1 Materials and reagents
The L. crocea roe was provided by Fujian Yuehai Aquatic Food Ltd (Fujian, China). Alkaline protease, activated carbon, activated clay, and standard mixtures of 37 fatty acid methyl esters were purchased from Solarbio Ltd (Beijing, China). All reagents used were of analytical grade and were purchased from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China).2.2 Extraction of crude L. crocea roe oil by enzymatic hydrolysis
The crude L. crocea roe oil was extracted using the method by Oliveira,15 with little modification. First, 100 g roe of L. crocea was thawed and stirred in water at a solid-to-liquid ratio of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to obtain a homogeneous mass. Afterward, hydrolysis was conducted using Alcalase (4 g enzyme to 100 g of the substrate) at 58.9 °C and pH 12 for 126 min under constant stirring. Finally, the enzyme was inactivated at 90 °C for 5 min after the hydrolysis completion, and then the hydrolysate L. crocea roe oil was subjected to centrifugation for 20 min under 5000 rpm (DL-5-B, Anting Scientific Instrument Factory, Shanghai).
1 to obtain a homogeneous mass. Afterward, hydrolysis was conducted using Alcalase (4 g enzyme to 100 g of the substrate) at 58.9 °C and pH 12 for 126 min under constant stirring. Finally, the enzyme was inactivated at 90 °C for 5 min after the hydrolysis completion, and then the hydrolysate L. crocea roe oil was subjected to centrifugation for 20 min under 5000 rpm (DL-5-B, Anting Scientific Instrument Factory, Shanghai).
2.3 Refining of L. crocea roe crude oil
The refining of the L. crocea roe crude oil included four steps: degumming, deacidification, decolorization, and deodorization, according to the method reported by Chakraborty.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The obtained oil samples were washed with distilled warm water and centrifuged. The top layer was deacidified fish oil.
000 rpm. The obtained oil samples were washed with distilled warm water and centrifuged. The top layer was deacidified fish oil.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The oil samples were stirred at 75 °C for 15 min. After being cooled, the refined L. crocea roe oil samples were separated by centrifugation (15 min, 5000 rpm).
1. The oil samples were stirred at 75 °C for 15 min. After being cooled, the refined L. crocea roe oil samples were separated by centrifugation (15 min, 5000 rpm).2.4 Physicochemical characterization of the crude and refined L. crocea roe oils
The crude and refined L. crocea roe oils were characterized according to the American Oil Chemists' Society (AOCS, 1997) to determine the SV (method Cd 3-25), PV (method Cd 8-53), AV index (method Ca 5a-40), and IV (method Cd 1c-85 method).The AV index was determined as follows: first, 3.00 ± 0.01 g of L. crocea roe oil was dissolved in 50 mL of an ethyl ether–isopropyl alcohol mixture; then, 3 mL of an indicator (1% of a phenolphthalein solution in 95% ethanol) was added to the above solution, which was titrated with 0.1 M standardized NaOH. Each analysis was repeated three times.
2.5 Fatty acids analysis
Prior to GC analysis, the crude and refined L. crocea roe oils were subjected to methyl esterification according to the method by Li Chongchong.16 First, about 0.1 g of samples was dissolved in 1 mL of 2 mol L−1 methanolic sodium hydroxide solution and incubated at 60 °C for 2 min in a water bath. Next, 1 mL of 2 mol L−1 methanolic hydrochloride solution was added into the mixture, and the resulting mixture was incubated for 5 min. The reaction was stopped by the addition of 2 mL n-hexane at room temperature for 1 h. The n-hexane, which contained fatty acid methyl ester, was collected and desiccated by anhydrous sodium sulfate.The fatty acid methyl esters were analyzed using a gas chromatograph (Agilent 7890A) equipped with a capillary column (CNW CD-2560, 100 m × 0.25 mm I.D., film thickness 0.20 μm, Agilent Technologies Co. Ltd, State of Delaware, USA), with analytical nitrogen (flow rate was 1.0 mL min−1 at 88 kPa) as the carrier gas. The n-heptane solution (1 L) was injected into the chromatograph with a split ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The initial temperature was 140 °C, which was held for 1 min; then the temperature was raised to 190 °C at a rate of 5 °C min−1 and sustained for 10 min; finally, the temperature was increased to 220 °C at 5 °C min−1 and sustained for 10 min.2 Fatty acids were identified by comparing the retention time with that of standard purified fatty acids.
1. The initial temperature was 140 °C, which was held for 1 min; then the temperature was raised to 190 °C at a rate of 5 °C min−1 and sustained for 10 min; finally, the temperature was increased to 220 °C at 5 °C min−1 and sustained for 10 min.2 Fatty acids were identified by comparing the retention time with that of standard purified fatty acids.
2.6 Volatile compound analysis of crude and refined L. crocea roe oils
The L. crocea roe oil sample volatile compounds were quantitatively analyzed by GC-MS using the HP-INNOWAX capillary column (30 m × 0.25 mm I.D., 0.32 μm film, Agilent Technologies Co. Ltd, State of Delaware, USA). Helium was used as the carrier gas, and a flow rate of 0.8 mL min−1 was maintained. Each injection was conducted in the splitless mode. The GC oven initial temperature was 40 °C for 5 min, increased to 120 °C at a rate of 5 °C min−1 for 3 min, raised to 180 °C through intervals of 5 °C min−1, and then sustained for 3 min; the temperature was then raised to 210 °C, applying the same temperature interval, and sustained for 5 min. The injection port temperature was 250 °C. The mass spectrometer operated in the electron impact mode at 70 eV in the range of 35–500 m/z (mass–charge ratio) with the source temperature of 200 °C.
First, 1 g of oil sample was weighed and placed into a 20 mL headspace glass sampling vial and subsequently incubated at 60 °C for 10 min. A headspace volume of 500 μL was sampled at a speed of 500 μL s−1 and a syringe temperature of 85 °C to avoid condensation effects. To avoid cross-contamination, the syringe was automatically rinsed with gaseous nitrogen for 2 min before each analysis.
Then the samples were driven into a CNW CD-2560 capillary column (60 °C isothermal conditions for 30 min) by nitrogen at a programmed flow as follows: 5 mL min−1 for 10 min, then the flow rate was linearly increased to 150 mL min−1 within 5 min. When the gas-chromatograph completely separated the samples, the analytes were ionized in an IMS ionization chamber, whose detector temperature was 45 °C, and the ions were generated by a 3H ionization source (300 MBq activity). The drift tube length was 20 cm, and the drift tube was operated at a constant voltage of 400 V cm−1 and a temperature of 40 °C, with a nitrogen flow of 150 mL min−1.18
N-Ketones C4–C9 (Sinopharm Chemical Reagent Beijing Co., Ltd, China) were used as external references to calculate the retention index (RI) of volatile compounds. The volatile compounds were identified by comparing the RI and the drift time (the time it takes for ions to reach the collector through the drift tube, in milliseconds) of the compounds with the standard in the GC-IMS library.
2.7 Statistical analysis
All of the analyses were conducted in triplicate, and the results were indicated as mean value ± standard deviation; the significant differences were determined by analysis of variance (ANOVA, P < 0.05). The statistical difference between groups of fatty acid relative contents was determined by Duncan's multiple comparison test.The instrumental analysis software included the Laboratory Analytical Viewer software platform and three plug-ins, as well as GC × IMS Library Search software, which can be used for sample analysis from different angles. The visual analysis and processing of the measured two-dimensional data were performed using the MATLAB R2009b and PRTools 5.0 toolkit.
3. Results and discussion
3.1 Physicochemical characterization
The physicochemical properties of oils can directly reflect their quality. In this study, the physicochemical properties of the crude and refined L. crocea roe oils were analyzed. The AV, PV, IV, and SV of the crude and refined oils of L. crocea roe obtained by enzymatic hydrolysis are listed in Table 1.| Sample | Physicochemical index | |||
|---|---|---|---|---|
| AV (mgKOH g−1) | IV (g/100 g) | PV (mmol kg−1) | SV (g/100 g) | |
| a Means followed by different letters in the same column differ according to the Student's t-test at 5% probability. Results are the average values of three replicates ± standard deviation. | ||||
| Crude roe oil | 4.55 ± 0.07a | 155.5 ± 0.7b | 7.3 ± 0.2a | 221.1 ± 0.7b |
| Refined roe oil | 2.86 ± 0.01b | 163.1 ± 0.8a | 4.44 ± 0.04b | 222.9 ± 0.7a |
The AV can reflect the amount of FFAs in oil and fat, which are easily oxidized to generate an unpleasant odor.19 Owing to the relatively high autolytic activity and high content of PUFAs, fish oils are prone to lipolysis and oxidation; thus, they usually contain high FFA content.20 The AV of the crude L. crocea roe oil was 4.55 ± 0.07 mgKOH g−1, which exceeded the general recommendation of the FFA of edible oils (≤3.0%). The AV of the refined oil (2.86 ± 0.01 mgKOH g−1) was lower than that of the crude.
The PV as the measurement index of the hydroperoxide production is not only used to evaluate the oil oxidation degree but is also an important basis to estimate the oil quality. The PV of the refined oil was 4.44 ± 0.04 mmol kg−1, lower (P < 0.05) than that of the crude oil (7.3 ± 0.2 mmol kg−1); the refined oil PV accords with the allowable limit for fish oils for human consumption (≤5 mmol kg−1).21
The IV can reflect the fatty acid unsaturation degree of oil, and the greater the unsaturation degree, the greater the IV. The IV of the refined fish oil increased, which indicates that the impurities in the crude fish oil were removed by the refining process, thus increasing the fatty acid unsaturation degree of the fish oil.9 The IV of the refined L. crocea roe oil (163.1 ± 0.8 g iodine/100 g oil) was significantly higher (P = 0.014 < 0.05) than that of the crude (155.5 ± 0.7 g iodine/100 g oil), due to the higher monounsaturated fatty acids (MUFAs) content of the refined oil. The reported IVs of tilapia (82.4690 g iodine/100 g) and hybrid catfish (80.0408 g iodine/100 g oil) refined oils were lower than those of the L. crocea roe refined oil.22
The SV can indicate the relative molecular weight of oil; the higher the SV, the smaller the average relative molecular weight, the shorter the average chain length of fatty acids, and the higher the oil utilization rate. The SVs of the crude and refined L. crocea roe oils (221.0 ± 0.7 mgKOH g−1 and 222.9 ± 0.7 mgKOH g−1, respectively) were similar to that of reported by-products of processed tuna and anchovy oil (224.5 ± 0.2 mgKOH g−1).17
In the deacidification process, sodium hydroxide was added to neutralize most of the free fatty acids, so that the AV of L. crocea roe oil was greatly reduced, and the AV was further reduced in the later decolorization process due to the action of an adsorbent. At the same time, the PV also decreased significantly in the deacidification stage, which was due to the production of a large number of soapstock, and the adsorption of soapstock enabled the removal of a large number of peroxide in the oil. The IV of L. crocea roe oil increased mainly due to the impurity was removed continuously by soapstock adsorption during the deacidification stage, decoloring phase decoloring agent also has strong adsorption. With the process of refining, more and more impurities were removed, and the SV of L. crocea roe oil showed an upward trend. Especially in the deacidification stage, a large number of free fatty acids are neutralized by alkali, and the soapstock has a strong adsorption capacity so that the SV increases significantly. After the oil was refined, the PV and AV decreased, while the IV and SV increased; all indexes of the refined L. crocea roe oil reached the tolerance levels set by the industrial standard of China, which indicates that the quality of the L. crocea roe oil could be improved by the refining process.
3.2 Fatty acid analysis
To assess the effect of chemical refining on the fatty acid profile of L. crocea roe oil, the fatty acid compositions of the crude and refined oils were analyzed, and the fatty acid compositions and relative contents are presented in Table 2. The crude L. crocea roe oil contained 23 kinds of fatty acids, while the refined L. crocea roe oil contained 22 species; among them, myristic acid C14:1 had an increased content (0.1 ± 0.01%), while the contents of C21:0 and C22:0, two saturated fatty acids (SFAs), were decreased. The SFA proportions in the crude and refined L. crocea roe oils were 15.8 ± 0.1% and 13.7 ± 0.2%, respectively.| Fatty acid composition | Crude roe oil | Refined roe oil |
|---|---|---|
| a ∑SFA: sum of the saturated fatty acids; ∑MFA: sum of the monounsaturated fatty acids; ∑PUFA: sum of the polyunsaturated fatty acids.b Means indicated by different letters in the same column differ according to the Student's t-test at a 5% probability. Results are the average values of three replicates ± standard deviation. | ||
| C14:0 | 2.80 ± 0.02a | 1.90 ± 0.06b |
| C15:0 | 0.4 ± 0.0b | 0.80 ± 0.01a |
| C16:0 | 9.70 ± 0.03a | 9.20 ± 0.02b |
| C17:0 | 0.70 ± 0.02a | 0.40 ± 0.01b |
| C18:0 | 1.70 ± 0.04 | 1.10 ± 0.06 |
| C20:0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| C21:0 | 0.10 ± 0.01a | 0 |
| C22:0 | 0.1 ± 0.0 | 0 |
| C23:0 | 0.20 ± 0.01 | 0.2 ± 0.0 |
| ∑SFA | 15.8 ± 0.1 | 13.7 ± 0.2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| C14:1 | 0 | 0.10 ± 0.01a |
| C16:1 | 10.9 ± 0.2b | 13.4 ± 0.3a |
| C17:1 | 0.50 ± 0.01b | 0.7 ± 0.1a |
| C18:1 | 12.9 ± 0.2b | 16.5 ± 0.2a |
| C20:1 | 0.50 ± 0.03 | 0.6 ± 0.1 |
| C22:1 | 0.1 ± 0.1 | 0.20 ± 0.04 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| ∑MUFA | 24.9 ± 0.5 | 31.5 ± 0.7 |
| C18:2 | 5.7 ± 0.0 | 5.5 ± 0.1 |
| C18:3 (n6) | 3.4 ± 0.0a | 2.2 ± 0.0b |
| C18:3 (n3) | 3.3 ± 0.0b | 3.90 ± 0.02a |
| C20:2 | 0.20 ± 0.02 | 0.20 ± 0.01 |
| C20:3 (n6) | 0.20 ± 0.01 | 0.10 ± 0.02 |
| C20:3 (n3) | 0.2 ± 0.0 | 0.1 ± 0.0 |
| C20:4 | 1.00 ± 0.03 | 0.70 ± 0.02 |
| C20:5 (EPA) | 3.5 ± 0.0a | 2.60 ± 0.01b |
| C22:6 (DHA) | 12.6 ± 0.2 | 12.6 ± 0.4 |
| ∑PUFA | 30.2 ± 0.3 | 28.0 ± 0.5 |
Palmitic acid (C16:0) was the dominant fatty acid among the SFAs of L. crocea roe oils; it increased after the refining process, accounting for 61.39% and 67.15% of the total SFAs in the crude and refined oil, respectively. This result agrees with the percentage reported for several marine fish species; for example, 50.67–74.64% palmitic acid in the total SFAs of 34 marine water fish species from the Mediterranean Sea has been reported.23 From the group of MUFAs, the oleic acid (C18:1n9c) contents in the crude and refined L. crocea roe oils were 12.9 ± 0.2% and 16.5 ± 0.2%, respectively, while the total MUFAs amount increased from 24.9 ± 0.5% (crude oil) to 31.5 ± 0.7% (refined oil). Generally, because of the removal of oil components such as waste and soaps, the total MUFAs in the refined oil increased, while the total SFAs reduced, compared with those in the crude oil. Meanwhile, the higher the unsaturated fatty acids (UFAs) content, the higher the oil utilization value.22 As expected, docosahexaenoic acid (DHA) accounted for the highest percentage of the PUFAs in the crude and refined L. crocea roe oils (41.7% and 45%, respectively). Docosahexaenoic acid, the most important PUFAs, is deemed capable of preventing the occurrence of coronary heart diseases and inflammatory and autoimmune disorders, as well as promoting the formation of brain and retina phospholipid membrane cells.5,6 Thus, the percentage of PUFAs can be a useful indicator for determining the nutritional values of various fish oils.
3.3 Volatile fraction profile
| Volatile compounds | Relative content of volatile compounds/% | ||
|---|---|---|---|
| Crude roe oil | Refined roe oil | ||
| a The superscript letters indicate significant levels among the oil samples tested (p < 0.05).b —, not detected. | |||
| Alkane (15) | Pentane | 9.5 ± 0.2a | — |
| Nonane | 0.45 ± 0.07a | — | |
| Decane | 0.50 ± 0.06a | — | |
| Hendecane | 0.75 ± 0.09a | 0.34 ± 0.05b | |
| Dodecane | 0.98 ± 0.06a | 0.27 ± 0.05b | |
| Tridecane | 0.78 ± 0.02a | — | |
| Tetradecane | 0.84 ± 0.03a | 0.12 ± 0.02b | |
| Pentadecane | 15.4 ± 0.2a | 0.95 ± 0.07b | |
| Hexadecane | 0.07 ± 0.01a | — | |
| Heptadecane | 0.17 ± 0.01a | 0.07 ± 0.01b | |
| 2,6,10,14-Tetramethyl-pentadecane | 0.33 ± 0.02a | — | |
| 1-Chloro-dodecane | — | 0.07 ± 0.01a | |
| 1-Methyldecahydronaphthalene | 0.31 ± 0.03a | — | |
| 2-Ethyldecahydro-naphthalene | 0.06 ± 0.01a | — | |
| Decahydro-2,6-dimethyl-naphthalene | 0.15 ± 0.04a | — | |
| Total | 30.3 ± 0.8a | 1.8 ± 0.2b | |
| Olefin (17) | 1,3,5,7-Cyclooctatetraene | 1.1 ± 0.3 | 1.01 ± 0.02 |
| 3,5,5-Trimethyl-2-hexene | 1.4 ± 0.2a | 1.02 ± 0.03b | |
| D-Limonene | 0.47 ± 0.03b | 0.65 ± 0.03a | |
| Trans-5,6-diethenyl-cyclooctene | 1.3 ± 0.1 | 1.3 ± 0.2 | |
| (Z)-3-Tetradecene | 0.20 ± 0.05a | — | |
| 1-Tridecene | 1.4 ± 0.1a | 0.27 ± 0.05b | |
| Alpha-cedrene | 0.08 ± 0.00 | 0.09 ± 0.01 | |
| Caryophyllene | 0.08 ± 0.01 | 0.03 ± 0.00 | |
| (E)-9-Octadecene | 0.15 ± 0.00a | — | |
| 1-Pentadecene | 0.16 ± 0.01a | 0.01 ± 0.00b | |
| (E)-1,3-Nonadiene | — | 0.04 ± 0.01 | |
| (E,E)-2,4-heptadienal | — | 0.41 ± 0.05a | |
| 3-Methyl-1,4-heptadiene | — | 0.37 ± 0.02a | |
| 1,2,3,4-Tetramethyl-5-methylene-1,3-cyclopentadiene | — | 0.15 ± 0.01a | |
| (E,E,E)-1,4,8-Dodecatriene | — | 0.13 ± 0.05a | |
| Aromandendrene | — | 0.06 ± 0.00a | |
| (Z,Z,Z)-1,8,11,14-Heptadecatetraene | — | 0.04 ± 0.00 | |
| Total | 6.3 ± 0.8 | 5.6 ± 0.4 | |
| Alkyne (3) | 1-Dodecen-3-yne | 0.8 ± 0.1 | 0.6 ± 0.2 |
| 1-Tetradecen-3-yne | 1.3 ± 0.1 | 1.3 ± 0.1 | |
| (E)-6-Hexadecen-4-yne | 0.74 ± 0.06b | 1.5 ± 0.2a | |
| Total | 2.8 ± 0.3 | 3.4 ± 0.3 | |
| Aldehyde (10) | Heptanal | — | 1.52 ± 0.03a |
| Octanal | — | 0.57 ± 0.02a | |
| (E)-2-Octenal | — | 0.63 ± 0.08a | |
| (Z,Z)-3,6-Nonadienal | — | 0.4 ± 0.1a | |
| (E,Z)-2,6-Nonadienal | — | 0.18 ± 0.02a | |
| (E)-2-Nonenal | — | 0.17 ± 0.02a | |
| Decanal | — | 0.06 ± 0.02 | |
| Acetaldehyde | — | 0.05 ± 0.01 | |
| (Z)-2-Decenal | — | 0.09 ± 0.01a | |
| Tetradecanal | — | 0.10 ± 0.02a | |
| Total | 3.7 ± 0.4a | ||
| Ketone (3) | Acetophenone | — | 0.1 ± 0.0a |
| 2,2-Dimethyl-3-heptanone | — | 0.35 ± 0.07a | |
| trans-beta-ionone | — | 0.04 ± 0.01 | |
| Total | — | 0.49 ± 0.08a | |
| Alcohol (11) | 2-Ethyl-hexanol | 0.26 ± 0.05bb | 0.32 ± 0.09a |
| 2-Methyl-5-(1-methylethyl)-(1.alpha.,2.alpha.,5.alpha.)-bicyclo[3.1.0]hex-3-en-2-ol | 0.15 ± 0.02a | — | |
| (E)-2-octen-1-ol | 0.19 ± 0.03a | — | |
| 2-Methylene-cyclopentanepropanol | 0.45 ± 0.06 | 0.57 ± 0.07 | |
| Cyclooctyl alcohol | 0.07 ± 0.02 | — | |
| Myristic alcohol | 1.6 ± 0.2a | 1.0 ± 0.2b | |
| Cetyl alcohol | 0.83 ± 0.03 | 0.82 ± 0.02 | |
| 4-Ethyl-1-octyn-3-ol | — | 0.26 ± 0.01a | |
| 3,7-Dimethyl-1,7-octadien-3-ol | — | 1.02 ± 0.00a | |
| 11-Tridecyn-1-ol | — | 0.26 ± 0.03a | |
| Decyl alcohol | — | 0.04 ± 0.01 | |
| Total | 3.5 ± 0.4 | 4.3 ± 0.4 | |
| Aromatic compounds (12) | 1,3-Dimethyl-benzene | 0.78 ± 0.07a | 0.19 ± 0.01b |
| 1,2,3-Trimethyl-benzene | 0.3 ± 0.1a | — | |
| 5-Ethyl-3,5-dimethyl-benzene | 0.46 ± 0.04a | 0.04 ± 0.01b | |
| 1,2,4,5-Tetramethyl-benzene | 0.35 ± 0.01a | — | |
| 1,3-Diethyl-5-methyl-benzene | 0.11 ± 0.01a | — | |
| 1-Ethyl-3,5-dimethyl-benzene | 0.18 ± 0.01a | — | |
| 10-Methyl-1-undecene | 0.24 ± 0.01a | — | |
| 9-Methyl-1-undecene | 0.13 ± 0.00a | — | |
| Butylated hydroxytoluene | 0.37 ± 0.02a | 0.22 ± 0.03b | |
| o-Xylene | — | 0.28 ± 0.01a | |
| 2,4-Diethyl-1-methyl-benzene | — | 0.06 ± 0.01a | |
| 1,1-Dimethylpropyl-benzene | — | 0.05 ± 0.00a | |
| Total | 3.0 ± 0.3a | 0.84 ± 0.07b | |
| Carboxylic acids (2) | Acetic acid | 54.4 ± 1.4b | 75.6 ± 2.8a |
| Nonanoic acid | — | 0.86 ± 0.01a | |
| Total | 54.4 ± 1.4b | 76.5 ± 2.8a | |
| Ester (1) | Propanoic acid, 2,2-dimethyl-, 2-phenylethyl ester | — | 0.50 ± 0.03a |
| Heterocyclic compound (1) | 2-Ethyl-3,5-dimethyl-pyrazine | 0.35 ± 0.04a | — |
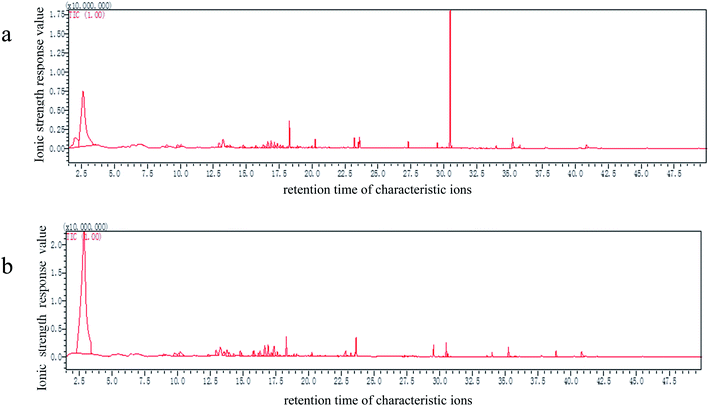
Aldehydes, which are essential indicators of the oxidation in fish oils, have a highly intense odor and an overwhelming impact on the overall aroma, due to their lower odor thresholds; the threshold of unsaturated aldehydes is lower than that of the saturated.25,26 In this study, aldehydes, as well as ketones, were undetected in the crude L. crocea roe oil; however, the refined L. crocea roe oil contained a very small aldehyde content (3.7 ± 0.4%), less than the results reported by Song et al. (6.5 ± 0.3%).27 Drumm et al.28 found that the products of oil and fat oxidation were nonanal and octanal compounds; thus, aldehydes may be the compounds formed by the UFAs oxidation. Given that the oil may be slightly oxidized during the refining process, the refined L. crocea roe oil is expected to have a higher aldehyde content than the crude oil.
Ketones are mainly derived from lipid oxidative degradation or PUFAs autoxidation via hydroperoxides; their threshold values are much higher than those of their aldehydes isomers; thus, their influence on fishy smell substances are much smaller.29 Generally, ketones have oily flavor, fruity flavor, flower fragrance, and roasty flavor, and the flavor is more intense with the growth of the carbon chain. As with aldehydes, the ketone content is slightly increased in refined oils, accounted for 0.49 ± 0.08%; this may be because the ketones are mainly generated via lipid oxidative degradation or the autoxidation of PUFAs by hydroperoxides.30 It is necessary to control the temperature, heating time, and operating pressure to avoid lipid oxidation in the refining process.
Alcohols are divided into saturated and unsaturated alcohols, and the sensory threshold of saturated alcohols is higher than that of unsaturated alcohols; therefore, the saturated alcohols contribute less to the overall flavor.31,32 Both the crude and refined L. crocea roe oils had a high hydrocarbon content; hydrocarbon compounds have little odor activities owing to their high odor thresholds; nevertheless, a small number of volatile small molecular olefins, aromatic hydrocarbons, and heterocyclic hydrocarbons may have an auxiliary effect on the overall flavor of the oil.33,34 The acid compounds, which account for the largest percentage of all volatile compounds in the crude and refined L. crocea roe oils, have an insignificant effect on the oil flavor composition, as the thresholds of most of the compounds are higher than 1000 μg kg−1. In this study, ester was added to the refined L. crocea roe oil, and the oil showed light fruity fragrance, but the threshold of esters is generally high, and thus, the ester addition had little effect on the oil flavor. Heterocyclic compounds exist in flavor mixtures in trace amounts, among which pyrazines are common. Some heterocyclic compounds have an extremely high flavor intensity and an extremely low flavor threshold, the lowest of which can reach 0.002 g kg−1. It is speculated that these compounds had a great influence on the flavor of the L. crocea roe oil, while no heterocyclic compounds were detected in the refined L. crocea roe oil, indicating that the refining improved the L. crocea roe oil flavor.
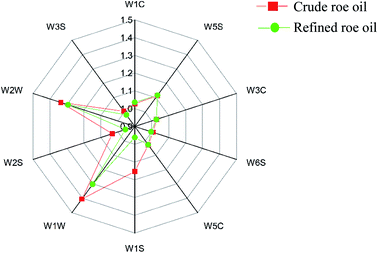
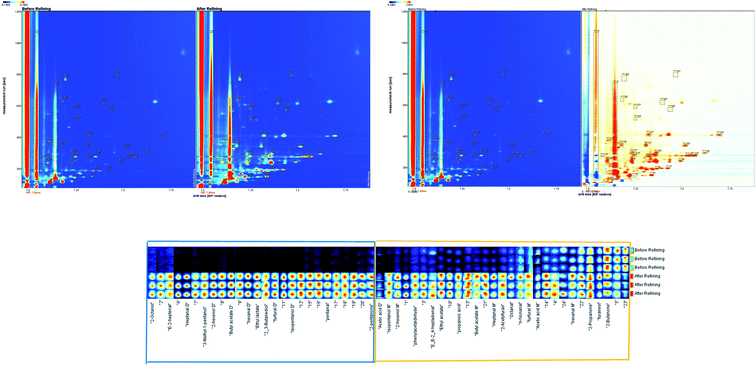
The difference image of the crude and refined L. crocea roe oil GC ion migration spectra is shown in Fig. 3B; the topographic plot of the crude L. crocea roe oil was selected as a reference, and the refined L. crocea roe oil was deduced from the reference.35,36 A white background color after offsetting indicates that the volatile compounds were uniform, while blue indicates that the compound concentration was lower than the reference, and red indicates that the compound concentration was higher than the reference. In Fig. 3B, most migration points are red, by different degrees, and only a few are white or blue; this illustrates that compared with the crude L. crocea roe oil, the refined L. crocea roe oil had a considerable amount of new volatile substances produced in the retention time of 116–800 s and the drift time of 1.0–1.75 s; correspondingly, the concentrations of most volatile compounds in the crude L. crocea roe oil were weakened to varying degrees.
As in previous studies, the information on the whole spectral fingerprint was considered to comprehensively compare the differences in volatile compounds between the crude and refined oils according to the difference spectroscopy technique.12,37 A total of 55 volatile compounds were presented in the fingerprint, among which 26 volatile compounds, which were in the blue frame region, only existed in the refined L. crocea roe oil, and 29 volatile substances, in the yellow frame region, existed in both the crude and refined L. crocea roe oils. A significant difference existed between the samples (Fig. 3C). The contents of the most volatile compounds in the refined L. crocea roe oil were much higher than those in crude L. crocea roe oil; among these compounds, 2,3-butanediol, 3-methyl-1-pentanol, 2-octanol, 2-pentanone, E-2-heptenal, pentanal, ethyl lactate, and other substances merely existed in the refined L. crocea roe oil; only few compounds, including 2-butanone, had a higher content in the crude L. crocea roe oil.
The characteristic volatiles fingerprints of the crude and refined L. crocea roe oils were successfully established through HS-GC-IMS, so that the different samples could be remarkably distinguished. Furthermore, the selected compounds in different samples could serve as biological markers used for differentiating crude and refined L. crocea roe oils.
4. Conclusion
In this study, the physicochemical quality of L. crocea roe oil was improved after refining. The total UFAs increased, and the DHA content in the total PUFAs increased. In addition, the differences in the volatile compounds and fingerprints of the crude and refined L. crocea roe oils were evaluated by HS-SPME-GC-MS and HS-GC-IMS. Based on the assessment result, L. crocea roe oil contains large amounts of eicosapentaenoic acid (EPA, C20:5 n-3) and docosahexaenoic acid (DHA, C22:6 n-3) which have the functions of preventing the incidence of coronary heart diseases, inflammatory, autoimmune disorders, and cancer. Thus, the L. crocea roe oil holds more potential applications in the future. Our finding suggests that L. crocea roe oil can be obtained through enzymatic hydrolysis followed by refining through a chemical method. However, the PUFAs were reasonably removed during refining, which shows the need for further research to improve the L. crocea roe oil quality.Credit authorship contribution statement
Lingyun Zhang: writing – original draft, investigation, conceptualization. Wei Wei: conceptualization, formal analysis, investigation, writing – review & editing. Luyao Huang: conceptualization, formal analysis, investigation. Tingting Zheng: conceptualization, formal analysis, investigation. Rongbin Zhong: conceptualization, formal analysis, investigation. Jie Pang: resources, writing – review & editing, supervision. Lijiao Chen: resources, writing – review & editing, supervision. Wenjian Cheng: resources, writing – review & editing, supervision. Peng Liang: resources, writing – review & editing, supervision.Conflicts of interest
The authors (Lingyun Zhang, Wei Wei, Luyao Huang, Tingting Zheng, Rongbin Zhong, Jie Pang, Lijiao Chen, Wenjian Cheng and Peng Liang) declared that they have no conflicts of interest to this work.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 31801465) and the Outstanding Young Scientific Research Talent Program of Fujian Agriculture and Forestry University (grant no. XJQ201808).References
- G. Hui, W. Liu, H. Feng, J. Li and Y. Gao, Food Chem., 2016, 203, 276–282 CrossRef CAS PubMed.
- P. Liang, R. Li, H. Sun, M. Zhang, W. Cheng and L. Chen, Food Chem., 2018, 245, 806–811 CrossRef CAS PubMed.
- China Fishery Statistical Yearbook, Ministry of Agriculture, China Agriculture Press, Beijing, 2020 Search PubMed.
- P. Liang, M. Zhang, W. Cheng, W. Lin and L. Chen, J. Agric. Food Chem., 2017, 65, 5107–5113 CrossRef CAS PubMed.
- Q. Wang, C. Xue, Z. Li and J. Xu, J. Food Compos. Anal., 2008, 21, 356–359 CrossRef CAS.
- A. Rosa, P. Scano, A. Atzeri, M. Deiana, S. Mereu and M. A. Dessì, J. Food Sci., 2012, 77, C107–C114 CrossRef CAS PubMed.
- X. Lu, R. Zhong, H. Sun, B. Zheng, L. Chen and S. Miao, Mar. Drugs, 2019, 17, 485 CrossRef CAS PubMed.
- K. Chakraborty and D. Joseph, J. Agric. Food Chem., 2015, 63, 998–1009 CrossRef CAS PubMed.
- V. T. Crexi, M. L. Monte, L. A. De Souza Soares and L. A. A. Pinto, Food Chem., 2010, 119, 945–950 CrossRef CAS.
- F. Donglu, Y. Wenjian, B. M. Kimatu, Z. Liyan, A. Xinxin and H. Qiuhui, Food Chem., 2017, 232, 1–9 CrossRef PubMed.
- A. A. Shvartsburg, Ion Mobility Spectrometry (IMS) and Mass Spectrometry, Pacific Northwest National Lab, PNNL, 2010 Search PubMed.
- N. Arroyo-Manzanares, A. Martín-Gómez, N. Jurado-Campos, R. Garrido-Delgado, C. Arce and L. Arce, Food Chem., 2018, 246, 65–73 CrossRef CAS PubMed.
- R. Garrido-Delgado, M. E. Muñoz-Pérez and L. Arce, Food Control, 2018, 85, 292–299 CrossRef CAS.
- R. Garrido-Delgado, M. Del Mar Dobao-Prieto, L. Arce and M. Valcárcel, Food Chem., 2015, 187, 572–579 CrossRef CAS PubMed.
- D. A. De Oliveira, M. G. Minozzo, S. Licodiedoff and N. Waszczynskyj, Food Chem., 2016, 207, 187–194 CrossRef CAS PubMed.
- C. LI, Z. LI, Y. LIU and X. QI, Food Sci., 2015, 36, 190–193 Search PubMed.
- G. Song, M. Zhang, X. Peng, X. Yu, Z. Dai and Q. Shen, LWT–Food Sci. Technol., 2018, 96, 560–567 CrossRef CAS.
- N. Gerhardt, S. Schwolow, S. Rohn, P. R. Pérez-Cacho, H. Galán-Soldevilla and L. Arce, Food Chem., 2019, 278, 720–728 CrossRef CAS PubMed.
- R. Abuzaytoun and F. Shahidi, J. Agric. Food Chem., 2006, 54, 8253–8260 CrossRef CAS PubMed.
- G. Özyurt, A. Şimşek, M. Etyemez and A. Polat, J. Aquat. Food Prod. Technol., 2013, 22, 322–329 CrossRef.
- Codex Alimentarius Commission, Proposed draft standard for fish oils, Langkawi, Malaysia, 2013, vol. 21, pp. 1–5, CCFO23 CRD Search PubMed.
- M. L. Menegazzo, M. E. Petenuci and G. G. Fonseca, Food Chem., 2014, 157, 100–104 CrossRef CAS PubMed.
- Y. Özogul, Fh. Özogul, E. Çi˙ çek, A. Polat and E. Kuley, Int. J. Food Sci. Nutr., 2009, 60, 464–475 CrossRef PubMed.
- A. Oliveira and M. R. Miller, Nutrients, 2014, 6, 2059–2076 CrossRef PubMed.
- D. B. Josephson, R. C. Lindsay and D. A. Stuiber, J. Food Sci., 1985, 50, 5–9 CrossRef CAS.
- S. Kochhar, Food Taints and Off-Flavours, Springer, Boston, MA, 1996, pp. 168–225, DOI:10.1007/978-1-4615-2151-8_6.
- G. Song, Z. Dai, Q. Shen, X. Peng and M. Zhang, Eur. J. Lipid Sci. Technol., 2018, 120, 1700219 CrossRef.
- T. D. Drumm and A. M. Spanier, J. Agric. Food Chem., 1991, 39, 336–343 CrossRef CAS.
- V. Varlet, C. Prost and T. Serot, Food Chem., 2007, 105, 1536–1556 CrossRef CAS.
- A. Giri, K. Osako and T. Ohshima, Food Chem., 2010, 120, 621–631 CrossRef CAS.
- M. Wurzenberger and W. Grosch, Biochim. Biophys. Acta, Lipids Lipid Metab., 1984, 795, 163–165 CrossRef CAS.
- V. Varlet, C. Knockaert, C. Prost and T. Serot, J. Agric. Food Chem., 2006, 54, 3391–3401 CrossRef CAS PubMed.
- D. B. Josephson, R. C. Lindsay and D. A. Stuiber, J. Food Sci., 1987, 52, 596–600 CrossRef CAS.
- T. Sérot, C. Regost and J. Arzel, J. Sci. Food Agric., 2002, 82, 636–643 CrossRef.
- B. V. Hollingsworth, S. E. Reichenbach, Q. Tao and A. Visvanathan, J. Chromatogr. A, 2006, 1105, 51–58 CrossRef CAS PubMed.
- Z. Sheng, X. Y. Jiang and W. Zhen, Adv. Mater. Res., 2011, 403–408, 1618–1621, DOI:10.4028/www.scientific.net/amr.403-408.1618.
- D. Cavanna, S. Zanardi, C. Dall'Asta and M. Suman, Food Chem., 2019, 271, 691–696 CrossRef CAS PubMed.
Footnote |
| † Equally contributing authors. |
| This journal is © The Royal Society of Chemistry 2021 |