Open Access Article
Open Access ArticleExploring synthetic and therapeutic prospects of new thiazoline derivatives as aldose reductase (ALR2) inhibitors†
Muhammad Tariq Shehzad‡
a,
Aqeel Imran‡ b,
Abdul Hameed
b,
Abdul Hameed cg,
Mariya al Rashida
cg,
Mariya al Rashida c,
Marium Bibid,
Maliha Uroose,
Asnuzilawati Asarif,
Shafia Iftikharg,
Habsah Mohamadh,
Muhammad Nawaz Tahiri,
Zahid Shafiq*a and
Jamshed Iqbal
c,
Marium Bibid,
Maliha Uroose,
Asnuzilawati Asarif,
Shafia Iftikharg,
Habsah Mohamadh,
Muhammad Nawaz Tahiri,
Zahid Shafiq*a and
Jamshed Iqbal *b
*b
aInstitute of Chemical Sciences, Bahauddin Zakariya University, Multan, 60800, Pakistan. E-mail: zahidshafiq@bzu.edu.pk
bCentre for Advanced Drug Research, COMSATS University Islamabad, Abbottabad Campus, Abbottabad, 22060, Pakistan. E-mail: drjamshed@ciit.net.pk; jamshediqb@googlemail.com
cDepartment of Chemistry, Forman Christian College (A Chartered University), Ferozepur Road, Lahore, 54600, Pakistan
dDepartment of Biosciences, 90 and 100 Clifton, Shaheed Zulfikar Ali Bhutto Institute of Science and Technology, Block 5, Clifton, Karachi, 75600, Pakistan
eInstitute of Chemistry, University of the Punjab, Lahore, 54590, Pakistan
fFaculty of Science and Marine Environment, Universiti Malaysia Terengganu, 21030, Kuala Nerus, Terengganu, Malaysia
gDepartment of Chemistry, University of Sahiwal, Sahiwal, 57000, Pakistan
hInstitute of Marine Biotechnology, Universiti Malaysia Terengganu, 21030, Kuala Nerus, Terengganu, Malaysia
iDepartment of Physics, University of Sargodha, Sargodha, Pakistan
First published on 11th May 2021
Abstract
Inhibition of aldose reductase (ALR2) by using small heterocyclic compounds provides a viable approach for the development of new antidiabetic agents. With our ongoing interest towards aldose reductase (ALR2) inhibition, we have synthesized and screened a series of thiazoline derivatives (5a–k, 6a–f, 7a–1 & 8a–j) to find a lead as a potential new antidiabetic agent. The bioactivity results showed the thiazoline-based compound 7b having a benzyl substituent and nitrophenyl substituent-bearing compound 8e were identified as the most potent molecules with IC50 values of 1.39 ± 2.21 μM and 1.52 ± 0.78 μM respectively compared with the reference sorbinil with an IC50 value of 3.14 ± 0.02 μM. Compound 7b with only 23.4% inhibition for ALR1 showed excellent selectivity for the targeted ALR2 to act as a potential lead for the development of new therapeutic agents for diabetic complications.
1 Introduction
The incidence of Diabetes mellitus (DM) disease is increasing alarmingly and more than 400 million people are affected all over the world.1 Diabetes complications affect about 25% of the elderly population over the age of 65, and this proportion is steadily growing.2 The majority of the population affected with DM belongs to under-developed or developing regions of the world.3–6 According to a recent study between COVID-19 and diabetes, the COVID-19 patients with diabetes have a two-fold higher risk of mortality and disease incidence than COVID-19 patients without diabetes [00]. In case of progression of this disease severe diabetic complications result such as neuropathy, nephropathy, mood disorders, diabetic retinopathy. These complications are generally a result of hyperglycemia7 which initiates the polyol pathway due to non-insulin dependent glucose uptake. This pathway primarily involves NADPH dependent reduction of glucose to sorbitol, the enzyme responsible for this reduction is aldose reductase (AR; ALR2; EC 1.1.1.21) belonging to aldo-keto reductase enzyme superfamily. The sorbitol in turn converts metabolically via another enzyme sorbitol dehydrogenase into fructose, resulting in increase in the glucose flux.8 High glucose level in diabetes promotes the combination of glucose to ALR2 and metabolized about one third of the total glucose to sorbitol via the polyol pathway in tissues such as retina, lens, peripheral nerves and kidneys. As a result, the regulated polyol pathway accumulates the sorbitol in cells causing swelling of cell, osmotic imbalance and changes in permeability of membrane. Sorbitol does not penetrate the cellular membranes especially that of eye lens. Moreover, the drastic lessening of NAD+ and NADPH alters the cellular redox potentials and weakens the enzymatic activities like that of glutathione reductase and nitric oxide synthase (NOS); worsening the intracellular oxidative stress level. Stress level is also increased via free radicals produced from a number of radical precursors like protein kinase C (PKC) isomer, advanced glycation end products (AGEs), poly-ADP-ribose polymerase (PARP) and mitogen-activated protein kinase (MAPK). High level of free radicals damages a number of tissues. Hence, all the oxidative stress reactions mediated by ALR2 along with the polyol pathway represent important pathogenesis of diabetic complications.9From aldo-keto reductase (AKR), those reducing the aldehydes are called aldehyde reductases (ALR1, EC 1.1.1.2) while those involved in the reduction of ketones are termed as ketoreductases (also belonging to AKR family). Both enzymes have almost similar structure differing just in their active sites.10 The one accurately capable to reduce the aldehyde functionality of glucose in polyol pathway is ALR1. This enzyme is also involved in metabolism of 3-deoxyglucosone and methyl glyoxal causing toxic glycation end products. In contrast, it also assists the reductive detoxification of reactive aldehydes. An example is the reduction of aldehyde phospholipids to regulate the pro-inflammatory response.11
There are a number of studies reported the aldose reductase inhibitors (ARIs).12–17 Up to yet now, only one ARI drug; Epalrestat, ONO Pharmaceutical, Osaka, Japan has been marketed.18,19 Though the polyol pathway inhibition is more challenging to reduce the complications of diabetes; some isolated natural products have been used as potent ARIs. Some synthesized compounds have same active functionalities as that of potent natural product and have been entered in to clinical trials (Fig. 1). It is of utmost importance to develop potent and selective ARIs (ALR1 and ALR2 share about 65% sequence homology), which can regulate the polyol pathway and combat secondary diabetic complications.20,21 The present work focuses on the synthesis of a series of novel thiazoline based inhibitors and their evaluation as ARIs.
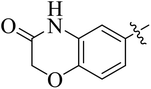
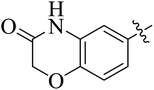
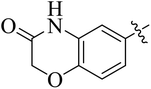
The compounds containing benzoxazinone, adamantyl, benzodioxane and indole nuclei have been synthesized and investigated for their diverse biological activities as many of these moieties are also the part of bioactive natural products.22–33 Incorporation or conjugation of thiazoline moieties with another biologically important nucleus is expected to enhance their biological potential. In view of this and in search of novel bioactive molecules, the study was designed and aimed to prepare a number of various thiazolines possessing benzoxazinone, adamantyl, benzodioxane and indole moieties to evaluate their enzyme inhibition potential with an expectation that they may display more potent activity and thus result into the development of different compounds of medicinal interest.
2. Results and discussion
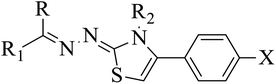
2.1 Chemistry of thiazolines derivatives 5a–k, 6a–f, 7a–i and 8a–j
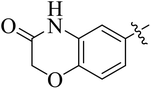
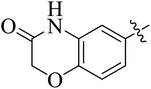
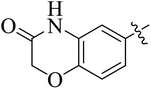
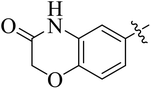
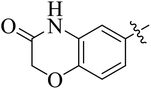
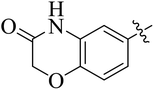
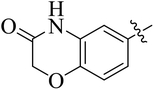
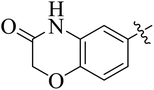
In the current study, the thiazoline derivatives 5–8 were designed and prepared in variable yields (76–92%) by using four different types of carbonyl group bearing compounds 1 i.e. 6-acetyl-2H-benzo[b][1,4]oxazin-3(4H)-one, 1-acetyl adamantane, 1,4-benzodioxan-6-yl methyl ketone and indole-3-carboxaldehyde. The starting materials bearing carbonyl group were treated in equimolar quantities with N-substituted thiosemicarbazides 2 in methanol as solvent. The reaction was catalyzed by using glacial acetic acid as catalyst to get thiosemicarbazones 3 as intermediate.34,35 Further, the thiosemicarbazone derivatives 3 were reacted with a range of 4-substituted 2-bromoacetophenones 4 in solvent ethanol along with sodium acetate. The resulting mixture was heated at reflux till the complete consumption of starting material, monitored by TLC analysis. The pure product was obtained via recrystallization from absolute ethanol (Scheme 1).The structures of thiazoline derivatives (5a–k, 6a–f, 7a–i and 8a–j) were confirmed by using different spectroscopic techniques that include IR spectra, NMR spectroscopy and microanalysis (CHN). The infrared spectra of a typical thiazolines from 5a–k series showed a stretching band of NH group at 3184–3338 cm−1, carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of lactam moiety at 1663–1748 cm−1 and imine group C
O) of lactam moiety at 1663–1748 cm−1 and imine group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bands were appeared at 1578–1593 cm−1 regions consequently, the compounds in thiazoline series 6a–f and 7a–i, showed imine group (C
N bands were appeared at 1578–1593 cm−1 regions consequently, the compounds in thiazoline series 6a–f and 7a–i, showed imine group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) in the range of 1558–1617 cm−1. Furthermore, the indole-based thiazolines 8a–j, showed NH stretching band at 3125–3444 cm−1 while C
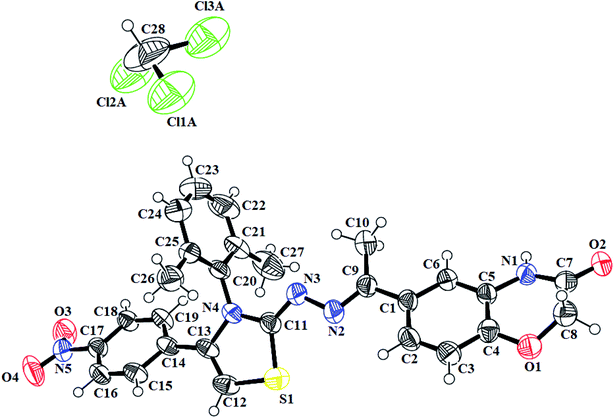
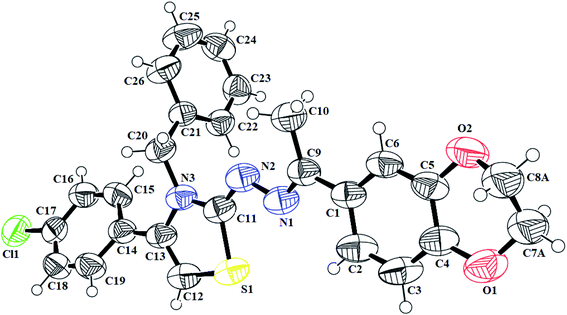
N) in the range of 1558–1617 cm−1. Furthermore, the indole-based thiazolines 8a–j, showed NH stretching band at 3125–3444 cm−1 while C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond in the range of 1603–1615 cm−1. The proton 1H-NMR spectra of different thiazoline series 5a–k and 8a–j, displayed broad singlets for lactam NH and indole NH group in the range from δH 10.78–10.83 and δH 11.51–10.55 ppm, respectively. The singlet of thiazoline –CH– appeared in the range of δH 5.79–7.01 ppm. The other of different protons in all the series of thiazolines were in well agreement to confirm the structures of desired compounds. Moreover, the crystal structure of the compounds 5h and 7i further confirm the structure of thiazoline derivatives (Fig. 2, 3) (Table 1).
N bond in the range of 1603–1615 cm−1. The proton 1H-NMR spectra of different thiazoline series 5a–k and 8a–j, displayed broad singlets for lactam NH and indole NH group in the range from δH 10.78–10.83 and δH 11.51–10.55 ppm, respectively. The singlet of thiazoline –CH– appeared in the range of δH 5.79–7.01 ppm. The other of different protons in all the series of thiazolines were in well agreement to confirm the structures of desired compounds. Moreover, the crystal structure of the compounds 5h and 7i further confirm the structure of thiazoline derivatives (Fig. 2, 3) (Table 1).
2.2 Biological activity
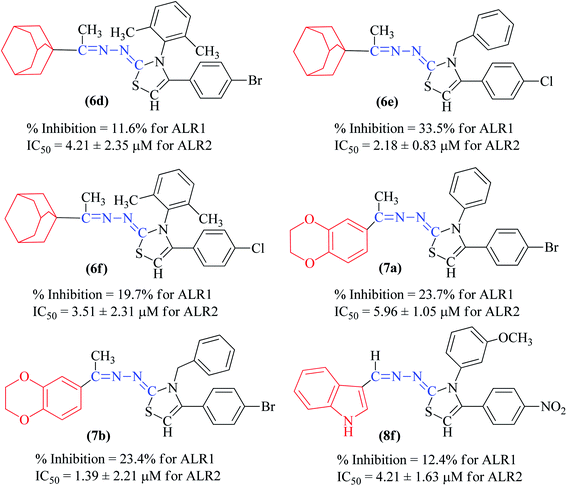
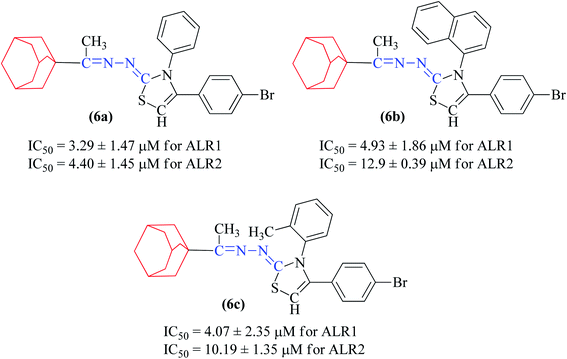
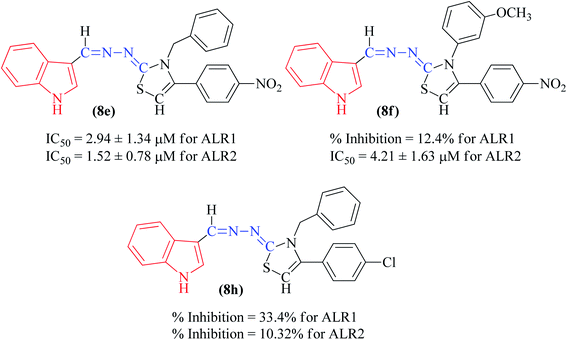
The synthetic thiazoline derivatives (5a–k, 6a–f, 7a–1 & 8a–j) were tested against aldehyde reductase enzyme (ALR1), and their anti-diabetic potential by evaluating inhibitory activity against aldose reductase (ALR2). Results indicated that out of thirty six compounds tested, eight of them, 5f, 6a, 6b, 6c, 7g, 7h, 7i, and 8e were found active inhibitors of ALR2 and ALR1 enzymes (Table 2). However, compound 6d, 6e, 6f, 7a, 7b, 7c and 8f were identified as selective ALR2 inhibitors (Fig. 4).| Code | Structure | ALR2 | ALR1 |
|---|---|---|---|
| IC50(μM) ± SEMa/Percent inhibition | |||
| a Half maximal inhibitory concentration.b Standard inhibitor. | |||
| 5a | 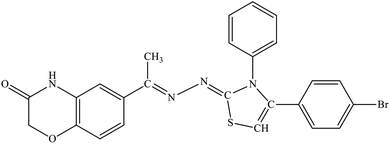 |
13.4% | 22.6% |
| 5b | 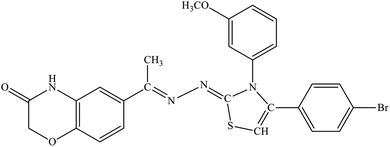 |
27.4% | 31.9% |
| 5c | 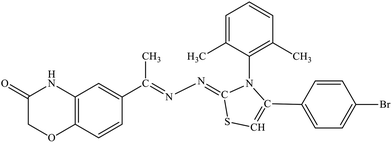 |
22.7% | 12.4% |
| 5d | 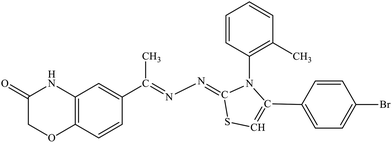 |
19.5% | 6.7% |
| 5e | 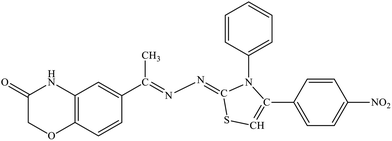 |
20.83% | 31.4% |
| 5f | 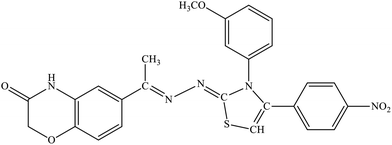 |
3.13 ± 1.45 | 3.24 ± 2.72 |
| 5g | 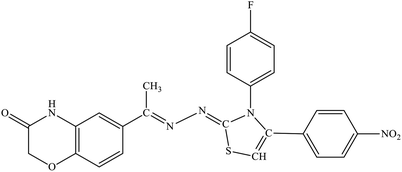 |
31.73% | 24.7% |
| 5h | 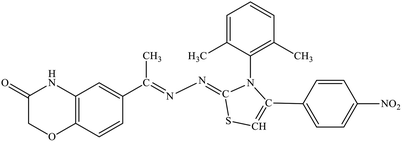 |
18% | 19.6% |
| 5i | 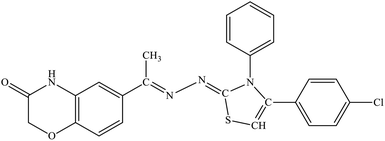 |
16.77% | 31.3% |
| 5j | 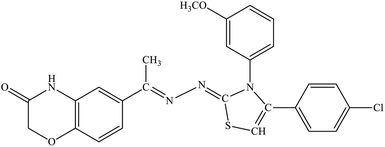 |
5.16% | 32.8% |
| 5k | 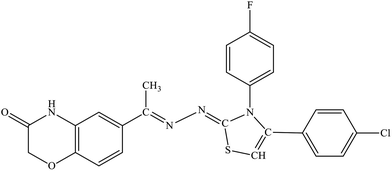 |
35.19% | 22.6% |
| 6a | 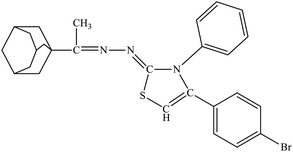 |
4.40 ± 1.45 | 3.29 ± 1.47 |
| 6b | 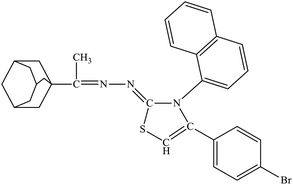 |
12.9 ± 0.39 | 4.93 ± 1.86 |
| 6c | 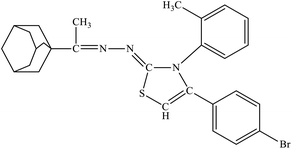 |
10.19 ± 1.35 | 4.07 ± 2.35 |
| 6d | 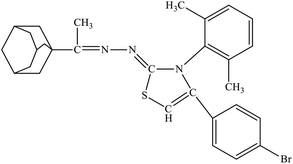 |
4.21 ± 2.35 | 11.6% |
| 6e | 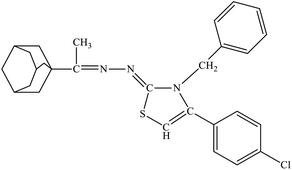 |
2.18 ± 0.83 | 33.5% |
| 6f | 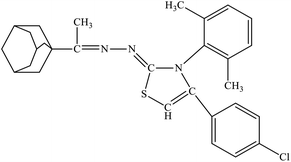 |
3.51 ± 2.31 | 19.7% |
| 7a | 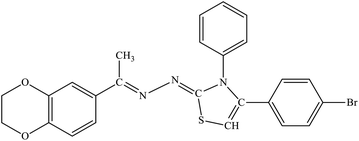 |
5.96 ± 1.05 | 23.7% |
| 7b | 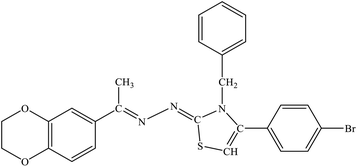 |
1.39 ± 2.21 | 23.4% |
| 7c | 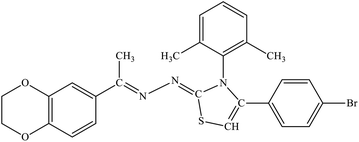 |
3.14 ± 1.87 | 33.4% |
| 7d | 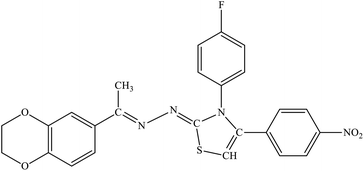 |
11% | 4.5% |
| 7e | 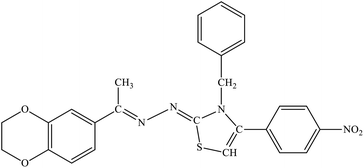 |
14% | 23.6% |
| 7f | 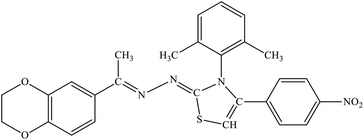 |
23% | 21.7% |
| 7g | 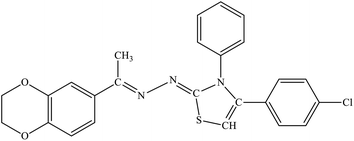 |
14.49 ± 1.49 | 3.14 ± 0.41 |
| 7h | 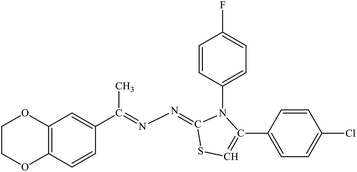 |
9.63 ± 1.21 | 2.94 ± 1.73 |
| 7i | 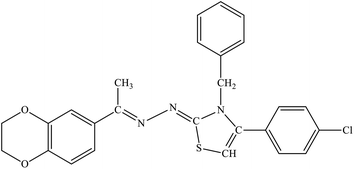 |
29.62 ± | 2.20 ± 0.92 |
| 8a | 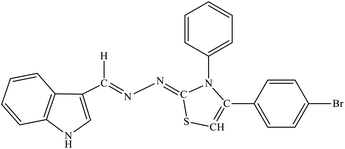 |
13.4% | 15.6% |
| 8b | 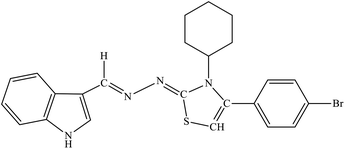 |
37.4% | 29.4% |
| 8c | 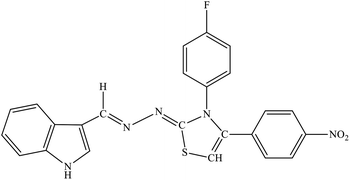 |
6.74% | 35.4% |
| 8d | 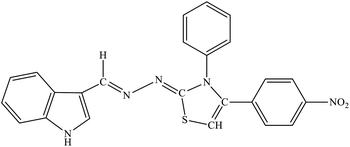 |
31% | 18.6% |
| 8e | 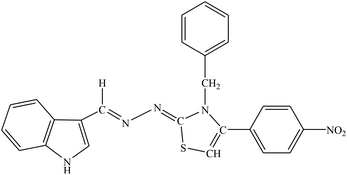 |
1.52 ± 0.78 | 2.94 ± 1.34 |
| 8f | 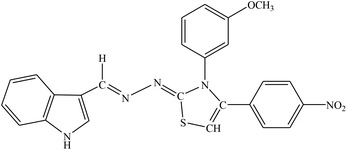 |
4.21 ± 1.63 | 12.4% |
| 8g | 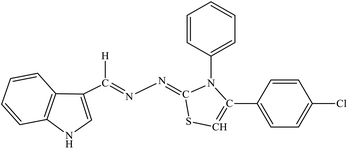 |
5.16% | 23.5% |
| 8h | 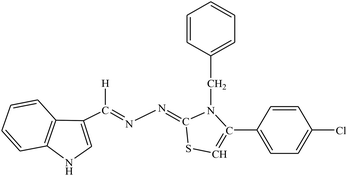 |
10.32% | 33.4% |
| 8i | 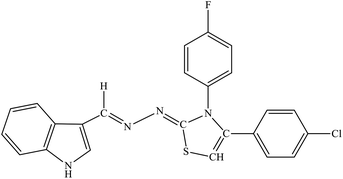 |
23.22% | 12.5% |
| 8j | 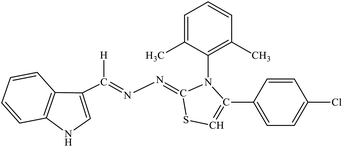 |
34.83% | 27.8% |
| Valproic acidb | — | 57.4 ± 0.89 | |
| Sorbinilb | 3.14 ± 0.02 | — | |
Compound 5f, one of the 2H-1,4-benzoxazin-3(4H)-one bearing derivatives were active against ALR1 and ALR2 having IC50 value of 3.13 ± 1.45 μM and 3.24 ± 2.72 μM, respectively. The substitution of nitrophenyl with bromophenyl or chlorophenyl, as in compound 5b and 5j, showed weak activity against both ARL1 and ARL2 enzymes in comparison to sorbinil and valproic acid with respective IC50 values of 3.14 ± 0.02 μM and 57.4 ± 0.89 μM (Table 2).
In general, compounds (6a–6f) containing adamantane substituent demonstrated the most promising activity among all the derivatives. Out of six, three compounds 6d, 6e and 6f showed a good inhibitory activity and selectively against ALR2 with IC50 values 4.21 ± 2.35 μM, 2.18 ± 0.83 μM and 3.51 ± 2.31 μM respectively. Compounds 6a, 6b and 6c were also found to be active against ALR1 and ALR2 enzymes (Fig. 5).
Among the series, compound 7b showed high inhibition potential against ALR2 (IC50 = 1.39 ± 2.21 μM). However, compound 7b was found to have considerably selective activity against ARL2 exhibiting only 23.4% inhibition against ALR1. The inhibition potential of chlorophenyl substituted thiazoline derivative 7i against ALR2 was much lower than the afore-mentioned compound with IC50 of 38.2 ± 1.43 μM. Furthermore, an improved inhibitor potency of compound 7i was also observed against ALR1 (IC50 4.01 ± 0.39 μM).
Among the indolyl substituted thiazoline derivatives, compound 8e having a nitrophenyl moiety showed high inhibitory potency against ARL2 and ARL1, with IC50 values of 1.52 ± 0.78 μM and 2.94 ± 1.34 μM respectively. However, the inhibitory activity of chlorophenyl substituted thiazoline derivative 8h, was weakened for both enzymes ALR2 and ALR1 demonstrating 10.32% and 33.4% inhibition respectively. Furthermore, the compound 8f was a selective ALR2 inhibitor than ALR1 exhibiting only 12.4% inhibition (Fig. 6). The other indolyl substituted compounds (8a, 8b, 8c, 8d, 8g, 8h, 8i, & 8j) were found inactive with less than 50% inhibitory activity against ALR2 as well against ARL1 enzymes.
3. Docking studies
3.1 Molecular docking studies of ALR1 and ALR2 inhibitors
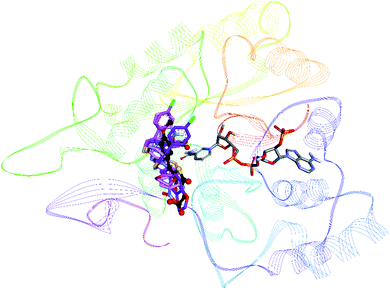
To rationalize the mode of binding and nature of binding site interactions, molecular docking studies were carried out using BioSolveIT's LeadIT software.36 For each inhibitor, the top 10 docked conformations were further evaluated for their binding free energy using HYDE utility (part of LeadIT software), the conformation with most favorable binding free energy was retained for further analysis The crystal structures of porcine ALR1 (ref. 37) and human ALR2 (ref. 38) were downloaded from the Protein Data Bank [PDB ids: 3FX4 at 1.99 Å and 1US0 at 0.66 Å respectively].39 Docking protocol was validated by re-docking of the co-crystallized ligand. The docking protocol was able to reproduce the experimentally bound conformation of co-crystallized ligand (FX4) with an rmsd of 1.03. For docking against ALR1, three of the most active inhibitors 7h, 7i and 8e were selected. All compounds were found to bind in the same area of the binding pocket as the co-crystallized inhibitor FX4 (Fig. 7).By analyzing the binding site interactions of the co-crystallized ligand (ALR1 inhibitor), FX4 ([5-(3-carboxymethoxy-4-methoxybenzylidene)-2,4-dioxothiazolidin-3-yl]acetic acid) it can be seen that the amino acid residues that are important for binding are Arg312, Phe298, Trp220, Trp22, Arg309, and Ala219. When docking studies of ALR1 inhibitors (7h, 7i, 8e) were carried out, same amino acids were found to be involved in binding these inhibitors (Table S1†). Fig. S1† shows the docked conformation of compound 7h. The oxygen atom of the benzodioxane ring was making hydrogen bond with Met302. The nitrogen atom of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N moiety next to the thiazole ring was making a hydrogen bond with Arg312. The OH group of Tyr50 was acting as a hydrogen bond donor towards the fluorine atom. The carbonyl oxygen atom of Tyr 50 was acting as a halogen bond acceptor towards the chlorine atom. A number of hydrophobic interactions were also observed. Phe125 was making pi–pi stacked interactions with the thiazole and the phenyl ring attached to thiazole ring. Ile299 was making alkyl and pi-alkyl interactions with the methyl group and the phenyl ring of benzodioxane ring respectively. Ile49 was making pi-alkyl interactions with both chlorophenyl and fluorophenyl rings, whereas Trp114 was making pi-alkyl interaction with the methyl group.
N moiety next to the thiazole ring was making a hydrogen bond with Arg312. The OH group of Tyr50 was acting as a hydrogen bond donor towards the fluorine atom. The carbonyl oxygen atom of Tyr 50 was acting as a halogen bond acceptor towards the chlorine atom. A number of hydrophobic interactions were also observed. Phe125 was making pi–pi stacked interactions with the thiazole and the phenyl ring attached to thiazole ring. Ile299 was making alkyl and pi-alkyl interactions with the methyl group and the phenyl ring of benzodioxane ring respectively. Ile49 was making pi-alkyl interactions with both chlorophenyl and fluorophenyl rings, whereas Trp114 was making pi-alkyl interaction with the methyl group.
Docking of compound 7i revealed hydrogen bonded interactions between the oxygen atom of the benzodioxane ring and Val300, and between the nitrogen atom of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group and Met302 (Fig. S2†). A number of hydrophobic interactions were also observed. Ile299 was making alkyl and pi-alkyl interactions with the methyl group and phenyl ring of benzodioxane ring respectively. Pro301 was making also alkyl interaction with the methyl group. Lys23 was alkyl interaction with the chlorine atom and pi-alkyl interaction with chlorophenyl ring. Arg218 was making pi-alkyl interaction with the phenyl ring of benzodioxane ring, whereas Ala219 was making pi-alkyl interaction with the thiazole ring.
N group and Met302 (Fig. S2†). A number of hydrophobic interactions were also observed. Ile299 was making alkyl and pi-alkyl interactions with the methyl group and phenyl ring of benzodioxane ring respectively. Pro301 was making also alkyl interaction with the methyl group. Lys23 was alkyl interaction with the chlorine atom and pi-alkyl interaction with chlorophenyl ring. Arg218 was making pi-alkyl interaction with the phenyl ring of benzodioxane ring, whereas Ala219 was making pi-alkyl interaction with the thiazole ring.
Docking of compound 8e revealed two hydrogen bonds of Arg309 and Arg312 with nitrogen atom of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and oxygen atom of the nitro group (Fig. S3†). Notable hydrophobic interactions include pi-sigma and pi-alkyl interaction of Ile49 with indole phenyl ring and pyrrole ring of indole respectively. Tyr50 was making pi–pi stacked interaction with pyrrole ring of indole. Ile299 and Met302 were making pi-alkyl interactions with thiazole ring and nitro phenyl ring respectively.
N bond and oxygen atom of the nitro group (Fig. S3†). Notable hydrophobic interactions include pi-sigma and pi-alkyl interaction of Ile49 with indole phenyl ring and pyrrole ring of indole respectively. Tyr50 was making pi–pi stacked interaction with pyrrole ring of indole. Ile299 and Met302 were making pi-alkyl interactions with thiazole ring and nitro phenyl ring respectively.
Docking studies of ALR2 inhibitors were also carried out for most active inhibitors 6e, 7b and 8e. Prior to docking, the docking protocol was verified by re-docking the co-crystallized ligand LDT ({2-[(4-bromo-2-fluorobenzyl)carbamothioyl]-5-fluorophenoxy}acetic acid) from the ALR2 (PDB id: 1su0). The docking protocol was able to reproduce the experimentally observed conformation of LDT with rmsd of <2. Moreover all compounds were found to bind at the same region of the active site as that of the co-crystallized inhibitor LDT (Fig. S4†).
Docked conformation of compound 6e is shown in Fig. S5.† The nitrogen atom of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N group was making a hydrogen bond with Trp20. A number of hydrophobic interactions were observed that are deemed necessary for efficient binding. Leu300 was making a pi-sigma interaction with the chloro phenyl ring. Trp20 was making pi–pi stacked and pi-alkyl interactions with the benzyl ring and the methyl group respectively. Trp111 was making a pi-stacked interaction with the chloro phenyl ring and a pi-alkyl interaction with the chloro group. Two pi-sulfur interactions were also observed. Trp219 was making a pi-sulfur contact with the sulfur atom of the thiazole ring, whereas the sulfur atom of Cys298 was making pi-sulfur contact with the benzyl ring.
N group was making a hydrogen bond with Trp20. A number of hydrophobic interactions were observed that are deemed necessary for efficient binding. Leu300 was making a pi-sigma interaction with the chloro phenyl ring. Trp20 was making pi–pi stacked and pi-alkyl interactions with the benzyl ring and the methyl group respectively. Trp111 was making a pi-stacked interaction with the chloro phenyl ring and a pi-alkyl interaction with the chloro group. Two pi-sulfur interactions were also observed. Trp219 was making a pi-sulfur contact with the sulfur atom of the thiazole ring, whereas the sulfur atom of Cys298 was making pi-sulfur contact with the benzyl ring.
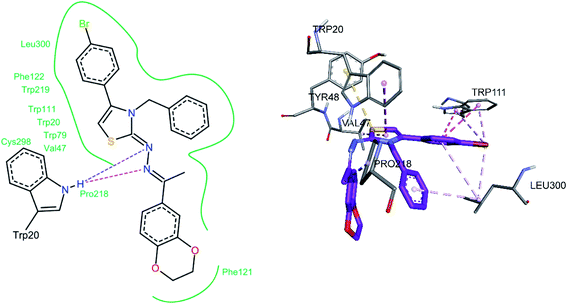
For compound 7b (Fig. 8), similar interactions were observed. Trp20 was within hydrogen bond distance (1.99 Å and 2.12 Å) of both nitrogen atoms of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups. Hydrophobic interactions include Trp111 making a pi–pi stacked interaction with the bromo phenyl ring and a pi-alkyl interaction with the bromine atom. Trp20 was making a pi–pi T-shaped interaction with the thiazole ring. Pro218 was making a pi-alkyl interaction with the methyl group. Leu300 was making pi-alkyl interaction with both benzyl and bromo phenyl ring, whereas Val47 was making pi-alkyl interaction with the thiazole ring. A pi-sulfur interaction was also observed between Tyr48 and the sulfur atom of thiazole ring.
N groups. Hydrophobic interactions include Trp111 making a pi–pi stacked interaction with the bromo phenyl ring and a pi-alkyl interaction with the bromine atom. Trp20 was making a pi–pi T-shaped interaction with the thiazole ring. Pro218 was making a pi-alkyl interaction with the methyl group. Leu300 was making pi-alkyl interaction with both benzyl and bromo phenyl ring, whereas Val47 was making pi-alkyl interaction with the thiazole ring. A pi-sulfur interaction was also observed between Tyr48 and the sulfur atom of thiazole ring.
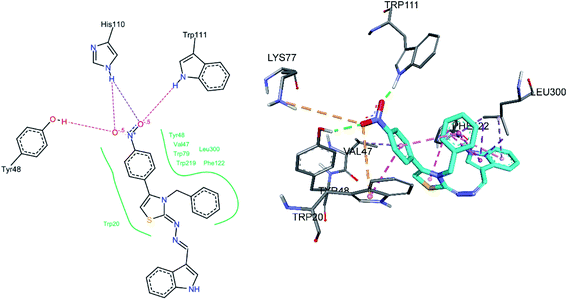
Docking of compound 8e was also carried out, its docked conformation along with binding site interactions are shown in Fig. 9. Both oxygen atoms of the nitro group were making hydrogen bonds with Trp111 and Tyr48, His110 is also within hydrogen bond distance to the nitro group. It is important to note that Trp111, Tyr48 and His110 are the same amino acids that are involved in binding the carboxylate group of standard inhibitor LDT. Leu300 was making hydrophobic interactions, pi-sigma and pi-alkyl with pyrrole ring of indole, and the phenyl indole ring respectively. Phe122 was making pi–pi T-shaped interaction with both thiazole and indole rings. Another pi–pi T-shaped interaction was observed between Trp20 and nitro phenyl ring. Moreover, an intramolecular pi–pi T shaped contact was also observed between the nitro phenyl and benzyl ring, this orientation may additionally stabilize the binding of inhibitor. The nitro phenyl ring was also found to be involved in a pi-alkyl interaction with Val47. An electrostatic attractive interaction was observed between Lys77 and the oxygen atom of the nitro group. Another electrostatic (pi-anion) interaction was observed between Trp20 and same oxygen atom of the nitro group.
4. Molecular dynamics simulation
The conformational stabilities of the apoenzymes (ALR1 and ALR2) and their protein + ligand complexes, both cognate and test compounds, were performed to simulate protein flexibility. The structure of proteins (apoproteins) were first subjected to MD run of 50 ns and then docked poses of cognate ligands and selected ligands (holoenzymes) were submitted to MD run for 50 ns. The time evolution of the radius of gyration of 3FX4 and 1US0, apoenzymes and holoenzymes, exposed in the applied electric fields are shown in Fig. S6† and 10, respectively. The graph indicating that the average value of Rg slightly fluctuating between 1.92–1.97 nm for ALR1 (3FX4), while for ALR2 (1US0), the radius of gyration lies between 1.89 and 1.93 nm, signifying a fine degree of compactness. The average values of RMSD over a period 50 ns for 3FX4 and 1US0, apoenzymes and holoenzymes, exhibited minute fluctuations, reaffirming stable complex formation between enzymes and test compounds, as shown in Fig. S7† and 11, respectively. RMSF analyzes the portions of structure that are fluctuating from their mean structure the most (or least). The (RMSF) of ALR1 and ALR2 were examined, it was observed that residues were found stable in both the cases (Fig. S8,† and 12).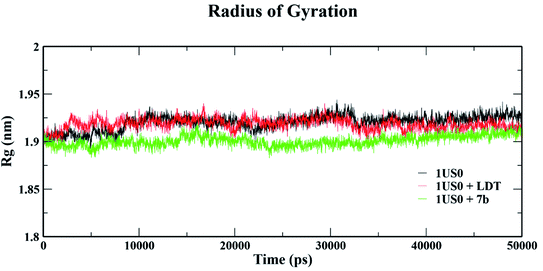 | ||
| Fig. 10 Radius of gyration (Rg) of 1US0, protein plus cognate ligand (LDT) and protein plus selective compound (7b) during 50 ns MD-simulation run. | ||
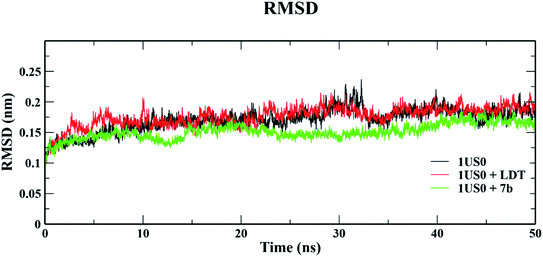 | ||
| Fig. 11 Root mean square deviation (RMSD) of 1US0, protein plus cognate ligand (LDT) and protein plus selective compound (7b) during 50 ns MD-simulation run. | ||
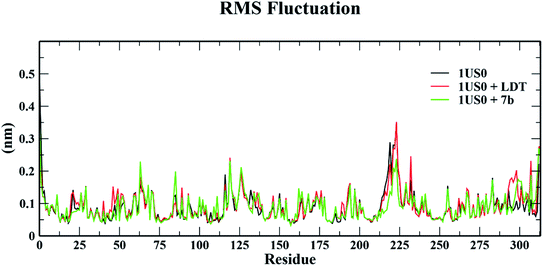 | ||
| Fig. 12 Root mean square fluctuation (RMSF) of 1US0, protein plus cognate ligand (LDT) and protein plus selective compound (7b) during 50 ns MD-simulation run. | ||
5. Conclusions
In this research, we have synthesized thiazoline derivatives (5a–k, 6a–f, 7a–1 & 8a–j), which were tested against aldehyde reductase (ALR1), and aldose reductase (ALR2) enzymes to study their anti-diabetic potential. The results demonstrated that compounds containing adamantyl substituent (6a–6f) have most promising activity among all the derivatives. The compound 7b (with benzyl substituent) among the series was found significantly selective against ARL2 with IC50 value of 1.39 ± 2.21 μM compare to sorbinil, a reference inhibitor, with IC50 values of 3.14 ± 0.02 μM. Furthermore, the compounds 6e also showed potency against ALR2 with IC50 values of 2.18 ± 0.83 μM, whilst 6f presented slightly higher with IC50 value of 3.51 ± 2.31 μM when compared with standard sorbinil. The compound 8e (with nitrophenyl substituent) demonstrated high potency and selectivity against ALR2 enzyme with IC50 values of 1.52 ± 0.78 μM. In silico molecular docking study was also performed to further study the putative binding of active compounds with the target enzyme to find lead compound for further steps of drug development.6 Experimental section
6.1 General procedure for the synthesis of thiosemicarbazones (3)
A solution of corresponding aldehyde or ketone 1 (6-acetyl-2H-benzo[b][1,4]oxazin-3(4H)-one, 1-acetyl adamantane, 1,4-benzodioxan-6-yl methyl ketone and indole-3-carboxaldehyde; 0.01 mol) in methanol (10 mL) was added to a hot stirred solution of appropriate N4-substituted thiosemicarbazide 2 (0.01 mol) in methanol (10 mL). After adding few drops of glacial acetic acid as catalyst, the reaction mixture was heated under reflux for 2–6 h. Upon completion of reaction, monitored through TLC, the hot reaction mixture was cooled to room temperature. The solid product obtained in each case was filtered, washed several times with hot methanol and dried under vacuum to afford the desired thiosemicarbazones 3 in pure form. The resultant thiosemicarbazone derivatives were used as such in the next step without any further purification.6.2 General procedure for the synthesis of thiazoline derivatives (5–8)
A mixture of equimolar amounts of appropriate thiosemicarbazone derivative 3 (0.005 mol), 4-substituted (bromo, nitro, chloro) phenacyl bromide 4 (0.005 mol) and anhydrous sodium acetate (0.005 mol) in absolute ethanol (25 mL) was heated under reflux with continuous stirring for 12–24 h. The reaction mixture was then partially concentrated on a rotary evaporator and left overnight. The precipitate formed in each case was filtered off, washed with warm diethyl ether, dried and recrystallized from absolute ethanol to furnish the target thiazoline derivatives 5–8 in pure form.The different compounds are characterized as under:
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1581 (C
O), 1581 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1539, 1506 (Ar-C
N), 1539, 1506 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.13 (s, 3H, CH3–C
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.13 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.60 (s, 2H, O–CH2–CO), 6.73 (s, 1H, CH-S), 6.96 (d, 1H, J = 8.4 Hz, Ar-H), 7.12 (d, 2H, J = 8.4 Hz, Ar-H), 7.28–7.36 (m, 4H, Ar-H), 7.38–7.39 (m, 2H, Ar-H), 7.43–7.47 (m, 3H, Ar-H), 10.80 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.82 (CH3), 67.25 (OCH2), 103.11 (S-CH
N), 4.60 (s, 2H, O–CH2–CO), 6.73 (s, 1H, CH-S), 6.96 (d, 1H, J = 8.4 Hz, Ar-H), 7.12 (d, 2H, J = 8.4 Hz, Ar-H), 7.28–7.36 (m, 4H, Ar-H), 7.38–7.39 (m, 2H, Ar-H), 7.43–7.47 (m, 3H, Ar-H), 10.80 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.82 (CH3), 67.25 (OCH2), 103.11 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 113.79 (Ar-C), 116.30 (Ar-C), 121.58 (Ar-C), 122.14 (Ar-C), 127.55 (Ar-C), 128.21 (Ar-C), 128.88 (Ar-C), 129.29 (Ar-C), 130.53 (Ar-C), 131.73 (Ar-C), 133.19 (Ar-C), 138.11 (Ar-C), 138.79 (Ar-C), 144.59 (N–C
), 113.79 (Ar-C), 116.30 (Ar-C), 121.58 (Ar-C), 122.14 (Ar-C), 127.55 (Ar-C), 128.21 (Ar-C), 128.88 (Ar-C), 129.29 (Ar-C), 130.53 (Ar-C), 131.73 (Ar-C), 133.19 (Ar-C), 138.11 (Ar-C), 138.79 (Ar-C), 144.59 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 155.60 (C
), 155.60 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 165.13 (N–C
N–N), 165.13 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 168.69 (HN–C
N), 168.69 (HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C25H19BrN4O2S (519.41): C, 57.81; H, 3.69; N, 10.79; found: C, 57.88; H, 3.65; N, 10.85.
O); anal. calcd for C25H19BrN4O2S (519.41): C, 57.81; H, 3.69; N, 10.79; found: C, 57.88; H, 3.65; N, 10.85.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1585 (C
O), 1585 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1536, 1509 (Ar-C
N), 1536, 1509 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.15 (s, 3H, CH3–C
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.15 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 3.69 (s, 3H, OCH3), 4.59 (s, 2H, O–CH2–CO), 6.70 (s, 1H, CH–S), 6.75–6.77 (m, 1H, Ar-H), 6.86–6.88 (dd, 1H, J = 0.8 Hz, 8.4 Hz, Ar-H), 6.94–6.97 (m, 2H, Ar-H), 7.14 (d, 2H, J = 2.0 Hz, Ar-H), 7.25 (t, 1H, J = 8.0 Hz, Ar-H), 7.34 (dd, 1H, J = 2.4 Hz, 8.8 Hz, Ar-H), 7.42 (d, 1H, J = 2.0 Hz, Ar-H), 7.47 (d, 2H, J = 2.0 Hz, Ar-H), 10.78 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.87 (CH3), 55.77 (OCH3), 67.25 (O–CH2), 103.14 (S-CH
N), 3.69 (s, 3H, OCH3), 4.59 (s, 2H, O–CH2–CO), 6.70 (s, 1H, CH–S), 6.75–6.77 (m, 1H, Ar-H), 6.86–6.88 (dd, 1H, J = 0.8 Hz, 8.4 Hz, Ar-H), 6.94–6.97 (m, 2H, Ar-H), 7.14 (d, 2H, J = 2.0 Hz, Ar-H), 7.25 (t, 1H, J = 8.0 Hz, Ar-H), 7.34 (dd, 1H, J = 2.4 Hz, 8.8 Hz, Ar-H), 7.42 (d, 1H, J = 2.0 Hz, Ar-H), 7.47 (d, 2H, J = 2.0 Hz, Ar-H), 10.78 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.87 (CH3), 55.77 (OCH3), 67.25 (O–CH2), 103.14 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 113.81 (Ar-C), 113.98 (Ar-C), 114.74 (Ar-C), 116.31 (Ar-C), 120.87 (Ar-C), 122.13 (Ar-C), 127.56 (Ar-C), 129.87 (Ar-C), 130.43 (Ar-C), 130.71 (Ar-C), 131.73 (Ar-C), 133.19 (Ar-C), 138.79 (Ar-C), 139.09 (Ar-C), 144.61 (Ar-C), 155.70 (N–C
), 113.81 (Ar-C), 113.98 (Ar-C), 114.74 (Ar-C), 116.31 (Ar-C), 120.87 (Ar-C), 122.13 (Ar-C), 127.56 (Ar-C), 129.87 (Ar-C), 130.43 (Ar-C), 130.71 (Ar-C), 131.73 (Ar-C), 133.19 (Ar-C), 138.79 (Ar-C), 139.09 (Ar-C), 144.61 (Ar-C), 155.70 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 159.75 (C
), 159.75 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 165.13 (N–C
N–N), 165.13 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 168.55 (HN–C
N), 168.55 (HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C26H21BrN4O3S (549.44): C, 56.84; H, 3.85; N, 10.20; found: C, 56.80; H, 3.89; N, 10.24.
O); anal. calcd for C26H21BrN4O3S (549.44): C, 56.84; H, 3.85; N, 10.20; found: C, 56.80; H, 3.89; N, 10.24.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1583 (C
O), 1583 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1541, 1487 (Ar-C
N), 1541, 1487 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.70 (s, 6H, 2×CH3-Ar), 2.29 (s, 3H, CH3–C
C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.70 (s, 6H, 2×CH3-Ar), 2.29 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.76 (s, 2H, O–CH2–CO), 6.35 (s, 1H, CH–S), 7.06–7.10 (m, 3H, Ar-H), 7.17–7.19 (m, 2H, Ar-H), 7.27–7.31 (m, 1H, Ar-H), 7.42–7.44 (m, 2H, Ar-H), 7.49–7.51 (m, 1H, Ar-H), 7.54 (d, 1H, J = 2.0 Hz, Ar-H), 7.86 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.45 (CH3), 18.25 (2×CH3-Ar), 67.43 (OCH2), 100.90 (S-CH
N), 4.76 (s, 2H, O–CH2–CO), 6.35 (s, 1H, CH–S), 7.06–7.10 (m, 3H, Ar-H), 7.17–7.19 (m, 2H, Ar-H), 7.27–7.31 (m, 1H, Ar-H), 7.42–7.44 (m, 2H, Ar-H), 7.49–7.51 (m, 1H, Ar-H), 7.54 (d, 1H, J = 2.0 Hz, Ar-H), 7.86 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.45 (CH3), 18.25 (2×CH3-Ar), 67.43 (OCH2), 100.90 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 113.29 (Ar-C), 116.37 (Ar-C), 122.43 (Ar-C), 125.81 (Ar-C), 128.51 (Ar-C), 128.74 (Ar-C), 128.85 (Ar-C), 129.85 (Ar-C), 131.48 (Ar-C), 134.07 (Ar-C), 136.54 (Ar-C), 139.23 (Ar-C), 144.28 (N–C
), 113.29 (Ar-C), 116.37 (Ar-C), 122.43 (Ar-C), 125.81 (Ar-C), 128.51 (Ar-C), 128.74 (Ar-C), 128.85 (Ar-C), 129.85 (Ar-C), 131.48 (Ar-C), 134.07 (Ar-C), 136.54 (Ar-C), 139.23 (Ar-C), 144.28 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 155.54 (C
), 155.54 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 164.94 (N–C
N–N), 164.94 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 166.68 (HN–C
N), 166.68 (HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C27H23BrN4O2S (547.47): C, 59.23; H, 4.23; N, 10.23; found: C, 59.30; H, 4.25; N, 10.20.
O); anal. calcd for C27H23BrN4O2S (547.47): C, 59.23; H, 4.23; N, 10.23; found: C, 59.30; H, 4.25; N, 10.20.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590 (C
O), 1590 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1570, 1510 (Ar-C
N), 1570, 1510 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.06 (s, 3H, CH3-Ar), 2.17 (s, 3H, CH3–C
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.06 (s, 3H, CH3-Ar), 2.17 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.59 (s, 2H, O–CH2–CO), 6.73 (s, 1H, CH–S), 6.96 (d, 1H, J = 8.4 Hz, Ar-H), 7.08–7.10 (m, 2H, Ar-H), 7.17–7.19 (m, 2H, Ar-H), 7.30–7.34 (m, 3H, Ar-H), 7.41–7.44 (m, 3H, Ar-H), 10.78 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.66 (CH3), 18.05 (CH3), 67.25 (OCH2), 102.49 (S-CH
N), 4.59 (s, 2H, O–CH2–CO), 6.73 (s, 1H, CH–S), 6.96 (d, 1H, J = 8.4 Hz, Ar-H), 7.08–7.10 (m, 2H, Ar-H), 7.17–7.19 (m, 2H, Ar-H), 7.30–7.34 (m, 3H, Ar-H), 7.41–7.44 (m, 3H, Ar-H), 10.78 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.66 (CH3), 18.05 (CH3), 67.25 (OCH2), 102.49 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 113.76 (Ar-C), 116.29 (Ar-C), 127.16 (Ar-C), 127.53 (Ar-C), 129.19 (Ar-C),, 129.72 (Ar-C), 130.31 (Ar-C), 130.35 (Ar-C), 131.33 (Ar-C), 131.74 (Ar-C), 133.22 (Ar-C), 136.49 (Ar-C), 137.32 (Ar-C), 139.00 (Ar-C), 144.53 (N–C
), 113.76 (Ar-C), 116.29 (Ar-C), 127.16 (Ar-C), 127.53 (Ar-C), 129.19 (Ar-C),, 129.72 (Ar-C), 130.31 (Ar-C), 130.35 (Ar-C), 131.33 (Ar-C), 131.74 (Ar-C), 133.22 (Ar-C), 136.49 (Ar-C), 137.32 (Ar-C), 139.00 (Ar-C), 144.53 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 155.32 (C
), 155.32 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 165.12 (N–C
N–N), 165.12 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 167.92 (HN–C
N), 167.92 (HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C26H21BrN4O2S (533.44): C, 58.54; H, 3.97; N, 10.50; found: C, 58.50; H, 3.96; N, 10.54.
O); anal. calcd for C26H21BrN4O2S (533.44): C, 58.54; H, 3.97; N, 10.50; found: C, 58.50; H, 3.96; N, 10.54.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1578 (C
O), 1578 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1544, 1494 (Ar-C
N), 1544, 1494 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.15 (s, 3H, CH3–C
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.15 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.60 (s, 2H, O–CH2–CO), 6.96 (d, 1H, J = 8.4 Hz, Ar-H),7.00 (s, 1H, CH–S), 7.32–7.36 (m, 4H, Ar-H), 7.36–7.49 (m, 5H, Ar-H), 8.09–8.11 (m, 2H, Ar-H), 10.80 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.90 (CH3), 67.25 (OCH2), 106.36 (S-CH
N), 4.60 (s, 2H, O–CH2–CO), 6.96 (d, 1H, J = 8.4 Hz, Ar-H),7.00 (s, 1H, CH–S), 7.32–7.36 (m, 4H, Ar-H), 7.36–7.49 (m, 5H, Ar-H), 8.09–8.11 (m, 2H, Ar-H), 10.80 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.90 (CH3), 67.25 (OCH2), 106.36 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 113.83 (Ar-C), 116.32 (Ar-C), 119.49 (Ar-C), 121.66 (Ar-C), 123.96 (Ar-C), 127.57 (Ar-C), 128.35 (Ar-C), 128.71 (Ar-C), 129.39 (Ar-C), 129.42 (Ar-C), 133.06 (Ar-C), 137.51 (Ar-C), 138.02 (Ar-C), 144.68 (Ar-C), 147.16 (N–C
), 113.83 (Ar-C), 116.32 (Ar-C), 119.49 (Ar-C), 121.66 (Ar-C), 123.96 (Ar-C), 127.57 (Ar-C), 128.35 (Ar-C), 128.71 (Ar-C), 129.39 (Ar-C), 129.42 (Ar-C), 133.06 (Ar-C), 137.51 (Ar-C), 138.02 (Ar-C), 144.68 (Ar-C), 147.16 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 156.13 (C
), 156.13 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 165.13 (N–C
N–N), 165.13 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 168.46 (HN–C
N), 168.46 (HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C25H19N5O4S (485.51): C, 61.85; H, 3.94; N, 11.42; found: C, 61.80; H, 3.98; N, 11.36.
O); anal. calcd for C25H19N5O4S (485.51): C, 61.85; H, 3.94; N, 11.42; found: C, 61.80; H, 3.98; N, 11.36.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1593 (C
O), 1593 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1561, 1485 (Ar-C
N), 1561, 1485 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.13 (s, 3H, CH3–C
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.13 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 3.62 (s, 3H, OCH3), 4.59 (s, 2H, O–CH2–CO), 6.62–6.65 (m, 1H, Ar-H), 6.90–6.98 (m, 3H, CH–S, Ar-H), 7.08 (t, 1H, J = 8.0 Hz, Ar-H), 7.35–7.43 (m, 2H, Ar-H), 7.79 (s, 1H, Ar-H), 7.82–7.84 (m, 2H, Ar-H), 8.12–8.14 (m, 2H, Ar-H), 10.82 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.49 (CH3), 55.95 (OCH3), 67.25 (O–CH2), 94.87 (S-CH
N), 3.62 (s, 3H, OCH3), 4.59 (s, 2H, O–CH2–CO), 6.62–6.65 (m, 1H, Ar-H), 6.90–6.98 (m, 3H, CH–S, Ar-H), 7.08 (t, 1H, J = 8.0 Hz, Ar-H), 7.35–7.43 (m, 2H, Ar-H), 7.79 (s, 1H, Ar-H), 7.82–7.84 (m, 2H, Ar-H), 8.12–8.14 (m, 2H, Ar-H), 10.82 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.49 (CH3), 55.95 (OCH3), 67.25 (O–CH2), 94.87 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 103.19 (Ar-C), 113.46 (Ar-C), 114.01 (Ar-C), 116.07 (Ar-C), 117.16 (Ar-C), 121.70 (Ar-C), 123.63 (Ar-C), 126.06 (Ar-C), 127.56 (Ar-C), 128.71 (Ar-C), 133.14 (Ar-C), 140.39 (Ar-C), 147.51 (Ar-C), 149.61 (Ar-C), 157.01 (Ar-C), 157.40 (Ar-C), 159.20 (N–C
), 103.19 (Ar-C), 113.46 (Ar-C), 114.01 (Ar-C), 116.07 (Ar-C), 117.16 (Ar-C), 121.70 (Ar-C), 123.63 (Ar-C), 126.06 (Ar-C), 127.56 (Ar-C), 128.71 (Ar-C), 133.14 (Ar-C), 140.39 (Ar-C), 147.51 (Ar-C), 149.61 (Ar-C), 157.01 (Ar-C), 157.40 (Ar-C), 159.20 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 164.92 (C
), 164.92 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 165.15 (N–C
N–N), 165.15 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 167.07 (HN–C
N), 167.07 (HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C26H21N5O5S (515.54): C, 60.57; H, 4.11; N, 13.58; found: C, 60.51; H, 4.15; N, 13.52.
O); anal. calcd for C26H21N5O5S (515.54): C, 60.57; H, 4.11; N, 13.58; found: C, 60.51; H, 4.15; N, 13.52.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589 (C
O), 1589 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1557, 1508 (Ar-C
N), 1557, 1508 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.10 (s, 3H, CH3–C
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.10 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.60 (s, 2H, O–CH2–CO), 6.97 (s, 1H, CH–S), 7.05 (t, 2H, J = 8.8 Hz, Ar-H), 7.31–7.38 (m, 3H, Ar-H), 7.43 (d, 1H, J = 2.4 Hz, Ar-H), 7.82 (s, 1H, Ar-H), 7.86 (dd, 2H, J = 1.6 Hz, 6.8 Hz, Ar-H), 8.15 (dd, 2H, J = 2.0 Hz, 7.2 Hz, Ar-H), 10.83 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.81 (CH3), 67.24 (OCH2), 94.60 (S-CH
N), 4.60 (s, 2H, O–CH2–CO), 6.97 (s, 1H, CH–S), 7.05 (t, 2H, J = 8.8 Hz, Ar-H), 7.31–7.38 (m, 3H, Ar-H), 7.43 (d, 1H, J = 2.4 Hz, Ar-H), 7.82 (s, 1H, Ar-H), 7.86 (dd, 2H, J = 1.6 Hz, 6.8 Hz, Ar-H), 8.15 (dd, 2H, J = 2.0 Hz, 7.2 Hz, Ar-H), 10.83 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.81 (CH3), 67.24 (OCH2), 94.60 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 113.91 (Ar-C), 115.28 (Ar-C), 115.51 (Ar-C), 116.33 (Ar-C), 121.69 (Ar-C), 123.65 (Ar-C), 127.55 (Ar-C), 128.78 (Ar-C), 130.48 (Ar-C), 130.57 (Ar-C), 133.13 (Ar-C), 135.53 (Ar-C), 144.75 (Ar-C), 147.56 (Ar-C), 149.36 (Ar-C), 157.06 (Ar-C), 159.27 (N–C
), 113.91 (Ar-C), 115.28 (Ar-C), 115.51 (Ar-C), 116.33 (Ar-C), 121.69 (Ar-C), 123.65 (Ar-C), 127.55 (Ar-C), 128.78 (Ar-C), 130.48 (Ar-C), 130.57 (Ar-C), 133.13 (Ar-C), 135.53 (Ar-C), 144.75 (Ar-C), 147.56 (Ar-C), 149.36 (Ar-C), 157.06 (Ar-C), 159.27 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 161.69 (C
), 161.69 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 165.15 (N–C
N–N), 165.15 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 167.40 (HN–C
N), 167.40 (HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C25H18 FN5O4S (503.50): C, 59.64; H, 3.60; N, 13.91; found: C, 59.68; H, 3.64; N, 13.85.
O); anal. calcd for C25H18 FN5O4S (503.50): C, 59.64; H, 3.60; N, 13.91; found: C, 59.68; H, 3.64; N, 13.85.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1587 (C
O), 1587 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1570, 1498 (Ar-C
N), 1570, 1498 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.07 (s, 3H, CH3–C
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.07 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.12 (s, 6H, 2×CH3-Ar), 4.60 (s, 2H, O–CH2–CO), 6.97 (d, 1H, J = 8.4 Hz, Ar-H), 7.06 (s, 1H, CH–S), 7.13–7.22 (m, 3H, Ar-H), 7.32–7.43 (m, 4H, Ar-H), 8.08 (dd, 2H, J = 2.0 Hz, 6.8 Hz, Ar-H), 10.78 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.69 (CH3), 18.22 (2×CH3-Ar), 67.24 (OCH2), 105.63 (S-CH
N), 2.12 (s, 6H, 2×CH3-Ar), 4.60 (s, 2H, O–CH2–CO), 6.97 (d, 1H, J = 8.4 Hz, Ar-H), 7.06 (s, 1H, CH–S), 7.13–7.22 (m, 3H, Ar-H), 7.32–7.43 (m, 4H, Ar-H), 8.08 (dd, 2H, J = 2.0 Hz, 6.8 Hz, Ar-H), 10.78 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.69 (CH3), 18.22 (2×CH3-Ar), 67.24 (OCH2), 105.63 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 112.75 (Ar-C), 113.78 (Ar-C), 116.30 (Ar-C), 121.60 (Ar-C), 124.08 (Ar-C), 127.53 (Ar-C), 128.58 (Ar-C), 129.46 (Ar-C), 133.07 (Ar-C), 136.47 (Ar-C), 136.90 (Ar-C), 138.03 (Ar-C), 143.19 (Ar-C), 144.59 (Ar-C), 147.39 (N–C
), 112.75 (Ar-C), 113.78 (Ar-C), 116.30 (Ar-C), 121.60 (Ar-C), 124.08 (Ar-C), 127.53 (Ar-C), 128.58 (Ar-C), 129.46 (Ar-C), 133.07 (Ar-C), 136.47 (Ar-C), 136.90 (Ar-C), 138.03 (Ar-C), 143.19 (Ar-C), 144.59 (Ar-C), 147.39 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 155.78 (C
), 155.78 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 165.11 (N–C
N–N), 165.11 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 166.58 (HN–C
N), 166.58 (HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); fnal calcd for C27H23N5O4S (513.57): C, 63.14; H, 4.51; N, 13.64; found: C, 63.18; H, 4.54; N, 13.57.
O); fnal calcd for C27H23N5O4S (513.57): C, 63.14; H, 4.51; N, 13.64; found: C, 63.18; H, 4.54; N, 13.57.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1587 (C
O), 1587 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1572, 1489 (Ar-C
N), 1572, 1489 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.09 (s, 3H, CH3–C
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.09 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.60 (s, 2H, O–CH2–CO), 6.59–7.08 (m, 2H, CH–S, Ar-H), 7.20 (t, 2H, J = 7.2 Hz, Ar-H), 7.28–7.43 (m, 6H, Ar-H), 7.53–7.56 (m, 3H, Ar-H), 10.82 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.81 (CH3), 67.24 (OCH2), 94.94 (S-CH
N), 4.60 (s, 2H, O–CH2–CO), 6.59–7.08 (m, 2H, CH–S, Ar-H), 7.20 (t, 2H, J = 7.2 Hz, Ar-H), 7.28–7.43 (m, 6H, Ar-H), 7.53–7.56 (m, 3H, Ar-H), 10.82 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.81 (CH3), 67.24 (OCH2), 94.94 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 113.87 (Ar-C), 116.31 (Ar-C), 121.63 (Ar-C), 126.28 (Ar-C), 127.53 (Ar-C), 128.20 (Ar-C), 128.39 (Ar-C), 129.16 (Ar-C), 132.96 (Ar-C), 133.23 (Ar-C), 139.57 (Ar-C), 141.29 (Ar-C), 144.67 (N–C
), 113.87 (Ar-C), 116.31 (Ar-C), 121.63 (Ar-C), 126.28 (Ar-C), 127.53 (Ar-C), 128.20 (Ar-C), 128.39 (Ar-C), 129.16 (Ar-C), 132.96 (Ar-C), 133.23 (Ar-C), 139.57 (Ar-C), 141.29 (Ar-C), 144.67 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 156.57 (C
), 156.57 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 165.16 (N–C
N–N), 165.16 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 167.36 (HN–C
N), 167.36 (HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C25H19ClN4O2S (474.96): C, 63.22; H, 4.03; N, 11.80; found: C, 63.25; H, 4.08; N, 10.76.
O); anal. calcd for C25H19ClN4O2S (474.96): C, 63.22; H, 4.03; N, 11.80; found: C, 63.25; H, 4.08; N, 10.76.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1593 (C
O), 1593 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1541, 1488 (Ar-C
N), 1541, 1488 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.13 (s, 3H, CH3–C
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.13 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 3.63 (s, 3H, OCH3), 4.60 (s, 2H, O–CH2–CO), 6.62–6.65 (m, 1H, Ar-H), 6.87–6.97 (m, 3H, CH–S, Ar-H), 7.08 (t, 1H, J = 8.0 Hz, Ar-H), 7.32–7.37 (m, 3H, Ar-H), 7.42 (d, 1H, J = 1.6 Hz, Ar-H), 7.53–7.56 (m, 2H, Ar-H), 7.57 (s, 1H,Ar-H), 10.82 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.89 (CH3), 55.43 (OCH3), 67.25 (O–CH2), 95.04 (S-CH
N), 3.63 (s, 3H, OCH3), 4.60 (s, 2H, O–CH2–CO), 6.62–6.65 (m, 1H, Ar-H), 6.87–6.97 (m, 3H, CH–S, Ar-H), 7.08 (t, 1H, J = 8.0 Hz, Ar-H), 7.32–7.37 (m, 3H, Ar-H), 7.42 (d, 1H, J = 1.6 Hz, Ar-H), 7.53–7.56 (m, 2H, Ar-H), 7.57 (s, 1H,Ar-H), 10.82 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.89 (CH3), 55.43 (OCH3), 67.25 (O–CH2), 95.04 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 111.65 (Ar-C), 113.89 (Ar-C), 114.01 (Ar-C), 116.31 (Ar-C), 120.33 (Ar-C), 121.66 (Ar-C), 127.54 (Ar-C), 128.41 (Ar-C), 128.89 (Ar-C), 129.11 (Ar-C), 132.97 (Ar-C), 133.22 (Ar-C), 140.62 (Ar-C), 141.33 (Ar-C), 144.69 (Ar-C), 156.69 (N–C
), 111.65 (Ar-C), 113.89 (Ar-C), 114.01 (Ar-C), 116.31 (Ar-C), 120.33 (Ar-C), 121.66 (Ar-C), 127.54 (Ar-C), 128.41 (Ar-C), 128.89 (Ar-C), 129.11 (Ar-C), 132.97 (Ar-C), 133.22 (Ar-C), 140.62 (Ar-C), 141.33 (Ar-C), 144.69 (Ar-C), 156.69 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 159.11 (C
), 159.11 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 165.15 (N–C
N–N), 165.15 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 167.17 (HN–C
N), 167.17 (HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C26H21ClN4O3S (504.99): C, 61.84; H, 4.19; N, 11.09; found: C, 61.80; H, 4.24; N, 11.13.
O); anal. calcd for C26H21ClN4O3S (504.99): C, 61.84; H, 4.19; N, 11.09; found: C, 61.80; H, 4.24; N, 11.13.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1587 (C
O), 1587 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1529, 1496 (Ar-C
N), 1529, 1496 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.08 (s, 3H, CH3–C
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.08 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.59 (s, 2H, O–CH2–CO), 6.54–7.06 (m, 3H, CH–S, Ar-H), 7.28–7.34 (m, 5H, Ar-H), 7.41–7.42 (m, 1H, Ar-H), 7.54–7.57 (m, 3H, Ar-H), 10.81 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.78 (CH3), 67.25 (O–CH2), 94.76 (S-CH
N), 4.59 (s, 2H, O–CH2–CO), 6.54–7.06 (m, 3H, CH–S, Ar-H), 7.28–7.34 (m, 5H, Ar-H), 7.41–7.42 (m, 1H, Ar-H), 7.54–7.57 (m, 3H, Ar-H), 10.81 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.78 (CH3), 67.25 (O–CH2), 94.76 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 100.04 (Ar-C), 113.89 (Ar-C), 115.34 (Ar-C), 116.31 (Ar-C), 127.54 (Ar-C), 128.45 (Ar-C), 129.18 (Ar-C), 130.41 (Ar-C), 130.49 (Ar-C), 133.20 (Ar-C), 141.08 (Ar-C), 144.70 (Ar-C), 156.74 (Ar-C), 159.48 (N–C
), 100.04 (Ar-C), 113.89 (Ar-C), 115.34 (Ar-C), 116.31 (Ar-C), 127.54 (Ar-C), 128.45 (Ar-C), 129.18 (Ar-C), 130.41 (Ar-C), 130.49 (Ar-C), 133.20 (Ar-C), 141.08 (Ar-C), 144.70 (Ar-C), 156.74 (Ar-C), 159.48 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 161.62 (C
), 161.62 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 165.15 (N–C
N–N), 165.15 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 167.50 (HN–C
N), 167.50 (HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); anal. calcd for C25H18ClFN4O2S (492.95): C, 60.91; H, 3.68; N, 11.37; found: C, 60.95; H, 3.65; N, 11.32.
O); anal. calcd for C25H18ClFN4O2S (492.95): C, 60.91; H, 3.68; N, 11.37; found: C, 60.95; H, 3.65; N, 11.32.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1541, 1487 (Ar-C
N), 1541, 1487 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.67–1.75 (m, 6H, adamantane –CH2), 1.81 (m, 6H, adamantane –CH2), 1.85 (s, 3H, CH3–C
C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.67–1.75 (m, 6H, adamantane –CH2), 1.81 (m, 6H, adamantane –CH2), 1.85 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.01–2.02 (bs, 3H, adamantane –CH2), 6.11 (s, 1H, CH–S), 6.95 (d, 2H, J = 8.8 Hz, Ar-H), 7.19–7.21 (m, 3H, Ar-H), 7.27–7.31 (m, 4H, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 12.48 (CH3), 28.46 (adamantane-C), 36.98 (adamantane-C), 39.94 (adamantane-C), 40.38 (adamantane-C), 102.08 (S-CH
N), 2.01–2.02 (bs, 3H, adamantane –CH2), 6.11 (s, 1H, CH–S), 6.95 (d, 2H, J = 8.8 Hz, Ar-H), 7.19–7.21 (m, 3H, Ar-H), 7.27–7.31 (m, 4H, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 12.48 (CH3), 28.46 (adamantane-C), 36.98 (adamantane-C), 39.94 (adamantane-C), 40.38 (adamantane-C), 102.08 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 122.24 (Ar-C), 127.39 (Ar-C), 128.21 (Ar-C), 128.69 (Ar-C), 129.45 (Ar-C), 130.55 (Ar-C), 131.43 (Ar-C), 136.08 (Ar-C), 137.97 (N–C
), 122.24 (Ar-C), 127.39 (Ar-C), 128.21 (Ar-C), 128.69 (Ar-C), 129.45 (Ar-C), 130.55 (Ar-C), 131.43 (Ar-C), 136.08 (Ar-C), 137.97 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 167.51 (C
), 167.51 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 169.44 (N–C
N–N), 169.44 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C27H28BrN3S (506.50): C, 64.03; H, 5.57; N, 8.30; found: C, 64.10; H, 5.52; N, 8.34.
N); anal. calcd for C27H28BrN3S (506.50): C, 64.03; H, 5.57; N, 8.30; found: C, 64.10; H, 5.52; N, 8.34.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1545, 1507 (Ar-C
N), 1545, 1507 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.54 (s, 3H, CH3–C
C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.54 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1.65–1.70 (m, 6H, adamantane –CH2), 1.73–1.77 (bs, 6H, adamantane –CH2), 2.00 (bs, 3H, adamantane –CH2), 6.18 (s, 1H, CH–S), 6.89 (dd, 2H, J = 2.0 Hz, 6.8 Hz, Ar-H), 7.13 (dd, 2H, J = 2.0 Hz, 6.8 Hz, Ar-H), 7.22 (dd, 1H, J = 1.2 Hz, 7.2 Hz, Ar-H), 7.36 (t, 1H, J = 7.6 Hz, Ar-H), 7.47–7.50 (m, 2H, Ar-H), 7.77–7.89 (m, 3H, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 12.24 (CH3), 28.43 (adamantane-C), 36.96 (adamantane-C), 39.86 (adamantane-C), 40.26 (adamantane-C), 97.91 (S-CH
N), 1.65–1.70 (m, 6H, adamantane –CH2), 1.73–1.77 (bs, 6H, adamantane –CH2), 2.00 (bs, 3H, adamantane –CH2), 6.18 (s, 1H, CH–S), 6.89 (dd, 2H, J = 2.0 Hz, 6.8 Hz, Ar-H), 7.13 (dd, 2H, J = 2.0 Hz, 6.8 Hz, Ar-H), 7.22 (dd, 1H, J = 1.2 Hz, 7.2 Hz, Ar-H), 7.36 (t, 1H, J = 7.6 Hz, Ar-H), 7.47–7.50 (m, 2H, Ar-H), 7.77–7.89 (m, 3H, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 12.24 (CH3), 28.43 (adamantane-C), 36.96 (adamantane-C), 39.86 (adamantane-C), 40.26 (adamantane-C), 97.91 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 123.84 (Ar-C), 125.34 (Ar-C), 126.31 (Ar-C), 126.72 (Ar-C), 127.18 (Ar-C), 128.26 (Ar-C), 128.82 (Ar-C), 129.04 (Ar-C), 131.26 (Ar-C), 134.27 (Ar-C), 135.09 (Ar-C), 136.87 (Ar-C), 155.38 (N–C
), 123.84 (Ar-C), 125.34 (Ar-C), 126.31 (Ar-C), 126.72 (Ar-C), 127.18 (Ar-C), 128.26 (Ar-C), 128.82 (Ar-C), 129.04 (Ar-C), 131.26 (Ar-C), 134.27 (Ar-C), 135.09 (Ar-C), 136.87 (Ar-C), 155.38 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 166.17 (C
), 166.17 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 169.88 (N–C
N–N), 169.88 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C31H30BrN3S (556.56): C, 66.90; H, 5.43; N, 7.55; found: C, 66.95; H, 5.45; N, 7.52.
N); anal. calcd for C31H30BrN3S (556.56): C, 66.90; H, 5.43; N, 7.55; found: C, 66.95; H, 5.45; N, 7.52.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1581, 1514 (Ar-C
N), 1581, 1514 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.56 (bs, 6H, CH3–C
C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.56 (bs, 6H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, CH3-Ar), 1.66–1.72 (m, 6H, adamantane –CH2), 1.75–1.80 (bs, 6H, adamantane –CH2), 2.00–2.02 (bs, 3H, adamantane –CH2), 6.12 (s, 1H, CH–S), 6.91–6.94 (m, 2H, Ar-H),7.02 (d, 1H, J = 7.6 Hz, Ar-H), 7.09–7.13 (m, 1H, Ar-H), 7.19–7.21 (m, 2H, Ar-H), 7.27–7.29 (m, 2H, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 13.00 (CH3), 18.21 (CH3-Ar), 28.44 (adamantane-C), 36.96 (adamantane-C), 39.86 (adamantane-C), 40.36 (adamantane-C), 93.39 (S-CH
N, CH3-Ar), 1.66–1.72 (m, 6H, adamantane –CH2), 1.75–1.80 (bs, 6H, adamantane –CH2), 2.00–2.02 (bs, 3H, adamantane –CH2), 6.12 (s, 1H, CH–S), 6.91–6.94 (m, 2H, Ar-H),7.02 (d, 1H, J = 7.6 Hz, Ar-H), 7.09–7.13 (m, 1H, Ar-H), 7.19–7.21 (m, 2H, Ar-H), 7.27–7.29 (m, 2H, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 13.00 (CH3), 18.21 (CH3-Ar), 28.44 (adamantane-C), 36.96 (adamantane-C), 39.86 (adamantane-C), 40.36 (adamantane-C), 93.39 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 126.51 (Ar-C), 127.32 (Ar-C), 128.11 (Ar-C), 128.84 (Ar-C), 129.23 (Ar-C), 130.86 (Ar-C), 131.00 (Ar-C), 131.33 (Ar-C), 131.39 (Ar-C), 143.59 (N–C
), 126.51 (Ar-C), 127.32 (Ar-C), 128.11 (Ar-C), 128.84 (Ar-C), 129.23 (Ar-C), 130.86 (Ar-C), 131.00 (Ar-C), 131.33 (Ar-C), 131.39 (Ar-C), 143.59 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 166.18 (C
), 166.18 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 167.53 (N–C
N–N), 167.53 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C28H30BrN3S (520.53): C, 64.61; H, 5.81; N, 8.07; found: C, 64.58; H, 5.85; N, 8.12.
N); anal. calcd for C28H30BrN3S (520.53): C, 64.61; H, 5.81; N, 8.07; found: C, 64.58; H, 5.85; N, 8.12.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1547, 1509 (Ar-C
N), 1547, 1509 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.56 (s, 6H, CH3–C
C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.56 (s, 6H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, CH3-Ar), 1.69–1.71 (m, 6H, adamantane –CH2), 1.77–1.80 (bs, 6H, adamantane –CH2), 2.00–2.02 (bs, 3H, adamantane –CH2), 2.15 (s, 3H, CH3-Ar), 6.13 (s, 1H, CH–S), 6.93 (d, 2H, J = 8.4 Hz, Ar-H), 7.02 (d, 2H, J = 7.2 Hz, Ar-H), 7.12 (t, 1H, J = 7.2 Hz, Ar-H), 7.28 (d, 2H, J = 8.8 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 12.17 (CH3), 18.17 (2×CH3–Ar), 28.27 (adamantane-C), 36.79 (adamantane-C), 39.67 (adamantane-C), 40.08 (adamantane-C), 100.18 (S-CH
N, CH3-Ar), 1.69–1.71 (m, 6H, adamantane –CH2), 1.77–1.80 (bs, 6H, adamantane –CH2), 2.00–2.02 (bs, 3H, adamantane –CH2), 2.15 (s, 3H, CH3-Ar), 6.13 (s, 1H, CH–S), 6.93 (d, 2H, J = 8.4 Hz, Ar-H), 7.02 (d, 2H, J = 7.2 Hz, Ar-H), 7.12 (t, 1H, J = 7.2 Hz, Ar-H), 7.28 (d, 2H, J = 8.8 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 12.17 (CH3), 18.17 (2×CH3–Ar), 28.27 (adamantane-C), 36.79 (adamantane-C), 39.67 (adamantane-C), 40.08 (adamantane-C), 100.18 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 128.22 (Ar-C), 128.32 (Ar-C), 129.60 (Ar-C), 134.01 (Ar-C), 136.01 (Ar-C), 136.53 (Ar-C), 138.77 (Ar-C), 143.56 (N–C
), 128.22 (Ar-C), 128.32 (Ar-C), 129.60 (Ar-C), 134.01 (Ar-C), 136.01 (Ar-C), 136.53 (Ar-C), 138.77 (Ar-C), 143.56 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 164.19 (C
), 164.19 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 168.63 (N–C
N–N), 168.63 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C29H32BrN3S (534.55): C, 65.16; H, 6.03; N, 7.86; found: C, 65.20; H, 6.09; N, 7.80.
N); anal. calcd for C29H32BrN3S (534.55): C, 65.16; H, 6.03; N, 7.86; found: C, 65.20; H, 6.09; N, 7.80.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1556, 1488 (Ar-C
N), 1556, 1488 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.63–1.68 (m, 6H, adamantane –CH2), 1.73–1.74 (m, 6H, adamantane –CH2), 1.81 (s, 3H, CH3–C
C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.63–1.68 (m, 6H, adamantane –CH2), 1.73–1.74 (m, 6H, adamantane –CH2), 1.81 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1.98 (bs, 3H, adamantane –CH2), 4.96 (s, 2H, CH2), 6.31 (s, 1H, CH–S), 6.98–7.00 (m, 2H, Ar-H), 7.18–7.26 (m, 3H, Ar-H), 7.35 (dd, 2H, J = 2.0 Hz, 6.4 Hz, Ar-H), 7.46 (dd, 2H, J = 2.0 Hz, 6.8 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 12.48 (CH3), 28.26 (adamantane-C), 36.90 (adamantane-C), 39.19 (adamantane-C), 40.67 (adamantane-C), 101.07 (S-CH
N), 1.98 (bs, 3H, adamantane –CH2), 4.96 (s, 2H, CH2), 6.31 (s, 1H, CH–S), 6.98–7.00 (m, 2H, Ar-H), 7.18–7.26 (m, 3H, Ar-H), 7.35 (dd, 2H, J = 2.0 Hz, 6.4 Hz, Ar-H), 7.46 (dd, 2H, J = 2.0 Hz, 6.8 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 12.48 (CH3), 28.26 (adamantane-C), 36.90 (adamantane-C), 39.19 (adamantane-C), 40.67 (adamantane-C), 101.07 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 127.08 (Ar-C), 127.55 (Ar-C), 128.84 (Ar-C), 129.18 (Ar-C), 130.23 (Ar-C), 130.90 (Ar-C), 134.25 (Ar-C), 137.63 (Ar-C), 139.11 (N–C
), 127.08 (Ar-C), 127.55 (Ar-C), 128.84 (Ar-C), 129.18 (Ar-C), 130.23 (Ar-C), 130.90 (Ar-C), 134.25 (Ar-C), 137.63 (Ar-C), 139.11 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 166.81 (C
), 166.81 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 167.41 (N–C
N–N), 167.41 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C28H30ClN3S (476.08): C, 70.64; H, 6.35; N, 8.83; found: C, 70.68; H, 6.30; N, 8.87.
N); anal. calcd for C28H30ClN3S (476.08): C, 70.64; H, 6.35; N, 8.83; found: C, 70.68; H, 6.30; N, 8.87.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1541, 1493 (Ar-C
N), 1541, 1493 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.65–1.69 (m, 6H, adamantane –CH2), 1.74 (s, 3H, CH3–C
C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.65–1.69 (m, 6H, adamantane –CH2), 1.74 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1.78–1.79 (bs, 6H, adamantane –CH2), 2.00 (bs, 3H, adamantane –CH2), 2.13 (s, 6H, 2×CH3–Ar), 6.08 (s, 1H, CH–S), 6.96–7.00 (m, 4H, Ar-H), 7.08–7.10 (m, 3H, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 12.37 (CH3), 18.36 (2×CH3–Ar), 28.46 (adamantane-C), 36.98 (adamantane-C), 39.86 (adamantane-C), 40.28 (adamantane-C), 100.37 (S-CH
N), 1.78–1.79 (bs, 6H, adamantane –CH2), 2.00 (bs, 3H, adamantane –CH2), 2.13 (s, 6H, 2×CH3–Ar), 6.08 (s, 1H, CH–S), 6.96–7.00 (m, 4H, Ar-H), 7.08–7.10 (m, 3H, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 12.37 (CH3), 18.36 (2×CH3–Ar), 28.46 (adamantane-C), 36.98 (adamantane-C), 39.86 (adamantane-C), 40.28 (adamantane-C), 100.37 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 128.41 (Ar-C), 128.47 (Ar-C), 128.51 (Ar-C), 129.79 (Ar-C), 134.21 (Ar-C), 136.21 (Ar-C), 136.72 (Ar-C), 138.96 (N–C
), 128.41 (Ar-C), 128.47 (Ar-C), 128.51 (Ar-C), 129.79 (Ar-C), 134.21 (Ar-C), 136.21 (Ar-C), 136.72 (Ar-C), 138.96 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 164.39 (C
), 164.39 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 168.82 (N–C
N–N), 168.82 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C29H32ClN3S (490.10): C, 71.07; H, 6.58; N, 8.57; found: C, 71.01; H, 6.62; N, 8.53.
N); anal. calcd for C29H32ClN3S (490.10): C, 71.07; H, 6.58; N, 8.57; found: C, 71.01; H, 6.62; N, 8.53.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1539, 1498 (Ar-C
N), 1539, 1498 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.23 (s, 3H, CH3–C
C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.23 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.27 (s, 4H, –OCH2CH2O–), 6.16 (s, 1H, CH–S), 6.85 (d, 1H, J = 8.4 Hz, Ar-H), 6.96–7.00 (m, 2H, Ar-H), 7.22–7.27 (m, 3H, Ar-H), 7.30–7.33 (m, 4H, Ar-H), 7.34–7.38 (m, 1H, Ar-H), 7.40 (d, 1H, J = 2.4 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.70 (CH3), 64.33 (O–CH2), 64.56 (O–CH2), 102.25 (S-CH
N), 4.27 (s, 4H, –OCH2CH2O–), 6.16 (s, 1H, CH–S), 6.85 (d, 1H, J = 8.4 Hz, Ar-H), 6.96–7.00 (m, 2H, Ar-H), 7.22–7.27 (m, 3H, Ar-H), 7.30–7.33 (m, 4H, Ar-H), 7.34–7.38 (m, 1H, Ar-H), 7.40 (d, 1H, J = 2.4 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.70 (CH3), 64.33 (O–CH2), 64.56 (O–CH2), 102.25 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 115.33 (Ar-C), 116.85 (Ar-C), 119.86 (Ar-C), 122.37 (Ar-C), 127.40 (Ar-C), 128.30 (Ar-C), 128.72 (Ar-C), 129.53 (Ar-C), 131.48 (Ar-C), 132.75 (Ar-C), 137.90 (Ar-C), 138.90 (Ar-C), 143.14 (Ar-C), 144.47 (N–C
), 115.33 (Ar-C), 116.85 (Ar-C), 119.86 (Ar-C), 122.37 (Ar-C), 127.40 (Ar-C), 128.30 (Ar-C), 128.72 (Ar-C), 129.53 (Ar-C), 131.48 (Ar-C), 132.75 (Ar-C), 137.90 (Ar-C), 138.90 (Ar-C), 143.14 (Ar-C), 144.47 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 156.72 (C
), 156.72 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 168.36 (N–C
N–N), 168.36 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C25H20BrN3O2S (506.41): C, 59.29; H, 3.98; N, 8.30; found: C, 59.35; H, 3.95; N, 8.34.
N); anal. calcd for C25H20BrN3O2S (506.41): C, 59.29; H, 3.98; N, 8.30; found: C, 59.35; H, 3.95; N, 8.34.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1533, 1496 (Ar-C
N), 1533, 1496 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.30 (s, 3H, CH3–C
C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.30 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.28 (s, 4H, –OCH2CH2O–), 5.03 (s, 2H, –CH2–), 5.95 (s, 1H, CH–S), 6.85 (d, 1H, J = 8.4 Hz, Ar-H), 7.07–7.10 (m, 4H, Ar-H), 7.22–7.26 (m, 3H, Ar-H), 7.37–7.47 (m, 4H, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.45 (CH3), 49.30 (CH2), 64.34 (O–CH2), 64.56 (O–CH2), 100.75 (S-CH
N), 4.28 (s, 4H, –OCH2CH2O–), 5.03 (s, 2H, –CH2–), 5.95 (s, 1H, CH–S), 6.85 (d, 1H, J = 8.4 Hz, Ar-H), 7.07–7.10 (m, 4H, Ar-H), 7.22–7.26 (m, 3H, Ar-H), 7.37–7.47 (m, 4H, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.45 (CH3), 49.30 (CH2), 64.34 (O–CH2), 64.56 (O–CH2), 100.75 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 115.23 (Ar-C), 116.86 (Ar-C), 119.76 (Ar-C), 123.36 (Ar-C), 127.15 (Ar-C), 128.43 (Ar-C), 130.27 (Ar-C), 130.56 (Ar-C), 131.77 (Ar-C), 132.92 (Ar-C), 137.17 (Ar-C), 139.49 (Ar-C), 143.16 (Ar-C), 144.34 (Ar-C), 155.69 (N–C
), 115.23 (Ar-C), 116.86 (Ar-C), 119.76 (Ar-C), 123.36 (Ar-C), 127.15 (Ar-C), 128.43 (Ar-C), 130.27 (Ar-C), 130.56 (Ar-C), 131.77 (Ar-C), 132.92 (Ar-C), 137.17 (Ar-C), 139.49 (Ar-C), 143.16 (Ar-C), 144.34 (Ar-C), 155.69 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 163.82 (C
), 163.82 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 168.55 (N–C
N–N), 168.55 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C26H22BrN3O2S (520.44): C, 60.00; H, 4.26; N, 8.07; found: C, 60.07; H, 4.22; N, 8.12.
N); anal. calcd for C26H22BrN3O2S (520.44): C, 60.00; H, 4.26; N, 8.07; found: C, 60.07; H, 4.22; N, 8.12.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1589, 1483 (Ar-C
N), 1589, 1483 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.15 (s, 3H, CH3–C
C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.15 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.16 (s, 3H, CH3-Ar), 2.17 (s, 3H, CH3-Ar), 4.27 (s, 4H, –OCH2CH2O–), 6.19 (s, 1H, CH–S), 6.83 (d, 1H, J = 8.4 Hz, Ar-H), 6.94–6.97 (m, 2H, Ar-H), 7.03–7.05 (m, 2H, Ar-H), 7.13–7.15 (m, 1H, Ar-H), 7.28–7.30 (m, 2H, Ar-H), 7.35–7.39 (m, 2H, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.52 (CH3), 18.29 (2×CH3-Ar), 64.32 (O–CH2), 64.55 (O–CH2), 100.99 (S-CH
N), 2.16 (s, 3H, CH3-Ar), 2.17 (s, 3H, CH3-Ar), 4.27 (s, 4H, –OCH2CH2O–), 6.19 (s, 1H, CH–S), 6.83 (d, 1H, J = 8.4 Hz, Ar-H), 6.94–6.97 (m, 2H, Ar-H), 7.03–7.05 (m, 2H, Ar-H), 7.13–7.15 (m, 1H, Ar-H), 7.28–7.30 (m, 2H, Ar-H), 7.35–7.39 (m, 2H, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.52 (CH3), 18.29 (2×CH3-Ar), 64.32 (O–CH2), 64.55 (O–CH2), 100.99 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 115.23 (Ar-C), 116.84 (Ar-C), 119.74 (Ar-C), 122.61 (Ar-C), 128.46 (Ar-C), 128.63 (Ar-C), 128.82 (Ar-C), 130.02 (Ar-C), 131.43 (Ar-C), 132.78 (Ar-C), 136.62 (Ar-C), 139.03 (Ar-C), 143.13 (Ar-C), 144.37 (N–C
), 115.23 (Ar-C), 116.84 (Ar-C), 119.74 (Ar-C), 122.61 (Ar-C), 128.46 (Ar-C), 128.63 (Ar-C), 128.82 (Ar-C), 130.02 (Ar-C), 131.43 (Ar-C), 132.78 (Ar-C), 136.62 (Ar-C), 139.03 (Ar-C), 143.13 (Ar-C), 144.37 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 156.32 (C
), 156.32 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 166.19 (N–C
N–N), 166.19 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C27H24BrN3O2S (534.47): C, 60.68; H, 4.53; N, 7.86; found: C, 60.74; H, 4.57; N, 7.81.
N); anal. calcd for C27H24BrN3O2S (534.47): C, 60.68; H, 4.53; N, 7.86; found: C, 60.74; H, 4.57; N, 7.81.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1577, 1545 (Ar-C
N), 1577, 1545 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.14 (s, 3H, CH3–C
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.14 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.25 (s, 4H, –OCH2CH2O–), 6.88 (d, 1H, J = 8.4 Hz, Ar-H), 6.93 (s, 1H, CH–S), 7.21–7.30 (m, 4H, Ar-H), 7.36–7.45 (m, 4H, Ar-H), 8.12 (dd, 2H, J = 2.0 Hz, 7.2 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.84 (CH3), 64.51 (O–CH2), 64.75 (O–CH2), 106.12 (S-CH
N), 4.25 (s, 4H, –OCH2CH2O–), 6.88 (d, 1H, J = 8.4 Hz, Ar-H), 6.93 (s, 1H, CH–S), 7.21–7.30 (m, 4H, Ar-H), 7.36–7.45 (m, 4H, Ar-H), 8.12 (dd, 2H, J = 2.0 Hz, 7.2 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.84 (CH3), 64.51 (O–CH2), 64.75 (O–CH2), 106.12 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 115.03 (Ar-C), 116.19 (Ar-C), 116.42 (Ar-C), 117.27 (Ar-C), 119.84 (Ar-C), 124.01 (Ar-C), 129.52 (Ar-C), 130.98 (Ar-C), 132.03 (Ar-C), 134.28 (Ar-C), 137.33 (Ar-C), 137.91 (Ar-C), 143.54 (Ar-C), 145.02 (Ar-C), 147.24 (N–C
), 115.03 (Ar-C), 116.19 (Ar-C), 116.42 (Ar-C), 117.27 (Ar-C), 119.84 (Ar-C), 124.01 (Ar-C), 129.52 (Ar-C), 130.98 (Ar-C), 132.03 (Ar-C), 134.28 (Ar-C), 137.33 (Ar-C), 137.91 (Ar-C), 143.54 (Ar-C), 145.02 (Ar-C), 147.24 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 156.45 (C
), 156.45 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 168.29 (N–C
N–N), 168.29 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C25H19FN4O4S (490.51): C, 61.22; H, 3.90; N, 11.42; found: C, 61.27; H, 3.85; N, 11.45.
N); anal. calcd for C25H19FN4O4S (490.51): C, 61.22; H, 3.90; N, 11.42; found: C, 61.27; H, 3.85; N, 11.45.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1538, 1497 (Ar-C
N), 1538, 1497 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.24 (s, 3H, CH3–C
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.24 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.26 (s, 4H, –OCH2CH2O–), 5.12 (s, 2H, –CH2–), 6.66 (s, 1H, CH–S), 6.88 (d, 1H, J = 8.8 Hz, Ar-H), 7.02 (d, 2H, J = 7.2 Hz, Ar-H), 7.19–7.24 (m, 4H, Ar-H), 7.31 (s, 1H, Ar-H), 7.67 (dd, 2H, J = 2.0 Hz, 6.8 Hz, Ar-H), 8.25 (dd, 2H, Ar-H, J = 1.6 Hz, 6.8 Hz); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.67 (CH3), 49.07 (CH2), 64.50 (O–CH2), 64.74 (O–CH2), 104.16 (S-CH
N), 4.26 (s, 4H, –OCH2CH2O–), 5.12 (s, 2H, –CH2–), 6.66 (s, 1H, CH–S), 6.88 (d, 1H, J = 8.8 Hz, Ar-H), 7.02 (d, 2H, J = 7.2 Hz, Ar-H), 7.19–7.24 (m, 4H, Ar-H), 7.31 (s, 1H, Ar-H), 7.67 (dd, 2H, J = 2.0 Hz, 6.8 Hz, Ar-H), 8.25 (dd, 2H, Ar-H, J = 1.6 Hz, 6.8 Hz); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.67 (CH3), 49.07 (CH2), 64.50 (O–CH2), 64.74 (O–CH2), 104.16 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 114.95 (Ar-C), 117.26 (Ar-C), 119.72 (Ar-C), 124.31 (Ar-C), 127.17 (Ar-C), 127.75 (Ar-C), 128.97 (Ar-C), 130.20 (Ar-C), 132.26 (Ar-C), 137.23 (Ar-C), 137.47 (Ar-C), 138.73 (Ar-C), 143.53 (Ar-C), 144.83 (Ar-C), 147.92 (N–C
), 114.95 (Ar-C), 117.26 (Ar-C), 119.72 (Ar-C), 124.31 (Ar-C), 127.17 (Ar-C), 127.75 (Ar-C), 128.97 (Ar-C), 130.20 (Ar-C), 132.26 (Ar-C), 137.23 (Ar-C), 137.47 (Ar-C), 138.73 (Ar-C), 143.53 (Ar-C), 144.83 (Ar-C), 147.92 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 155.26 (C
), 155.26 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 168.42 (N–C
N–N), 168.42 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C26H22N4O4S (486.54): C, 64.18; H, 4.56; N, 11.52; found: C, 64.22; H, 4.50; N, 11.55.
N); anal. calcd for C26H22N4O4S (486.54): C, 64.18; H, 4.56; N, 11.52; found: C, 64.22; H, 4.50; N, 11.55.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1557, 1482 (Ar-C
N). 1557, 1482 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.05 (s, 3H, CH3–C
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.05 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 2.12 (s, 6H, 2×CH3-Ar), 4.25 (s, 4H, –OCH2CH2O–), 6.88 (d, 1H, J = 9.2 Hz, Ar-H), 7.01 (s, 1H, CH–S), 7.13–7.21 (m, 3H, Ar-H), 7.26–7.29 (m, 2H, Ar-H), 7.37 (dd, 2H, J = 2.0 Hz, 7.2 Hz, Ar-H), 8.08 (dd, 2H, J = 2.0 Hz, 7.2 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.63 (CH3), 18.22 (2×CH3-Ar), 64.50 (O–CH2), 64.73 (O–CH2), 105.56 (S-CH
N), 2.12 (s, 6H, 2×CH3-Ar), 4.25 (s, 4H, –OCH2CH2O–), 6.88 (d, 1H, J = 9.2 Hz, Ar-H), 7.01 (s, 1H, CH–S), 7.13–7.21 (m, 3H, Ar-H), 7.26–7.29 (m, 2H, Ar-H), 7.37 (dd, 2H, J = 2.0 Hz, 7.2 Hz, Ar-H), 8.08 (dd, 2H, J = 2.0 Hz, 7.2 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.63 (CH3), 18.22 (2×CH3-Ar), 64.50 (O–CH2), 64.73 (O–CH2), 105.56 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 110.17 (Ar-C), 114.96 (Ar-C), 117.26 (Ar-C), 119.73 (Ar-C), 124.06 (Ar-C), 128.57 (Ar-C), 129.06 (Ar-C), 129.43 (Ar-C), 132.04 (Ar-C), 136.47 (Ar-C), 136.93 (Ar-C), 138.03 (Ar-C), 143.52 (Ar-C), 144.90 (Ar-C), 147.37 (N–C
), 110.17 (Ar-C), 114.96 (Ar-C), 117.26 (Ar-C), 119.73 (Ar-C), 124.06 (Ar-C), 128.57 (Ar-C), 129.06 (Ar-C), 129.43 (Ar-C), 132.04 (Ar-C), 136.47 (Ar-C), 136.93 (Ar-C), 138.03 (Ar-C), 143.52 (Ar-C), 144.90 (Ar-C), 147.37 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 155.95 (C
), 155.95 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 166.42 (N–C
N–N), 166.42 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C27H24N4O4S (500.57): C, 64.78; H, 4.83; N, 11.19; found: C, 64.72; H, 4.85; N, 11.15.
N); anal. calcd for C27H24N4O4S (500.57): C, 64.78; H, 4.83; N, 11.19; found: C, 64.72; H, 4.85; N, 11.15.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1545, 1501 (Ar-C
N), 1545, 1501 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.21 (s, 3H, CH3–C
C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.21 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.25 (s, 4H, –OCH2CH2O–), 6.13 (s, 1H, CH–S), 6.83 (d, 1H, J = 8.4 Hz, Ar-H), 7.01–7.03 (m, 2H, Ar-H), 7.13–7.15 (m, 2H, Ar-H), 7.20–7.23 (m, 3H, Ar-H), 7.28–7.36 (m, 3H, Ar-H), 7.39 (d, 1H, J = 2.0 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.72 (CH3), 64.33 (O–CH2), 64.56 (O–CH2), 102.19 (S-CH
N), 4.25 (s, 4H, –OCH2CH2O–), 6.13 (s, 1H, CH–S), 6.83 (d, 1H, J = 8.4 Hz, Ar-H), 7.01–7.03 (m, 2H, Ar-H), 7.13–7.15 (m, 2H, Ar-H), 7.20–7.23 (m, 3H, Ar-H), 7.28–7.36 (m, 3H, Ar-H), 7.39 (d, 1H, J = 2.0 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.72 (CH3), 64.33 (O–CH2), 64.56 (O–CH2), 102.19 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 115.33 (Ar-C), 116.86 (Ar-C), 119.86 (Ar-C), 127.40 (Ar-C), 128.31 (Ar-C), 128.53 (Ar-C), 128.72 (Ar-C), 129.29 (Ar-C), 129.93 (Ar-C), 132.75 (Ar-C), 137.89 (Ar-C), 143.13 (Ar-C), 144.47 (N–C
), 115.33 (Ar-C), 116.86 (Ar-C), 119.86 (Ar-C), 127.40 (Ar-C), 128.31 (Ar-C), 128.53 (Ar-C), 128.72 (Ar-C), 129.29 (Ar-C), 129.93 (Ar-C), 132.75 (Ar-C), 137.89 (Ar-C), 143.13 (Ar-C), 144.47 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 156.69 (C
), 156.69 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 168.40 (N–C
N–N), 168.40 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C25H20ClN3O2S (461.96): C, 65.00; H, 4.36; N, 9.10; found: C, 65.04; H, 4.31; N, 9.16.
N); anal. calcd for C25H20ClN3O2S (461.96): C, 65.00; H, 4.36; N, 9.10; found: C, 65.04; H, 4.31; N, 9.16.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1560, 1517 (Ar-C
N), 1560, 1517 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.12 (s, 3H, CH3–C
C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.12 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.25 (s, 4H, –OCH2CH2O–), 6.13 (s, 1H, CH–S), 6.82 (d, 1H, J = 8.4 Hz, Ar-H), 6.96–7.02 (m, 4H, Ar-H), 7.15–7.19 (m, 4H, Ar-H), 7.34 (dd, 1H, J = 2.4 Hz, 8.8 Hz, Ar-H) 7.39 (d, 1H, J = 2.0 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.69 (CH3), 64.33 (O–CH2), 64.56 (O–CH2), 102.21 (S-CH
N), 4.25 (s, 4H, –OCH2CH2O–), 6.13 (s, 1H, CH–S), 6.82 (d, 1H, J = 8.4 Hz, Ar-H), 6.96–7.02 (m, 4H, Ar-H), 7.15–7.19 (m, 4H, Ar-H), 7.34 (dd, 1H, J = 2.4 Hz, 8.8 Hz, Ar-H) 7.39 (d, 1H, J = 2.0 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.69 (CH3), 64.33 (O–CH2), 64.56 (O–CH2), 102.21 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 115.34 (Ar-C), 115.62 (Ar-C), 115.84 (Ar-C), 116.88 (Ar-C), 119.87 (Ar-C), 128.67 (Ar-C), 129.35 (Ar-C), 129.65 (Ar-C), 129.94 (Ar-C), 130.03 (Ar-C), 132.63 (Ar-C), 143.15 (Ar-C), 144.54 (Ar-C), 156.92 (Ar-C), 160.08 (N–C
), 115.34 (Ar-C), 115.62 (Ar-C), 115.84 (Ar-C), 116.88 (Ar-C), 119.87 (Ar-C), 128.67 (Ar-C), 129.35 (Ar-C), 129.65 (Ar-C), 129.94 (Ar-C), 130.03 (Ar-C), 132.63 (Ar-C), 143.15 (Ar-C), 144.54 (Ar-C), 156.92 (Ar-C), 160.08 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 162.54 (C
), 162.54 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 168.33 (N–C
N–N), 168.33 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C25H19ClFN3O2S (479.95): C, 62.56; H, 3.99; N, 8.76; found: C, 62.63; H, 3.92; N, 8.84.
N); anal. calcd for C25H19ClFN3O2S (479.95): C, 62.56; H, 3.99; N, 8.76; found: C, 62.63; H, 3.92; N, 8.84.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1578, 1507 (Ar-C
N), 1578, 1507 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.27 (s, 3H, CH3–C
C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.27 (s, 3H, CH3–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 4.25 (s, 4H, –OCH2CH2O–), 5.00 (s, 2H, –CH2–), 5.92 (s, 1H, CH–S), 6.82 (d, 1H, J = 8.4 Hz, Ar-H), 7.05–7.07 (m, 2H, Ar-H), 7.10–7.12 (m, 2H, Ar-H),7.19–7.23 (m, 3H, Ar-H), 7.26–7.28 (m, 2H, Ar-H) 7.37 (dd, 1H, J = 2.0 Hz, 8.4 Hz, Ar-H) 7.40 (d, 1H, J = 2.4 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.46 (CH3), 49.28 (CH2), 64.34 (O–CH2), 64.56 (O–CH2), 100.74 (S-CH
N), 4.25 (s, 4H, –OCH2CH2O–), 5.00 (s, 2H, –CH2–), 5.92 (s, 1H, CH–S), 6.82 (d, 1H, J = 8.4 Hz, Ar-H), 7.05–7.07 (m, 2H, Ar-H), 7.10–7.12 (m, 2H, Ar-H),7.19–7.23 (m, 3H, Ar-H), 7.26–7.28 (m, 2H, Ar-H) 7.37 (dd, 1H, J = 2.0 Hz, 8.4 Hz, Ar-H) 7.40 (d, 1H, J = 2.4 Hz, Ar-H); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 14.46 (CH3), 49.28 (CH2), 64.34 (O–CH2), 64.56 (O–CH2), 100.74 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 115.23 (Ar-C), 116.87 (Ar-C), 119.76 (Ar-C), 127.16 (Ar-C), 127.26 (Ar-C), 128.43 (Ar-C), 128.81 (Ar-C), 130.33 (Ar-C), 132.92 (Ar-C), 137.18 (Ar-C), 139.45 (Ar-C), 143.16 (Ar-C), 144.33 (N–C
), 115.23 (Ar-C), 116.87 (Ar-C), 119.76 (Ar-C), 127.16 (Ar-C), 127.26 (Ar-C), 128.43 (Ar-C), 128.81 (Ar-C), 130.33 (Ar-C), 132.92 (Ar-C), 137.18 (Ar-C), 139.45 (Ar-C), 143.16 (Ar-C), 144.33 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 155.67 (C
), 155.67 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 168.58 (N–C
N–N), 168.58 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C26H22ClN3O2S (475.99): C, 65.61; H, 4.66; N, 8.83; found; C, 65.69; H, 4.61; N, 8.87.
N); anal. calcd for C26H22ClN3O2S (475.99): C, 65.61; H, 4.66; N, 8.83; found; C, 65.69; H, 4.61; N, 8.87.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1572, 1497 (Ar-C
N), 1572, 1497 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 6.66 (s, 1H, CH–S), 7.12–7.21 (m, 4H, Ar-H), 7.29–7.33 (m, 3H, Ar-H), 7.38–7.46 (m, 5H, Ar-H), 7.68 (s, 1H, CH
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 6.66 (s, 1H, CH–S), 7.12–7.21 (m, 4H, Ar-H), 7.29–7.33 (m, 3H, Ar-H), 7.38–7.46 (m, 5H, Ar-H), 7.68 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.27–8.29 (m, 1H, Ar-H), 8.36 (s, 1H, indole CH), 11.51 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 102.50 (S-CH
N), 8.27–8.29 (m, 1H, Ar-H), 8.36 (s, 1H, indole CH), 11.51 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 102.50 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 112.30 (Ar-C), 113.01 (Ar-C), 120.91 (Ar-C), 122.05 (Ar-C), 122.52 (Ar-C), 122.95 (Ar-C), 124.96 (Ar-C), 128.18 (Ar-C), 129.15 (Ar-C), 129.40 (Ar-C), 130.52 (Ar-C), 128.75 (Ar-C), 130.72 (Ar-C), 131.69 (Ar-C), 137.57 (Ar-C), 138.24 (Ar-C), 138.79 (N–C
), 112.30 (Ar-C), 113.01 (Ar-C), 120.91 (Ar-C), 122.05 (Ar-C), 122.52 (Ar-C), 122.95 (Ar-C), 124.96 (Ar-C), 128.18 (Ar-C), 129.15 (Ar-C), 129.40 (Ar-C), 130.52 (Ar-C), 128.75 (Ar-C), 130.72 (Ar-C), 131.69 (Ar-C), 137.57 (Ar-C), 138.24 (Ar-C), 138.79 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 149.27 (C
), 149.27 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 167.09 (N–C
N–N), 167.09 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C24H17BrN4S (473.39): C, 60.89; H, 3.62; N, 11.84; found: C, 60.82; H, 3.68; N, 11.89.
N); anal. calcd for C24H17BrN4S (473.39): C, 60.89; H, 3.62; N, 11.84; found: C, 60.82; H, 3.68; N, 11.89.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1533, 1489 (Ar-C
N), 1533, 1489 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.03–1.56 (m, 3H, cyclohexyl), 1.60–1.74 (m, 5H, cyclohexyl), 2.58–2.67 (m, 2H, cyclohexyl), 3.60–3.66 (m, 1H, cyclohexyl), 5.79 (s, 1H, CH–S), 7.14–7.22 (m, 4H, Ar-H), 7.30–7.36 (m, 2H, Ar-H), 7.48–7.51 (m, 2H, Ar-H), 8.25 (s, 1H, CH
C); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.03–1.56 (m, 3H, cyclohexyl), 1.60–1.74 (m, 5H, cyclohexyl), 2.58–2.67 (m, 2H, cyclohexyl), 3.60–3.66 (m, 1H, cyclohexyl), 5.79 (s, 1H, CH–S), 7.14–7.22 (m, 4H, Ar-H), 7.30–7.36 (m, 2H, Ar-H), 7.48–7.51 (m, 2H, Ar-H), 8.25 (s, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 8.41–8.43 (m, 1H, Ar-H), 8.54 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 25.09 (cyclohexyl-C), 26.15 (cyclohexyl-C), 29.06 (cyclohexyl-C), 59.31 (cyclohexyl-C), 100.37 (S-CH
N), 8.41–8.43 (m, 1H, Ar-H), 8.54 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 25.09 (cyclohexyl-C), 26.15 (cyclohexyl-C), 29.06 (cyclohexyl-C), 59.31 (cyclohexyl-C), 100.37 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 110.96 (Ar-C), 114.96 (Ar-C), 121.29 (Ar-C), 123.07 (Ar-C), 123.24 (Ar-C), 125.08 (Ar-C), 127.15 (Ar-C), 130.46 (Ar-C), 130.53 (Ar-C), 131.86 (Ar-C), 131.96 (Ar-C), 136.88 (Ar-C), 140.21 (N–C
), 110.96 (Ar-C), 114.96 (Ar-C), 121.29 (Ar-C), 123.07 (Ar-C), 123.24 (Ar-C), 125.08 (Ar-C), 127.15 (Ar-C), 130.46 (Ar-C), 130.53 (Ar-C), 131.86 (Ar-C), 131.96 (Ar-C), 136.88 (Ar-C), 140.21 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 146.94 (C
), 146.94 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 167.77 (N–C
N–N), 167.77 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C24H23BrN4S (479.44): C, 60.12; H, 4.84; N, 11.69; found: C, 60.17; H, 4.80; N, 11.62.
N); anal. calcd for C24H23BrN4S (479.44): C, 60.12; H, 4.84; N, 11.69; found: C, 60.17; H, 4.80; N, 11.62.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1542, 1502 (Ar-C
N), 1542, 1502 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 6.93 (s, 1H, CH–S), 7.18–7.28 (m, 4H, Ar-H), 7.39–7.48 (m, 5H, CH
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 6.93 (s, 1H, CH–S), 7.18–7.28 (m, 4H, Ar-H), 7.39–7.48 (m, 5H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, Ar-H), 7.72 (d, 1H, J = 2.4 Hz, Ar-H), 8.13 (dd, 2H, J = 2.0 Hz, 6.8 Hz, Ar-H), 8.26–8.28 (m, 1H, Ar-H), 8.39 (s, 1H, indole CH), 11.55 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 105.80 (S-CH
N, Ar-H), 7.72 (d, 1H, J = 2.4 Hz, Ar-H), 8.13 (dd, 2H, J = 2.0 Hz, 6.8 Hz, Ar-H), 8.26–8.28 (m, 1H, Ar-H), 8.39 (s, 1H, indole CH), 11.55 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 105.80 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 112.86 (Ar-C), 116.55 (Ar-C), 120.85 (Ar-C), 120.94 (Ar-C), 122.49 (Ar-C), 123.07 (Ar-C), 124.01 (Ar-C), 124.87 (Ar-C), 129.49 (Ar-C), 131.04 (Ar-C), 131.22 (Ar-C), 134.49 (Ar-C), 137.25 (Ar-C), 137.64 (Ar-C), 138.00 (Ar-C), 147.25 (Ar-C), 149.89 (N–C
), 112.86 (Ar-C), 116.55 (Ar-C), 120.85 (Ar-C), 120.94 (Ar-C), 122.49 (Ar-C), 123.07 (Ar-C), 124.01 (Ar-C), 124.87 (Ar-C), 129.49 (Ar-C), 131.04 (Ar-C), 131.22 (Ar-C), 134.49 (Ar-C), 137.25 (Ar-C), 137.64 (Ar-C), 138.00 (Ar-C), 147.25 (Ar-C), 149.89 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 160.20 (C
), 160.20 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 166.88 (N–C
N–N), 166.88 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C24H16FN5 O2S (457.48): C, 63.01; H, 3.53; N, 15.31; found: C, 63.07; H, 3.48; N, 15.35.
N); anal. calcd for C24H16FN5 O2S (457.48): C, 63.01; H, 3.53; N, 15.31; found: C, 63.07; H, 3.48; N, 15.35.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1553, 1499 (Ar-C
N), 1553, 1499 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (Acetone + DMSO-d6, 400 MHz) δ ppm: 6.93 (s, 1H, CH–S), 7.18–7.22 (m, 2H, Ar-H), 7.33–7.35 (m, 3H, Ar-H), 7.39–7.46 (m, 5H, Ar-H, CH
C); 1H-NMR (Acetone + DMSO-d6, 400 MHz) δ ppm: 6.93 (s, 1H, CH–S), 7.18–7.22 (m, 2H, Ar-H), 7.33–7.35 (m, 3H, Ar-H), 7.39–7.46 (m, 5H, Ar-H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 7.71 (d, 1H, J = 2.8 Hz, Ar-H), 8.10 (dd, 2H, J = 2.0 Hz, 7.2 Hz, Ar-H), 8.26–8.29 (m, 1H, Ar-H), 8.39 (s, 1H, indole CH), 11.54 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 105.86 (S-CH
N), 7.71 (d, 1H, J = 2.8 Hz, Ar-H), 8.10 (dd, 2H, J = 2.0 Hz, 7.2 Hz, Ar-H), 8.26–8.29 (m, 1H, Ar-H), 8.39 (s, 1H, indole CH), 11.54 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 105.86 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 112.34 (Ar-C), 112.90 (Ar-C), 120.98 (Ar-C), 122.49 (Ar-C), 123.00 (Ar-C), 123.92 (Ar-C), 124.92 (Ar-C), 128.32 (Ar-C), 128.96 (Ar-C), 129.34 (Ar-C), 129.54 (Ar-C), 130.97 (Ar-C), 137.58 (Ar-C), 137.66 (Ar-C), 138.05 (Ar-C), 138.14 (Ar-C), 147.10 (N–C
), 112.34 (Ar-C), 112.90 (Ar-C), 120.98 (Ar-C), 122.49 (Ar-C), 123.00 (Ar-C), 123.92 (Ar-C), 124.92 (Ar-C), 128.32 (Ar-C), 128.96 (Ar-C), 129.34 (Ar-C), 129.54 (Ar-C), 130.97 (Ar-C), 137.58 (Ar-C), 137.66 (Ar-C), 138.05 (Ar-C), 138.14 (Ar-C), 147.10 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 149.73 (C
), 149.73 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 166.81 (N–C
N–N), 166.81 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C24H17N5O2S (439.49): C, 65.59; H, 3.90; N, 15.94; found: C, 65.55; H, 3.95; N, 15.90.
N); anal. calcd for C24H17N5O2S (439.49): C, 65.59; H, 3.90; N, 15.94; found: C, 65.55; H, 3.95; N, 15.90.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1536, 1491 (Ar-C
N), 1536, 1491 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (Acetone + DMSO-d6, 400 MHz) δ ppm: 5.12 (s, 2H, –CH2–), 6.65 (s, 1H, CH–S), 7.02 (d, 2H, J = 6.8 Hz, Ar-H), 7.19–7.26 (m, 5H, Ar-H, CH
C); 1H-NMR (Acetone + DMSO-d6, 400 MHz) δ ppm: 5.12 (s, 2H, –CH2–), 6.65 (s, 1H, CH–S), 7.02 (d, 2H, J = 6.8 Hz, Ar-H), 7.19–7.26 (m, 5H, Ar-H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 7.44–7.46 (m, 1H, Ar-H), 7.66 (dd, 2H, J = 2.0 Hz, 6.8 Hz, Ar-H), 7.74 (d, 1H, J = 2.4 Hz, Ar-H), 8.23 (dd, 2H, J = 2.0 Hz, 7.2 Hz, Ar-H), 8.27–8.30 (m, 1H, Ar-H), 8.47 (s, 1H, indole CH), 11.53 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 48.82 (CH2), 103.76 (S-CH
N), 7.44–7.46 (m, 1H, Ar-H), 7.66 (dd, 2H, J = 2.0 Hz, 6.8 Hz, Ar-H), 7.74 (d, 1H, J = 2.4 Hz, Ar-H), 8.23 (dd, 2H, J = 2.0 Hz, 7.2 Hz, Ar-H), 8.27–8.30 (m, 1H, Ar-H), 8.47 (s, 1H, indole CH), 11.53 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 48.82 (CH2), 103.76 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 112.32 (Ar-C), 113.01 (Ar-C), 120.90 (Ar-C), 122.52 (Ar-C), 122.97 (Ar-C), 124.28 (Ar-C), 124.95 (Ar-C), 126.90 (Ar-C), 127.68 (Ar-C), 129.01 (Ar-C), 129.99 (Ar-C), 130.66 (Ar-C), 137.37 (Ar-C), 137.57 (Ar-C), 137.58 (Ar-C), 138.75 (Ar-C), 147.83 (N–C
), 112.32 (Ar-C), 113.01 (Ar-C), 120.90 (Ar-C), 122.52 (Ar-C), 122.97 (Ar-C), 124.28 (Ar-C), 124.95 (Ar-C), 126.90 (Ar-C), 127.68 (Ar-C), 129.01 (Ar-C), 129.99 (Ar-C), 130.66 (Ar-C), 137.37 (Ar-C), 137.57 (Ar-C), 137.58 (Ar-C), 138.75 (Ar-C), 147.83 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 148.94 (C
), 148.94 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 166.94 (N–C
N–N), 166.94 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C25H19N5O2S (453.52): C, 66.21; H, 4.22; N, 15.44; found: C, 66.15; H, 4.19; N, 15.48.
N); anal. calcd for C25H19N5O2S (453.52): C, 66.21; H, 4.22; N, 15.44; found: C, 66.15; H, 4.19; N, 15.48.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1545, 1496 (Ar-C
N), 1545, 1496 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (Acetone + DMSO-d6, 400 MHz) δ ppm: 3.71 (s, 3H, OCH3), 6.82–6.84 (m, 1H, Ar-H), 6.89–6.92 (m, 2H, Ar-H, CH–S), 7.00 (t, 1H, J = 2.0 Hz, Ar-H), 7.18–7.20 (m, 2H, Ar-H), 7.26 (t, 1H, J = 8.4 Hz, Ar-H), 7.44–7.50 (m, 3H, Ar-H, CH
C); 1H-NMR (Acetone + DMSO-d6, 400 MHz) δ ppm: 3.71 (s, 3H, OCH3), 6.82–6.84 (m, 1H, Ar-H), 6.89–6.92 (m, 2H, Ar-H, CH–S), 7.00 (t, 1H, J = 2.0 Hz, Ar-H), 7.18–7.20 (m, 2H, Ar-H), 7.26 (t, 1H, J = 8.4 Hz, Ar-H), 7.44–7.50 (m, 3H, Ar-H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 7.72 (d, 1H, J = 2.8 Hz, Ar-H), 8.12 (d, 2H, J = 8.8 Hz, Ar-H), 8.26–8.28 (m, 1H, Ar-H), 8.41 (s, 1H, indole CH), 11.55 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 55.80 (OCH3), 105.82 (S-CH
N), 7.72 (d, 1H, J = 2.8 Hz, Ar-H), 8.12 (d, 2H, J = 8.8 Hz, Ar-H), 8.26–8.28 (m, 1H, Ar-H), 8.41 (s, 1H, indole CH), 11.55 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 55.80 (OCH3), 105.82 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 112.35 (Ar-C), 112.90 (Ar-C), 113.88 (Ar-C), 115.09 (Ar-C), 120.98 (Ar-C), 122.49 (Ar-C), 123.00 (Ar-C), 123.94 (Ar-C), 124.92 (Ar-C), 129.24 (Ar-C), 130.19 (Ar-C), 130.98 (Ar-C), 137.58 (Ar-C), 137.77 (Ar-C), 138.05 (Ar-C), 139.12 (Ar-C), 147.12 (Ar-C), 149.80 (N–C
), 112.35 (Ar-C), 112.90 (Ar-C), 113.88 (Ar-C), 115.09 (Ar-C), 120.98 (Ar-C), 122.49 (Ar-C), 123.00 (Ar-C), 123.94 (Ar-C), 124.92 (Ar-C), 129.24 (Ar-C), 130.19 (Ar-C), 130.98 (Ar-C), 137.58 (Ar-C), 137.77 (Ar-C), 138.05 (Ar-C), 139.12 (Ar-C), 147.12 (Ar-C), 149.80 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 159.95 (C
), 159.95 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 166.73 (N–C
N–N), 166.73 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C25H19N5O3S (469.52): C, 63.95; H, 4.08; N, 14.92; found: C, 63.90; H, 4.12; N, 14.87.
N); anal. calcd for C25H19N5O3S (469.52): C, 63.95; H, 4.08; N, 14.92; found: C, 63.90; H, 4.12; N, 14.87.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1577, 1506 (Ar-C
N), 1577, 1506 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 6.65 (s, 1H, CH–S), 7.18–7.21 (m, 4H, Ar-H), 7.28–7.32 (m, 5H, Ar-H), 7.37–7.46 (m, 3H, CH
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 6.65 (s, 1H, CH–S), 7.18–7.21 (m, 4H, Ar-H), 7.28–7.32 (m, 5H, Ar-H), 7.37–7.46 (m, 3H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, Ar-H), 7.68 (d, 1H, J = 2.8 Hz, Ar-H), 8.27–8.30 (m, 1H, Ar-H), 8.36 (s, 1H, indole CH), 11.52 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 102.47 (S-CH
N, Ar-H), 7.68 (d, 1H, J = 2.8 Hz, Ar-H), 8.27–8.30 (m, 1H, Ar-H), 8.36 (s, 1H, indole CH), 11.52 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 102.47 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 112.31 (Ar-C), 113.00 (Ar-C), 120.93 (Ar-C), 122.53 (Ar-C), 122.96 (Ar-C), 124.95 (Ar-C), 128.18 (Ar-C), 128.78 (Ar-C), 129.15 (Ar-C), 129.40 (Ar-C), 130.28 (Ar-C), 130.34 (Ar-C), 130.72 (Ar-C), 133.39 (Ar-C), 137.57 (Ar-C), 138.22 (Ar-C), 138.73 (N–C
), 112.31 (Ar-C), 113.00 (Ar-C), 120.93 (Ar-C), 122.53 (Ar-C), 122.96 (Ar-C), 124.95 (Ar-C), 128.18 (Ar-C), 128.78 (Ar-C), 129.15 (Ar-C), 129.40 (Ar-C), 130.28 (Ar-C), 130.34 (Ar-C), 130.72 (Ar-C), 133.39 (Ar-C), 137.57 (Ar-C), 138.22 (Ar-C), 138.73 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 149.25 (C
), 149.25 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 167.12 (N–C
N–N), 167.12 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C24H17ClN4S (428.94): C, 67.20; H, 3.99; N, 13.06; found: C, 67.27; H, 3.92; N, 13.14.
N); anal. calcd for C24H17ClN4S (428.94): C, 67.20; H, 3.99; N, 13.06; found: C, 67.27; H, 3.92; N, 13.14.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1564, 1520 (Ar-C
N), 1564, 1520 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 5.03 (s, 2H, –CH2–), 6.41 (s, 1H, CH–S), 7.01 (d, 2H, J = 7.2 Hz, Ar-H), 7.15–7.27 (m, 5H, Ar-H), 7.35–7.46 (m, 5H, CH
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 5.03 (s, 2H, –CH2–), 6.41 (s, 1H, CH–S), 7.01 (d, 2H, J = 7.2 Hz, Ar-H), 7.15–7.27 (m, 5H, Ar-H), 7.35–7.46 (m, 5H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, Ar-H), 7.70 (d, 1H, J = 2.8 Hz, Ar-H), 8.28 (d, 1H, J = 6.8 Hz, Ar-H), 8.43 (s, 1H, indole CH), 11.50 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 48.54 (CH2), 101.10 (S-CH
N, Ar-H), 7.70 (d, 1H, J = 2.8 Hz, Ar-H), 8.28 (d, 1H, J = 6.8 Hz, Ar-H), 8.43 (s, 1H, indole CH), 11.50 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 48.54 (CH2), 101.10 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 112.29 (Ar-C), 113.10 (Ar-C), 120.86 (Ar-C), 122.55 (Ar-C), 122.93 (Ar-C), 124.97 (Ar-C), 126.56 (Ar-C), 126.81 (Ar-C), 127.57 (Ar-C), 128.95 (Ar-C), 129.19 (Ar-C), 129.29 (Ar-C), 130.11 (Ar-C), 130.87 (Ar-C), 134.31 (Ar-C), 137.60 (Ar-C), 139.39 (N–C
), 112.29 (Ar-C), 113.10 (Ar-C), 120.86 (Ar-C), 122.55 (Ar-C), 122.93 (Ar-C), 124.97 (Ar-C), 126.56 (Ar-C), 126.81 (Ar-C), 127.57 (Ar-C), 128.95 (Ar-C), 129.19 (Ar-C), 129.29 (Ar-C), 130.11 (Ar-C), 130.87 (Ar-C), 134.31 (Ar-C), 137.60 (Ar-C), 139.39 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 148.52 (C
), 148.52 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 166.94 (N–C
N–N), 166.94 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C25H19 ClN4S (442.96): C, 67.79; H, 4.32; N, 12.65; found; C, 67.86; H, 4.36; N, 12.72.
N); anal. calcd for C25H19 ClN4S (442.96): C, 67.79; H, 4.32; N, 12.65; found; C, 67.86; H, 4.36; N, 12.72.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1561, 1498 (Ar-C
N), 1561, 1498 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (Acetone + DMSO-d6, 400 MHz) δ ppm: 6.65 (s, 1H, CH–S), 7.17–7.25 (m, 6H, Ar-H), 7.33–7.37 (m, 4H, Ar-H, CH
C); 1H-NMR (Acetone + DMSO-d6, 400 MHz) δ ppm: 6.65 (s, 1H, CH–S), 7.17–7.25 (m, 6H, Ar-H), 7.33–7.37 (m, 4H, Ar-H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 7.45 (dd, 1H, J = 1.6 Hz, 6.8 Hz, Ar-H), 7.69 (d, 1H, J = 2.8 Hz, Ar-H), 8.26–8.28 (m, 1H, Ar-H), 8.36 (s, 1H, indole CH), 11.53 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 102.36 (S-CH
N), 7.45 (dd, 1H, J = 1.6 Hz, 6.8 Hz, Ar-H), 7.69 (d, 1H, J = 2.8 Hz, Ar-H), 8.26–8.28 (m, 1H, Ar-H), 8.36 (s, 1H, indole CH), 11.53 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 102.36 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 112.32 (Ar-C), 112.96 (Ar-C), 116.18 (Ar-C), 116.40 (Ar-C), 120.94 (Ar-C), 122.51 (Ar-C), 122.97 (Ar-C), 124.93 (Ar-C), 128.86 (Ar-C), 130.15 (Ar-C), 130.42 (Ar-C), 130.80 (Ar-C), 131.28 (Ar-C), 131.37 (Ar-C), 133.52 (Ar-C), 137.56 (Ar-C), 138.62 (N–C
), 112.32 (Ar-C), 112.96 (Ar-C), 116.18 (Ar-C), 116.40 (Ar-C), 120.94 (Ar-C), 122.51 (Ar-C), 122.97 (Ar-C), 124.93 (Ar-C), 128.86 (Ar-C), 130.15 (Ar-C), 130.42 (Ar-C), 130.80 (Ar-C), 131.28 (Ar-C), 131.37 (Ar-C), 133.52 (Ar-C), 137.56 (Ar-C), 138.62 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 149.37 (C
), 149.37 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 167.11 (N–C
N–N), 167.11 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C24H16 ClFN4S (446.93): C, 64.50; H, 3.61; N, 12.54; found: C, 64.58; H, 3.58; N, 12.57.
N); anal. calcd for C24H16 ClFN4S (446.93): C, 64.50; H, 3.61; N, 12.54; found: C, 64.58; H, 3.58; N, 12.57.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1565, 1503 (Ar-C
N), 1565, 1503 (Ar-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.11 (s, 6H, 2×CH3-Ar), 6.72 (s, 1H, CH–S), 7.11–7.13 (m, 4H, Ar-H), 7.16–7.20 (m, 3H, Ar-H), 7.29–7.31 (m, 2H, Ar-H, CH
C); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.11 (s, 6H, 2×CH3-Ar), 6.72 (s, 1H, CH–S), 7.11–7.13 (m, 4H, Ar-H), 7.16–7.20 (m, 3H, Ar-H), 7.29–7.31 (m, 2H, Ar-H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 7.44 (d, 1H, J = 6.8 Hz, Ar-H), 7.65 (d, 1H, J = 2.8 Hz, Ar-H), 8.25–8.28 (m, 1H, Ar-H), 8.30 (s, 1H, indole CH), 11.50 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 18.25 (2×CH3-Ar), 101.92 (S-CH
N), 7.44 (d, 1H, J = 6.8 Hz, Ar-H), 7.65 (d, 1H, J = 2.8 Hz, Ar-H), 8.25–8.28 (m, 1H, Ar-H), 8.30 (s, 1H, indole CH), 11.50 (s, 1H, NH); 13C NMR (DMSO-d6, 100 MHz) δ ppm: 18.25 (2×CH3-Ar), 101.92 (S-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 112.24 (Ar-C), 112.95 (Ar-C), 120.87 (Ar-C), 122.47 (Ar-C), 123.04 (Ar-C), 124.89 (Ar-C), 128.87 (Ar-C), 129.24 (Ar-C), 129.46 (Ar-C), 129.88 (Ar-C), 130.59 (Ar-C), 130.64 (Ar-C), 133.72 (Ar-C), 136.69 (Ar-C), 137.55 (Ar-C), 137.63 (Ar-C), 138.65 (N–C
), 112.24 (Ar-C), 112.95 (Ar-C), 120.87 (Ar-C), 122.47 (Ar-C), 123.04 (Ar-C), 124.89 (Ar-C), 128.87 (Ar-C), 129.24 (Ar-C), 129.46 (Ar-C), 129.88 (Ar-C), 130.59 (Ar-C), 130.64 (Ar-C), 133.72 (Ar-C), 136.69 (Ar-C), 137.55 (Ar-C), 137.63 (Ar-C), 138.65 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 149.01 (C
), 149.01 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 165.34 (N–C
N–N), 165.34 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N); anal. calcd for C26H21ClN4S (456.99): C, 68.33; H, 4.63; N, 12.26; found: C, 68.41; H, 4.66; N, 12.31.
N); anal. calcd for C26H21ClN4S (456.99): C, 68.33; H, 4.63; N, 12.26; found: C, 68.41; H, 4.66; N, 12.31.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Z. S. is thankful to Higher Education Commission, Islamabad, Pakistan through Project No. NRPU/6975 for financial support.References
- R. Khursheed, S. K. Singh, S. Wadhwa, B. Kapoor, M. Gulati, R. Kumar, A. K. Ramanunny, A. Awasthi and K. Dua, Treatment strategies against diabetes: Success so far and challenges ahead, Eur. J. Pharmacol., 2019, 862, 172625 CrossRef CAS PubMed.
- A. D. Association, 12. Older adults: standards of medical care in diabetes—2019, Diabetes Care, 2019, 42, S139–S147 CrossRef PubMed.
- M. Blair, Diabetes Mellitus Review, Urol. Nurs., 2016, 36, 27–36 CrossRef PubMed.
- Y. Shi and F. B. Hu, The global implications of diabetes and cancer, Lancet, 2014, 383, 1947 CrossRef.
- D. R. Whiting, L. Guariguata, C. Weil and J. Shaw, IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030, Diabetes Res. Clin. Pract., 2011, 94, 311–321 CrossRef PubMed.
- H. A. Bhatti, Y. Tehseen, K. Maryam, M. Uroos, B. S. Siddiqui, A. Hameed and J. Iqbal, Identification of new potent inhibitor of aldose reductase from Ocimum basilicum, Bioorg. Chem., 2017, 75, 62–70 CrossRef CAS PubMed.
- M. Majekova, J. Ballekova, M. Prnova and M. Stefek, Structure optimization of tetrahydropyridoindole-based aldose reductase inhibitors improved their efficacy and selectivity, Bioorg. Med. Chem., 2017, 25, 6353–6360 CrossRef CAS PubMed.
- H. Andleeb, Y. Tehseen, F. Jabeen, I. Khan, J. Iqbal and S. Hameed, Exploration of thioxothiazolidinone–sulfonate conjugates as a new class of aldehyde/aldose reductase inhibitors: A synthetic and computational investigation, Bioorg. Chem., 2017, 75, 1–15 CrossRef CAS PubMed.
- X. Hao, Z. Han, Y. Li, C. Li, X. Wang, X. Zhang, Q. Yang, B. Ma and C. Zhu, Synthesis and structure–activity relationship studies of phenolic hydroxyl derivatives based on quinoxalinone as aldose reductase inhibitors with antioxidant activity, Bioorg. Med. Chem. Lett., 2017, 27, 887–892 CrossRef CAS PubMed.
- O. El-Kabbani and A. Podjarny, Selectivity determinants of the aldose and aldehyde reductase inhibitor-binding sites, Cell. Mol. Life Sci., 2007, 64, 1970–1978 CrossRef CAS PubMed.
- A. Saeed, Y. Tehseen, H. Rafique, N. Furtmann, J. Bajorath, U. Flörke and J. Iqbal, Benzothiazolyl substituted iminothiazolidinones and benzamido-oxothiazolidines as potent and partly selective aldose reductase inhibitors, MedChemComm, 2014, 5, 1371–1380 RSC.
- O. Bozdağ-Dündar, N. D. Evcimen, M. Ceylan-Ünlüsoy, R. Ertan and M. Sarıkaya, Some new thiazolyl thiazolidinedione derivatives as aldose reductase inhibitors, Med. Chem. Res., 2008, 16, 39–47 CrossRef.
- N. Zaher, I. Nicolaou and V. J. Demopoulos, Pyrrolylbenzothiazole derivatives as aldose reductase inhibitors, J. Enzyme Inhib. Med. Chem., 2002, 17, 131–135 CrossRef CAS PubMed.
- Y. Demir, P. Taslimi, Ü. M. Koçyiğit, M. Akkuş, M. S. Özaslan, H. E. Duran, Y. Budak, B. Tüzün, M. B. Gürdere and M. Ceylan, Determination of the inhibition profiles of pyrazolyl–thiazole derivatives against aldose reductase and α-glycosidase and molecular docking studies, Arch. Pharm., 2020, 353, 2000118 CrossRef CAS PubMed.
- B. Sever, M. D. Altıntop, Y. Demir, G. A. Çiftçi, Ş. Beydemir and A. Özdemir, Design, synthesis, in vitro and in silico investigation of aldose reductase inhibitory effects of new thiazole-based compounds, Bioorg. Chem., 2020, 102, 104110 CrossRef CAS PubMed.
- N. U. Güzeldemirci, S. Cimok, D.-E. Net and M. Sarikaya, Synthesis and aldose reductase inhibitory effect of some new hydrazinecarbothioamides and 4-thiazolidinones bearing an imidazo [2, 1-b] thiazole moiety, Turk. J. Pharm. Sci., 2019, 16, 1 CrossRef PubMed.
- B. Sever, M. D. Altıntop, Y. Demir, M. Pekdoğan, G. A. Çiftçi, Ş. Beydemir and A. Özdemir, An extensive research on aldose reductase inhibitory effects of new 4H-1, 2, 4-triazole derivatives, J. Mol. Struct., 2021, 1224, 129446 CrossRef CAS.
- S. Miyamoto, Recent advances in aldose reductase inhibitors: potential agents for the treatment of diabetic complications, Expert Opin. Ther. Pat., 2002, 12, 621–631 CrossRef CAS.
- K. Šturm, L. Levstik, V. J. Demopoulos and A. Kristl, Permeability characteristics of novel aldose reductase inhibitors using rat jejunum in vitro, Eur. J. Pharm. Sci., 2006, 28, 128–133 CrossRef PubMed.
- M. T. Shehzad, A. Imran, G. S. S. Njateng, A. Hameed, M. Islam, M. al-Rashida, M. Uroos, A. Asari, Z. Shafiq and J. Iqbal, Benzoxazinone-thiosemicarbazones as antidiabetic leads via aldose reductase inhibition: Synthesis, biological screening and molecular docking study, Bioorg. Chem., 2019, 87, 857–866 CrossRef CAS PubMed.
- M. Kumar, S. Choudhary, P. K. Singh and O. Silakari, Addressing selectivity issues of aldose reductase 2 inhibitors for the management of diabetic complications, Future Med. Chem., 2020, 12, 1327–1358 CrossRef CAS PubMed.
- F. C. Wouters, J. Gershenzon and D. G. Vassão, Benzoxazinoids: reactivity and modes of action of a versatile class of plant chemical defenses, J. Braz. Chem. Soc., 2016, 27, 1379–1397 CAS.
- W. G. Lima, F. J. dos Santos, A. Cristina Soares, F. A. Macías, J. M. Molinillo, J. Maria Siqueira Ferreira and J. Máximo de Siqueira, Synthesis and antimicrobial activity of some benzoxazinoids derivatives of 2-nitrophenol and 3-hydroxy-2-nitropyridine, Synth. Commun., 2019, 49, 286–296 CrossRef CAS.
- B. P. Marasini, F. Rahim, S. Perveen, A. Karim, K. M. Khan and M. I. Choudhary, Synthesis, structure-activity relationships studies of benzoxazinone derivatives as α-chymotrypsin inhibitors, Bioorg. Chem., 2017, 70, 210–221 CrossRef CAS PubMed.
- N. Kolocouris, G. Zoidis, G. B. Foscolos, G. Fytas, S. R. Prathalingham, J. M. Kelly, L. Naesens and E. De Clercq, Design and synthesis of bioactive adamantane spiro heterocycles, Bioorg. Med. Chem. Lett., 2007, 17, 4358–4362 CrossRef CAS PubMed.
- G. Zoidis, N. Kolocouris, J. M. Kelly, S. R. Prathalingam, L. Naesens and E. De Clercq, Design and synthesis of bioactive adamantanaminoalcohols and adamantanamines, Eur. J. Med. Chem., 2010, 45, 5022–5030 CrossRef CAS PubMed.
- L. I. Pilkington and D. Barker, Synthesis and biology of 1, 4-benzodioxane lignan natural products, Nat. Prod. Rep., 2015, 32, 1369–1388 RSC.
- L. I. Pilkington, J. Wagoner, S. J. Polyak and D. Barker, Enantioselective synthesis, stereochemical correction, and biological investigation of the rodgersinine family of 1, 4-benzodioxane neolignans, Org. Lett., 2015, 17, 1046–1049 CrossRef CAS PubMed.
- A. Özdemir, M. D. Altıntop, G. Turan-Zitouni, G. A. Çiftçi, İ. Ertorun, Ö. Alataş and Z. A. Kaplancıklı, Synthesis and evaluation of new indole-based chalcones as potential antiinflammatory agents, Eur. J. Med. Chem., 2015, 89, 304–309 CrossRef PubMed.
- S. D. Shamon and M. I. Perez, Blood pressure-lowering efficacy of reserpine for primary hypertension, Cochrane Database Syst. Rev., 2016, 12, CD007655 Search PubMed.
- E. Westermann, ANN, Cumulative effects of reserpine on the pituitary-adrenocortical and sympathetic nervous system, Drugs and Enzymes, 2013, 3, 381–392 Search PubMed.
- S. Bindu, K. Rameshkumar, B. Kumar, A. Singh and C. Anilkumar, Distribution of reserpine in Rauvolfia species from India–HPTLC and LC–MS studies, Ind. Crops Prod., 2014, 62, 430–436 CrossRef CAS.
- M. T. Shehzad, A. Khan, M. Islam, S. A. Halim, M. Khiat, M. U. Anwar, J. Hussain, A. Hameed, A. R. Pasha, F. A. Khan, A. Al-Harrasi and Z. Shafiq, Synthesis, characterization and molecular docking of some novel hydrazonothiazolines as urease inhibitors, Bioorg. Chem., 2020, 94, 103404 CrossRef CAS PubMed.
- M. T. Shehzad, A. Hameed, M. Al-Rashida, A. Imran, M. Uroos, A. Asari, H. Mohamad, M. Islam, S. Iftikhar, Z. Shafiq and J. Iqbal, Exploring antidiabetic potential of adamantyl-thiosemicarbazones via aldose reductase (ALR2) inhibition, Bioorg. Chem., 2019, 92, 103244 CrossRef CAS PubMed.
- M. T. Shehzad, A. Khan, M. Islam, A. Hameed, M. Khiat, S. A. Halim, M. U. Anwar, S. R. Shah, J. Hussain, R. Csuk, S. Khan, A. Al-Harrasi and Z. Shafiq, Synthesis and urease inhibitory activity of 1, 4-benzodioxane-based thiosemicarbazones: Biochemical and computational approach, J. Mol. Struct., 2020, 1209, 127922 CrossRef CAS.
- J. Gao, Z. Tian and X. Yang, Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies, BioSci. Trends, 2020, 14(1), 72–73 CrossRef CAS PubMed.
- V. Carbone, M. Giglio, R. Chung, T. Huyton, J. Adams, R. Maccari, R. Ottana, A. Hara and O. El-Kabbani, Structure of aldehyde reductase in ternary complex with a 5-arylidene-2, 4-thiazolidinedione aldose reductase inhibitor, Eur. J. Med. Chem., 2010, 45, 1140–1145 CrossRef CAS PubMed.
- E. Howard, R. Sanishvili, R. Cachau, A. Mitschler, B. Chevrier, P. Barth, V. Lamour, M. Van Zandt, E. Sibley and C. Bon, Ultrahigh resolution drug design I: Details of interactions in human aldose reductase–inhibitor complex at 0.66 Å, Proteins: Struct., Funct., Bioinf., 2004, 55, 792–804 CrossRef CAS PubMed.
- RCSB Protein Data Bank, in RCSB Protein Data Bank, http://www.rcsb.org, (accessed June, 2018) Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Supplementary information includes the bioactivity protocol, docking protocol and NMR spectra of some compounds. See DOI:10.1039/d1ra01716k |
| ‡ Both authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |