Open Access Article
Open Access ArticleA sensitive and selective BINOL based ratiometric fluorescence sensor for the detection of cyanide ions†
Sathishkumar Munusamy,
Sathish Swaminathan,
Dhanapal Jothi,
Vivek Panyam Muralidharan and
Sathiyanarayanan Kulathu Iyer *
*
Department of Chemistry, School of Advanced Sciences, Vellore Institute of Technology, Vellore-632014, India. E-mail: sathiya_kuna@hotmail.com
First published on 27th April 2021
Abstract
A highly selective, novel BINOL based sensor BBCN has been developed for the fluorescent ratiometric detection of cyanide ions (CN−). The optical study revealed that BBCN exhibited unique spectral changes only with cyanide ions in the presence of other competing ions. Besides, an apparent fluorescent colour change from green to blue was observed. A clear linear relationship was observed between the fluorescence ratiometric ratio of BBCN and the concentration of CN− with a reasonably low detection limit (LOD) of 189 nM (507 ppb). The optical response was due to the nucleophilic addition of CN− to the dicyanovinyl group of the sensor, which compromises the probe's intramolecular charge transfer. This mechanism was well confirmed by Job's plot, 1H-NMR and ESI-MS studies. BBCN showed immediate spectral response towards (1 second) CN− and detection could be realized in a broad pH window. Furthermore, the practical utility of BBCN was studied by test paper-based analysis and the detection of CN− in various water resources.
Introduction
The participation of anions in biological systems and chemical processes is inevitable.1–4 Over the past decade, constant effort has been made by the scientific community to understand the fundamental principles of interaction between a host and a negatively charged guest.5–13 Though some anions (phosphate, sulphate and carboxylate) are crucial for biological function, cyanide exposure is extremely poisonous to living organisms. The stable complexation of cyanide ion to the active site of cytochrome C has a cascading effect of oxygen transfer inhibition and hypoxiation. Besides, the accumulation of cyanide in the body can lead to cardiac arrest and coma. However, CN− has widespread application in many fields (electroplating, metallurgy and mining) and cannot be avoided. Hence, the development of a reliable and accurate analytical method is in great demand for various situations. In this regard, development of fluorescent dosimeter for the detection of cyanide ion will be a better choice.14–27 Among the fluorescent probes, ratiometric probes have a unique advantage of emission at two different wavelengths providing a built-in correction for environmental effects.28–40The integral property of fluorescence and chirality of 1,1′-binaphthol (BINOL) and its derivatives have made them potential candidates for asymmetric catalysis as well as fluorescent chemosensors.41–48 Additionally, the fluorescence property and the host–guest interaction of these molecules can be manipulated by adding suitable substitution in the major or minor groove of BINOL. Despite significant reports on the asymmetric synthesis and fluorescent chiral recognition of BINOL, the development of fluorescence sensors involving BINOL for detecting potential small molecules remains very limited. Nevertheless, some BINOL based probes were developed and reported during the last decade. For example, Cheng et al., and Nandhakumar et al., have separately developed BINOL fluorescent derivatives for the selective fluorescent recognition of Hg2+.42,49 To detect Al3+, Peng's group linked amino alcohol in the minor groove of BINOL as the reaction site to catch Al3+ with turn-on fluorescent responses at low limit of detection (16 ppb).50 In 2016, the Yi et al., developed colourimetric and fluorescent off–on sensors for Fe3+ based on BINOL–rhodamine derivatives.51 Besides, quite a few molecular BINOL-derived probes have been designed to sense anions and biologically vital molecules.52,53
In continuation of our research in the development of probes for the molecular recognition44–46 in the current article, we report ‘turn on’ and ratiometric colourimetric, fluorescent probes for cyanide anion, namely, BBCN. The synthesized probes have BINOL–dicyanovinyl based platform. BINOL is chosen as the fluorescent core due to its excellent photophysical features and photo stability, whereas, dicyanovinyl group acts as a reaction subunit selective for cyanide ion where it is often employed. Also, the presence of more than one sensing sites in a probe improve the detection scope. Probe BBCN as such exhibits green colour fluorescence and turns into blue-emitting probe over the addition of CN−. The detailed analytical study revealed that this probe displayed rapid response, highly selective and offered sensitive recognition for tetrabutylammonium cyanide over other competitive anions (F−, Cl−, Br−, I−, H2PO4−, HSO4−, ClO4−, and OH−). The sensing behaviour was analyzed by UV-vis, fluorescence and NMR spectroscopy.
Experimental section
Reagents and equipment
Solvents and reagents were commercially available and procured from commercial sources. Nuclear magnetic resonance spectra were recorded on Bruker Ascent 400 MHz spectrometer. Chemical shift values are reported in δ notation of parts per million using tetramethylsilane (TMS) as the standard. Absorption spectra were recorded on Shimadzu 3600 spectrophotometer while emission spectra were recorded on PerkinElmer LS-55 luminescence spectrometer.Synthesis and characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to afford the pure BBCN. The structure of the compound BBCN was confirmed by their spectral analysis (1H NMR and mass spectral analysis). Yellow solid; mp-225 °C; 1H NMR (400 MHz, DMSO-d6)™: δ 6.70 (s, 1H, CH), 7.30 (m, 7H, J = 8.8 Hz, Ar-CH), 7.461 (q, 4H, J = 5.2 Hz, Ar-CH), 7.54 (q, 2H, J = 5.2 Hz, Ar-CH), 7.71 (t, 1H, J = 5.6 Hz, Ar-CH) 13C NMR (DMSO-d6, 100 MHz): δ 94.53, 100.04, 115.68, 115.77, 115.89, 115.99, 116.34, 116.55, 118.01, 129.87, 131.11, 132.13, 132.22, 132.56, 132.64, 133.13, 133.37, 140.63, 142.00, 147.15, 161.73, 163.92, 164.17. FT-IR (cm−1): 2249, 1629, 1222, 1053, 1024, 1004, 819, 758, 623. HRMS for C56H30N4O2: calculated [M+] m/z 790.2369, found 790.2365.
3) to afford the pure BBCN. The structure of the compound BBCN was confirmed by their spectral analysis (1H NMR and mass spectral analysis). Yellow solid; mp-225 °C; 1H NMR (400 MHz, DMSO-d6)™: δ 6.70 (s, 1H, CH), 7.30 (m, 7H, J = 8.8 Hz, Ar-CH), 7.461 (q, 4H, J = 5.2 Hz, Ar-CH), 7.54 (q, 2H, J = 5.2 Hz, Ar-CH), 7.71 (t, 1H, J = 5.6 Hz, Ar-CH) 13C NMR (DMSO-d6, 100 MHz): δ 94.53, 100.04, 115.68, 115.77, 115.89, 115.99, 116.34, 116.55, 118.01, 129.87, 131.11, 132.13, 132.22, 132.56, 132.64, 133.13, 133.37, 140.63, 142.00, 147.15, 161.73, 163.92, 164.17. FT-IR (cm−1): 2249, 1629, 1222, 1053, 1024, 1004, 819, 758, 623. HRMS for C56H30N4O2: calculated [M+] m/z 790.2369, found 790.2365.Optical selectivity and titration experiments
Absorption and fluorescence experiments were carried out in 10 mm quartz cuvettes at ambient temperature. For fluorescence measurements, the excitation and emission slit widths were set at 5 nm. The spectral recording was taken immediately after the addition of CN− as the reaction between CN− and BBCN is rapid. The required quantity of pure BBCN was dissolved in acetonitrile solvent (spectroscopic grade) to afford the stock solution (2 × 10−2 M), which was diluted with the solvent medium of CH3CN/HEPES in water buffer pH 7.2 (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) for further studies. Anions were dissolved in deionized water to prepare a stock solution.
3 v/v) for further studies. Anions were dissolved in deionized water to prepare a stock solution.
Test paper strips of BBCN
BBCN coated filter papers were prepared by dipping the test strips in the solution of (2 × 10−5 M) and then air-dried in an air oven at 40 °C for 30 minutes. Then cyanide and various other anions were tested one by one on BBCN coated test papers.Quantum yield calculation
The quantum yield was calculated using quinine sulfate (Φ = 0.54 in 0.1 M H2SO4) as a standard reference using the following formulawhere Φs represents the quantum yield, F stands for integrated emission area of corresponding fluorescence spectrum, and A denote absorption intensity of the spectrum. The subscripts ‘s’ and ‘u’ stand for standard and sample, respectively.
Results and discussion
Design of sensor BBCN
1,1′-Bi-2-naphthol is a stable fluorophore with conformational stability. Its fluorescence property can be modified by introducing suitable substitution at minor groove (3, 3′ position) or phenolic –OH group. In this contribution, BINOL based cyanide sensing was designed and synthesized. The synthetic route and molecular structure of sensor BBCN are given in Scheme 1. Compound BBCN was achieved in three steps by Sonogashira coupling and Knoevenagel condensation.The probe structure consisted of dicyanovinyl as signalling unit for selective sensing of cyanide ion, and the major groove was incorporated with π extension unit to enhance the optical property. Compound BBCN was attained in good yield, which was confirmed by 1H-NMR, 13C-NMR, FT-IR, HRMS and HPLC analysis (see ESI; Fig S1–S6†). Probe BBCN is soluble in common polar organic solvents such as CH2Cl2, THF, MeOH, and CH3CN. In this work, CH3CN was preferred for further analytical studies due to its water miscibility and low toxicity. Compound BBCN has very low solubility in water, but with the 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (CH3CN
3 (CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) volume ratio with CH3CN, complete solubility was achieved, and the same solvent composition was used for the analytical studies.
H2O) volume ratio with CH3CN, complete solubility was achieved, and the same solvent composition was used for the analytical studies.
Optical properties of the probes towards CN−
Compound BBCN was dissolved in a mixture of CH3CN/HEPES in water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
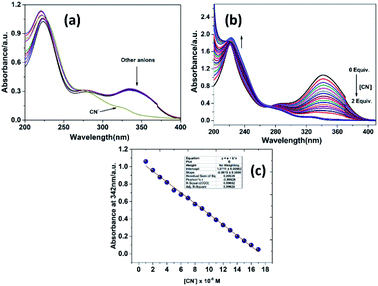
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) at pH 7.2. It showed two distinct absorption peaks at around 340 nm and 221 nm. The absorption is mainly originated from π–π* electron transition and moderate intramolecular charge transfer (ICT) process. While excess equivalence of anions such as F−, Cl−, Br−, I−, H2PO4−, HSO4−, ClO4−, OH−, S2−, CO32−, AcO−, SCN− and N3− did not generate any change in the absorption spectral pattern (Fig. 1a), the addition of CN− to the probe showed significant changes in the absorption spectra. On constant increment of CN−, the intensity of the peak at around 342 nm decreased remarkably, and the peak at about 222 nm showed an increase in peak intensity BBCN (Fig. 1b). Furthermore, the absorption spectra of the probes showed a blue shift with increasing concentration of CN−. An isosbestic point spot observed at 274 nm was indicative of new product formed between BBCN and CN−. The decrease in absorption intensity value at A342 nm of BBCN varied linearly as a function of the cyanide concentration and reached plateau almost at 2 equiv. of CN−. The linearity of the equation was calculated to be y = −0.0651 + 1.0711 with the coefficient of determination R2 value, which was found to be 0.9965 (Fig. 1c).
3 v/v) at pH 7.2. It showed two distinct absorption peaks at around 340 nm and 221 nm. The absorption is mainly originated from π–π* electron transition and moderate intramolecular charge transfer (ICT) process. While excess equivalence of anions such as F−, Cl−, Br−, I−, H2PO4−, HSO4−, ClO4−, OH−, S2−, CO32−, AcO−, SCN− and N3− did not generate any change in the absorption spectral pattern (Fig. 1a), the addition of CN− to the probe showed significant changes in the absorption spectra. On constant increment of CN−, the intensity of the peak at around 342 nm decreased remarkably, and the peak at about 222 nm showed an increase in peak intensity BBCN (Fig. 1b). Furthermore, the absorption spectra of the probes showed a blue shift with increasing concentration of CN−. An isosbestic point spot observed at 274 nm was indicative of new product formed between BBCN and CN−. The decrease in absorption intensity value at A342 nm of BBCN varied linearly as a function of the cyanide concentration and reached plateau almost at 2 equiv. of CN−. The linearity of the equation was calculated to be y = −0.0651 + 1.0711 with the coefficient of determination R2 value, which was found to be 0.9965 (Fig. 1c).
Similarly, the fluorescence behaviour of the probe BBCN was also studied with and without the addition of CN− in the solvent medium of CH3CN/HEPES in water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) buffer at pH 7.2. As shown in Fig. 2a, when the probe BBCN is excited at 274 nm, a clear emission peak appeared at the 395 nm (Φ = 0.18), which could be attributed to the emission of BINOL core and the ICT emission, respectively. Upon the addition of 2 equiv. of CN− ion, the intensity of the emission peak at 395 nm got decreased significantly with the simultaneous appearance of a blue-shifted peak at 315 nm (Φ = 0.23). The intensity of the new peak at 315 nm was found to be 180% higher than the peak at 395 nm. To ascertain the fluorescence selectivity of BBCN towards various competitive anions, including highly nucleophilic F− and SCN− was tested. As shown in the Fig. 2a, except CN− other anions had almost no effect on the fluorescence behaviour of BBCN. This selectivity is indicated in the bar plot in Fig. S7,† which represents the change in fluorescence ratio (I315/I395) in the presence of various anions.
3 v/v) buffer at pH 7.2. As shown in Fig. 2a, when the probe BBCN is excited at 274 nm, a clear emission peak appeared at the 395 nm (Φ = 0.18), which could be attributed to the emission of BINOL core and the ICT emission, respectively. Upon the addition of 2 equiv. of CN− ion, the intensity of the emission peak at 395 nm got decreased significantly with the simultaneous appearance of a blue-shifted peak at 315 nm (Φ = 0.23). The intensity of the new peak at 315 nm was found to be 180% higher than the peak at 395 nm. To ascertain the fluorescence selectivity of BBCN towards various competitive anions, including highly nucleophilic F− and SCN− was tested. As shown in the Fig. 2a, except CN− other anions had almost no effect on the fluorescence behaviour of BBCN. This selectivity is indicated in the bar plot in Fig. S7,† which represents the change in fluorescence ratio (I315/I395) in the presence of various anions.
To study the quantitative detection of CN−, fluorescence titration experiment of probe BBCN (20 μM) over the incremental addition of CN− (0–40 μM) was carried out as shown in Fig. 2b. As can be seen, the intensity of fluorescence peak at 395 nm decreased, while a new emission peak at 315 nm emerged and increased gradually with increasing concentration of CN−. Notably, an isoemission point at 365 nm was observed, which indicates a straightforward reaction process between CN− and BBCN. The plot of fluorescence intensity ratio (I315/I395) against the concentration of CN− was found to be linear with the coefficient of determination R2 value of 0.9957, which indicated the determination of CN− concentration ratiometrically (Fig. 2c). Limit of detection (LOD) is the most critical attribute of an exemplary sensor. LOD of BBCN was calculated based on the fluorescence titration method using 3σ/K method. To calculate the standard deviation (σ), the emission intensity of BBCN at 315 nm was measured ten times, and the standard deviation was calculated. Slope (K) was calculated from the fluorescence titration profile at 315 nm versus the concentration of CN−. From these values, the LOD was found to be 189 nM (507 ppb) (Fig. S8†). The LOD was far below the maximum allowed content in drinking water (1.9 μM) set by the World Health Organization (WHO), and the results were compared with previous chemosensors for CN− (Table S1†). Additionally, when visualized under a UV-lamp of 365 nm source, the apparent green emission of BBCN changed to a blue fluorescence only in the presence of cyanide ions, while the emission remained same over the addition of the anions (Fig. 2d).
The probe's ability to detect CN− ion in the presence of other relevant competitive ions was studied. As shown in the Fig. 3a, CN− sensing of BBCN is not affected by other anions despite introduction in higher equivalents and also the found fluorescence intensity is as significant as BBCN–CN−. This competitive binding experiment clearly demonstrates the excellent selectivity of BBCN toward CN−, which is due to strong nucleophilicity of CN−. Besides, the influence of pH on the emission of the property of BBCN and BBCN–CN− was studied with the range of pH solutions (2 to 11) as shown in the Fig. 3b. As the pH of the medium was adjusted between 2 and 11, the fluorescence intensity of BBCN and BBCN–CN− was not affected, which indicates that BBCN can be used in the broader range of pH to detect CN− of the physiological and biological samples. In general, chemosensors with short response time are highly preferred than those with delayed responses. To check the kinetics of CN− detection by BBCN, the time course of fluorescence emission intensity of BBCN over the addition of CN− was studied. As shown in the Fig. 3c, the fluorescence intensity ratio (I315/I395) reached its maximum within 1 second, indicating fluorescence response of BBCN towards is sensitive and rapid.
Sensing mechanism
The spectral response phenomena of probe BBCN to cyanide can result from CN− nucleophilic addition of CN− to the dicyanovinyl group as expected. This reaction process can sufficiently hinder the efficiency of the intramolecular charge transfer. The Job's plot measured using fluorescent titration method indicated that the maximum fluorescence intensity was achieved when the molar fraction of BBCN and CN− was around 0.75, showing the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding event (Fig. 4a). The sensing mechanism was further explored by recording and comparing the 1H NMR of BBCN and BBCN + CN− reaction mixture. 1H NMR spectra of BBCN, and the resultant product is shown in the Fig. 4b. The resonance signal at δ = 8.33 ppm was ascribed to the vinylic proton (Ha). After adding an excess of CN− the resonance signal corresponding to vinylic proton (Ha) at 8.33 ppm completely disappeared, while a new signal of the α-proton (Hb) appeared at 4.46 ppm, which can be attributed to dicyano ethyl proton. As a result, the aromatic proton displayed a more significant upfield shift than those probes due to the broken conjugated bridge. These observations indicated that the cyanide anion was added to the vinyl group via nucleophilic addition reaction. The formation of new nucleophilic addition product was further confirmed by recording ESI-MS spectra. The peak observed at m/z = 842.19 corresponded to BBCN + CN− (Fig. S9†). The results confirmed the selective detection of CN− based on nucleophilic addition of CN− ion in the π-conjugated position of dicyanovinyl moiety.16,54–62 In addition, the structure and the binding mechanism were further analysed by FT-IR analysis of free BBCN and BBCN–CN− (Fig. S10†). BBCA revealed clear and strong characteristic absorption bands for disubstituted alkene C
2 binding event (Fig. 4a). The sensing mechanism was further explored by recording and comparing the 1H NMR of BBCN and BBCN + CN− reaction mixture. 1H NMR spectra of BBCN, and the resultant product is shown in the Fig. 4b. The resonance signal at δ = 8.33 ppm was ascribed to the vinylic proton (Ha). After adding an excess of CN− the resonance signal corresponding to vinylic proton (Ha) at 8.33 ppm completely disappeared, while a new signal of the α-proton (Hb) appeared at 4.46 ppm, which can be attributed to dicyano ethyl proton. As a result, the aromatic proton displayed a more significant upfield shift than those probes due to the broken conjugated bridge. These observations indicated that the cyanide anion was added to the vinyl group via nucleophilic addition reaction. The formation of new nucleophilic addition product was further confirmed by recording ESI-MS spectra. The peak observed at m/z = 842.19 corresponded to BBCN + CN− (Fig. S9†). The results confirmed the selective detection of CN− based on nucleophilic addition of CN− ion in the π-conjugated position of dicyanovinyl moiety.16,54–62 In addition, the structure and the binding mechanism were further analysed by FT-IR analysis of free BBCN and BBCN–CN− (Fig. S10†). BBCA revealed clear and strong characteristic absorption bands for disubstituted alkene C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (C
C bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending, 758 cm−1) and geminal C–CN bond (C–CN bending, 819 cm−1). Both of these peaks completely disappeared for BBCN–CN− and a new strong peak appeared in the finger print region at 952 cm−1 which is due to C–C bending which corroborates well with the structure of BBCN–CN−.
C bending, 758 cm−1) and geminal C–CN bond (C–CN bending, 819 cm−1). Both of these peaks completely disappeared for BBCN–CN− and a new strong peak appeared in the finger print region at 952 cm−1 which is due to C–C bending which corroborates well with the structure of BBCN–CN−.
Real-time application
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) followed by drying in air. When the test strips were immersed in various anions, colour change from green to blue was observed only with CN− (Fig. 5a).
3 v/v) followed by drying in air. When the test strips were immersed in various anions, colour change from green to blue was observed only with CN− (Fig. 5a).
Besides, to get a direct quantitative detection, test strips were dipped in different vials, increasing the cyanide ion concentration, gradual colour transformation from green to blue was observed (Fig. 5b). These observations indicate that BBCN coated test strip could be conveniently used to detect cyanide ion for sensitive and rapid detection of cyanide ions in practical samples without using expensive equipment.
| S. no. | Water samplesa | CN− spiked (μM) | CN− found (μM) | Recovery (%) | R.S.Db (n = 3) (%) |
|---|---|---|---|---|---|
a Water samples were collected from in and around VIT campus, Vellore.b Relative standard deviation conditions: 10 μM BBCN in a mixed solution of CH3CN/water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). 3). |
|||||
| 1 | Tap water | 1 × 10−6 | 0.98 × 10−6 | 98 | 0.2 |
| 2 | Lake water | 1 × 10−6 | 0.92 × 10−6 | 92 | 1.2 |
| 3 | Well water | 1 × 10−6 | 0.97 × 10−6 | 97 | 0.23 |
| 4 | Mineral water | 1 × 10−6 | 0.95 × 10−6 | 95 | 0.3 |
| 5 | Distilled water | 1 × 10−6 | 0.99 × 10−6 | 99 | 0.19 |
| 6 | Purified water | 1 × 10−6 | 0.97 × 10−6 | 97 | 0.23 |
Conclusions
In summary, a new efficient BINOL based fluorescent ratiometric sensor BBCN for the highly sensitive and selective detection of CN− has been developed. BBCN undergoes remarkable hypochromic shift both in the absorbance and emission spectra occurs over the addition of CN− ion as a result of ICT suppression. Spectral changes of BBCN have excellent selectivity towards CN− ion with rapid response time. LOD was found to be in the range of 189 nm (507 ppb). This probe was successfully utilized to detect CN− in real time water samples collected from various water bodies with satisfactory results. Moreover, BBCN based test strips can quickly and conveniently developed and for selective and sensitive detection of CN−.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Sathish S and Dhanapal J thank VIT University for providing financial support through research associateship. The DST-FIST NMR facility at VIT University is duly acknowledged. The authors thank Dr R. Srinivasan, SSL-VIT for language editing.References
- R. Sakai, T. Satoh and T. Kakuchi, Polym. Rev., 2017, 57, 159–174 CrossRef CAS.
- R. Martínez-Máñez and F. Sancenón, Chem. Rev., 2003, 103, 4419–4476 CrossRef PubMed.
- Y. Liu, Y. Hu, S. Lee, D. Lee and J. Yoon, Bull. Korean Chem. Soc., 2016, 37, 1661–1678 CrossRef CAS.
- P. Molina, F. Zapata and A. Caballero, Chem. Rev., 2017, 117, 9907–9972 CrossRef CAS PubMed.
- C. J. Serpell and P. D. Beer, in Comprehensive Supramolecular Chemistry II, ed. J. L. Atwood, Elsevier, Oxford, 2017, pp. 351–385 Search PubMed.
- Q. Lin, F. Zheng, T.-T. Lu, J. Liu, H. Li, T.-B. Wei, H. Yao and Y.-M. Zhang, Sens. Actuators, B, 2017, 251, 250–255 CrossRef CAS.
- R. Arumugaperumal, V. Srinivasadesikan, M. V. Ramakrishnam Raju, M.-C. Lin, T. Shukla, R. Singh and H.-C. Lin, ACS Appl. Mater. Interfaces, 2015, 7, 26491–26503 CrossRef CAS PubMed.
- M. J. Langton, Y. Xiong and P. D. Beer, Chem.–Eur. J., 2015, 21, 18910–18914 CrossRef CAS PubMed.
- P. A. Gale, N. Busschaert, C. J. E. Haynes, L. E. Karagiannidis and I. L. Kirby, Chem. Soc. Rev., 2013, 43, 205–241 RSC.
- N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Chem. Rev., 2015, 115, 8038–8155 CrossRef CAS PubMed.
- A. Brown and P. D. Beer, Chem. Commun., 2016, 52, 8645–8658 RSC.
- D. M. Gillen, C. S. Hawes and T. Gunnlaugsson, J. Org. Chem., 2018, 83, 10398–10408 CrossRef CAS PubMed.
- M. I. Rednic, R. A. Varga, A. Bende, I. G. Grosu, M. Miclăuş, N. D. Hădade, A. Terec, E. Bogdan and I. Grosu, Chem. Commun., 2016, 52, 12322–12325 RSC.
- M. H. Chua, H. Zhou, T. T. Lin, J. Wu and J. Xu, J. Mater. Chem. C, 2017, 5, 12194–12203 RSC.
- F. Wang, L. Wang, X. Chen and J. Yoon, Chem. Soc. Rev., 2014, 43, 4312–4324 RSC.
- T. M. Ebaston, G. Balamurugan and S. Velmathi, Anal. Methods, 2016, 8, 6909–6915 RSC.
- Y.-L. Leng, J.-H. Zhang, Q. Li, Y.-M. Zhang, Q. Lin, H. Yao and T.-B. Wei, New J. Chem., 2016, 40, 8607–8613 RSC.
- Y. Zhang, D. Li, Y. Li and J. Yu, Chem. Sci., 2014, 5, 2710–2716 RSC.
- X. Chen, S.-W. Nam, G.-H. Kim, N. Song, Y. Jeong, I. Shin, S. K. Kim, J. Kim, S. Park and J. Yoon, Chem. Commun., 2010, 46, 8953–8955 RSC.
- B. Aydıner, Ö. Şahin, D. Çakmaz, G. Kaplan, K. Kaya, Ü. Ö. Özdemir, N. Seferoğlu and Z. Seferoğlu, New J. Chem., 2020, 44, 19155–19165 RSC.
- S. Li, F. Huo, K. Ma, Y. Zhang and C. Yin, New J. Chem., 2021, 45, 1216–1220 RSC.
- S. Malkondu, S. Erdemir and S. Karakurt, Dyes Pigm., 2020, 174, 108019 CrossRef CAS.
- A. Popczyk, Y. Cheret, A. El-Ghayoury, B. Sahraoui and J. Mysliwiec, Dyes Pigm., 2020, 177, 108300 CrossRef CAS.
- G. Sun, W. Chen, Y. Liu, X. Jin, Z. Zhang and J. Su, Dyes Pigm., 2020, 176, 108224 CrossRef CAS.
- Y.-D. Lin, Y.-S. Pen, W. Su, K.-L. Liau, Y.-S. Wen, C.-H. Tu, C.-H. Sun and T. J. Chow, Chem.–Asian J., 2012, 7, 2864–2871 CrossRef CAS PubMed.
- Y. Liu, J. s. Du, S. l. Qi, L. b. Zhu, Q. b. Yang, H. Xu and Y. x. Li, Luminescence, 2021, 36, 336–344 CrossRef CAS PubMed.
- W.-J. Qu, W.-T. Li, H.-L. Zhang, T.-B. Wei, Q. Lin, H. Yao and Y.-M. Zhang, J. Heterocycl. Chem., 2018, 55, 879–887 CrossRef CAS.
- L. Yang, X. Li, J. Yang, Y. Qu and J. Hua, ACS Appl. Mater. Interfaces, 2013, 5, 1317–1326 CrossRef CAS PubMed.
- L. Long, X. Yuan, S. Cao, Y. Han, W. Liu, Q. Chen, Z. Han and K. Wang, ACS Omega, 2019, 4, 10784–10790 CrossRef CAS PubMed.
- X. Cheng, R. Tang, H. Jia, J. Feng, J. Qin and Z. Li, ACS Appl. Mater. Interfaces, 2012, 4, 4387–4392 CrossRef CAS PubMed.
- W.-C. Lin, S.-K. Fang, J.-W. Hu, H.-Y. Tsai and K.-Y. Chen, Anal. Chem., 2014, 86, 4648–4652 CrossRef CAS PubMed.
- P. S. Kumar, P. R. Lakshmi and K. P. Elango, New J. Chem., 2019, 43, 675–680 RSC.
- X. Sun, Y. Wang, X. Deng, J. Zhang and Z. Zhang, RSC Adv., 2016, 6, 10266–10271 RSC.
- L. Hou, F. Li, J. Guo, X. Zhang, X. Kong, X. T. Cui, C. Dong, Y. Wang and S. Shuang, J. Mater. Chem. B, 2019, 7, 4620–4629 RSC.
- J. Kang, F. Huo, Y. Zhang, J. Chao, T. E. Glass and C. Yin, Spectrochim. Acta, Part A, 2019, 209, 95–99 CrossRef CAS PubMed.
- Y. Hao, K. H. Nguyen, Y. Zhang, G. Zhang, S. Fan, F. Li, C. Guo, Y. Lu, X. Song, P. Qu, Y.-N. Liu and M. Xu, Talanta, 2018, 176, 234–241 CrossRef CAS PubMed.
- L. Lan, T. Li, T. Wei, H. Pang, T. Sun, E. Wang, H. Liu and Q. Niu, Spectrochim. Acta, Part A, 2018, 193, 289–296 CrossRef CAS PubMed.
- W.-C. Lin, J.-W. Hu and K.-Y. Chen, Anal. Chim. Acta, 2015, 893, 91–100 CrossRef CAS PubMed.
- G.-L. Fu and C.-H. Zhao, Tetrahedron, 2013, 69, 1700–1704 CrossRef CAS.
- A. S. Gupta, K. Paul and V. Luxami, Inorg. Chim. Acta, 2016, 443, 57–63 CrossRef CAS.
- X. Feng, Y. Wang, W. Feng and Y. Peng, Chin. Chem. Lett., 2020, 31, 2960–2964 CrossRef CAS.
- K. Velmurugan and R. Nandhakumar, J. Lumin., 2015, 162, 8–13 CrossRef CAS.
- C. Monterde, R. Navarro, M. Iglesias and F. Sánchez, J. Catal., 2019, 377, 609–618 CrossRef CAS.
- S. Munusamy and S. Kulathu Iyer, Tetrahedron: Asymmetry, 2016, 27, 492–497 CrossRef CAS.
- S. Munusamy, V. P. Muralidharan and S. K. Iyer, Sens. Actuators, B, 2017, 250, 244–249 CrossRef CAS.
- S. K. Munusamy, K. Thirumoorthy, V. P. Muralidharan, U. Balijapalli and S. K. Iyer, Sens. Actuators, B, 2017, 244, 175–181 CrossRef CAS.
- L. Pu, Acc. Chem. Res., 2012, 45, 150–163 CrossRef CAS PubMed.
- M.-Q. Wang, K. Li, J.-T. Hou, M.-Y. Wu, Z. Huang and X.-Q. Yu, J. Org. Chem., 2012, 77, 8350–8354 CrossRef CAS PubMed.
- X. Liu, X. Yang, Y. Fu, C. Zhu and Y. Cheng, Tetrahedron, 2011, 67, 3181–3186 CrossRef CAS.
- M. Dong, Y.-M. Dong, T.-H. Ma, Y.-W. Wang and Y. Peng, Inorg. Chim. Acta, 2012, 381, 137–142 CrossRef CAS.
- C. Fan, X. Huang, L. Han, Z. Lu, Z. Wang and Y. Yi, Sens. Actuators, B, 2016, 224, 592–599 CrossRef CAS.
- J. Li, C. Yin, F. Huo, K. Xiong, J. Chao and Y. Zhang, Sens. Actuators, B, 2016, 231, 547–551 CrossRef CAS.
- F. Li, G. Wei, Y. Sheng, Y. Quan, Y. Cheng and C. Zhu, Polymer, 2014, 55, 5689–5694 CrossRef CAS.
- E. Thanayupong, K. Suttisintong, M. Sukwattanasinitt and N. Niamnont, New J. Chem., 2017, 41, 4058–4064 RSC.
- Q. Zou, X. Li, Q. Xu, H. Ågren, W. Zhao and Y. Qu, RSC Adv., 2014, 4, 59809–59816 RSC.
- H. Li, X. Wu, Y. Xu, H. Tong and L. Wang, Polym. Chem., 2014, 5, 5949–5956 RSC.
- R. K. Konidena and K. R. J. Thomas, RSC Adv., 2014, 4, 22902–22910 RSC.
- W. Chen, Z. Zhang, X. Li, H. Ågren and J. Su, RSC Adv., 2015, 5, 12191–12201 RSC.
- E. Jeong, S. Yoon, H. S. Lee, A. Kumar and P. S. Chae, Dyes Pigm., 2019, 162, 348–357 CrossRef CAS.
- A. Tigreros, J.-C. Castillo and J. Portilla, Talanta, 2020, 215, 120905 CrossRef CAS PubMed.
- A. Ozdemir and S. Erdemir, J. Photochem. Photobiol. Chem., 2020, 390, 112328 CrossRef CAS.
- E. Ramachandran, S. A. A. Vandarkuzhali, G. Sivaraman and R. Dhamodharan, Chem.–Eur. J., 2018, 24, 11042–11050 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01213d |
| This journal is © The Royal Society of Chemistry 2021 |