Open Access Article
Open Access ArticleTin(IV) chloride mediated (3 + 2) annulation of trans-2-aroyl-3-styrylcyclopropane-1,1-dicarboxylates with nitriles: diastereoselective access to 5-vinyl-1-pyrroline derivatives†
Murugesan Thangamani,
Subaramaniam Thangamalar and
Kannupal Srinivasan *
*
School of Chemistry, Bharathidasan University, Tiruchirappalli-620024, Tamil Nadu, India. E-mail: srinivasank@bdu.ac.in; Fax: +91-431-2407043; Tel: +91-431-2407053
First published on 21st April 2021
Abstract
A tin(IV) chloride promoted (3 + 2) annulation of trans-2-aroyl-3-styrylcyclopropane-1,1-dicarboxylates with nitriles is reported. The transformation involves the Lewis acid assisted formation of 1,5-dipolar intermediates from the cyclopropane dicarboxylates and nitriles followed by cyclization. The reactions proceed in a highly diastereoselective manner and afford 5-vinyl-1-pyrroline derivatives in 60–88% yields.
Introduction
The annulation (formal cycloaddition) reactions of donor–acceptor (D–A) cyclopropanes are one of the efficient tools for the construction of various carbocyclic and heterocyclic compounds.1 The merits of the methodology include excellent stereoselectivity, atom economy and formation of products in good yields with diverse functionality. Due to their merits, few of the methods have been employed as key steps in the total synthesis of various biologically important natural products.21-Pyrrolines are an important class of heterocyclic compounds as the core is present in a notable number of natural products and biologically relevant compounds.3 They also serve as versatile synthetic intermediates for the access of pharmaceutically important compounds.4 So numerous approaches have been developed for the synthesis of 1-pyrrolines.5 The (3 + 2) annulation of D–A cyclopropanes with nitriles is a versatile strategy for the stereoselective synthesis of 1-pyrrolines.6
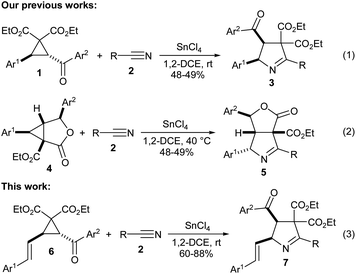
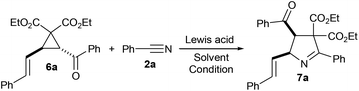
Few years back, we reported that aroyl substituted D–A cyclopropanes 1 undergo SnCl4-promoted (3 + 2) annulation with nitriles 2 to give 1-pyrrolines 3 diastereoselectively (Scheme 1, eqn (1)).7 Recently, we reported a similar approach for the access of γ-butyrolactone-fused 1-pyrrolines 5 from γ-butyrolactone-fused D–A cyclopropanes 4 (Scheme 1, eqn (2)).8 Meanwhile, we have also synthesized and explored the synthetic potential of a similar class of aroyl substituted D–A cyclopropanes having aryl vinyl donor group, namely, trans-2-aroyl-3-styrylcyclopropane-1,1-dicarboxylates 6.9 Naturally, we became interested in exploring the (3 + 2) annulation of 6 with various nitriles with a view to obtain vinyl-substituted 1-pyrroline derivatives 7 (Scheme 1, eqn (3)). It is interesting to note that the vinyl pyrroline core is present in alkaloids isolated from the venom of the myrmicine ant Megalomyrmex foreli of Costa Rica.10
Results and discussion
To identify optimal reaction conditions for the (3 + 2) annulation of trans-2-aroyl-3-styrylcycyopropane-1,1-dicarboxylates with nitriles, we selected cyclopropane 6a and benzonitrile (2a) as model substrates and reacted under the optimized conditions previously reported for similar D–A cyclopropanes 1 (Table 1, entry 1).7 Accordingly, when 1 equiv. of 6a was treated with 5 equiv. of 2a in the presence of 1 equiv. of SnCl4 in 1,2-dichloroethane (1,2-DCE) at room temperature for 12 h, the expected vinyl 1-pyrroline product was produced in 86% yield (entry 1). Normally, an excess amount of nitrile (2.5 to 5 equiv.) was used in cyclopropane-nitrile annulations for achieving better yields.6 In the present case, we observed that the use of 2.5 equiv. of 2a was enough to obtain the same yield (entry 2). So, in the subsequent experiments, we used only 2.5 equiv. of 2a. Next, we reduced the amount of SnCl4 to 0.2 or 0.5 equiv., but the yield of 7a was also decreased to 10 and 58%, respectively (entries 3 and 4). When the amount of SnCl4 was increased to 1.5 or 2 equiv., again the yield of 6a was decreased owing to the formation of more impurities (entries 5 and 6). The yield of 7a also decreased when the reaction was carried out at 0 °C or 60 °C (entries 7 and 8). Switching the solvent to dichloromethane, nitromethane or toluene also gave only a lower yield of 7a while the reaction did not take place in THF (entries 9–12). We also investigated the suitability of other tin sources for the transformation. When SnCl4·5H2O was used, the reaction gave a complicated mixture of products (entry 13) while the reaction did not take place with SnCl2 (entry 14). We also tested other Lewis acids for the transformation. The use of AlCl3 reduced the yield of 7a to 70% while TiCl4 gave only trace amount of 7a (entries 15 and 16). Upon using BF3·OEt2, the cyclopropane 6a did not react with 2a; instead, it underwent fragmentation to give cinnamaldehyde and phenacyl malonate (entry 17).9a When other Lewis acids such as InCl3, In(OTf)3, Cu(OTf)2, Sc(OTf)3 and Yb(OTf)3 were used, the transformation did not take place (entries 18–22). Also, the reaction did not work when a Bronsted acid, viz., p-TSOH was used (entry 23). So we chose treating 1 equiv. of 6a with 2.5 equiv. of 2a in the presence of 1 equiv. of SnCl4 in 1,2-DCE at room temperature as optimal condition for the formation of 7a in a better yield.| Entry | Reagents (equiv.) and conditionsa | Yield of 7ab (%) |
|---|---|---|
| a The reaction was conducted with 1a (1 equiv.), 2a (2.5 equiv.), Lewis acid (n equiv.) and solvent (3 mL).b Isolated yield.c No reaction.d Complicated mixture of products.e 6a underwent fragmentation to give cinnamaldehyde and phenacyl malonate.9a | ||
| 1 | SnCl4 (1.0), 1,2-DCE, rt, 12 h [using 2a (5 equiv.)] | 86 |
| 2 | SnCl4 (1.0), 1,2-DCE, rt, 12 h | 86 |
| 3 | SnCl4 (0.2), 1,2-DCE, rt, 12 h | 10 |
| 4 | SnCl4 (0.5), 1,2-DCE, rt, 12 h | 58 |
| 5 | SnCl4 (1.5), 1,2-DCE, rt, 12 h | 65 |
| 6 | SnCl4 (2.0), 1,2-DCE, rt, 12 h | 62 |
| 7 | SnCl4 (1.0), 1,2-DCE, 0 °C, 12 h | 55 |
| 8 | SnCl4 (1.0), 1,2-DCE, 60 °C, 12 h | 46 |
| 9 | SnCl4 (1.0), CH2Cl2, rt, 12 h | 30 |
| 10 | SnCl4 (1.0), MeNO2, rt, 12 h | 10 |
| 11 | SnCl4 (1.0), PhMe, rt, 12 h | 30 |
| 12 | SnCl4 (1.0), THF, rt, 24 h | NRc |
| 13 | SnCl4·5H2O (1.0), 1,2-DCE, rt, 12 h | c.m.d |
| 14 | SnCl2 (1.0), 1,2-DCE, rt, 24 h | NRc |
| 15 | AlCl3 (1.0), 1,2-DCE, rt, 12 h | 70 |
| 16 | TiCl4 (1.0), 1,2-DCE, rt, 12 h | Trace |
| 17 | BF3·Et2O (1.0), 1,2-DCE, rt, 12 h | —e |
| 18 | InCl3 (1.0), 1,2-DCE, rt, 24 h | NRc |
| 19 | In(OTf)3 (1.0), 1,2-DCE, rt, 24 h | NRc |
| 20 | Cu(OTf)2 (1.0), 1,2-DCE, rt, 24 h | NRc |
| 21 | Sc(OTf)3 (1.0), 1,2-DCE, rt, 24 h | NRc |
| 22 | Yb(OTf)3 (1.0), 1,2-DCE, rt, 24 h | NRc |
| 23 | p-TsOH (1.0), 1,2-DCE, rt, 24 h | NRc |
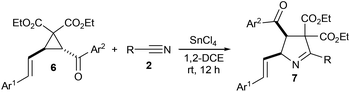
Next, we examined the scope of the transformation for various vinyl D–A cyclopropanes and ntriles and the results are summarized in Table 2. Initially, we tested the reactions of cyclopropane 6a with aromatic nitriles 2a–d having electron donating and electron withdrawing substituents on the aryl ring (entries 1–4). Except for the reaction in entry 4, in which the expected product was not detected, all other reactions afforded the corresponding 5-vinyl-1-pyrroline derivatives 7a–c in 66–75% yields. When 6a was reacted with nitriles 2e and 2f having bulky 1-naphthyl and heteroaromatic 2-thienyl rings, vinyl 1-pyrrolines 7d and 7e were formed in 76 and 80% yields, respectively (entries 5 and 6). We also reacted cyclopropane 6a with an aliphatic nitrile, viz., acetonitrile (2g) and obtained the corresponding 1-pyrroline derivative 7f in 88% yield (entry 7). Next, we reacted cyclopropanes 6b–g having different aromatic rings as Ar1 or Ar2 with benzonitrile (2a) and obtained the respective 1-pyrrolines 7g–l in 63–78% yields (entries 8–13). Finally, we reacted various substrates having different Ar1, Ar2 or R groups and obtained the corresponding 1-pyrrolines 7m–q in 60–86% yields (entries 14–18).
| Entry | Ar1, Ar2 | R | Yield of 7a (%) |
|---|---|---|---|
| a Isolated yield.b Not detected. | |||
| 1 | Ph, Ph (6a) | Ph (2a) | 86 (7a) |
| 2 | Ph, Ph (6a) | 4-MeC6H4 (2b) | 75 (7b) |
| 3 | Ph, Ph (6a) | 4-MeOC6H4 (2c) | 66 (7c) |
| 4 | Ph, Ph (6a) | 4-O2NC6H4 (2d) | n.d.b |
| 5 | Ph, Ph (6a) | 1-Naphthyl (2e) | 76 (7d) |
| 6 | Ph, Ph (6a) | 2-Thienyl (2f) | 80 (7e) |
| 7 | Ph, Ph (6a) | Me (2g) | 88 (7f) |
| 8 | 4-MeOC6H4, Ph (6b) | Ph (2a) | 78 (7g) |
| 9 | 2-Naphthyl, Ph (6c) | Ph (2a) | 76 (7h) |
| 10 | 2-Thienyl, Ph (6d) | Ph (2a) | 72 (7i) |
| 11 | Ph, 4-MeC6H4 (6e) | Ph (2a) | 69 (7j) |
| 12 | Ph, 4-MeOC6H4 (6f) | Ph (2a) | 63 (7k) |
| 13 | Ph, 4-O2NC6H4 (6g) | Ph (2a) | 68 (7l) |
| 14 | 4-MeC6H4, Ph (6h) | 4-MeC6H4 (2b) | 78 (7m) |
| 15 | 4-MeC6H4, Ph (6h) | 4-BrC6H4 (2h) | 60 (7n) |
| 16 | 4-MeC6H4, Ph (6h) | Me (2g) | 80 (7o) |
| 17 | 4-O2NC6H4, Ph (6i) | 2-Thienyl (2f) | 65 (7p) |
| 18 | 4-MeC6H4, 4-MeC6H4 (6j) | Me (2g) | 86 (7q) |
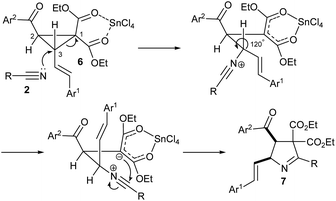
We propose a mechanism depicted in Scheme 2 for the formation of vinyl 1-pyrrolines 7 from D–A cyclopropanes 6 and nitriles 2 based on earlier reports.7,8,11 Accordingly, the Lewis acid (SnCl4) complexes with malonate unit of 6, which facilitates the nucleophilic attack of 2 on 6 at the carbon (C-3) attached to vinyl unit. In the resulting 1,5-dipolar intermediate, the groups attached to C-3 undergo 120° rotation which brings the nitrile carbon and the malonate carbanion in close proximity for cyclization. It may be noted that the rotation also brings Ar1 and Ar2 groups to a cis-position. So the product 1-pyrroline 7 is formed in a diastereoselective manner.
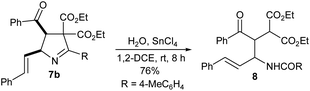
The vinyl 1-pyrroline products synthesized in the present study could serve as potential synthetic precursors for other compounds. To prove the point, we treated vinyl 1-pyrroline 7b with water in the presence of 1 equiv. of SnCl4 in 1,2-DCE for 8 h. It underwent nucleophilic addition of water followed by ring-opening to furnish the multifunctional malonate 8 in 76% yield (Scheme 3; the structure of 8 was confirmed by X-ray analysis12).
Conclusions
In summary, we have developed a convenient method for the generation of vinyl 1-pyrroline derivatives from trans-2-aroyl-3-styrylcyclopropane-1,1-dicarboxylates and nitriles. The method involves a [3 + 2] annulation between the reactants and affords the products in good yields with excellent diastereoselectivity. The products could serve as potential precursors for other compounds as exemplified by the formation of a multifunctional malonate from one of the products.Experimental section
General procedure for the synthesis of vinyl 1-pyrrolines 7
To a solution of 2-aroyl-3-styrylcyclopropane-1,1-dicarboxylate 6 (1.0 mmol) and nitrile 2 (2.5 mmol) in 1,2-dichloroethane (3 mL) was added SnCl4 (1.0 mmol, 0.261 g) and the reaction mixture was stirred at room temperature. After completion of the reaction (12 h), the reaction mixture was quenched with water and extracted with dichloromethane. The organic layer was washed with brine (2 × 10 mL), dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was purified by column chromatography using EtOAc/hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) to afford vinyl 1-pyrroline 7.
9) to afford vinyl 1-pyrroline 7.
Diethyl 4-benzoyl-2-phenyl-5-styryl-4,5-dihydropyrrole-3,3-dicarboxylate (7a)
Yellow oily liquid. Yield: 427 mg (86%). 1H NMR (400 MHz, CDCl3): δ 7.91 (d, J = 8.0 Hz, 4H), 7.62–7.38 (m, 10H), 7.14–7.02 (m, 3H), 6.37 (q, J = 16.0 Hz, 1H), 5.50 (t, J = 6.6 Hz, 1H), 5.01, (d, J = 6.0 Hz, 1H), 4.31–4.24 (m, 2H), 4.00–3.94 (m, 2H), 1.21 (t, J = 7.2 Hz, 3H), 1.01 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (400 MHz, CDCl3): δ 196.5, 167.8, 166.0, 161.0, 147.8, 137.5, 136.8, 133.8, 133.7, 132.7, 132.5, 130.9, 130.0, 129.0, 128.8, 128.6, 128.3, 127.8, 127.7, 127.4, 124.5, 75.0, 74.7, 63.3, 62.4, 59.7, 13.8, 13.5 ppm. HRMS (ESI-TOF) m/z: [M + MeOH + H]+ calcd for C32H34NO6, 528.2375; found: 528.2380.Diethyl 4-benzoyl-5-styryl-2-p-tolyl-4,5-dihydropyrrole-3,3-dicarboxylate (7b)
Light yellow liquid. Yield: 382 mg (75%). 1H NMR (400 MHz,CDCl3): δ 7.84–7.80 (m, 4H), 7.30–7.11 (m, 11H), 6.49 (d, J = 16.0 Hz, 1H), 6.15–6.09 (m, 1H), 5.48–5.44 (m, 1H), 5.25 (d, J = 8.8 Hz, 1H), 4.36–4.20 (m, 4H), 2.43 (s, 3H), 1.22 (t, J = 7.2 Hz, 3H), 1.17 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (400 MHz, CDCl3): δ 195.5, 168.3, 168.2, 167.7, 144.2, 140.7, 136.8, 135.0, 132.1, 131.1, 129.9, 129.0, 128.53, 128.49, 128.3, 127.5, 126.6, 126.6, 75.0, 73.3, 62.6, 62.1, 59.1, 21.7, 13.8, 13.7 ppm. MS (ESI-TOF): m/z 544.19 [M + Cl]−. Anal. calcd for C32H31NO5: C 75.42, H 6.13, N 2.75; found: C 75.78, H 6.25, N 2.68.Diethyl 4-benzoyl-2-(4-methoxyphenyl)-5-styryl-4,5-dihydro-pyrrole-3,3-dicarboxylate (7c)
Pale yellow liquid. Yield: 347 mg (66%). 1H NMR (400 MHz, CDCl3): δ 7.91 (d, J = 7.2 Hz, 2H), 7.73 (d, J = 8.8 Hz, 2H), 7.50–7.13 (m, 8H), 6.80 (d, J = 9.2 Hz, 2H), 6.61 (d, J 15.6 Hz, 1H), 6.27 (q, J = 16.0 Hz, 1H), 5.38 (t, J = 7.0 Hz, 1H), 4.81 (d, J = 7.6 Hz, 1H), 4.11–3.91 (m, 4H), 3.75 (s, 3H), 1.03 (t, J = 7.2 Hz, 3H), 0.95 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 191.3, 168.9, 167.4, 159.4, 142.3, 142.1, 136.4, 133.4, 130.6, 129.6, 128.6, 126.2, 113.7, 76.7, 71.5, 64.4, 62.1, 60.5, 55.3, 13.74, 13.70 ppm. MS (ESI-TOF): m/z 524.21 [M − H]−. Anal. calcd for C32H31NO6: C 73.13, H 5.94, N 2.66; found: C 73.35, H 5.85, N 2.72.Diethyl 4-benzoyl-2-naphthalen-1-yl-5-styryl-4,5-dihydro-pyrrole-3,3-dicarboxylate (7d)
Yellow oily liquid. Yield: 414 mg (76%). 1H NMR (400 MHz, CDCl3): δ 8.09 (d, J = 8.4 Hz, 1H), 7.90–7.76 (m, 4H), 7.49–7.36 (m, 3H), 7.17–7.00 (m, 5H), 6.51 (d, J = 15.6 Hz, 1H), 5.85 (s, 1H), 5.00 (d, J = 8.4 Hz, 1H), 4.82 (t, J = 7.6 Hz, 1H), 3.97–3.25 (m, 4H), 1.19 (t, J = 7.2 Hz, 3H), 0.80 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 192.7, 172.14, 172.09, 167.5, 144.3, 143.7, 138.5, 136.5, 133.4, 129.7, 128.58, 128.57, 128.43, 128.40, 128.1, 127.7, 114.8, 114.3, 81.9, 72.6, 72.58, 63.0, 61.4, 60.0, 13.8, 13.4, ppm. MS (ESI-TOF): m/z 566.18 [M + Na–2H]−. Anal. calcd for C35H31NO5: C 77.04, H 5.73, N 2.57; found: C 77.28, H 5.67, N 2.69.Diethyl 4-benzoyl-5-styryl-2-thiophen-2-yl-4,5-dihydro-pyrrole-3,3-dicarboxylate (7e)
Brown oily liquid. Yield: 401 mg (80%). 1H NMR (400 MHz, CDCl3): δ 8.01 (d, J = 7.2 Hz, 2H), 7.92–7.36 (m, 11H), 7.14–7.02 (m, 1H), 6.40–6.34 (m, 1H), 5.50 (t, J = 6.8 Hz, 1H), 5.01 (d, J = 6.0 Hz, 1H), 4.31–4.24 (m, 2H), 3.97 (q, J = 7.2 Hz, 2H), 1.21 (t, J = 7.2 Hz, 3H), 1.01 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 198.0, 168.3, 167.8, 150.2, 142.0, 141.1, 135.6, 134.0, 129.6, 128.5, 128.3, 126.7, 126.5, 125.9, 124.89, 124.86, 124.0, 82.7, 81.5, 69.7, 62.5, 62.0, 57.9, 13.8, 13.5 ppm. MS (ESI-TOF): m/z 502.18 [M + H]+. Anal. calcd for C29H27NO5S: C 69.44, H 5.43, N 2.79; found: C 69.76, H 5.55, N 2.88.Diethyl 4-benzoyl-2-methyl-5-styryl-4,5-dihydropyrrole-3,3-dicarboxylate (7f)
Yellow oily liquid. Yield: 381 mg (88%). 1H NMR (400 MHz, CDCl3): δ 7.78 (d, J = 7.6 Hz, 2H), 7.45–7.06 (m, 8H), 6.93–6.91 (m, 2H), 6.42 (d, J = 16.0 Hz, 1H), 5.79 (q, J = 15.6 Hz, 1H), 5.19 (t, J = 9.2 Hz, 1H), 5.08 (d, J = 7.2 Hz, 1H), 4.25–4.04 (m, 4H), 2.43 (s, 3H), 1.24 (t, J = 7.2 Hz, 3H), 1.01 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (400 MHz, CDCl3): δ 197.8, 166.9, 166.7, 137.6, 136.6, 133.3, 133.2, 128.8, 128.7, 128.6, 128.5, 128.2, 127.5, 126.5, 126.5, 75.1, 74.1, 62.9, 61.8, 56.9, 18.9, 14.0, 13.7 ppm. MS (ESI-TOF): m/z 512.27 [M + DMSO + H]+. Anal. calcd for C26H27NO5: C 72.04, H 6.28, N 3.23; found: C 72.33, H 6.40, N 3.36.Diethyl 4-benzoyl-5-[2-(4-methoxyphenyl)-vinyl]-2-phenyl-4,5-dihydropyrrole-3,3-dicarboxylate (7g)
Yellow oily liquid. Yield: 410 mg (78%). 1H NMR (400 MHz, CDCl3): δ 7.99 (d, J = 7.2 Hz, 2H), 7.60–7.26 (m, 11H), 6.95 (d, J = 8.8 Hz, 1H), 6.62 (d, J = 15.2 Hz, 1H), 6.27 (q, J = 16.0 Hz, 1H), 5.19 (t, J = 7.6 Hz, 1H), 4.75 (q, J = 8.8 Hz, 1H), 4.30–4.16 (m, 4H), 3.86 (s, 3H), 1.27 (t, J = 7.2 Hz, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ 192.3, 170.7, 168.2, 158.9, 145.3, 142.7, 137.7, 132.6, 131.5, 129.9, 129.4, 128.6, 128.4, 113.5, 72.2, 61.8, 61.2, 55.3, 51.0, 40.0, 13.9, 13.7 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C32H31NO6, 526.2224; found: 526.2235.Diethyl 4-benzoyl-5-(2-naphthalen-2-yl-vinyl)-2-phenyl-4,5-dihydropyrrole-3,3-dicarboxylate (7h)
Pale yellow liquid. Yield: 414 mg (76%). 1H NMR (400 MHz, CDCl3): δ 7.81–7.60 (m, 11H), 7.49–7.43 (m, 6H), 6.34 (q, J = 7.2 Hz, 1H), 6.15 (q, J = 5.6 Hz, 1H), 5.47 (t, J = 4.4 Hz, 1H), 4.36–4.20 (m, 4H), 3.46 (q, J = 14.8 Hz, 1H), 3.20 (q, J = 11.2 Hz, 1H), 1.31 (t, J = 8.4 Hz, 3H), 0.58 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 194.0, 173.1, 169.5, 166.8, 153.0, 134.6, 134.0, 131.9, 129.2, 129.1, 129.0, 128.8, 128.6, 128.1, 126.9, 126.4, 126.0, 124.6, 81.6, 62.8, 51.8, 50.8, 14.1, 14.0 ppm. MS (ESI-TOF): m/z 577.23 [M + MeOH]+. Anal. calcd for C35H31NO5: C 77.04, H 5.73, N 2.57; found: C 77.15, H 5.88, N 2.69.Diethyl 4-benzoyl-2-phenyl-5-(2-thiophen-2-yl-vinyl)-4,5-dihydropyrrole-3,3-dicarboxylate (7i)
Yellow oily liquid. Yield: 361 mg (72%). 1H NMR (400 MHz, CDCl3): δ 8.29 (d, J = 8.8 Hz, 3H), 8.05 (d, J = 8.4 Hz, 3H), 7.27–7.19 (m, 8H), 6.56 (d, J = 2.4 Hz, 1H), 5.03–5.01 (m, 1H), 4.27 (q, J = 7.2 Hz, 2H), 3.75–3.70 (m, 2H), 3.57–3.52 (m, 1H), 1.25 (t, J = 7.2 Hz, 3H), 0.77 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 191.0, 171.8, 167.5, 150.5, 144.8, 143.8, 141.3, 138.0, 130.5, 128.5, 128.4, 127.9, 123.8, 82.0, 72.7, 63.2, 61.7, 60.0, 13.8, 13.4. ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C29H27NO5S, 502.1683; found: 502.1676.Diethyl 4-(4-methylbenzoyl)-2-phenyl-5-styryl-4,5-dihydro-pyrrole-3,3-dicarboxylate (7j)
Pale yellow liquid. Yield: 351 mg (69%). 1H NMR (400 MHz, CDCl3): δ 8.06 (d, J = 7.2 Hz, 2H), 7.58–7.26 (m, 11H), 6.66–6.57 (m, 2H), 6.21 (q, J = 16.0 Hz, 1H), 4.86 (q, J = 10.0 Hz, 1H), 4.24–4.09 (m, 4H), 2.40 (s, 3H), 1.26 (t, J = 7.2 Hz, 3H), 1.18 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 191.4, 171.7, 167.6, 144.0, 143.8, 139.8, 134.8, 132.0, 131.0, 129.9, 128.9, 128.6, 128.4, 127.7, 119.2, 109.2, 81.6, 72.3, 62.9, 61.5, 59.9, 21.8, 13.8, 13.5 ppm. MS (ESI-TOF): m/z 542.16 [M + MeOH + H]+. Anal. calcd for C32H31NO5: C 75.42, H 6.13, N 2.75; found: C 75.59, H 6.22, N 2.70.Diethyl 4-(4-methoxybenzoyl)-2-phenyl-5-styryl-4,5-dihydro-pyrrole-3,3-dicarboxylate (7k)
Yellow oily liquid. Yield: 331 mg (63%). 1H NMR (400 MHz, CDCl3): δ 7.95 (d, J = 7.2 Hz, 2H), 7.94–7.37 (m, 5H), 6.89 (d, J = 8.8 Hz, 2H), 6.52 (d, J = 12.0 Hz, 1H), 5.43 (q, J = 8.0 Hz, 1H), 4.24–3.95 (m, 5H), 3.81 (s, 3H), 1.11 (t, J = 7.2 Hz, 3H), 1.03 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 198.7, 170.0, 169.5, 158.0, 136.4, 135.8, 133.5, 131.3, 130.2, 129.1, 128.6, 113.4, 69.1, 61.4, 61.2, 60.4, 56.0, 55.3, 13.7, 13.4 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C32H31NO6, 526.2224; found: 526.2202.Diethyl-4-(4-nitro-benzoyl)-2-phenyl-5-styryl-4,5-dihydro-pyrrole-3,3-dicarboxylate (7l)
Brown oily liquid. Yield: 367 mg (68%). 1H NMR (400 MHz, CDCl3): δ 7.94 (d, J = 7.6 Hz, 2H), 7.51–7.46 (m, 9H), 6.54 (d, J = 16.0 Hz, 1H), 6.44 (d, J = 7.2 Hz, 1H), 5.00 (d, J = 8.0 Hz, 1H), 4.83 (d, J = 7.6 Hz, 1H), 3.96–3.2 (m, 4H), 0.77 (t, J = 7.2 Hz, 3H), 0.63 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 199.1, 168.7, 168.0, 137.3, 136.9, 136.1, 134.0, 133.8, 130.2, 128.9, 128.7, 128.6, 128.5, 128.7, 128.6, 128.5, 128.1, 127.9, 127.3, 126.9, 126.3, 85.3, 88.0, 70.3, 61.9, 61.5, 57.5, 13.33, 13.25 ppm. MS (ESI-TOF): m/z 579.35 [M + K]+. Anal. calcd for C31H28N2O7: C 68.88, H 5.22, N 5.18; found: C 68.79, H 5.31, N 5.30.Diethyl 4-(4-methylbenzoyl)-2-(1-methylene-pent-2-enyl)-5-styryl-4,5-dihydropyrrole-3,3-dicarboxylate (7m)
Yellow oily liquid. Yield: 408 mg (78%). 1H NMR (400 MHz, CDCl3): δ 7.72–7.68 (m, 4H), 7.46–7.00 (m, 9H), 6.37 (d, J = 15.6 Hz, 1H), 6.00 (d, J = 7.2 Hz, 1H), 5.34 (t, J = 8.4 Hz, 1H), 5.13 (d, J = 8.4 Hz, 1H), 4.19–4.11 (m, 4H), 2.32 (s, 3H), 2.30 (s, 3H), 1.10 (t, J = 7.2 Hz, 3H), 1.05 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 195.6, 168.3, 168.1, 167.7, 144.2, 140.6, 136.8, 135.0, 133.8, 132.1, 131.0, 129.9, 129.4, 129.0, 128.51, 128.49, 128.3, 127.5, 129.7, 126.6, 75.0, 73.3, 62.6, 62.1, 59.1, 21.7, 21.5, 13.8, 13.7 ppm. HRMS (ESI-TOF) m/z: [M + NH4]+ calcd for C33H33NO5, 541.2697; found: 541.2700.Diethyl 4-benzoyl-2-(4-bromophenyl)-5-(2-p-tolyl-vinyl)-4,5-dihydropyrrole-3,3-dicarboxylate (7n)
Brown liquid. Yield: 355 mg (60%). 1H NMR (400 MHz, CDCl3): δ 7.84 (d, J = 8.4 Hz, 2H), 7.44–7.15 (m, 15H), 6.49–6.48 (m, 1H), 4.24–4.09 (m, 3H), 2.31 (s, 3H), 1.18 (t, J = 7.2 Hz, 3H), 0.76 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 196.8, 168.3, 166.5, 166.4, 143.8, 140.8, 137.2, 136.6, 133.6, 132.0, 129.9, 128.7, 128.6, 128.5, 126.6, 119.2, 109.2, 75.0, 74.7, 62.9, 62.4, 60.3, 21.8, 13.7, 13.6 ppm. HRMS (ESI-TOF) m/z: [M + NH4]+ calcd for C32H30BrNO5, 605.1646; found: 605.1646.Diethyl 4-benzoyl-2-methyl-5-(2-p-tolyl-vinyl)-4,5-dihydro-pyrrole-3,3-dicarboxylate (7o)
Pale yellow liquid. Yield: 358 mg (80%). 1H NMR (400 MHz, CDCl3): δ 7.76 (d, J = 8.0 Hz, 2H), 7.30–6.99 (m, 7H), 6.49 (d, J = 15.2 Hz, 1H), 5.86 (q, J = 15.2 Hz, 1H), 5.22–5.10 (m, 2H), 4.32–4.10 (m, 4H), 2.49 (s, 3H), 2.36 (s, 3H), 1.31 (t, J = 7.2 Hz, 3H), 1.08 (t, J = 7.0 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 197.2, 166.9, 166.8, 144.1, 136.7, 135.7, 135.2, 133.2, 129.3, 128.6, 128.2, 127.4, 126.7, 126.5, 75.1, 74.2, 62.8, 61.7, 56.8, 21.6, 18.8, 14.0, 13.7 ppm. MS (ESI-TOF): m/z 465.12 [M + NH4]+. Anal. calcd for C27H29NO5: C 72.46, H 6.53, N 3.13; found: C 72.33, H 6.64, N 3.27.Diethyl 4-benzoyl-5-[2-(4-nitrophenyl)-vinyl]-2-thiophen-2-yl-4,5-dihydropyrrole-3,3-dicarboxylate (7p)
Yellow oily liquid. Yield: 354 mg (65%). 1H NMR (400 MHz, CDCl3): δ 8.01 (d, J = 7.2 Hz, 2H), 7.62–7.38 (m, 5H), 7.14–7.02 (m, 1H), 6.40–6.34 (m, 1H), 5.51– (t, J = 6.8 Hz, 1H), 5.01 (d, J = 6.0 Hz, 1H), 4.31–4.24 (m, 2H), 3.97 (q, J = 7.2 Hz, 2H), 1.21 (t, J = 7.2 Hz, 3H), 1.01 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 196.5, 167.8, 166.3, 161.0, 147.8, 137.47, 136.8, 133.75, 133.67, 133.14, 132.67, 132.5, 130.8, 129.0, 128.8, 128.6, 128.3, 127.8, 127.7, 127.4, 124.5, 75.0, 74.7, 63.3, 62.4, 59.7, 13.8, 13.5 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C29H26N2O7S, 547.1533; found: 547.1537.Diethyl 2-methyl-4-(4-methyl-benzoyl)-5-(2-p-tolyl-vinyl)-4,5-dihydropyrrole-3,3-dicarboxylate (7q)
Pale yellow liquid. Yield: 396 mg (86%). 1H NMR (400 MHz, CDCl3): δ 7.35 (d, J = 8.0 Hz, 2H), 7.00 (d, J = 8.0 Hz, 3H), 6.90 (d, J = 8.0 Hz, 3H), 6.79 (d, J = 7.6 Hz, 2H), 5.57 (d, J = 6.0 Hz, 1H), 5.34 (d, J = 8.0 Hz, 1H), 4.33–4.02 (m, 4H), 2.58 (s, 3H), 2.31 (s, 3H), 2.12 (s, 3H), 1.33 (t, J = 7.2 Hz, 3H), 0.99 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 198.3, 169.5, 166.9, 166.7, 143.2, 136.7, 135.4, 134.5, 128.6, 128.3, 128.2, 128.0, 75.7, 75.6, 62.9, 61.5, 57.8, 21.6, 21.0, 18.8, 14.0, 13.6 ppm. MS (ESI-TOF): m/z 494.28 [M + MeOH + H]+. Anal. calcd for C28H31NO5: C 72.86, H 6.77, N 3.03; found: C 72.92, H 6.87, N 3.11.Synthesis of diethyl 2-[2-benzoylamino-1-(4-methylbenzoyl)-4-phenyl-but-3-enyl]malonate (8)
To a solution of vinyl 1-pyrroline 7b (1.0 mmol, 510 mg) in 1,2-dichloroethane (5 mL) was added SnCl4 (1.0 mmol, 260 mg). After 15 min, water (1.0 mmol, 18 μL) was added and the reaction mixture was allowed to stir at room temperture for 8 h. After completion of the reaction, the reaction mixture was extracted with dichloromethane. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was purified by column chromatography using EtOAc/hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) to give 8. Pale yellow liquid. Yield: 400 mg (76%). 1H NMR (400 MHz, CDCl3): δ 7.83 (d, J = 8.4 Hz, 2H), 7.44–7.37 (m, 5H), 7.22–7.15 (m, 8H), 6.49 (d, J = 1.6 Hz, 1H), 5.02 (s, J = 1H), 4.24–(m, 3H), 3.74–3.54 (m, 3H), 2.31 (s, 3H), 1.18 (t, J = 7.2 Hz, 3H), 0.76 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 191.4, 171.7, 167.6, 144.0, 143.8, 139.8, 134.8, 132.0, 131.0, 129.9, 128.9, 128.6, 128.4, 127.7, 119.2, 109.2, 72.3, 62.9, 61.5, 59.9, 21.8, 13.8, 13.5 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C32H33NO6, 528.2381; found: 528.2376.
9) to give 8. Pale yellow liquid. Yield: 400 mg (76%). 1H NMR (400 MHz, CDCl3): δ 7.83 (d, J = 8.4 Hz, 2H), 7.44–7.37 (m, 5H), 7.22–7.15 (m, 8H), 6.49 (d, J = 1.6 Hz, 1H), 5.02 (s, J = 1H), 4.24–(m, 3H), 3.74–3.54 (m, 3H), 2.31 (s, 3H), 1.18 (t, J = 7.2 Hz, 3H), 0.76 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ 191.4, 171.7, 167.6, 144.0, 143.8, 139.8, 134.8, 132.0, 131.0, 129.9, 128.9, 128.6, 128.4, 127.7, 119.2, 109.2, 72.3, 62.9, 61.5, 59.9, 21.8, 13.8, 13.5 ppm. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C32H33NO6, 528.2381; found: 528.2376.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Science and Engineering Research Board (SERB), India for financial support and DST-FIST for instrumentation facilities at School of Chemistry, Bharathidasan University. M. T. thanks the University Grants Commission (UGC) for a BSR-RFSMS fellowship.Notes and references
- (a) H.-U. Reissig and R. Zimmer, Chem. Rev., 2003, 103, 1151–1196 CrossRef CAS PubMed; (b) M. Yu and B. L. Pagenkopf, Tetrahedron, 2005, 61, 321–347 CrossRef CAS; (c) T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504–5523 CrossRef CAS PubMed; (d) F. de Nanteuil, F. de Simone, R. Frei, F. Benfatti, E. Serrano and J. Waser, Chem. Commun., 2014, 50, 10912–10928 RSC; (e) M. A. Kerr, Isr. J. Chem., 2016, 56, 476–487 CrossRef CAS; (f) O. A. Ivanova and I. V. Trushkov, Chem. Rec., 2019, 19, 2189–2208 CrossRef CAS PubMed; (g) P. Singh, R. K. Varshnaya, R. Dey and P. Banerjee, Adv. Synth. Catal., 2020, 362, 1447–1484 CrossRef CAS; (h) D. B. Werz and A. T. Biju, Angew. Chem., Int. Ed., 2020, 59, 3385–3398 CrossRef CAS PubMed.
- (a) I. S. Young and M. A. Kerr, J. Am. Chem. Soc., 2007, 129, 1465–1469 CrossRef CAS PubMed; (b) C. L. Morales and B. L. Pagenkopf, Org. Lett., 2008, 10, 157–159 CrossRef CAS PubMed; (c) M. J. Campbell and J. S. Johnson, J. Am. Chem. Soc., 2009, 131, 10370–10371 CrossRef CAS PubMed; (d) A. F. G. Goldberg and B. M. Stoltz, Org. Lett., 2011, 13, 4474–4476 CrossRef CAS PubMed; (e) S. J. Gharpure and L. N. Nanda, Tetrahedron Lett., 2017, 58, 711–720 CrossRef CAS.
- (a) S. Schann, V. Bruban, K. K. Pompermayer, J. Feldman, B. Pfeiffer, P. Renard, E. Scalbert, P. Bousquet and J.-D. Ehrhardt, J. Med. Chem., 2001, 44, 1588–1593 CrossRef CAS PubMed; (b) M. Kitajima, N. Kogure, K. Yamaguchi, H. Takayama and N. Aimi, Org. Lett., 2003, 5, 2075–2078 CrossRef CAS PubMed; (c) T. H. Jones, V. E. Zottig, H. G. Robertson and R. R. Snelling, J. Chem. Ecol., 2003, 29, 2721–2727 CrossRef CAS PubMed; (d) R. P. Mason, Free Radicals Biol. Med., 2004, 36, 1214–1223 CrossRef CAS PubMed; (e) Y.-K. Xu, S.-P. Yang, S.-G. Liao, H. Zhang, L.-P. Lin, J. Ding and J.-M. Yue, J. Nat. Prod., 2006, 69, 1347–1350 CrossRef CAS PubMed; (f) T. Harada, J. Shimokawa and T. Fukuyama, Org. Lett., 2016, 18, 4622–4625 CrossRef CAS PubMed; (g) R. Miyauchi, C. Ono, T. Ohnuki and Y. Shiba, Appl. Environ. Microbiol., 2016, 82, 6414–6422 CrossRef CAS PubMed.
- G. Dannhardt and W. Kiefer, Arch. Pharm. Pharm. Med. Chem., 2001, 334, 183–188 CrossRef CAS PubMed.
- (a) P. J. Campos, A. Soldevilla, D. Sampedro and M. A. Rodriguez, Org. Lett., 2001, 3, 4087–4089 CrossRef CAS PubMed; (b) S. Peddibhotla and J. J. Tepe, J. Am. Chem. Soc., 2004, 126, 12776–12777 CrossRef CAS PubMed; (c) M. P. Sibi, T. Soeta and C. P. Jasperse, Org. Lett., 2009, 11, 5366–5369 CrossRef CAS PubMed; (d) V. B. R. Iska, V. Verdolino, O. Wiest and P. Helquist, J. Org. Chem., 2010, 75, 1325–1328 CrossRef CAS PubMed; (e) D.-S. Wang, Z.-S. Ye, Q.-A. Chen, Y.-G. Zhou, C.-B. Yu, H.-J. Fan and Y. Duan, J. Am. Chem. Soc., 2011, 133, 8866–8869 CrossRef CAS PubMed; (f) M. Strohmeier, K. Leach and M. A. Zajac, Angew. Chem., Int. Ed., 2011, 50, 12335–12338 CrossRef CAS PubMed; (g) Z.-W. Guo, X. Huang, J.-M. Mao, W.-D. Zhu and J.-W. Xie, RSC Adv., 2013, 3, 25103–25109 RSC; (h) X. Zhu and S. Chiba, Chem. Commun., 2016, 52, 2473–2476 RSC; (i) X. Bao, Q. Wang and J. Zhu, Angew. Chem., Int. Ed., 2017, 56, 9577–9581 CrossRef CAS PubMed; (j) H. Jiang and A. Studer, Angew. Chem., Int. Ed., 2017, 56, 12273–12276 CrossRef CAS PubMed; (k) V. Kanchupalli and S. Katukojvala, Angew. Chem., Int. Ed., 2018, 57, 5433–5437 CrossRef CAS PubMed; (l) N. S. Medra, A. La-Venia and S. A. Testero, RSC Adv., 2019, 9, 6804–6844 RSC; (m) J. Aleman, R. I. Rodriguez and L. Mollari, Angew. Chem., Int. Ed., 2021, 60, 4555–4560 CrossRef PubMed.
- (a) M. Yu and B. L. Pagenkopf, J. Am. Chem. Soc., 2003, 125, 8122–8123 CrossRef CAS PubMed; (b) A. O. Chagarovskiy, E. M. Budynina, O. A. Ivanova and I. V. Trushkov, Chem. Heterocycl. Compd., 2010, 46, 120–122 CrossRef CAS; (c) B. Cui, J. Ren and Z. Wang, J. Org. Chem., 2014, 79, 790–796 CrossRef CAS PubMed; (d) B. L. Pagenkopf and N. Vemula, Eur. J. Org. Chem., 2017, 2017, 2561–2567 CrossRef CAS.
- G. Sathishkannan and K. Srinivasan, Org. Lett., 2011, 13, 6002–6005 CrossRef CAS PubMed.
- V. J. Tamilarasan and K. Srinivasan, J. Org. Chem., 2019, 84, 8782–8787 CrossRef CAS PubMed.
- (a) M. Thangamani and K. Srinivasan, J. Org. Chem., 2018, 83, 571–577 CrossRef CAS PubMed; (b) M. Thangamani and K. Srinivasan, J. Org. Chem., 2021, 86, 1172–1177 CrossRef CAS PubMed.
- T. H. Jones, P. J. Devries and P. Escoubas, J. Chem. Ecol., 1991, 17, 2507–2518 CrossRef CAS PubMed.
- G. Yang, Y. Shen, K. Li, Y. Sun and Y. Hua, J. Org. Chem., 2011, 76, 229–233 CrossRef CAS PubMed.
- CCDC 2048692 for compound 8. See the ESI† for details.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra of all products and X-ray structural information of 8. CCDC 2048692. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ra01194d |
| This journal is © The Royal Society of Chemistry 2021 |