Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A green metal-free “one-pot” microwave assisted synthesis of 1,4-dihydrochromene triazoles†
Tânia M. F. Alves,
Guilherme A. M. Jardim and
Marco A. B. Ferreira
and
Marco A. B. Ferreira *
*
Centre for Excellence for Research in Sustainable Chemistry (CERSusChem), Department of Chemistry, Federal University of São Carlos – UFSCar, Rodovia Washington Luís, Km 235, SP-310, São Carlos, São Paulo, 13565-905, Brazil. E-mail: marco.ferreira@ufscar.br
First published on 9th March 2021
Abstract
The synthesis of several 4-aryl-1,4-dihydrochromene-triazoles was achieved via a metal-free “one-pot” procedure using PEG400 as the sole solvent in an eco-friendly process. Using microwave irradiation, the triazole derivatives were obtained in good yields and short reaction times starting from readily accessible building blocks.
Novel green technologies play a pivotal role in modern society economics and way of life, and are being considered an important engine of transformation by directly affecting people's daily lives.1 In this sense, the “Click Chemistry” philosophy encompasses several features within the “Twelve Principles of Green Chemistry”, including mild reaction conditions, simple operation, simple product isolation procedures, solvent-free conditions, readily available starting materials and reagents, high yields and selectivity.2
Among the “Click” reaction arsenal, the formation of 1,2,3-triazoles emerged as a reliable approach for the synthesis of novel compounds, being present in several fields of science, particularly in biomedical chemistry and drug design.3 1,2,3-Triazoles are useful building blocks in organic chemistry owing to their stablily to moisture, oxidation, and metabolism in organisms.4 Moreover, these moieties are powerful pharmacophores, playing an important role in bio-media.5 Mainly represented by the Cu(I) catalyzed Huisgen cycloaddition over the last years, the majority of reports englobes the use of sodium/organic azides and alkynes.6
Green and sustainable triazole synthesis approaches have gained the attention of chemists more recently, with highlights to metal-free conditions,7 heterogeneous reactions employing an immobilized catalyst8 and microwave irradiation.9 Regarding green protocols that use nitroolefins as dipolarophiles,10 the metal-free acid-catalyzed approach by Guan et al.11 as well as the use of palladium- and copper-supported mesoporous polyaromatic hydrocarbons by Banerjee et al.12 represent important advances in this field. However, due the lack of solubility of azides, DMSO and DMF are the solvents of choice for those transformations.
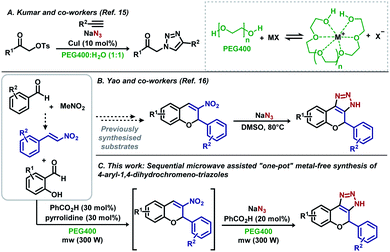
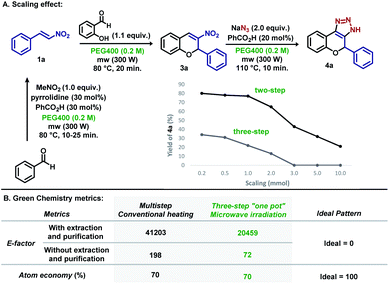
The use of water as a benign solvent in organic reactions is always appreciated as it is one of the most abundant, cheapest, and greener solvents.13 As another important green alternative, polyethylene glycol 400 (PEG400) has been used to enhance the solubility of organic compounds. An important feature of PEG400 is its ability to act as a crown ether,14 enhancing the solubility of metal-based salts such as sodium azide (Scheme 1A).
Kumar and Varma demonstrated that aqueous PEG400 is an excellent solvent for Cu(I)-catalysed Huisgen cycloadditions (Scheme 1A).15 In the same year, Yao et al. reported the synthesis of 4-aryl-1,4-dihydrochromene-triazoles from 3-nitrochromens in DMSO (Scheme 1B).16 In recent years, nitroolefins have been efficiently explored, allowing the direct obtention of 1H-1,2,3-triazoles using sodium azides with concomitant aromatization,10–14,16 and HNO2 release, which assists in the decomposition of residual NaN3.10f Aiming for a more sustainable process, based on microwave irradiation and sequential metal-free “one-pot” reactions, we developed a methodology starting from simple building blocks such as substituted benzaldehydes and nitromethane, avoiding the manipulation of potentially toxic nitroalkenes, and using PEG400 as the sole solvent for the synthesis of aryl-1,4-dihydrochromene 1H-1,2,3-triazoles (Scheme 1C). We believe that this novel procedure can open new possibilities for the synthesis of such an important scaffold in medicinal chemistry.
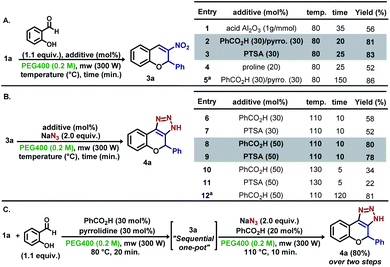
Our study began with the optimization of a reaction model consisting of 1a, sodium azide and PEG400 as the solvent under microwave irradiation conditions (300 W) (Table 1-entry 1). With a reaction time of 10 min at 120 °C, a complex mixture of products was obtained. In the second attempt, temperature was maintained at 80 °C for 20 min, and 2a was obtained in 37% yield (entry 2). Based on previous reports,11 catalytic amounts of acids were employed, aiming at reducing the formation of 1,3,5-triphenylbenzene. The addition of p-toluene sulfonic acid (PTSA) resulted in a slightest yield improvement, while the use of camphor sulfonic acid (CSA) led to a decrease in yield (entries 3–4). Although an increase in the yield was not observed by increasing the concentration of the reaction medium (entry 5), a better result was observed when catalytic amounts of several acids were used under the same conditions (entry 6–9), and the best result was found for benzoic acid (62%, entry 9), with a minor increase in the reaction time. Previous results showed similar performance for acetic acid as the solvent.10f Next, an increase in the amount of benzoic acid led to a decrease in the yield (entries 10–11) and attempts of using mixtures of solvents resulted in the reaction inhibition (entries 12–13). Furthermore, doubling the reaction concentration, decreasing the reaction temperature and raising the reaction time led to unsatisfactory results (entries 14–15).
| Entry | Solvent | Temp. | Time | 1a (M) | Additive (mol%) | Yielda (%) |
|---|---|---|---|---|---|---|
| a All yields were isolated. | ||||||
| 1 | PEG400 | 120 | 10 | 0.2 | — | Mixture |
| 2 | PEG400 | 80 | 20 | 0.2 | — | 37% |
| 3 | PEG400 | 80 | 20 | 0.2 | PTSA (10) | 40% |
| 4 | PEG400 | 80 | 20 | 0.2 | CSA (10) | 30% |
| 5 | PEG400 | 80 | 20 | 0.4 | — | 35% |
| 6 | PEG400 | 80 | 20 | 0.4 | PTSA (10) | 48% |
| 7 | PEG400 | 80 | 20 | 0.4 | CSA (10) | 54% |
| 8 | PEG400 | 80 | 20 | 0.4 | TFA (10) | 30% |
| 9 | PEG400 | 80 | 25 | 0.4 | PhCO2H (10) | 62% |
| 10 | PEG400 | 80 | 25 | 0.4 | PhCO2H (20) | 58% |
| 11 | PEG400 | 80 | 25 | 0.4 | PhCO2H (30) | 48% |
| 12 | PEG400/EtOH (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
80 | 35 | 0.4 | PhCO2H (10) | Trace |
| 13 | PEG400/H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
80 | 35 | 0.4 | PhCO2H (10) | Trace |
| 14 | PEG400 | 80 | 20 | 0.8 | PhCO2H (10) | 34% |
| 15 | PEG400 | 60 | 35 | 0.4 | PhCO2H (10) | Trace |
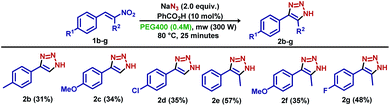
To demonstrate the effectiveness of the optimized reaction, model substrates 1b–g were previously synthesized and applied as starting materials, and resulted in derivatives 2b–g in yields lower than obtained by that the model substrate 1a (Scheme 2). Apparently, no differences were observed for para-substituted benzo nitroolefins with electron-donating and electron-withdrawing groups (derivatives 2b–d). The reaction proceeded smoothly for substrates bearing a methyl group in the olefin terminal carbon (derivatives 2e–g), with unexpected lower yields.
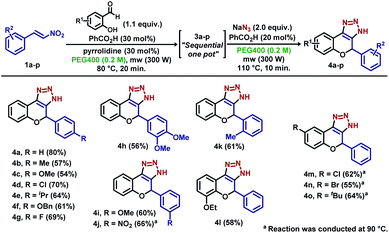
Inspired by the previous results, new experiments were planned to expand the methodology for the obtention of dihydrochromene-triazole moieties in a “one-pot” microwave procedure, which initially explored the individual elementary steps. For this endeavour, the synthesis of nitrochromene 3a was conducted using parameters obtained in the optimization steps (Scheme 3A). The reaction of 1a with salicylaldehyde at 80 °C in 35 min using acid Al2O3 gave 3a in 56% yield (entry 1). Best results were found using benzoic acid/pyrrolidine (pyrro.) (81%, entry 2) and PTSA as additives (83%, entry 3). As shown in entry 5, reaction carried out under conventional heating over 2.5 h resulted in minor yield improvements. Next, 3a was submitted to the reaction with sodium azide using different amounts of benzoic acid and PTSA to find the optimized quantity of the additives (Scheme 3B). For our delight, the reaction with 50 mol% of both acids afforded 4a in 80% and 78%, respectively after fine tuning the reaction temperature and time (10 min at 110 °C) (entries 8–9). Similar yields were obtained by performing the reaction under conventional heating over 2.5 h (entry 12). Starting from 1a, the “one pot” synthesis of 4a was accomplished in 80% yield over two steps, as exemplified in Scheme 3C.
 | ||
| Scheme 3 Optimization and fine tuning for the “one-pot” synthesis of 4a. aReaction conducted using conventional heating (oil bath). | ||
Next, the sequential procedure was used to synthesize a series of novel derivatives bearing different structural features (Scheme 4). Starting from phenyl-substituted nitroolefins 1a–k, the novel sequential “one-pot” reaction was executed, which afforded derivatives 4a–k in average to high yields in a two-step procedure. The reaction proceeded smoothly with electron-withdrawing groups at the para position, as showed for derivatives 4d and 4g, as well as derivative 4j, which bears a nitro group at the meta position. A slight decrease in the yield was observed for derivatives bearing electron-donating groups at the para position (4b–c, 4e–f), meta and ortho positions (4i and 4k), and same behaviour was observed for derivative 4h, which bears a methoxy group at both meta and para positions. Different salicylaldehyde derivatives were also employed, which afforded derivatives 4l–o in good yields. Slight reduction in the yield was observed for both electron-withdrawing and electron-donating groups, with major decrease for brominated derivative 4n (55%) and yields ranging from 55% to 64% (Scheme 4).
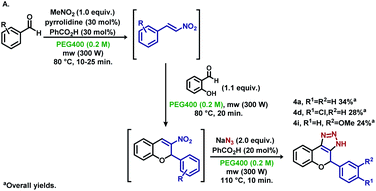
To expand the functionality of the methodology, the synthesis of substituted nitroolefins was conducted via microwave irradiation to verify the orthogonality of the reactants needed in the next steps. To our delight, substituted 2-nitrovinyl benzenes were detected via the TLC analysis, and further steps were carried out for the synthesis of aryl-1,4-dihydrochromeno-triazoles (Scheme 5A). Derivatives 4a, 4d and 4i were obtained in good yields per reaction step, showing that a three-step “one-pot” procedure is feasible for the synthesis of these triazole moieties.
The methodology was scaled to provide information about the robustness of microwave irradiation. As shown in Scheme 6A, scaling up to 0.2, 0.5 and 1 mmol (2.5× and 5× folder) provided 4a with almost no variation in the yield for the two-step process, although a decrease in the yield was observed for the three-step reaction. Scaling up to 2 mmol (10× folder) in the two-step reaction resulted in almost 20% less yield, and the decrease in yield was observed for larger amounts (3 mmol–15× folder, 5 mmol–25× folder and 10 mmol–50× folder). For the three-step procedure, no product was observed after scaling up to 3 mmol.
Next, two green chemistry metrics (E-factor and atom economy) were calculated for the conventional multistep procedure, and the three-step microwave irradiation method (see ESI†).17 As shown in Scheme 6B, E-factor was determined for the two procedures by comparing parameters regarding the extraction and purification in both cases. It is notable that the three-step microwave irradiation procedure shows the E-factor value closer to the ideality mostly because it “skips” two classical extraction and purification steps. When calculation was performed in the absence of these two parameters, the method presents an E-factor = 72, which is mostly due to the decrease in the amount of the solvent used in the reaction steps. In both cases, the atom economy is the same, since this green chemistry metric does not consider the other reagents, solvents and catalysts used in the purification and extraction steps.
Conclusions
In summary, a three step “one-pot” procedure was successfully developed for the synthesis of important biological motifs using PEG400 as the sole solvent in the process. All reaction steps were carefully investigated to determine the best reaction parameters encompassing the “one-pot” methodology. Considered an eco-friendly solvent, the use of PEG400, allied with microwave irradiation, provided a fast, efficient, and green reaction for obtaining dihydrochromene-triazole hybrids in good overall yields. Scaling experiments were conducted, showing the limit in which the reaction maintains its robustness. A quantitative comparison based on Green Chemistry metrics between the multistep conventional heating and “one-pot” microwave irradiation procedure showed that the second method presents advantages in terms of sustainability.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge FAPESP (grants 2014/50249-8; 2020/10246-0; and 2020/01255-6), GlaxoSmithKline, CAPES (Finance Code 001), PNPD/CAPES and CNPq for funding and fellowships.References
- M. J. Arévalo, O. López and M. V. Gil, Green Chemical Synthesis and Click Reactions, in Click React. Org. Synth., Wiley-VCH Verlag GmbH & Co. KGaA, 2016, vol. 77, pp. 77–97, DOI:10.1002/9783527694174.ch3.
- P. T. Anastas and J. C. Warner, Green chemistry: theory and practice, New York: Oxford University Press, 1998 Search PubMed.
- (a) X. Wang, B. Huang, X. Liu and P. Zhan, Drug Discovery Today, 2016, 21, 118 CrossRef CAS PubMed; (b) S. G. Agalave, S. R. Maujan and V. S. Pore, Chem. –Asian J., 2011, 6, 2696 CrossRef CAS PubMed; (c) J.-F. Lutz and Z. Zarafshani, Adv. Drug Delivery Rev., 2008, 60, 958 CrossRef CAS PubMed; (d) E. Kim and H. Koo, Chem. Sci., 2019, 10, 7835 RSC; (e) M. Duan, L. H. Zhang and J. Li, Chem. J. Chin. Univ., 2008, 29, 2118 CAS.
- (a) D. Dheer, V. Singh and R. Shankar, Bioorg. Chem., 2017, 71, 30 CrossRef CAS PubMed; (b) S. B. Ferreira, A. C. Sodero, M. F. Cardoso, E. S Lima, C. R. Kaiser, F. P. Silva and V. F. J. Ferreira, Med. Chem., 2010, 53, 2364 CrossRef CAS PubMed.
- R. kharb, P. C. Sharma and M. S. Yar, J. Enzym. Inhib. Med. Ch., 2011, 26, 1 CrossRef CAS PubMed.
- (a) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS; (b) H. C. Kolb and B. K. Sharpless, Drug Discov. Today, 2003, 8, 1128 CrossRef CAS PubMed.
- (a) V. M. Muzalevskiy, M. N. Mamedzade, V. A. Chertkov, V. A. Bakulev and V. G. Nenajdenko, Mendeleev Comum, 2018, 28, 17 CrossRef CAS; (b) M. K. Hussain, M. I. Ansari, R. Kant and K. Hajela, Org. Lett., 2014, 16, 560 CrossRef CAS PubMed; (c) D. Sahu, S. Dey, T. Pathak and B. Ganguly, Org. Lett., 2014, 16, 2100 CrossRef CAS PubMed.
- (a) B. Dervaux and F. D. Prez, Chem. Sci., 2012, 3, 959 RSC; (b) B. J. Borah, D. Dutta, P. P. Saikia, N. C. Barua and D. K. Dutta, Green Chem., 2011, 13, 3453 RSC; (c) P. Veerakumar, M. Velayudham, K.-L. Lub and S. Rajagopal, Catal. Sci. Technol., 2011, 1, 1512 RSC.
- (a) P. Bhuyan, P. Bhorali, A. J. Bhuyan and L. Saikia, Tetrahedron Lett., 2018, 59, 1587 CrossRef CAS; (b) E. C. Lindsay, F. H. John, S. P. Marshall and D. Y. Douglas, Bioorg. Med. Chem. Lett., 2018, 28, 81 CrossRef PubMed; (c) A. Sacchetti, E. Mauri, M. Sani, M. Mais and F. Rossi, Tetrahedron Lett., 2014, 55, 6817 CrossRef CAS.
- (a) D. R. Mishra, S. Nayak, B. P. Raiguru, S. Mohapatra, M. B. Podh, C. R. Sahoo and R. N. Padhy, J. Heterocyclic Chem., 2021, 58, 111 CrossRef CAS; (b) S. B. Autade and K. G. Akamanchi, Synth. Comumm., 2019, 49, 1947 CrossRef CAS; (c) R. Jianga, H.-B. Suna, S. Lia, K. Zhana, J. Zhoua, L. Liua, K. Zhanga, Q. Liangb and Z. Chena, Synth. Comumm., 2018, 48, 2652 CrossRef; (d) R. J. Reddy, Md. Waheed, T. Karthik and A. Shankar, New J. Chem., 2018, 42, 980 RSC; (e) L. Yang, Y. Wu, Y. Yang, C. Wen and J.-P. Wan, Beilstein J. Org. Chem., 2018, 14, 2348 CrossRef CAS PubMed; (f) D. Li, L. Liu, Y. Tian, Y. Ai, Z. Tang, H.-B. Sun and G. Zhang, Tetrahedron, 2017, 73, 3959 CrossRef CAS; (g) P. Sharma, N. P. Kumar, K. R. Senwar, O. Forero-Doria, F. M. Nachtigall, L. S. Santos and N. Shankaraiah, J. Braz. Chem. Soc., 2017, 28, 589 CAS; (h) V. Y. Korotaev, I. B. Kutyashev, A. Y. Barkov and V. Y. Sosnovskikh, Chem. Heterocycl. Compd., 2017, 53, 597 CrossRef CAS; (i) G. Schwendt and T. Glasnov, Monatsh. Chem., 2017, 148, 69 CrossRef CAS; (j) T. Wang, X.-C. Hu, X.-J. Huang, X.-S. Li and J.-W. Xie, J. Braz. Chem. Soc., 2012, 23, 1119 CrossRef CAS; (k) B. Quiclet-Sire and S. Z. Zard, Synthesis, 2005, 19, 3319 CrossRef; (l) R. Vroemans and W. Dehaen, The Chemistry of 3-Nitrochromenes, in Targets in Heterocyclic Systems, Scietá Chimica Italiana, 2018, vol. 2, p. 318 Search PubMed.
- X.-J. Quan, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Org. Lett., 2014, 16, 5728 CrossRef CAS PubMed.
- (a) A. Saha, C.-M. Wu, R. Peng, R. Koodali and S. Banerjee, Eur. J. Org. Chem., 2019, 104 CrossRef CAS; (b) S. Payra, A. Saha and S. Banerjee, Chem. Cat. Chem., 2018, 10, 5468 CAS.
- (a) V. Polshettiwar and R. S. Varma, Chem. Soc. Rev., 2008, 37, 1546 RSC; (b) V. Polshettiwar and R. S. Varma, Acc. Chem. Res., 2008, 41, 629 CrossRef CAS PubMed.
- J. Chen, K. pear, J. G. Huddleston and R. D. Rogers, Green Chem., 2005, 7, 64 RSC.
- D. Kumar, V. B. Reddy and R. S. Varma, Tetrahedron Lett., 2009, 50, 2065 CrossRef CAS.
- P. M. Habib, B. R. Raju, V. Kavala, C.-W. Kuo and C.-F. Yao, Tetrahedron, 2009, 65, 5799 CrossRef CAS.
- (a) B. M. Trost, Science, 1991, 254, 1471 CrossRef CAS PubMed; (b) B. M. Trost, Angew. Chem., Int. Ed., 1995, 34, 259 CrossRef CAS; (c) R. A. Sheldon, Chem. Ind., 1992, 903 CAS; (d) R. A. Sheldon, Green Chem., 2007, 9, 1273 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01169c |
| This journal is © The Royal Society of Chemistry 2021 |