Open Access Article
Open Access ArticleEnvironmental investigation of pollutants in coal mine operation and waste dump area monitored in Ordos Region, China
Ang Li ab,
Changkun Chen*a,
Jie Chena and
Peng Leia
ab,
Changkun Chen*a,
Jie Chena and
Peng Leia
aInstitute of Disaster Prevention Science and Safety Technology, Central South University, Changsha, 410075, China. E-mail: cckchen@csu.edu.cn
bCollege of Forestry, Inner Mongolia Agricultural University, Hohhot, 010019, China
First published on 10th March 2021
Abstract
The increasingly severe emissions of greenhouse and poisonous gases from environmentally unsafe stockpiled coal mine waste dumps have urged people from the academia as well as the industry to focus on environmental impact assessment. In this study, one-year air pollutant monitoring was conducted at the Qipanjing coalfield in Inner Mongolia of China for determining the distribution pattern statue of pollutant exposure and its main driving factors. We used FTIR spectroscopy to measure the inorganic compounds in particulate matter with a diameter of less than 2.5 μm. The spatial and temporal distribution characteristics of leading pollutants, including PM2.5, PM10, SO2, NO2, O3 and CO were analyzed. Firstly, the research showed that the temporal and spatial distribution of pollutants in the coal mine waste area is non-homogeneous. Secondly, some meteorological parameters, such as wind speed, relative humidity, temperature, and rainfall, were found to have significant effects on air pollutant distribution. Stable atmospheric conditions were unfavorable for the diffusion of pollutants and prolong the pollution process. Finally, in the vicinity of coalfields, SO2 and NO2 are present in high concentrations in air. Primary reasons for such high values are coal mining-related activities and active mine fires. This study will help to offer valuable and detailed information for understanding and interpreting the pollution source.
1. Introduction
The increasingly severe environmental pollution problems, such as haze and poisonous gas dust usually induced by coal mine waste fires from coal industrial processes, have gained an increasing amount of attention from both the academia and the industry.1 With the multiple release sources and multiple channels of active coalfield fires, it is difficult to control and eliminate toxic gases produced. Coalfield fires not only lead to severe air pollution but also pose a great threat to the residents of the mining area.2–4 Coal fires, which can happen in any coal producing country all over the world at any time, have resulted in damage to the atmospheric environment, which has evolved into a global disaster. Coal mine fires have been reported in China, India, the United States, Australia, Russia, Indonesia, Venezuela, South Africa, and other countries.5 A large number of opencast coal mines are distributed in the central and western region of China. The coal spontaneous combustion in coalfields is extremely common, particularly in the middle reaches of the Yellow River. The combustion of coal in the mining area could produce a large quantity of toxic gases, which can pollute the environment. According to the statistical data, the spontaneous combustion of coal seams and coal waste piles releases a lot of CO, SO2, H2S, NOX, benzopyrene, and other noxious and detrimental gases.6–8 Meanwhile, it has been proved that the content of Pb, Cr, Hg, F, and As in the emitted flue gas exceeded the industrial pollution standard in Inner Mongolia.4,6The severity of coal mine fires was disclosed and the origin of coal mine fires and related gases was described by Stracher.9 Many coal seams or gangue hills are piled up, which not only occupy land but also pollute the surrounding soil and groundwater. Spontaneous combustion and explosion would take place under the appropriate conditions of temperature exceeding approximately 80 °C and coal accumulation.10 This produces high pressure, high-temperature heat waves, dust, and shock waves, and most importantly, releases greenhouse and toxic gases to affect the health of residents in the mining area.11,12
Recent studies have mainly focused on the simulations of mercury and carbon emissions from coal seam fires.13 The distribution of the pollution sources, topography, and meteorological factors in the mining area would lead to a difference in the spatial and temporal distribution of atmospheric pollution. The temporal and spatial distribution of atmospheric pollution has a distinctive regional characteristic, and the temporal and spatial distribution of different pollutants varies considerably.14 Therefore, the research on air pollutant monitoring in coal mining areas is vital because it can be used to assess the potential hazards and combined effects to determine short-term and long-term pollutant dispersion, and to improve the relevant assessment standards and regulations of air quality.14,15
The research on air pollutant monitoring is of great significance to guide air quality management efforts, to assess potential comprehensive effects, to reasonably allocate the possible emission sources, to determine the short-term and long-term migration of pollutants, and to improve air quality control laws and regulations. In addition, mining and its related activities, such as near the railway branch line, burning coal in the open air, and heavy vehicle load, are the main reasons for the high concentration of air pollutants in coalfields.16 The method of spatio-temporal interpolation for air quality data was applied to the daily nitric oxide concentration measurement of monitoring stations around the city, and the temporal and spatial variability of NO observations could be analyzed.17,18 The geostatistical characterization of the nitrogen dioxide concentration in an urban area was mapped by the co-Kriging method to improve the estimation of seasonal or annual concentrations.19 The metal composition released by a forest fire is similar to that released by a coal fire.20 Numerous studies have focused on the spatial and temporal variations of primary fine particles with diameters smaller than 10 μm (PM10) and 2.5 μm (PM2.5), and harmful gases such as sulphur dioxide (SO2), nitrogen oxides (NOx), ammonia (NH3), and volatile organic compounds (VOCs).11,13–15,21 Substantial seasonal differences and long-term trends of air pollutant levels have been noticed in several regions around the world.22–24 The seasonal and trend disparities among the cities could be caused by several factors, including the diffusion of local pollution sources, the existence of distant pollution sources affecting the city, and the meteorological and topographical conditions of the area.25,26 Although previous studies have tried to give some data about potential gas emissions during coal mine fires, these studies have focused on underground coal mine fires. This paper mainly studies environmental pollution in the process of coal mine fire activities in coalfield fire areas, and determines the pollution sources of coal mine fire and their influences. This also provides some ideas for the management strategy of the Chinese government to limit the pollution in the coalfield fire area.
The present study was conducted based on the one-year study of 2018 air pollutant concentration monitoring the coal mine waste area in the Qipanjing town of the Inner Mongolia Autonomous Region, China. The aim of this study is to describe the origin of coal fires and related gases, such as PM10, PM2.5, NO2, SO2, CO, and O3, to explore the spatiotemporal variations, statistical distributions, and conventional air pollutants emitted by coal mine fires, and to analyze the influencing factors of the spatial and temporal emission distribution.
2. Materials and methods
2.1 Study area
The heavy industry gathering area in Ordos Region was selected as the study area, including Mengxi Industrial Park and Qipanjing Economic Development Zone, in which there are some large-scale industrial enterprises and power plants with serious pollution. It is located at 39°22′32′′ north latitude and 107°00′44′′ east longitude, and belong to a typical temperate continental monsoon climate. Moreover, it is a crucial traffic hub of the Ordos City, connecting the Ningxia Hui Autonomous Region and the Wuhai City. The entire planned area of the industrial zone is 85 square kilometres. The integral area of Qipanjing Town is 3614 square kilometres and the total population was 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 at the end of 2018.
000 at the end of 2018.
The annual sunshine hours are about 3000 hours, the annual average temperature is about 6.4 °C, the annual precipitation is about 250 mm, and the annual evaporation is about 3000 mm. The precipitation is mainly concentrated in July–September and the frost-free period is over 122 days.27 In particular, natural climatic conditions such as drought, frequent wind, and strong sunshine, provide the necessary conditions in this area that lead to a frequent occurrence of coal fires.
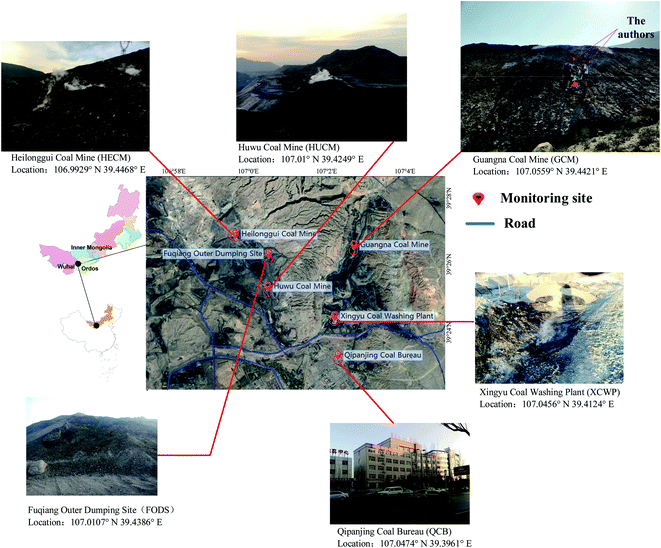
Based on the principle of convenient scheduling and sampling, six different sites were selected as the monitoring points of the air quality in the coalfield fire area to analyze the air pollution status. The location of monitoring points is shown in Fig. 1.
We used the micro-air quality sensors Microair A108, which are supplied by Fairsense (Beijing) Environmental Technology Co. Ltd, to measure PM10, PM2.5, SO2, NO2, O3, CO, temperature, humidity, and other parameters in the atmosphere. According to the ambient air quality standard GB3095-2012 (MEP, 2012), the pollutant concentration was calculated using the following methods. The NO2 concentration was determined by chemiluminescence and differential optical absorption spectroscopy (DOAS). In addition, UV fluorescence and DOAS were used for the determination of SO2 concentration. Ultraviolet absorption spectroscopy and DOAS determined the concentration of O3. The CO concentration was measured by the filtered infrared and non-dispersive infrared absorption methods. The measurement of the mass concentration of PM was conducted by the cone element oscillating microbalance and the gamma-ray method.
Samples of PM2.5 were collected on filters of HUCM, QCB, XCWP, and FODS monitoring sites at four sites. In a volume sampler at the inlet of PM2.5, PM2.5 aerosol was collected on a 3 cm × 3 cm glass fiber filter at a speed of 16.7 L min−1 (once every 4 hours). The drug NH4NO3 used in the experiment was analytically pure. The sampling period is from January 20, 2018 to January 25, 2018. In this experiment, all the samples were analyzed using a Fourier-transform infrared spectrometer (Thermo Nicolet 380) with smart single-bounce ATR and multiple-bounce ATR. The wavenumber of the sample spectrum is 400–4000 cm−1 and the resolution is 4 cm−1. By measuring the infrared spectrum of the sample film on the whole substrate, the linear relationship between the mass of sulfate, nitrate, and ammonium ions and its absorbance is established, and calibration is realized.
2.2 Data description and methods
The temporal heterogeneity data of the ruling air pollutants were analyzed and compared in terms of the dominant days of each pollutant. The number of days with PM10 as the dominant air pollutant was more than 80, which was the greatest among all the pollution days in spring at all the six sites. The number of days with PM2.5 as the dominant air pollutant was more than 68, which was the largest among all the pollution days in winter at all the sites. The sum of days on which SO2 was the dominant air pollutant was more than 20, which was the largest among all the seriously polluted days at each site in winter and spring.The Air Quality Index (AQI) was calculated using the following formula:
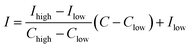 | (1) |
The correlation coefficient was defined as follows:
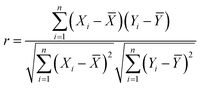 | (2) |
![[X with combining macron]](https://www.rsc.org/images/entities/i_char_0058_0304.gif) and Ȳ are the average values of X and Y, respectively, and i is the total number of variables. The terms “significant/strong”, “moderate”, and “insignificant” apply to correlation matrix analysis (CMA).
and Ȳ are the average values of X and Y, respectively, and i is the total number of variables. The terms “significant/strong”, “moderate”, and “insignificant” apply to correlation matrix analysis (CMA).
Principal component analysis (PCA) is a multivariate process, which reduces the dimension of the data sets containing a large number of air pollutant concentrations (PM2.5, PM10, SO2, NO2, CO, and O3). Therefore, factor analysis could be used to reduce the dimension of the variable. According to the theory of factor analysis, either of the following equations can be obtained.
 | (3) |
Through factor analysis, the contribution of various air pollutants to the main factors can be obtained. Therefore, the factor score of the monitoring sites could be calculated using eqn (4)
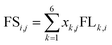 | (4) |
3. Results
3.1 Daily trends and FTIR analysis
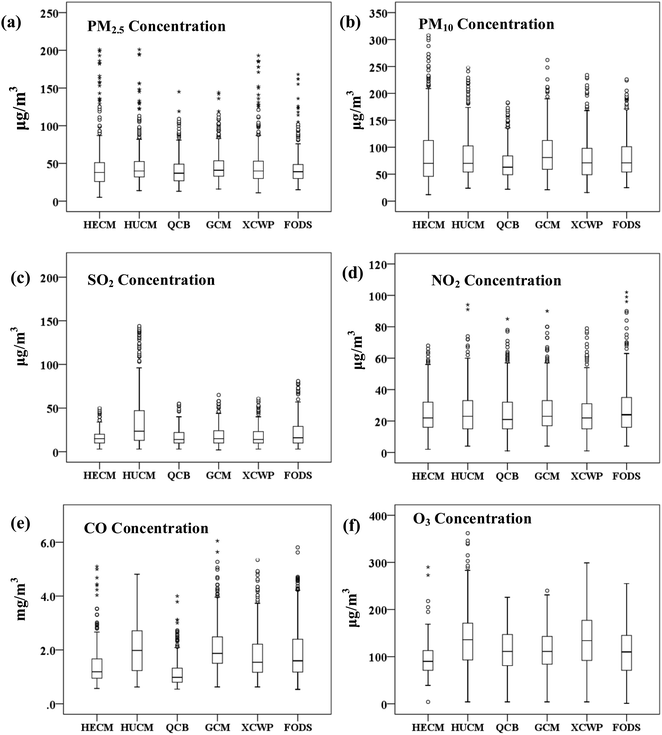
Through the analysis of the characteristics of pollutants at different observation points in the coalfield area, the trend chart of average pollutant concentration at each monitoring site in 2018 was obtained.On February 22, the concentrations of PM2.5 and PM10 were found to reach 331 μg m−3 and 672 μg m−3, respectively, at the monitoring site of Heilonggui Coal Mine (HECM). At the Huwu Coal Mine (HUCM) and Xingyu Coal Washing Plant (XCWP) monitoring sites, the PM2.5 concentrations on February 20 were seen to reach 724 μg m−3 and 674 μg m−3, respectively. It can be seen from Fig. 3 that the PM2.5 concentration is higher in spring and winter, and lower in summer and autumn.
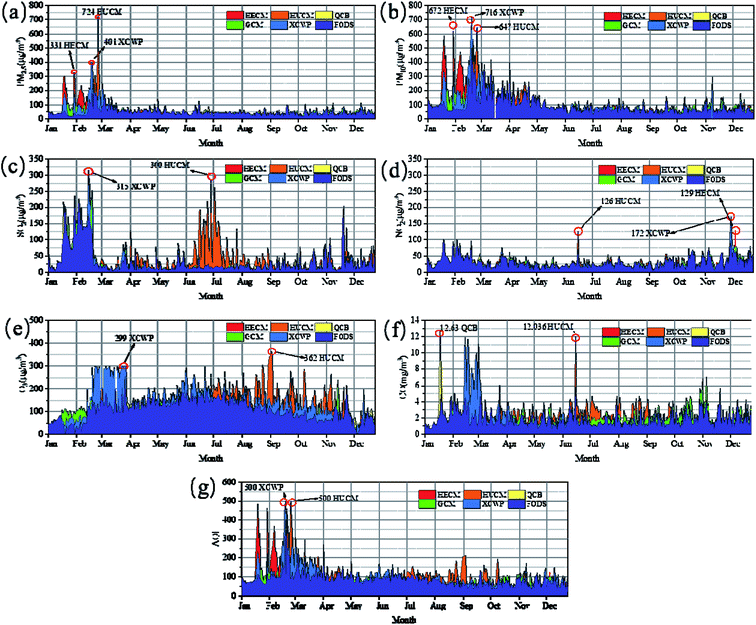 | ||
| Fig. 2 Monthly variation in concentration of (a) PM2.5, (b) PM10, (c) SO2, (d) NO2, (e) O3, (f) CO, and (g) AQI at six monitoring sites in 2018. | ||
The mean and range of O3 found resulted in it being the most prevalent gas recorded in the atmosphere at all the six stations, followed by NO2, SO2, and CO, which were clearly recorded at a higher concentration at HUCM with a mean value of 102.815 μg m−3 (4–362 μg m−3). The other gas that was also recorded at a very high concentration at HUCM was SO2, which had an average concentration of 42.899 μg m−3 (3–300 μg m−3).
According to the analysis of the AQI index, the highest average AQI index of HUCM was 102.8, followed by XCWP, which was 98. The average AQI index of QCB site was 77.4 and the average AQI index of the HUCM site was 1.3 times that of the QCB station. The average value of the AQI index of the other sites ranged from 84 to 88 (Table 1).
| Parameters | Site | Sample number | Minimum | Maximum | Average | Standard deviation | Median |
|---|---|---|---|---|---|---|---|
| PM2.5 (μg m−3) | HECM | 326 | 5 | 331 | 52.095 | 47.746 | 38.5 |
| HUCM | 357 | 14 | 724 | 52.165 | 52.349 | 40 | |
| QCB | 358 | 13 | 145 | 40.894 | 19.316 | 37 | |
| GCM | 356 | 16 | 144 | 46.346 | 21.574 | 41 | |
| XCWP | 357 | 11 | 401 | 52.936 | 45.98 | 40 | |
| FODS | 358 | 15 | 267 | 44.525 | 27.828 | 39 | |
| PM10 (μg m−3) | HECM | 324 | 12 | 672 | 106.154 | 98.415 | 72 |
| HUCM | 355 | 24 | 647 | 98.493 | 81.892 | 70 | |
| QCB | 357 | 22 | 292 | 73.036 | 37.21 | 63 | |
| GCM | 355 | 21 | 294 | 91.966 | 45.798 | 81 | |
| XCWP | 356 | 16 | 716 | 95.89 | 85.097 | 72 | |
| FODS | 356 | 25 | 559 | 92.126 | 67.642 | 71 | |
| SO2 (μg m−3) | HECM | 326 | 3 | 228 | 27.175 | 38.536 | 15 |
| HUCM | 357 | 3 | 300 | 42.899 | 49.439 | 24 | |
| QCB | 358 | 3 | 217 | 22.598 | 27.761 | 14 | |
| GCM | 356 | 2 | 289 | 26.537 | 36.07 | 15 | |
| XCWP | 356 | 3 | 315 | 27.587 | 41.335 | 14 | |
| FODS | 358 | 3 | 225 | 31.277 | 41.582 | 16 | |
| NO2 (μg m−3) | HECM | 326 | 2 | 129 | 25.331 | 13.979 | 22 |
| HUCM | 357 | 4 | 150 | 27.092 | 17.414 | 23 | |
| QCB | 358 | 1 | 127 | 25.187 | 15.619 | 21.5 | |
| GCM | 356 | 4 | 167 | 27.323 | 16.924 | 23 | |
| XCWP | 357 | 1 | 172 | 26.199 | 17.93 | 22 | |
| FODS | 358 | 4 | 103 | 28.399 | 17.521 | 24 | |
| CO(mg m−3) | HECM | 326 | 0.568 | 5.097 | 1.421 | 0.739 | 1.19 |
| HUCM | 357 | 0.622 | 12.036 | 2.111 | 1.13 | 1.981 | |
| QCB | 358 | 0.543 | 12.63 | 1.191 | 0.87 | 0.983 | |
| GCM | 356 | 0.626 | 7.062 | 2.128 | 0.978 | 1.873 | |
| XCWP | 357 | 0.625 | 11.875 | 2.066 | 1.629 | 1.581 | |
| FODS | 358 | 0.534 | 5.808 | 1.888 | 0.975 | 1.596 | |
| O3 (μg m−3) | HECM | 326 | 4 | 290 | 94.549 | 33.074 | 90 |
| HUCM | 357 | 4 | 362 | 136.891 | 62.166 | 136 | |
| QCB | 358 | 4 | 226 | 113.93 | 45.8 | 111 | |
| GCM | 356 | 4 | 240 | 113.093 | 41.632 | 111 | |
| XCWP | 349 | 4 | 299 | 140.923 | 67.24 | 134 | |
| FODS | 358 | 1 | 255 | 108.324 | 45.862 | 110 | |
| AQI | HECM | 325 | 29 | 485 | 84.849 | 60.15 | 65 |
| HUCM | 324 | 37 | 500 | 102.815 | 55.994 | 92 | |
| QCB | 325 | 31 | 193 | 77.425 | 24.024 | 75 | |
| GCM | 324 | 38 | 192 | 85.608 | 24.54 | 84 | |
| XCWP | 324 | 38 | 500 | 98.293 | 57.083 | 81 | |
| FODS | 325 | 34 | 459 | 88.858 | 41.348 | 84 |
As illustrated in Fig. 2, the seasonal variation trend of SO2 concentrations is winter (Dec, Jan, and Feb) > spring (Mar, Apr, and May) > summer (Jun, Jul, and Aug) > autumn (Sep, Oct, and Nov), which can be explained by the chemistry of SO2 because of the lower temperature in spring and winter, and the shorter light time, the lower concentration, the lower efficiency of SO2 conversion to SO42−, the higher concentration of SO2, and the lower concentration of SO42−. In summer and autumn, when the temperature is high and the illumination time is long, the situation is just the opposite.28 The mean concentration level in summer is the lowest but the outliers are higher than those in other seasons. The results showed that there was a high discretization in June (18.32 ± 15.965 μg m−3), July (27.78 ± 37.271 μg m−3), and August (19.26 ± 15.440 μg m−3), indicating that the control of sulfur dioxide is complicated.
The highest concentration of NO2 appeared in November (36.44 ± 17.491 μg m−3) and December (37.70 ± 21.465 μg m−3) in Fig. 2. The concentration of NO2 displayed a significant change with different seasons and locations.
From the average monthly scale change of the AQI index, the average AQI of HECM, HUCM, and XCWP stations in February is 300. By analyzing the seasonal variation characteristics of AQI, it was found that the AQI index is the highest in spring, reaching 120 on an average, while the AQI index in the other seasons is about 100 on an average. Except for spring, the AQI index is relatively high in autumn and lowest in winter, which is 89. It can be seen that the variation trend of PM2.5 and PM10 at six stations is more consistent with the trend of AQI, which indicates that the AQI has an obvious downward trend with the decrease in the fine particulate matter content.
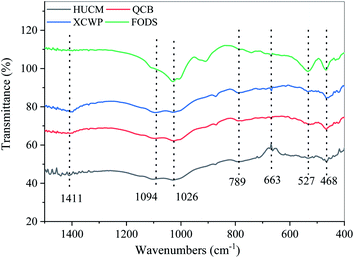
Fig. 3 shows the infrared spectral characteristics of the four samples. In this analysis, we mainly pay attention to the spectra of inorganic compounds (sulfate, nitrate, and ammonium ion) that inorganic species constitute a considerable part of PM2.5, and sulfate, nitrate and ammonium ion are the main ionic components of PM2.5.47,48
Peaks at wavenumbers of 1411 cm−1 and 1026 cm−1 are NO3− symmetric stretching vibration and asymmetric stretching vibration, respectively. Because SO42− in inorganic acid salt is tetrahedral, there are four vibration frequencies, which are asymmetric stretching vibration, symmetric stretching vibration, asymmetric deformation vibration, and symmetric deformation vibration, and the vibrational frequencies are located at 1094, 1026, 663, and 468 cm−1, respectively. In HSO4−, there are two bands of S–OH stretching vibration, near 1071 and 1018 cm−1, and the peaks at 1094 and 1026 cm−1 are their characteristic peaks, as shown in Fig. 3.
3.2 Meteorological factors
The data obtained by the air sensor were statisticed and analyzed. Multivariate analysis of variance was used to examine the effects of the monitoring sites, months, seasons, and their interactions on the concentration of different pollutants. The meteorological data from 2018, such as temperature, relative humidity, and average precipitation, were collected from the meteorological station of Qipanjing; they are expressed in Table 2. South (S) is the standard wind direction in spring (March, April, and May) and summer (June, July, and August), while north-west (N-W) and east (E) are the prevailing winds in autumn (September, October, and November) and winter (December, January, and February).| Season | Spring (Mar, Apr, and May) | Summer (Jun, Jul, and Aug) | Autumn (Sep, Oct, and Nov) | Winter (Dec, Jan, and Feb) |
|---|---|---|---|---|
| Average high (°C) | 11 | 30 | 26 | 3 |
| Daily mean (°C) | 4 | 23.5 | 19 | 3.5 |
| Average low (°C) | −3 | 17 | 12 | −10 |
| Average precipitation (mm) | 1 | 23 | 14 | 3 |
| Average relative humidity (%) | 30 | 41 | 46 | 44 |
3.3 Correlation of pollutants and meteorological factors
The relationship between air pollutants and meteorological conditions was further investigated through correlation analysis. The relative humidity in summers is negatively correlated with the concentrations of pollutants PM2.5, PM10, SO2, and NO2, which resulted from the higher proportional humidity in summer. Macromolecular water vapor in air can easily condense to form wet precipitation, which could prevent the diffusion of atmospheric pollution.29,30 Conversely, the humidity in winter is positively correlated with the concentration of PM2.5, PM10, SO2, and NO2. Therefore, the meteorological conditions of low temperature and high humidity are more conducive to liquid phase oxidation reactions and heterogeneous reactions so as to promote the formation of secondary inorganic components in PM2.5, PM10, SO2, and NO2, resulting in increased pollution.31 The low-altitude inversion layer is suspended over the near-surface layer, which seriously hinders air convection. It is difficult for soot, impurities, and harmful gases suspended in the atmosphere to spread upward.32 Wind is conducive for the diffusion and transmission of atmospheric pollutants and effectively reduces the concentration of local pollutants. Therefore, the wind speed in spring and winter has a negative correlation with the pollutants (P < 0.01). In summary, the pollution concentration in this area is closely related to the unfavorable meteorological conditions. Pearson's correlation coefficient (IBM SPSS21, bivariate correlation) was used to express the association between the contaminants in each season, and each data set included the records of PM2.5, PM10, SO2, NO2, CO, and O3. A two-sided correlation p also was adopted to test the local meteorological data and the results showed p < 0.01 for all the tests (Table 3).| Season | Meteorological elements | PM2.5 | PM10 | SO2 | NO2 | CO | O3 |
|---|---|---|---|---|---|---|---|
| a Significant correlation at the 0.01 level (bilateral).b Significant correlation at the 0.05 level (bilateral). | |||||||
| Spring | Relative humidity | −0.145 | −0.099 | −0.172 | −0.056 | −0.108 | 0.292a |
| Air temperature | 0.383a | −0.374a | −0.463a | −0.055 | 0.334a | 0.421a | |
| Average wind speed | −0.097 | 0.027 | −0.256b | 0.282a | 0.422a | 0.222b | |
| Summer | Relative humidity | −0.101 | −0.367a | −0.408a | −0.048 | 0.091 | 0.208b |
| Air temperature | −0.052 | −0.169 | 0.059 | 0.105 | −0.031 | 0.056 | |
| Average wind speed | 0.006 | −0.065 | 0.083 | −0.116 | –0.206b | 0.232b | |
| Autumn | Relative humidity | 0.387a | 0.444a | 0.165 | 0.142 | −0.006 | 0.504a |
| Air temperature | 0.004 | 0.055 | −0.12 | 0.388a | 0.333a | 0.729a | |
| Average wind speed | 0.271a | −0.228b | −0.324a | 0.401a | 0.432a | 0.085 | |
| Winter | Relative humidity | 0.357b | 0.364b | 0.508a | 0.310b | 0.343b | 0.269 |
| Air temperature | 0.401a | 0.478a | 0.632a | −0.277 | 0.442a | 0.497a | |
| Average wind speed | −0.134 | −0.211 | −0.142 | −0.014 | −0.096 | −0.003 | |
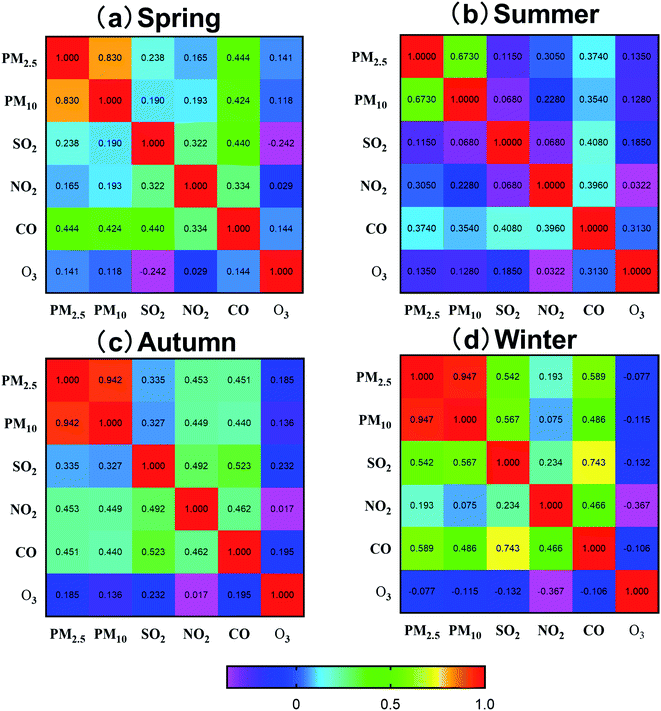
Pearson's correlation matrix of four seasons in Fig. 4 shows similar patterns and dependencies in the six different monitoring sites. There are high or moderate correlations (r) between PM2.5 or PM10 and NO2 or SO2 (PM2.5 with NO2: r = 0.16–0.45, mean r = 0.244; PM10 with NO2: r = 0.08–0.44, mean r = 0.235; PM2.5 with SO2: r = 0.11–0.52, mean r = 0.288; PM10 with SO2: r = 0.07–0.55, mean r = 0.225). The correlation (r) between PM2.5 or PM10 and CO is stable; however, it was interesting that the correlation between PM2.5 and CO is slightly higher (r = 0.37–0.59, mean r = 0.443) in the four seasons, and the correlation between PM10 and CO is the same (r = 0.35–0.49, mean r = 0.408). The correlation between PM2.5 or PM10 and O3, however, is different from other gaseous pollutants, which could be because O3 is related to secondary aerosols and not primary aerosols.28 The correlation between PM2.5 or PM10 and O3 was either weak or uncorrelated (PM2.5: r = −0.07–0.18, mean r = 0.09; PM10: r = −0.11–0.13, mean r = 0.06).
Due to the seasonal differences of the climatic conditions, there is a clear distinction between the correlation coefficients of the pollutants and different seasons (spring, winter, summer, and autumn). SO2 in the atmosphere is primarily derived from the combustion emission of sulfur-containing fuels used at the coal-fired sites and in coal-burning industries. From the correlation analysis of spring and winter, there is a high correlation coefficient between PM2.5 and PM10 (r = 0.67–0.94) and the correlation coefficient between SO2 and PM2.5 decreased from the spring and the winter to r > 0.4 compared to that of PM10 and CO, thereby indicating that these contaminants are from similar sources in the summer.
3.4 Spatial variation of the pollutants
To intuitively understand the spatial distribution characteristics of various atmospheric pollutant concentrations, the spatial interpolation analysis method is carried out for studying the pollutant concentration. The conditions in the whole studied area are illustrated in Fig. 5. It can be seen that the PM2.5 concentration is significantly higher in the east (48.43 ± 31.533 μg m−3) and west (47.43 ± 35.72 μg m−3) than that in the north (40.89 ± 19.31 μg m−3) and south (46.35 ± 21.57 μg m−3). One of the reasons for the high concentration of PM2.5 is that a lot of dust is produced while dumping the mining waste in the operation area. In addition, the coal dust of the coal preparation plant exhibits hygroscopicity, dispersibility, adsorption, suspension, and cohesion, which also results in the high concentration of PM2.5. It was found that coal fire is the primary source of fine particles and ultra-fine particles in the annual average concentration distribution of various pollutants.34 PM10 is also the primary pollutant in the coal field. The concentration of PM10 in the northern mining area (96.28 ± 76.07 μg m−3) and coal washing plant (93.76 ± 68.52 μg m−3) is higher than that in the southern office area (73.04 ± 37.21 μg m−3).
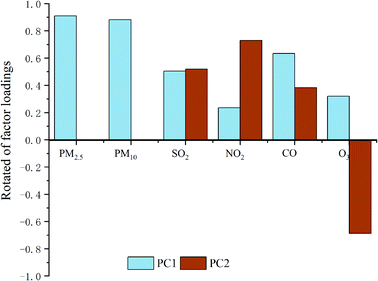
The air pollutant load factors are listed in Fig. 6 and Table 4. Also, there are significant characteristics in the main components, such as airborne particulate matter (PC1) and gaseous pollutants (PC2), of the pollutants at different coal mines. Therefore, all categories of pollution sources in a coal mine are identified from the marker species. The pollutants in the production areas of HUCM, GCM, and XCWP are the primary sources of PC1.
| Site name | PC1 | PC2 |
|---|---|---|
| HECM | −0.13 | 0.035 |
| HUCM | 0.268 | 0.005 |
| QCB | −0.348 | −0.123 |
| GCM | 0.105 | 0.118 |
| XCWP | 0.112 | −0.244 |
| FODS | −0.005 | 0.218 |
| Eigen value | 2.564 | 1.285 |
| Variance% | 42.74 | 21.42 |
| Cumulative% | 42.74 | 64.16 |
This is because the proportion of coarse particles in the air pollutants of the coal mining area is relatively high. In addition, it provides other evidence for the sources of air pollutants, i.e., the primary sources of PC2 are the active burning coal or mining operation from the HECM, HUCM, GCM, and FODS.
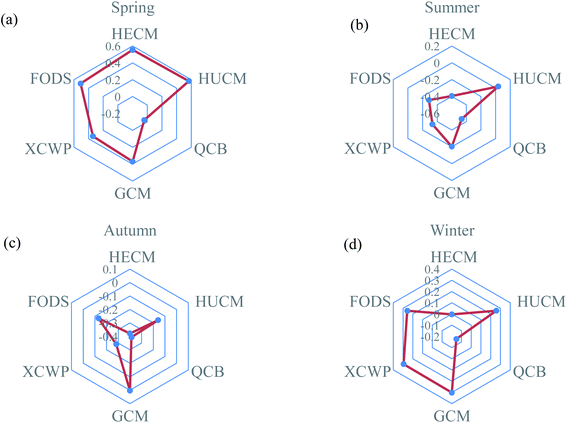
According to the principal component scores analysis of the six monitoring points in the four seasons from Fig. 7, the monitoring in HECM, HUCM, and GCM with the highest scores in spring is mainly due to the influence of particulate matter. The monitoring points of HUCM and GCM have high scores in summer, autumn, and winter, which are mainly caused by gaseous pollutants such as particles produced by coal spontaneous combustion. QCB scored very low in all the four seasons because the monitoring point was far away from the coal mine waste dumps. The high score of the monitoring point of XCWP in spring and winter is due to the very high atmospheric stability at the monitoring point, which is not conducive for the diffusion of pollutants.
4. Discussion
By analyzing the daily, monthly, and seasonal variations of atmospheric pollutant concentrations, the emissions, formation, and diffusion mechanisms of the pollutants are generalized. The higher PM2.5 concentration is due to a large amount of coal burned for spring and winter heating supply in the northern cities and the inadequate diffusion conditions in winter. In summer, the vegetation is the lushest, the leaf area of the green plants is significantly increased, and the leaves with a rough surface are favorable for the capture of PM2.5, which makes it easy to reduce the PM2.5 concentration.24 Previous studies have shown that meteorological factors, such as the temperature, rainfall, and wind speed have a significant impact on the spatial and temporal distribution of PM2.5 and PM10 concentrations in the atmosphere.23The coal seams or gangue hills burn away from the atmosphere by surface dry sedimentation or oxidation to sulphate. The oxidation of SO2 in the atmosphere can occur homogeneously in the gas phase and the aqueous phase (raindrops), heterogeneously on the surfaces of particles, or simultaneously through all three processes.36 The oxidation rate of SO2 from burning coal in summer is higher than that in winter but the dry deposition rate of SO2 in winter is higher than that in summer.22 The main component of NOx released from the coal fire area is NO, which then further reacts in the air to form NO2. In addition, automobile exhaust emissions in the mining area was paid close attention to and some NOx emissions produced during the ignition and combustion process of fuel.26 The seasonal variation in the NO2 concentration mainly depends on the meteorological conditions at different days in the year. Also, in the absence of sunlight, NO2 has a longer life in the atmosphere,37 which explains why NO2 is higher in winter. It is difficult to maintain gas mixing at their interface in winter, leading to an increase in the NO2 levels during this season.38
The large-scale use of coal in spring and winter has led to a significant increase in pollutant emissions. Meanwhile, severe weather conditions will further aggravate pollution.39 O3 has a negative correlation between the concentration of NO2 and CO, which is consistent with the previous findings, i.e., these pollutants, similar to some other volatile organic compounds, are considered as the chemical precursors of O3. Furthermore, the meteorological conditions also play a crucial role in the photochemical processes that affect the formation and destruction of O3.40,41 Pearson coefficient analysis shows that there is no significant difference between the PM2.5 and PM10 coefficients in the same season but the difference in the same gaseous pollutant in different seasons is very significant. There is a significant positive correlation between PM2.5 and PM10, while NO2 and SO2 all present a notable negative correlation with wind speed and the correlation degree is −0.05.
Wind speed, relative humidity, temperature, and rainfall are the control parameters for the seasonal variations of air pollutant concentration in the area. The average concentrations of airborne particulate matter and gaseous pollutants in the air were calculated based on the daily data of air pollutants monitored at the study sites. Since there were many fire hotspots from March 8 to 13 (Fig. 8), the distribution of the pollutants in the 5 days were analyzed. The PM2.5 concentration was higher than 100 μg m−3 from March 11 to 13. The number of fire points on the 10th reached 56, which was in good agreement with the most serious gas pollution in the area. The trend of CO was similar to PM2.5. On March 10, the concentration of CO reached 95 mg m−3 and the concentration of SO2 also reached 94 μg m−3. In the next few days, the concentration of SO2 remained above 60 μg m−3 because SO2 was the main gas emitted during the spontaneous combustion of the coalfield.
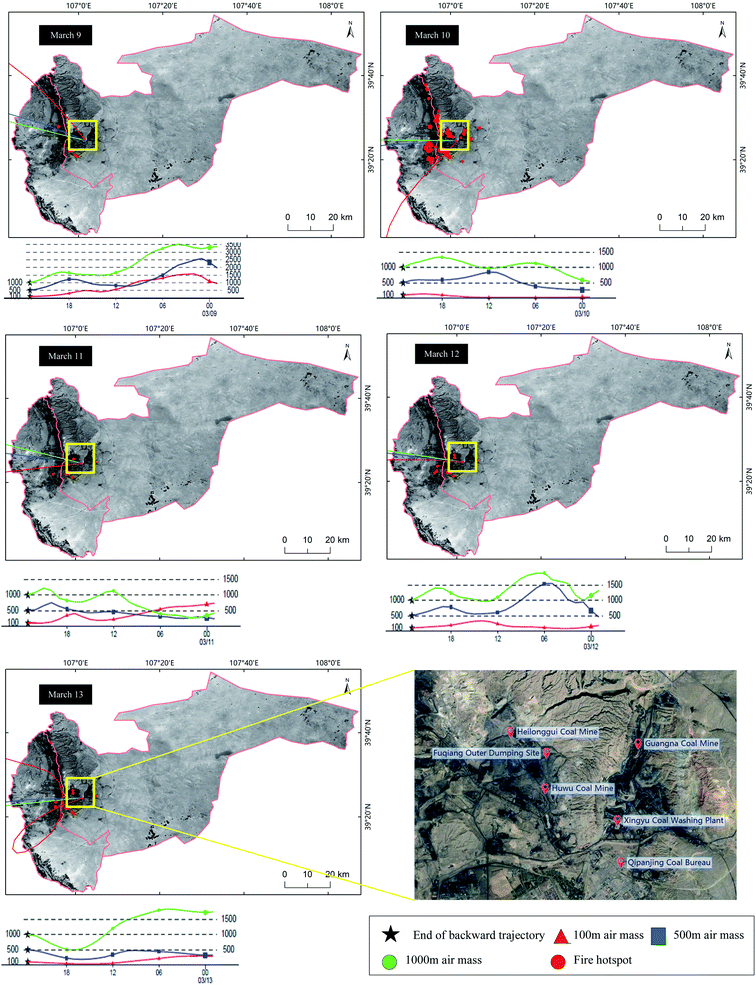 | ||
| Fig. 8 Fire hotspot distribution and HYSPLIT trajectory model from March 8 to 13 in the studied area. | ||
The HYSPLIT trajectory model was used to simulate the 24 hour transport of pollutants in the process of serious pollution from March 8 to 13, 2018. The height limits of the air masses are set at 100 m, 500 m, and 1000 m, representing the ascending range of the bottom, middle, and upper air masses, respectively. The latitude and longitude of the area is selected as the end point of the backward trajectory. The simulation results are shown in Fig. 8. It can be seen that the most of the bottom air masses come from the northwest and the air masses on March 10 originate from the south. The short trajectory of the air mass indicates the slow movement. The maximum distribution height of the air masses does not exceed 1000 m, indicating that the wind is less, the wind speed is slow, and the pressure difference is small, which prevents the vertical diffusion of pollutants. These air masses carry the pollutant gases and particulates produced by the spontaneous combustion of coal gangue, which further increases the pollution level in this area. By March 14, the three airflow trajectories were basically close to the situations on March 10 and the diffusion conditions were restored to their previous state. The concentration of pollutants in this area is greatly affected by the increase in the spontaneous combustion points and the transmission process of the pollutants inevitably affects this area.
The spatial distribution difference of SO2 concentration in the western area is higher than that in the eastern area. The generation of SO2 may be attributed to the active coal fires in the mining area and the hard coke industry. There was no significant difference in the total concentration of NO2 and that (28.19 ± 17.09 μg m−3) of the northern area was slightly higher than that of the southern area (24.90 ± 14.67 μg m−3). Similarly, the difference in the CO concentration in the various places on the studied area was not obvious. Only a slightly higher concentration of CO was found in the northeast; one of the main reasons was that there is a main road nearby, and more CO was produced by the emissions of motor vehicles on it. The concentration of O3 in the studied area showed a trapezoid, increasing gradually from north to south, and the relatively high concentration appeared in a high-altitude zone. The generation of O3 was influenced by the comprehensive factors, such as sectional illumination, temperature, and other meteorological conditions, as well as local emission sources and regional pollutant transport. The photochemical reaction was the main way to increase O3 in the near surfaces.42 Studies have shown that poisonous emissions and particulate matter are emitted by coal mine fires, resulting in poor air quality and having a direct effect on the people who normally live near coal mines. The data will need to be extrapolated to other mine fires with caution as the properties of coal can differ greatly spatially and temporally.43–46
5. Conclusion
In this study, taking the Qipanjing area of Ordos city as an example, the one-year monitoring of pollutants, including PM2.5, PM10, SO2, NO2, O3, and CO, was conducted based on the exposure level of coalfield fires and industrial mines. There is a positive correlation between PM2.5, PM10, and SO2, whereas the O3 concentrations are negatively correlated with these pollutants. Through principal component analysis, it was recognized that coal mining and active coal gangue fires (42.74% change) are significant contributors to the air pollutants of the studied region. Gaseous pollutant emissions (21.42% change) are another cause of the deterioration in the air quality around the coal mines. It is argued that reducing pollutant emission is the key to resolving the issues of coalfield pollution.Abbreviations
| FTIR | Fourier transform infrared spectroscopy |
| PM10 | Particulate matter 10 μm |
| PM2.5 | Particulate matter 2.5 μm |
| SO2 | Sulphur dioxide |
| NO2 | Nitrogen dioxide |
| CO | Carbon monoxide |
| O3 | Ozone |
| H2S | Hydrogen sulfide |
| NOx | Nitrogen oxides |
| NH3 | Ammonia |
| HECM | Heilonggui Coal Mine |
| HUCM | Huwu Coal Mine |
| GCM | Guangna Coal Mine |
| FODS | Fuqiang Outer Dumping Site |
| XCWP | Xingyu Coal Washing Plant |
| QCB | Qipanjing Coal Bureau |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The study is financially supported by Major Program of National Natural Science Foundation of China (No. 72091512 and 71790613). And Major science and technology project of Inner Mongolia Autonomous Region under Grant No. RZ190001148. The authors appreciate the supports deeply.References
- S. M. Melody and F. H. Johnston, Int. J. Coal Geol., 2015, 152, 1–14 CrossRef CAS.
- K. Brown, Science, 2003, 299, 1177 CrossRef CAS PubMed.
- C. Kuenzer, J. Zhang, A. Tetzlaff, P. van Dijk, S. Voigt, H. Mehl and W. Wagner, Appl. Geogr., 2007, 27, 42–62 CrossRef.
- Z. Song and C. Kuenzer, Int. J. Coal Geol., 2014, 133, 72–99 CrossRef CAS.
- P. Van Dijk, J. Zhang, W. Jun, C. Kuenzer and K.-H. J. Wolf, Int. J. Coal Geol., 2011, 86, 108–119 CrossRef CAS.
- X. Querol, M. Izquierdo, E. Monfort, E. Álvarez, O. Font, T. Moreno, A. Alastuey, X. Zhuang, W. Lu and Y. J. Wang, Int. J. Coal Geol., 2008, 75, 93–104 CrossRef CAS.
- R. Finkelman, Potential health impacts of burning coal beds and waste banks, 2004 Search PubMed.
- Y. Liang, H. Liang and S. Zhu, Atmos. Environ., 2014, 83, 176–184 CrossRef CAS.
- G. B. Stracher and T. P. Taylor, Int. J. Coal Geol., 2004, 59, 7–17 CrossRef CAS.
- C. Künzer, Demarcating coal fire risk areas based on spectral test sequences and partial unmixing using multi sensor remote sensing data, 2004 Search PubMed.
- Y. Zhao, J. Zhang, C. L. Chou, Y. Li, Z. Wang, Y. Ge and C. Zheng, Int. J. Coal Geol., 2008, 73, 52–62 CrossRef CAS.
- J. N. Carras, S. J. Day, A. Saghafi and D. J. Williams, Int. J. Coal Geol., 2009, 78, 161–168 CrossRef CAS.
- X. Hong, H. Liang, L. Shuai, Y. Jia, T. Zhao, W. Liang, X. Hong, H. Liang, L. Shuai and Y. Jia, Int. J. Coal Geol., 2017, 181, 78–86 CrossRef CAS.
- Ö. Özden, T. Döğeroğlu and S. Kara, Environ. Int., 2008, 34, 678–687 CrossRef PubMed.
- A. Bytnerowicz, B. Godzik, W. Fraczek, K. Grodzińska, M. Krywult, O. Badea, P. Barančok, O. Blum, M. Cerny, S. Godzik, B. Maňkovská, W. Manning, P. Moravcik, R. Musselman, J. Oszlanyi, D. Postelnicu, J. Szdzuj, M. Varsavova and M. Zota, Environ. Pollut., 2002, 116, 3–25 CrossRef CAS PubMed.
- M. Yadav, S. P. Sahu and N. K. Singh, J. Clean. Prod., 2019, 207, 97–110 CrossRef CAS.
- L. Matějíček, P. Engst and Z. Jaňour, Ecol. Model., 2006, 199, 261–277 CrossRef.
- R. Romanowicz, P. Young, P. Brown and P. Diggle, Environ Model Softw., 2006, 21, 759–769 CrossRef.
- C. de Fouquet, D. Gallois and G. Perron, Atmos. Environ., 2007, 41, 6701–6714 CrossRef CAS.
- R. Betha, M. Pradani, P. Lestari, U. Joshi, J. Reid and R. Balasubramanian, Atmos. Res., 2013, 122, 571–578 CrossRef CAS.
- A. Valavanidis, K. Fiotakis and T. Vlachogianni, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2008, 26, 339–362 CrossRef CAS PubMed.
- C. Pio and M. S. Feliciano, Phys. Chem. Earth, 1996, 21, 373–377 CrossRef.
- Z. Liu, B. Hu, L. Wang, F.-K. Wu, W. Gao and Y. Wang, Environ. Sci. Pollut. Res., 2015, 22, 627–642 CrossRef CAS PubMed.
- D. J. Nowak, S. Hirabayashi, A. Bodine and R. Hoehn, Environ. Pollut., 2013, 178, 395–402 CrossRef CAS PubMed.
- M. D. Petters and S. Kreidenweis, A single parameter representation of hygroscopic growth and cloud condensation nuclei activity, 2007 Search PubMed.
- Z. Hao, H. Yan, G. Mo, Z. Liao and K. Cen, J. Energy Inst., 2015, 90, S1743967115300520 Search PubMed.
- D. Xu, X. Kang, Z. Liu, D. Zhuang and J. Pan, Sci. China, Ser. D: Earth Sci., 2009, 52, 855–868 CrossRef.
- A. K. Tripathi and M. Gautam, J. Environ. Biol., 2007, 28, 127–132 CAS.
- F. J. Brechtel and S. M. Kreidenweis, J. Atmos. Sci., 2000, 57, 1872 CrossRef.
- M. Alghamdi, M. Khoder, R. Harrison, A. P. Hyvärinen, T. Hussein, H. Al-Jeelani, A. S. Abdelmaksoud, M. H. Goknil, I. Shabbaj, F. M. Almehmadi, H. Lihavainen and K. Hämeri, Atmos. Environ., 2014, 94, 205–214 CrossRef CAS.
- Y. L. Sun, Q. Zhang, J. J. Schwab, W. N. Chen, M. S. Bae, Y. C. Lin, H. M. Hung and K. L. Demerjian, Atmos. Chem. Phys., 2011, 11, 25751–25784 Search PubMed.
- S. Wang and L. M. Polvani, J. Geophys. Res.: Atmos., 2011, 116, D5 Search PubMed.
- S.-S. Tsai, C.-C. Chang, s.-h. Liou and C.-Y. Yang, Int. J. Environ. Res. Public Health, 2014, 11, 5081–5093 CrossRef CAS PubMed.
- R. S. Vedala, R. C. Karra, K. V. Nagesha, E. Muralidhar and M. Shoeb Mohiuddin, Procedia Earth Planet. Sci., 2015, 11, 303–311 CrossRef.
- J. Zhang, L.-y. Zhang, M. Du, W. Zhang, X. Huang, Y.-q. Zhang, Y.-y. Yang, J.-m. Zhang, S.-h. Deng and F. Shen, Atmos. Environ., 2016, 144, 37–46 CrossRef CAS.
- B. Pandey, M. Agrawal and S. Singh, Atmos. Pollut. Res., 2014, 5, 79–86 CrossRef.
- L. Li, C.-H. Chen, C. Huang, H.-Y. Huang, Z.-P. Li, J. Fu, C. J Jang and D. G. Streets, Regional air pollution characteristics simulation of O3 and PM10 over Yangtze River Delta region, 2008 Search PubMed.
- X. Yang, S. Wang, W. Zhang, D. Zhan and J. Li, The impact of anthropogenic emissions and meteorological conditions on the spatial variation of ambient SO2 concentrations: A panel study of 113 Chinese cities, 2016 Search PubMed.
- Y. Sarbassov, L. Duan, V. Manovic and E. Anthony, Greenhouse Gases: Sci. Technol., 2018, 8, 402–428 CrossRef CAS.
- R. García, A. Stohl, K. Karatzas, T. Bohler, P. James and J. Camaño, Environ Model Softw., 2005, 20, 587–593 CrossRef.
- Y. Yan, L. Peng, R. Li, Y. Li, L. Li and H. Bai, Environ. Pollut., 2017, 223, 295–304 CrossRef CAS PubMed.
- J.-d. Li, Q.-h. Deng, C. Lu and B.-l. Huang, J. Cent. South Univ. Technol., 2010, 17, 509–515 CrossRef CAS.
- S. Mondal, G. Singh and M. K. Jain, Environ. Monit. Assess., 2020, 192, 405 CrossRef CAS PubMed.
- J. Ribeiro, E. F. da Silva and D. Flores, Int. J. Coal Geol., 2010, 81, 359–372 CrossRef CAS.
- F. Reisen, R. Gillett, J. Choi, G. Fisher and P. Torre, Atmos. Environ., 2017, 151, 140–151 CrossRef CAS.
- X. Querol, M. Izquierdo, E. Monfort, E. Álvarez, O. Font, T. Moreno, A. Alastuey, X. Zhuang, W. Lu and Y. Wang, Int. J. Coal Geol., 2008, 75, 93–104 CrossRef CAS.
- A. Reff, B. J. Turpin, R. J. Porcja, R. Giovennetti, W. Cui and C. P. Weisel, et al., Indoor Air, 2005, 15(1), 53–61 CrossRef CAS PubMed.
- J. H. Liu, Y. H. Zhang, L. Y. Wang and Z. F. Wei, Spectrochim. Acta, Part A, 2005, 61(5), 893–899 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2021 |