Open Access Article
Open Access ArticleCopper based on diaminonaphthalene-coated magnetic nanoparticles as robust catalysts for catalytic oxidation reactions and C–S cross-coupling reactions†
Nasrin Yarmohammadi,
Mohammad Ghadermazi * and
Roya Mozafari
* and
Roya Mozafari
Department of Chemistry, University of Kurdistan, P.O. Box 66135-416, Sanandaj, Iran. E-mail: mghadermazi@yahoo.com; Fax: +98 87 3324133; Tel: +98 87 33624133
First published on 2nd March 2021
Abstract
In this work, the immobilization of copper(II) on the surface of 1,8-diaminonaphthalene (DAN)-coated magnetic nanoparticles provides a highly active catalyst for the oxidation reaction of sulfides to sulfoxides and the oxidative coupling of thiols to disulfides using hydrogen peroxide (H2O2). This catalyst was also applied for the one-pot synthesis of symmetrical sulfides via the reaction of aryl halides with thiourea as the sulfur source in the presence of NaOH instead of former strongly basic and harsh reaction conditions. Under optimum conditions, the synthesis yields of sulfoxides, symmetrical sulfides, and disulfides were about 99%, 95%, and 96% respectively with highest selectivity. The heterogeneous copper-based catalyst has advantages such as the easy recyclability of the catalyst, the easy separation of the product and the less wastage of products during the separation of the catalyst. This heterogeneous nanocatalyst was characterized by FESEM, FT-IR, VSM, XRD, EDX, ICP and TGA. Furthermore, the recycled catalyst can be reused for several runs and is economically effective.
1. Introduction
Catalysis is the master key to chemical transformations.1 Almost all biological reactions and more than 90% of industrial processes need catalysts.2 It is known that homogeneous catalysts, because of the solubility in the reaction medium, have good performance.3–5 However, these materials have disadvantages such as the generation of highly toxic wastes6 and time-consuming catalyst separation.7 Additionally, metal catalysts are recycled with difficulty, and they produce high amounts of wastes. In industrial applications, homogeneous catalysts in comparison with heterogeneous catalysts have a contribution of less than 20%.3 From the standpoint of green chemistry, using heterogeneous catalysts to run procedures for making fine chemical products has attracted remarkable attention from industrial and academic researchers. However, the catalytic activity of heterogeneous catalysts is reduced over time.2,8,9 Magnetic nanoparticle (MNP) catalysts can address the recovery and separation problems encountered in many catalytic reactions. Furthermore, these catalysts show not only high catalytic activity, but also a high degree of chemical stability. MNP catalysts can be recycled with an external magnetic field.10–13DAN has been widely utilized in the structure of inorganic and organometallic complexes as a suitable ligand because of having bidentate nucleophilic centers. This ligand has a unique structure and properties that make it an interesting choice for a diverse range of applications over recent years. DAN complex has been used in various fields such as optical devices, biological applications, conducting polymers,14 sensors, and electronic science.15 This ligand has been recently considered because of its unique properties in heterocyclic synthesis.16
Noble metals such as Pt, Cu, Au and Rh have been incorporated into the structure of nanoparticles.17–21 Among the mentioned metals, copper nanoparticles have been paid attention due to their catalytic activity, suitable treating costs, and high conductivity, which make them useful in nanoscience.22–24 Cu-based materials, because of the several oxidation states of this metal, can catalyze many oxidative reactions via both one- and two-electron pathways. The feasible modification of the properties of these materials via different synthetic policies and post-synthetic chemical treatments has engendered considerable interest in the field of catalysis. Furthermore, Cu's high boiling point makes it a good candidate for reactions under hard conditions such as high temperature and pressure.25
It is clear that sulfides are significant precursors for the synthesis of sulfoxides. Sulfoxides have been reported to possess widespread pharmaceutical functions and biological properties such as anticancer, antifungal and anti-atherosclerotic activities.26,27 Furthermore, they have been extensively utilized for the preparation of carbon–carbon bond and rearrangement reactions.28,29
Moreover, disulfides have important applications in biological and industrial fields such as protecting agents25 stabilization of protein structures,30 and vulcanizing entities.31,32 The catalytic formation of the C–S bond is a basic transformation in synthetic reactions. Their importance is based on the application in pharmaceutical industries and preparing a scaffold for aryl sulfides as intermediates in synthetic organic chemistry.33,34 Although the sulfide and disulfide oxidation reactions have been studied extensively,35–44 it is necessary to introduce procedures that are more simple, efficient and selective. Herein, a magnetically separable heterogeneous catalyst was fabricated by anchoring a Cu complex supported on functionalized CoFe2O4 nanoparticles. This catalyst exhibits efficient oxidation reactions of sulfides to sulfoxides and oxidative coupling of thiols to disulfides utilizing hydrogen peroxide. It is necessary to introduce procedures that are more simple, efficient and selective. Moreover, we used the prepared nanoparticle as a catalyst for the odorless C–S cross-coupling reaction of aryl halides under mild conditions. Furthermore, the catalyst is separated from the reaction media via magnetic decantation and the nanocatalyst can be recycled several times.
2. Experimental
2.1 Materials and methods
Chemical materials were supplied from Sigma-Aldrich and Merck and used as received. The X-ray powder diffraction (XRD) data of the CoFe2O4-DAN-Cu(II) nanocatalyst were obtained using a Co radiation source with a wavelength of λ = 1.78897 Å, 40 kV. The morphology of the nanocatalyst was investigated by FESEM-TESCAN MIRA3, operating at a voltage of 30 kV, which was gold-coated using a sputtering coater. FT-IR spectra of the prepared samples were recorded from a KBr disc using a VRTEX 70 model BRUKER FT-IR spectrophotometer, in the range of 400 and 4000 cm−1. 1H NMR spectra were recorded using a Bruker 400 MHz NMR AVANCE 300 spectrometer. TGA was performed using a Shimadzu DTG-60 instrument in the temperature range 25–800 °C. Energy-dispersive X-ray spectroscopy (EDX) was used for the elemental analysis. The copper content in the catalyst was evaluated by inductively coupled plasma-optical emission spectrometry (ICP-OES; 730-ES Varian). TEM analysis of the catalyst was performed using a Zeiss-EM10C transmission electron microscope.2.2 Preparation of CoFe2O4-DAN-Cu(II) nanocatalysts
First, the mixture of Fe(NO3)3·6H2O (16 mmol, 5.584 g) and CO(NO3)2·6H2O (8 mmol, 2.328 g) was dissolved in deionized water (20 mL) under vigorous stirring conditions until the components were dissolved. A brown precipitate was produced by adding sodium hydroxide (15 mL, 3 M) under magnetic stirring. After adjusting the pH to 11, the solution was heated at 80 °C for an hour. The obtained black magnetic nanoparticles were separated, washed five times with distilled water/ethanol mixture solution, and dried in an oven at 60 °C for 14 h. To prepare CoFe2O4–Cl, 1 g of MNP powder was dispersed in 50 mL of toluene under sonication for 30 min, then (1 mL) of 3-chloropropyltrimethoxysilane (CPTMS) was added to the reaction mixture and refluxed for 24 h. The obtained nanoparticles were separated by an external magnet, washed with ethanol/water mixture solution and dried in an oven at 80 °C. For the functionalization of CoFe2O4–Cl with 1,8-diaminonaphthalene, 1 g of the suspension of CoFe2O4–Cl in 70 mL of acetonitrile was added to a mixture of KI (6 mmol, 0.996 g) and K2CO3 (6 mmol, 0.828 g). The obtained mixture was stirred magnetically at room temperature for 35 min. Afterwards, 1,8-diaminonaphthalene (18 mmol, 2.84 g) was added to the solution and the mixture was refluxed for 24 h. The resulting mixture was then filtered, washed with 95% ethanol and dried at 60 °C to obtain CoFe2O4-DAN. Finally, the CoFe2O4-DAN-Cu(II) nanoparticles were afforded by mixing CoFe2O4-DAN (2 g) and CuCl2·2H2O (1 g) in 20 mL of ethanol and stirring under reflux for 18 h. The obtained product was then magnetically separated, washed with ethanol and dried at 50 °C.2.3 General procedure for oxidation of sulfides to sulfoxides with H2O2 in the presence of CoFe2O4-DAN-Cu(II)
In a 25 mL round-bottom flask equipped with a magnetic stirrer and heater, a suspension of 1 mmol sulfide and 0.025 g catalyst in ethanol (3 mL) was stirred for 2 min at room temperature. Then, H2O2 (0.5 mL, 4.90 mmol) was added dropwise to the reaction mixture and the obtained blend was stirred at 25 °C for appropriate time (Table 1). After reaction completion, ethyl acetate (2 × 5 mL) was added to the mixture, a heterogeneous catalyst was separated from the mixture using an external magnet. After that, the filtered solution was dried over Na2SO4 (2 g). The products were obtained by evaporating the organic solvent.| Entry | Catalyst (mg) | Oxidation agent | Solvent | Temp. (°C) | Time (min) | Yielda (%) |
|---|---|---|---|---|---|---|
| a Isolated yields. | ||||||
| 1 | 25 | H2O2 (0.5 mL) | Acetonitrile | 25 | 20 | 85 |
| 2 | 25 | H2O2 (0.5 mL) | n-Hexane | 25 | 120 | 25 |
| 3 | 25 | H2O2 (0.5 mL) | EtOAc | 25 | 25 | 80 |
| 4 | 25 | H2O2 (0.5 mL) | H2O | 25 | 30 | 75 |
| 5 | 25 | H2O2 (0.5 mL) | Ethanol | 25 | 15 | 99 |
| 6 | — | H2O2 (0.5 mL) | Ethanol | 25 | 140 | Trace |
| 7 | 25 | H2O2 (0.55 mL) | Ethanol | 25 | 15 | 98 |
| 8 | 25 | H2O2 (0.4 mL) | Ethanol | 25 | 25 | 85 |
| 9 | 25 | H2O2 (0.3 mL) | Ethanol | 25 | 25 | 79 |
| 10 | 25 | H2O2 (0.2 mL) | Ethanol | 25 | 25 | 68 |
| 11 | 25 | H2O2 (0.1 mL) | Ethanol | 25 | 25 | 43 |
| 12 | 25 | — | Ethanol | 25 | 140 | Trace |
| 13 | 7 | H2O2 (0.5 mL) | Ethanol | 25 | 25 | 55 |
| 14 | 9 | H2O2 (0.5 mL) | Ethanol | 25 | 25 | 68 |
| 15 | 10 | H2O2 (0.5 mL) | Ethanol | 25 | 25 | 85 |
| 16 | CuCl2·2H2O | H2O2 (0.5 mL) | Ethanol | 25 | 65 | 40 |
| 17 | 25 | NaIO4 (2 mmol) | CH3CN/H2O | 25 | 100 | 85 |
| 18 | 25 | Oxone (0.3689 g, 0.6 mmol) | Ethanol | 60 | 720 | 90 |
| 19 | 25 | O2 (2 MPa) | PEG | 100 | 720 | 10 |
2.4 Typical procedure for the synthesis of derivative sulfides in the presence of CoFe2O4-DAN-Cu(II)
In a 25 mL round-bottom flask equipped with a magnetic stirrer and heater, a mixture of the aryl halide (1 mmol), thiourea (1 mmol, 0.076 g), NaOH (1 mmol, 0.04 g), and CoFe2O4-DAN-Cu(II) (0.03 g) nanocatalyst were mixed under stirring at 130 °C in 3 mL DMSO for an appropriate time specified in Table 2. After reaction completion (monitored by TLC), CH2Cl2 was added to the reaction mixture, the heterogeneous catalyst was magnetically decanted. The filtered solution was washed with H2O and dehydrated over Na2SO4. After organic solvent volatilization, the corresponding sulfide was obtained.| Entry | Substrate | Product | Time (min) | Yieldb (%) | Mp (°C) |
|---|---|---|---|---|---|
| a Reaction conditions: catalyst (0.025 g), sulfide (1 mmol), 30% H2O2 (0.5 mL) and solvent (3 mL) at 25 °C.b Isolated yields. | |||||
| 1 | 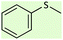 |
 |
15 | 99 | Oil51 |
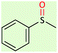
| 2 | 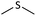 |
 |
15 | 95 | Oil52 |
| 3 |  |
 |
2 | 93 | Oil53 |
| 4 | 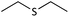 |
 |
27 | 95 | Oil54 |
| 5 |  |
 |
6 | 94 | Oil53 |
| 6 | 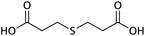 |
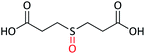 |
20 | 93 | 112–114 (ref. 11) |
| 7 |  |
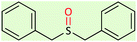 |
85 | 88 | 130–133 (ref. 55) |
| 8 | 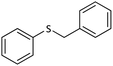 |
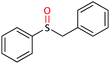 |
100 | 87 | 117–119 (ref. 55) |
| 9 | 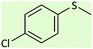 |
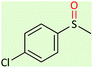 |
105 | 85 | Oil47 |
2.5 Typical procedure for oxidative coupling of thiols to disulfides with H2O2 in the presence of CoFe2O4-DAN-Cu(II)
In a typical experiment, thiol (1 mmol), CoFe2O4-DAN-Cu(II) (0.025 g) and ethanol (3 mL) were taken in a 25 mL round-bottom flask, equipped with a magnetic stirrer and heater. The solution was stirred for 2 min at room temperature. After that, H2O2 (0.4 mL, 3.92 mmol) was added dropwise to the reaction mixture and the obtained blend was stirred at 25 °C for an appropriate time (Table 3). The reaction process was monitored by TLC (ethyl acetate/n-hexane: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Upon completion of the reaction, the nanocatalyst was magnetically isolated and the product was extracted with ethyl acetate. The product was obtained, dried over anhydrous Na2SO4 and evaporated to yield pure products.
4). Upon completion of the reaction, the nanocatalyst was magnetically isolated and the product was extracted with ethyl acetate. The product was obtained, dried over anhydrous Na2SO4 and evaporated to yield pure products.
| Entry | Catalyst (mg) | Base | Thiourea (mmol) | Solvent | Temp. (°C) | Time (h) | Yielda (%) |
|---|---|---|---|---|---|---|---|
| a Isolated yields. | |||||||
| 1 | 30 | NaOH | 1 | H2O | 130 | 4 | N.R. |
| 2 | 30 | NaOH | 1 | PEG | 130 | 4 | N.R. |
| 3 | 30 | NaOH | 1 | DMF | 130 | 3.5 | 52 |
| 4 | 30 | NaOH | 1 | DMSO | 130 | 2.5 | 95 |
| 5 | 30 | NaOH | 1 | Toluene | 130 | 3 | 35 |
| 6 | — | NaOH | 1 | DMSO | 130 | 5 | Trace |
| 7 | 30 | NaOH | 0.5 | DMSO | 130 | 3.5 | 67 |
| 8 | 30 | NaOH | 0.8 | DMSO | 130 | 3.5 | 82 |
| 9 | 8 | NaOH | 1 | DMSO | 130 | 3.5 | 37 |
| 10 | 10 | NaOH | 1 | DMSO | 130 | 3.5 | 52 |
| 11 | 20 | NaOH | 1 | DMSO | 130 | 3.5 | 79 |
| 12 | 30 | NaOH | 1 | DMSO | 45 | 3.5 | 40 |
| 13 | 30 | NaOH | 1 | DMSO | 65 | 3.5 | 65 |
| 14 | 30 | NaOH | 1 | DMSO | 85 | 3.5 | 75 |
| 15 | 30 | NaOH | 1 | DMSO | 100 | 3.5 | 85 |
| 16 | 30 | KOH | 1 | DMSO | 130 | 3.5 | Trace |
| 17 | 30 | Na2CO3 | 1 | DMSO | 130 | 3.5 | Trace |
| 18 | CuCl2·2H2O | NaOH | 1 | DMSO | 130 | 4 | Trace |
2.6 Selected spectral data
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O): 1079.
O): 1079.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O): 1068.
O): 1068.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O): 1026–1089.
O): 1026–1089.2.7 Procedure for the recovery of the CoFe2O4-DAN-Cu(II) nanocatalyst
For recycling of the catalyst in the mentioned reactions, methyl phenyl sulfide, iodobenzene, and 4-methylthiophenol as model substrates were used under optimized conditions. After completion of the reaction, the CoFe2O4-DAN-Cu(II) nanocatalyst was separated from the reaction mixture by a magnet, washed, and dried at 80 C. The obtained nanocatalyst was used for the next reaction under optimum conditions. This procedure was repeated for nine runs. Selected spectral data using 1H-NMR spectra for sulfoxides, symmetrical sulfides, and disulfides are given in the ESI.†3. Results and discussions
CoFe2O4-DAN-Cu(II) nanoparticles were synthesized according to a three-step procedure (Scheme 1). Cobalt ferrite was prepared by the co-precipitation of aqueous Fe3+ and Co2+ salt solutions in the presence of a base at 80 °C.45 The functionalization of the hydroxyl groups of CoFe2O4 with CPTMS yielded CoFe2O4–Cl. Finally, CoFe2O4-DAN was treated with CuCl2·2H2O to give CoFe2O4-DAN-Cu(II) via an electrostatic interaction between copper ions and functional groups of CoFe2O4-DAN. The catalytic performance of the synthesized heterogeneous nanocatalyst was evaluated in the oxidation of sulfides, thiols and C–S cross-coupling reactions of aryl halides (Scheme 1).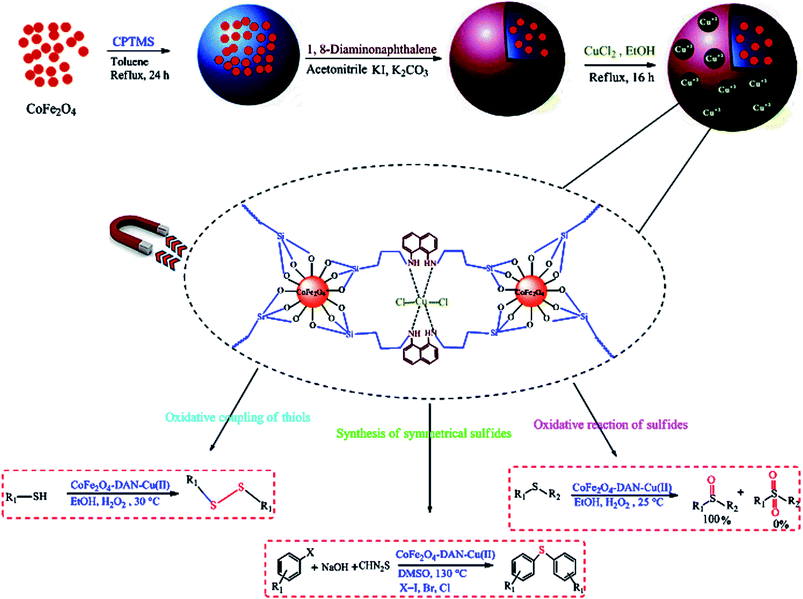 | ||
| Scheme 1 Preparation of CoFe2O4-DAN-Cu(II) nanocatalyst and its application in the oxidation of sulfides, thiols and C–S cross-coupling reactions. | ||
3.1 Catalyst characterization
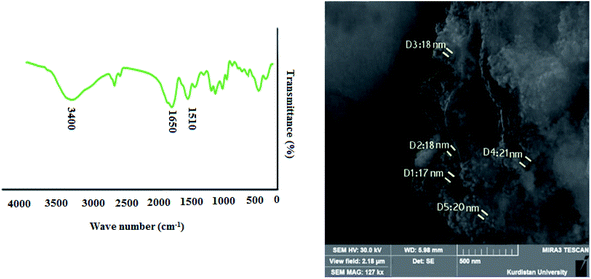
The obtained CoFe2O4-DAN-Cu(II) nanocatalyst was characterized by FESEM, FT-IR, EDX, TGA, VSM, XRD and ICP-OES analysis. Fig. 1 depicts the particle shape and surface morphology of CoFe2O4 and CoFe2O4-DAN-Cu(II). According to the SEM images, prepared nanoparticles were made up of homogeneous and quite spherical particles ranging about 21 nm in size. Moreover, grafting copper complex onto nanoparticles did not cause any significant change in the morphology and structure of CoFe2O4-DAN-Cu(II) nanoparticles. Transmission electron microscopic (TEM) study of the CoFe2O4-DAN-Cu(II) nanocatalyst is also shown in Fig. 1d. This image shows that CoFe2O4 was encapsulated by copper complexes, and the particles show an average diameter of about 21 nm, like the sizes resulting from the SEM measurements.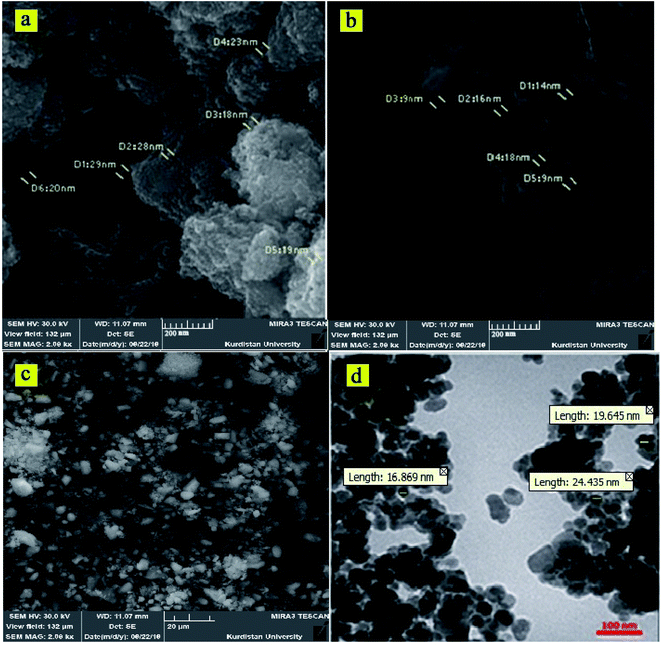 | ||
| Fig. 1 SEM images of CoFe2O4 at (a) 200 nm and CoFe2O4-DAN-Cu(II) at (b) 200 nm, (c) 20 μm, and TEM image of CoFe2O4-DAN-Cu(II) (d). | ||
The EDX spectrum can provide qualitative information about the types of different chemical elements in the catalyst. Fig. S1† shows an EDX spectrum of CoFe2O4-DAN-Cu(II) and Fe, Si, N, C, O, and Cu were detected. On the basis of these results, it is demonstrated that copper(II) was immobilized on the surface of diaminonaphthalene-coated MNPs. Moreover, the elemental mapping images indicate the uniform dispersion of copper in the nanocatalyst. This has been further confirmed from the EDX spectrum of the nanocatalyst. The ICP-OES technique was studied for the measurement of Cu amounts loaded onto the modified surface of the nanoparticles, from which the exact amount was found to be 0.43 mmol g−1.
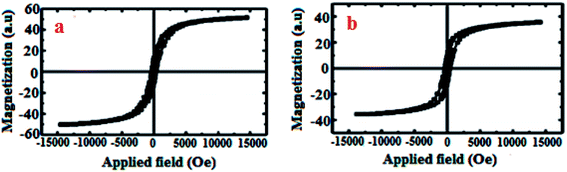
The magnetic properties of bare CoFe2O4 (a) and CoFe2O4-DAN-Cu(II) (b) were studied using a vibrating sample magnetometer (VSM) with a peak field of 15 kOe (Fig. 2). The saturation magnetization value (Ms) of these nanoparticles was found to be 53.7 and 36.6 emu g−1 respectively. As seen, the magnetization of the synthesized nanocatalyst is decreased because of the copper complex coated on CoFe2O4 MNPs; however, this reduction is insignificant for the separation of this nanocatalyst using an external magnetic field.
Fig. S2† shows the thermogravimetric analysis (TGA) curve of CoFe2O4-DAN-Cu(II) at a temperature ranging from 25 °C to 800 °C. The first weight loss was about 2 wt%, until the temperature of 150 °C, which is attributed to the removal of adsorbed solvents and surface hydroxyl groups.47 In the range of 200–500 °C, decomposition of the organic layer and Cu complex grafted onto the surface of magnetite nanoparticles was shown. On the basis of these results, CoFe2O4-DAN-Cu(II) has high thermal stability that spreads its application for several types of organic reactions.
The FT-IR spectra shown in Fig. S3† exhibit a comparison of bare CoFe2O4, CoFe2O4Cl, CoFe2O4-DAN and CoFe2O4-DAN-Cu(II) nanoparticles. The pure nanoparticles exhibit bands at 430 and 586 cm−1 characteristic of the Fe–O stretching vibration in the tetrahedral and octahedral sites of the CoFe2O4, respectively. The broad band at 3403 cm−1 can be related to characteristic –OH bands of CoFe2O4 (Fig. S3a†).48 The band located at 2986 cm−1 in the spectrum of CoFe2O4–Cl could be attributed to the stretching vibration of C–H (Fig. S3b†). The sharp peak at 1101 cm−1 is attributed to the Si–O stretching vibration, and these bands are assigned to the characteristic absorptions of the linker CPTMS attached on the cobalt ferrite surface (Fig. S3b†).49 The peaks at about 1440–1660 cm−1 are due to the amine group bending vibration of the 1,8-diaminonaphthalene ring and also the band at 3400 cm−1 is assigned to the amine stretching band of the synthesized nanoparticles (Fig. S3c†).50 In the spectrum of CoFe2O4-DAN-Cu(II), however, the shift absorption of amine to a lower wave number occurs, which is attributed to a robust interaction between the N group of the copper complex on CoFe2O4 (Fig. S3d†).
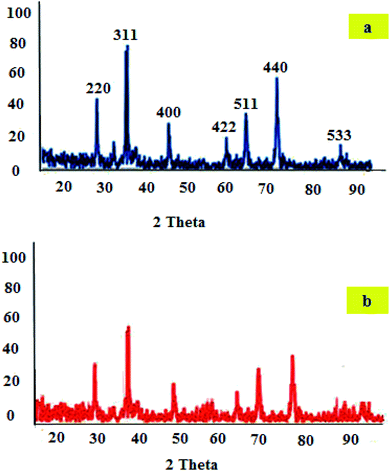
Fig. 3 represents the XRD patterns of CoFe2O4 and CoFe2O4-DAN-Cu(II). The diffraction peaks related to Bragg's reflections from the (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), (4 4 0), and (5 3 3) planes correspond to the standard spinel structure of CoFe2O4 (JCPDS 02-8517) (Fig. 3a).46 Furthermore, on the basis of the Debye–Scherrer equation, the crystallite size of CoFe2O4 was about 21 nm. The XRD pattern of CoFe2O4-DAN-Cu(II) exhibits characteristic peaks whose relative intensities match well with the standard XRD data of CoFe2O4. These results indicate that the spinel structure of the CoFe2O4 framework was well maintained during the process of catalyst preparation.
3.2 Catalytic activity
After synthesis and characterization of the nanocatalyst, we decided to study the catalytic activity in the oxidation of sulfides. Therefore, for optimizing the reaction conditions for the oxidation of sulfides, methyl phenyl sulfide was used as a model substrate and different amounts of the catalyst, H2O2 and various solvents were studied. The results are summed up in Table 1. In order to obtain the optimum solvents of the reaction, the oxidation reaction was performed in different solvents. It was observed that polar aprotic solvents such as acetonitrile, ethyl acetate, water and ethanol obtained 85%, 80%, 75% and 99% conversion of sulfide respectively (Table 1, entries 1 and 3–5). Moreover, the reaction was performed in non-polar solvents like n-hexane that gave only 25% yield (Table 1, entry 2). These results indicated that ethanol is the appropriate solvent for this reaction. Moreover, it is seen that selectivity remained the same in all solvents and sulfoxide is the only product of this system. Then, different oxidation agents such as NaIO4, oxone (2KHSO5·KHSO4·K2SO4) and O2 were studied (Table 1, entries 17–19). The oxidant H2O2 showed higher activity than other used oxidants, while the sulfoxide selectivity was kept above 100%. The results also indicate that the reaction did not perform in the absence of H2O2 and very low yield was obtained (Table 1, entry 12). The effect of changing catalyst loaded (0.007, 0.009, 0.010 and 0.025 g) on methyl phenyl sulfide conversion was studied (Table 1, entries 13–15 and 5), and it indicates that the existence of catalyst is vital for this reaction. The highest yield was obtained in the presence of 0.025 g CoFe2O4-DAN-Cu(II) nanocatalyst. It is shown that no detectable product was observed when the reaction was carried out in the absence of the CoFe2O4-DAN-Cu(II) catalyst (Table 1, entry 6). It should be mentioned that only 40% was obtained in the presence of CuCl2·2H2O used as a catalyst (Table 1, entry 16). As shown, using ethanol as the solvent in the presence of catalytic amounts of CoFe2O4-DAN-Cu(II) (0.025 g) and H2O2 (0.5 mL) at 25 °C was realized to be the optimized conditions (Table 1, entry 5).Because of the successful oxidation of methyl phenyl sulfide, the reactions of different sulfides under optimized conditions were examined (Table 2). Sulfoxides were prepared at excellent conversion in a short reaction time. It can be seen that phenyl sulfide with an electron-withdrawing group showed a longer reaction time and a lower yield (Table 2, entries 8 and 9). Researches show that in this case, the resonance of the sulfur electron pair with the aromatic ring is effective. Moreover, alkyl sulfides with longer alkyl chains proceeded in higher reaction times (Table 2, entries 4 and 6). These results are probably due to the substrate's insolubility in ethanol that decreased the substrate concentration in the reaction mixture and lowered the reaction rate. The selectivity of the catalyst is highly important in the industry and in the mentioned reaction, oxidation of sulfides with the optimal conditions is thoroughly selective and the sulfone was not observed as a by-product.
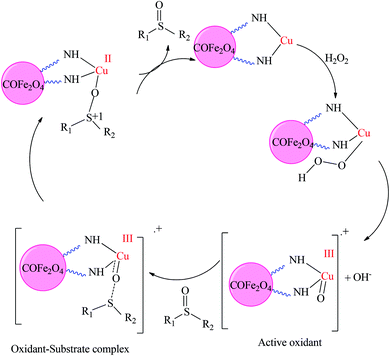
Sulfoxide usually acts as an electrophile, while sulfide is a nucleophilic reductant. This dual performance of the sulfur atom in the sulfide and the sulfoxide makes it an appropriate system to investigate nucleophilic behavior versus oxidant electrophilic behavior. H2O2 as a mild oxidizing agent reacts slowly and must be activated by homogeneous or heterogeneous catalysts. The catalytic cycle for the oxidation of sulfide by H2O2 catalyzed by the CoFe2O4-DAN-Cu(II) catalyst is proposed in Scheme 2. The explanation for this transformation is the formation of the intermediate A using the reaction of CoFe2O4-DAN-Cu(II) with H2O2, followed by the conversion of the intermediate A to the active oxidant. Then, nucleophile sulfide attacks active oxidants and with heterolytic cleavage of the Cu–O bond yields sulfoxide.56,57
In continuation of our experiment, the catalytic activity of CoFe2O4-DAN-Cu(II) was examined for the synthesis of symmetrical sulfides. The effect of catalyst dosage, the nature of the solvents and bases, and the reaction temperature were optimized (Table 3). Initially, the reaction of the coupling of iodobenzene (1 mmol), thiourea (1 mmol) as a sulfur source and NaOH was chosen for a model reaction. In order to investigate the influence of the solvents, we utilized DMF, toluene, water, DMSO and PEG as solvents (Table 3, entries 1–5). As shown, the reaction did not proceed in PEG, H2O and yielded only 35% in toluene (Table 3, entries 1, 2 and 5). The sulfide synthesis reaction was accomplished in excellent yields in DMSO as the reaction solvent (Table 3, entry 4), whereas the product yield prepared in DMF under the same condition was 52% (Table 3, entry 3). Additionally, different bases for the sulfide synthesis were checked (Table 3, entries 16, 17 and 4), and 0.4 g of NaOH was found to be the desired amount of the base (Table 3, entry 4). The reaction was carried out for catalytic amounts of thiourea (Table 3, entries 7, 8 and 4). The highest yield of diphenyl sulfide was achieved using 1 mmol of thiourea without increasing the catalyst loading (Table 3, entry 4). In order to gain the best reaction temperature, the mentioned reaction was investigated at several temperatures (Table 3, entries 12–15 and 4), and the results indicate that increasing temperature from 45 to 130 °C increased the yield. Therefore, the optimized temperature was found to be 130 °C. The effect of the catalyst amount was also checked (Table 3, entries 9–11 and 4), and the results show that with the increase in the amount of catalyst from 0.008 to 0.05, the yield also increased from 37% to 95%. The control experiment showed that catalyst-free conditions yielded trace amounts of products even after 5 hours (Table 3, entry 6). Furthermore, the reaction was carried out in the presence of CuCl2·2H2O as the catalyst, which afforded diphenyl sulfide in low yields (Table 3, entry 18).
Several derivatives of aryl halides with electron-donating and electron-withdrawing groups were examined under optimal conditions (Table 4). In the synthesis of symmetrical sulfides, used haloarenes follow ArI > ArBr > ArCl (Table 4, entries 1–3). Aromatic haloarenes with electron-withdrawing groups are more reactive in comparison to electron-donating groups (Table 4, entries 4 and 7). Selectivity is one of the most notable advantages of this system by which the coupling reaction of 1-bromo-4-nitrobenzene, 1-chloro-4-nitrobenzene, and 1-iodo-4-nitrobenzene resulted in the favorite product without extra changes.
| Entry | Substrate | Product | Time (h) | Yieldb (%) | MP (°C) |
|---|---|---|---|---|---|
| a Reaction conditions: catalyst (0.030 g), aryl halide (1 mmol), CH4N2S (1 mmol), base (0.040 g) and solvent (3 mL) at 130 °C.b Isolated yields. | |||||
| 1 | 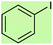 |
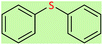 |
2.5 | 95 | Oil55 |
| 2 | 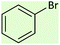 |
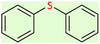 |
4 | 88 | Oil55 |
| 3 | 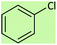 |
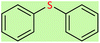 |
8 | 40 | Oil58 |
| 4 | 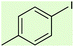 |
 |
7 | 69 | Oil58 |
| 5 |  |
 |
5.5 | 75 | 158–160 (ref. 56) |
| 6 |  |
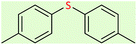 |
8 | 58 | Oil58 |
| 7 | 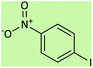 |
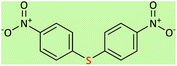 |
5 | 75 | 157–160 (ref. 58) |
| 8 | 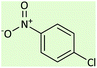 |
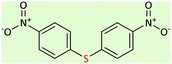 |
9.5 | 45 | 158–160 (ref. 59) |
| 9 | 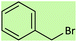 |
 |
4.5 | 78 | 44–46 (ref. 56) |
| 10 | 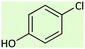 |
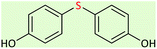 |
10.5 | 43 | 150–153 (ref. 26) |
A plausible mechanism for this transformation is outlined in the presence of the CoFe2O4-DAN-Cu(II) nanocatalyst (Scheme 3). First, the oxidative addition of Cu nanoparticles to the aryl halide forms intermediate (I). Then, the coupling reaction of the aryl halide with thiourea generates intermediate (II), which is transmitted to a thiol anion in the presence of NaOH. Finally, thiol anion reacts with intermediate (I) to produce the sulfide product as well as the initial catalyst.60
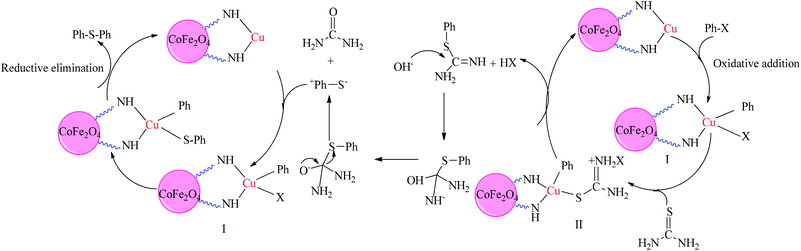 | ||
| Scheme 3 Plausible mechanism for the symmetrical sulfide synthesis by CoFe2O4-DAN-Cu(II) nanocatalysts. | ||
At last, the CoFe2O4-DAN-Cu(II) catalytic system was examined for oxidative coupling of thiols to disulfides using 4-methylthiophenol as the model substrate. To find out the optimized reaction conditions, the presence of various catalytic amounts of CoFe2O4-DAN-Cu(II) and several organic solvents using different amounts of H2O2 was tested (Table 5). Initially, the model reaction was carried out in several solvents such as acetonitrile, n-hexane, EtOAc, CH2Cl2, EtOH and H2O. Among them, acetonitrile (85%), EtOAc (87%) and EtOH (96%) showed better conversion for selective disulfide synthesis. However, because of the nontoxic and availability properties, EtOH was chosen as a green solvent (Table 5, entries 1–6). In order to study the effect of the oxidant nature, the reaction was also investigated in the presence of several oxidants such as NaClO2, NaIO4 and 1,3-dibromo-5,5-dimethyl-hydantoin (DBDMH) instead of H2O2 (Table 5, entries 17–19). Compared with the other oxidants, H2O2 showed higher activity. Then, the effect of the catalyst amount (0.008, 0.010, 0.020 and 0.025 g) on 4-methylthiophenol conversion was studied, and it was observed that the activity of the catalyst was affected by the amount of the catalyst (Table 5, entries 13–15 and 6). The highest yield was obtained in the presence of 0.025 g of CoFe2O4-DAN-Cu(II). The blank reaction showed that catalyst-free conditions produced trace amounts of products even after 140 minutes (Table 5, entry 7). When the catalyst CoFe2O4-DAN-Cu(II) was replaced with CuCl2·2H2O, the desired oxidation product was obtained in lower yields (45%) (Table 5, entry 16).
| Entry | Catalyst (mg) | Oxidation agent | Solvent | Temp. (°C) | Time (min) | Yielda (%) |
|---|---|---|---|---|---|---|
| a Isolated yields. | ||||||
| 1 | 25 | H2O2 (0.4 mL) | Acetonitrile | 25 | 35 | 85 |
| 2 | 25 | H2O2 (0.4 mL) | n-Hexane | 25 | 180 | 25 |
| 3 | 25 | H2O2 (0.4 mL) | CH2Cl2 | 25 | 40 | 60 |
| 4 | 25 | H2O2 (0.4 mL) | EtOAc | 25 | 35 | 87 |
| 5 | 25 | H2O2 (0.4 mL) | H2O | 25 | 30 | 75 |
| 6 | 25 | H2O2 (0.4 mL) | Ethanol | 25 | 20 | 96 |
| 7 | — | H2O2 (0.4 mL) | Ethanol | 25 | 140 | Trace |
| 8 | 25 | H2O2 (0.45 mL) | Ethanol | 25 | 25 | 97 |
| 9 | 25 | H2O2 (0.3 mL) | Ethanol | 25 | 40 | 80 |
| 10 | 25 | H2O2 (0.2 mL) | Ethanol | 25 | 40 | 75 |
| 11 | 25 | H2O2 (0.1 mL) | Ethanol | 25 | 40 | 65 |
| 12 | 25 | — | Ethanol | 25 | 140 | Trace |
| 13 | 8 | H2O2 (0.4 mL) | Ethanol | 25 | 40 | 58 |
| 14 | 10 | H2O2 (0.4 mL) | Ethanol | 25 | 40 | 72 |
| 15 | 20 | H2O2 (0.4 mL) | Ethanol | 25 | 25 | 86 |
| 16 | CuCl2·2H2O | H2O2 (0.4 mL) | Ethanol | 25 | 75 | 45 |
| 17 | 25 | NaClO2 (1 mmol, 0.090 g) | Methanol | 5 | 20 | 93 |
| 18 | 25 | NaIO4 (1 mmol, 0.213 g) | H2O | 25 | 25 | 100 |
| 19 | 25 | DBDMH (0.2 eq.) | CHCl3 | 25 | 2 | 96 |
Utilizing the optimum reaction condition, a variety of substituted thiols were selected for the synthesis of disulfides (Table 6). The results indicate that the CoFe2O4-DAN-Cu(II) nanocatalyst exhibited efficient catalytic activity with good to high yields in the reaction time. It was observed that aliphatic thiols oxidized more slowly than aromatic ones (Table 6, entries 1 and 9). It is also shown that aromatic thiols including electron-donating groups are more reactive than thiols with electron-withdrawing groups (Table 6, entries 1 and 7). This system shows good chemoselectivity by which the hydroxyl group of 2-mercaptoethanol remains constant, and a thiol functional group of these molecules was oxidized to disulfide. Furthermore, selectivity is one of the main important features of this system. Because of the selectivity of the mentioned heterogeneous catalyst, there is no overoxidation to thiosulfinates, disulfoxides, sulfinyl sulfones, or disulfones.
| Entry | Substrate | Product | Time (min) | Yieldb (%) | MP (°C) |
|---|---|---|---|---|---|
| a Reaction conditions: catalyst (0.025 g), thiol (1 mmol), 30% H2O2 (0.4 mL) and solvent (3 mL) at 25 °C.b Isolated yields. | |||||
| 1 | 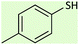 |
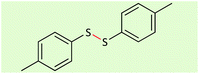 |
20 | 96 | 38–40 (ref. 26) |
| 2 |  |
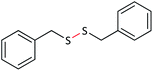 |
40 | 92 | 65–71 (ref. 55) |
| 3 | 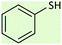 |
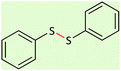 |
25 | 96 | 58–60 (ref. 53) |
| 4 | 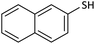 |
 |
30 | 95 | 134–136 (ref. 26) |
| 5 | 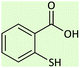 |
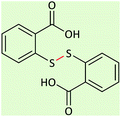 |
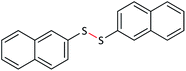
45 | 88 | 276–278 (ref. 26) |
| 6 | 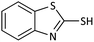 |
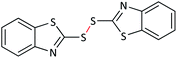 |
20 | 90 | Oil55 |
| 7 | 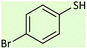 |
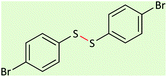 |
60 | 86 | 88–90 (ref. 26) |
| 8 | 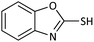 |
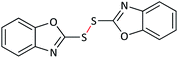 |
65 | 90 | 98–99 (ref. 55) |
| 9 |  |
 |
35 | 95 | Oil26 |
| 10 | 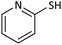 |
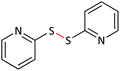 |
35 | 90 | 55–57 (ref. 55) |
A plausible mechanism for this transformation is shown in Scheme 4, on the basis of the literature works.23 A comparison for the efficiency of the catalytic activity of CoFe2O4-DAN-Cu(II) for the oxidation of sulfides to sulfoxides (Table 7, entries 1–7), synthesis of symmetrical sulfides (Table 7, entries 8–11), and oxidative coupling of thiols to disulfides (Table 7, entries 12–15) with several previously reported methods is presented. Recently, the use of Co@SiO2[(EtO)3Si–L3]/Mn(III) (Table 7, entry 1), FeNi3/SiO2 (Table 7, entry 3), Mn(III)-binapthyl Schiff base diamine-SBA-15 (Table 7, entry 4), K6H8[(SeV10O28(SeO3)3)2(M(H2O)4)]·24H2O (Table 7, entry 5), VO-TAPT-2,3-DHTA COF (Table 7, entry 6), CuFe2O4 (Table 7, entry 12), Pd-isatin Schiff base@KIT-6 (Table 7, entry 13), and TiO(O2CCF3)/NaI/thiol (Table 7, entry 14) was reported for oxidation reactions. These methods need toxic organic solvents with high reaction times. The use of Mn(III)-binapthyl Schiff base diamine-SBA-15 (Table 7, entry 4), K6H8[(SeV10O28(SeO3)3)2(M(H2O)4)]·24H2O (Table 7, entry 5), VO-TAPT-2,3-DHTA COF (Table 7, entry 6), and Pd-isatin Schiff base@KIT-6 (Table 7, entry 13) as the catalyst for oxidation reactions was reported, requiring high reaction times. Moreover, harsh conditions are found when using TiCl3(O3SCF3) (Table 7, entry 14), TiO(O2CCF3)2 (Table 7, entry 14), CuFe2O4 (Table 7, entry 12), and ethyl 2-oxocyclohexanecarboxylate as catalysts (Table 7, entry 8) in oxidation reactions and sulfide synthesis, respectively. FeNi3/SiO2 (Table 7, entry 3) causes to use of m-CPBA instead of green oxidant H2O2. Accordingly, this catalyst affords an absolutely green procedure, and these results clearly accentuate the efficiency of this applied methodology.
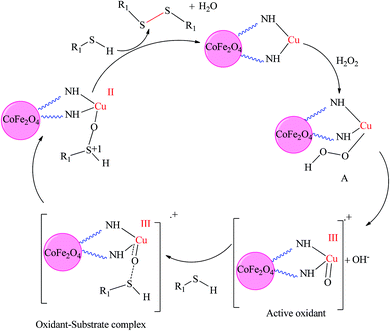 | ||
| Scheme 4 Plausible mechanism for the oxidative coupling of thiols by CoFe2O4-DAN-Cu(II) nanocatalysts. | ||
| Entry | Substrate | Catalyst | Time (min) | Catalyst loading | Condition | Yieldb (%) | Ref. |
|---|---|---|---|---|---|---|---|
| a Abbreviations: 1Schiff-base Mn(III) and Co(II) complexes coated on Co nanoparticles, 2tungstate ions loaded onto triazine-based ionic liquid-functionalized magnetic nanoparticle, 3FeNi3 nanoparticle conjugated tetraethyl orthosilicate; 4chloro(S,S)(−)[N-3-tert-butyl-5-chloromethylsalicylidene]-N′-[3′,5′-ditert-butylsalicylidene]1,1′-binapthyl-2,2′-diamine manganese(III) complex over modified surface of SBA-15, 5transition metal-sandwiched heteropolyoxovanadate complexes, 6complex vanadium of Schiff base of 2,4,6-tris(4-aminophenyl)-1,3,5-triazine–2,3-dihydroxyterephthaldehyde (TAPT–2,3-DHTA), 8ethyl 2-oxocyclohexanecarboxylate ligand in the presence of Cu2O as catalyst, 9nickel(II) complex supported on modified surface of Fe3O4. 10Magnetite/silica nanoparticles supported N-heterocyclic carbene nickel catalyst, 12copper ferrite nanoparticles, 13Pd(II)-isatin Schiff base complex immobilized into three-dimensional mesoporous silica KIT-6, 14TiCl3(O3SCF3) and TiO(O2CCF3)2 as the catalyst.b Isolated yields. | |||||||
| 1 | 1Ph–S–CH3 | Co@SiO2[(EtO)3Si–L3]/Mn(III) | 50 | 0.06 g | Methyl phenyl sulfide (0.5 mmol), acetonitrile, 90 μL H2O2 and 65 °C | 99 | 61 |
| 2 | 2Ph–S–CH3 | MNP@TA-IL/W | 60 | 0.4 mol% | H2O, 1.5 mmol H2O2 and 25 °C | 99 | 62 |
| 3 | 3Ph–S–CH3 | FeNi3/SiO2 | 60 | 0.04 | DCM (2.0 mL), m-CPBA (2.0 mmol) and r.t | 99 | 63 |
| 4 | 4Ph–S–CH3 | Mn(III)-binapthyl Schiff base diamine-SBA-15 | 300 | 50 mg | CH2Cl2 and 25 °C | 94 | 64 |
| 5 | 5Ph–S–CH3 | M2+-sandwiched POVs: K6H8[(SeV10O28(SeO3)3)2(M(H2O)4)]·24H2O | 60 | 2 μmol | CH3OH and 25 °C | 97.9 | 65 |
| 6 | 6Ph–S–CH3 | VO-TAPT-2,3-DHTA COF | 240 | 20 mg | CH3CN and 25 °C | 95 | 66 |
| 7 | Ph–S–CH3 | CoFe2O4-DAN-Cu(II) | 15 | 0.025 g | C2H5OH and 25 °C | 99 | This work |
| 8 | 8Iodobenzene | Ethyl 2-oxocyclohexanecarboxylate | 1200 | 0.1 mmol | 80 °C and under Ar | 96 | 67 |
| 9 | 9Iodobenzene | Fe3O4@SBTU@Ni(II) | 210 | 0.030 | DMSO and 130 °C | 94 | 60 |
| 10 | 10Iodobenzene | MNP-Si-NHC(Pyr)-Ni | 600 | 10 mol% | DMF, 100 °C, base (2 mmol) and thiol (1 mmol) | 92 | 68 |
| 11 | Iodobenzene | CoFe2O4-DAN-Cu(II) | 150 | 0.030 g | DMSO and 130 °C | 95 | This work |
| 12 | 12Thiophenol | CuFe2O4 | 24 h | 10 mol% | 1.80 mmol of halide, 2.0 eq. of base, 5 mL of 1,4-dioxane and under N2 atmosphere | 95 | 69 |
| 13 | 134-Methyl thiophenol | Pd-isatin Schiff base@KIT-6 | 30 | 0.025 g | CH3CN, 25 °C and 5 mmol H2O2 | 95 | 70 |
| 14 | 14Thiophenol | TiO(O2CCF3)2/NaI/thiol | 150 | 1 mmol | CH3CN and under reflux conditions | 100 | 71 |
| 15 | Thiophenol | CoFe2O4-DAN-Cu(II) | 20 | 0.025 g | C2H5OH and 25 °C | 96 | This work |
3.3 Catalyst recycling
Recycling as one of the main important properties of nanomagnetic catalysts was also investigated by the synthesis of methyl phenyl sulfoxide, diphenyl sulphide, and 1,2-di-p-tolyldisulfane under optimized conditions. After completion of the reactions, the CoFe2O4-DAN-Cu(II) nanocatalyst was removed from the reaction mixture by a magnet and used in the next cycle under mentioned optimum conditions. As shown in Fig. 4, the catalyst can be recycled for nine runs without any considerable loss of catalytic activity.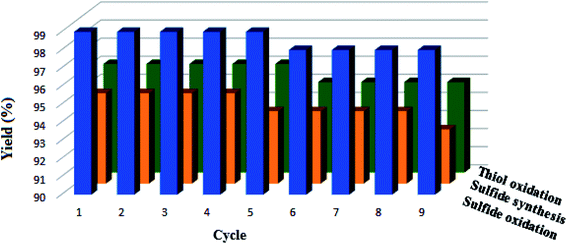 | ||
| Fig. 4 Reusability of the CoFe2O4-DAN-Cu(II) nanocatalyst in the sulfide oxidation, sulfide synthesis and oxidative coupling of thiols. | ||
In order to clarify the changes in the chemical structure of the prepared catalyst towards oxidation during the nine cycles, FE-SEM and FT-IR analyses were performed. SEM and FT-IR analyses of this catalyst after nine runs show no significant changes during the reaction time (Fig. 5). Moreover, the amount of copper on recovered CoFe2O4-DAN-Cu(II) was measured by ICP-OES analysis for the conversion of methyl phenyl sulfide to methyl phenyl sulfoxide in the fresh catalyst and after nine consecutive cycles, it was found to be 0.43 mmol g−1 and 0.32 mmol g−1, which indicates the minimum amount of Cu leaching in the catalytic process. Furthermore, AAS analysis of the reaction mixture after catalyst removal showed no considerable amount of copper. AAS analyses of the recycled CoFe2O4-DAN-Cu(II) catalyst also exhibited no significant (<0.001 mmol g−1 of Cu) differences compared with the fresh catalyst, presenting that no considerable copper leaching occurred during the catalytic processes. Moreover, regarding the heterogeneous nature of CoFe2O4-DAN-Cu(II), the oxidation of methyl phenyl sulfide has been examined by a hot filtration experiment under optimal reaction conditions. In this test, we found the yield of product in a half time of the reaction to be 65%. Then, the reaction was repeated and at half time of the reaction, the catalyst was separated using a magnet and allowed to react further. The yield of the reaction in this stage was 69%, which confirmed that the reaction proceeded heterogeneously and after hot filtration, the reaction of the residual mixture was completely stopped.
3.4 Catalyst poisoning test
Another heterogeneity test was performed with Hg poisoning. Hg(0) can poison catalysts, either by amalgamating the applied metal or adsorbing on the metal surface.72 The sulfide oxidation was conducted under the same mentioned conditions, except the addition of elemental Hg (1 mmol) to the reaction mixture at 50% conversion of methyl phenyl sulfide to methyl phenyl sulfoxide. No product was gained with this procedure, which is strong evidence that the catalyst nature is heterogeneous.4. Conclusion
An efficient CoFe2O4-DAN-Cu(II) nanocatalyst has been successfully synthesized using an anchored Cu complex on the stable and bidentate ligand 1,8-diaminonaphthalene-modified CoFe2O4 nanoparticles. Then, the prepared nanostructure has been characterized by techniques such as SEM, EDX, ICP-OES, TGA, XRD, VSM, FT-IR and TEM. The immobilized Cu complex exhibits remarkable catalytic activity in the oxidation of sulfides and thiols in a green catalytic system (C2H5OH as a solvent and H2O2 as an oxidant). All studied sulfides and thiols were oxidized to their corresponding sulfoxides and disulfides without production of any by-product. Moreover, the C–S cross-coupling reaction produced in the clean suspension of a variety of diarylsulfides appropriate to good yields. This system has many benefits such as high yields, chemical stability and ease of catalyst separation using an external magnet. The nanocatalyst can be recycled and reused for next runs without any significant change in its catalytic activity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to thank the financial support from the University of Kurdistan.Notes and references
- M. Kazemi, M. Ghobadi and A. Mirzaie, Nanotechnol. Rev., 2017, 7, 43–93 CrossRef.
- M. Kaushik and A. Moores, Curr. Opin. Green Sustain. Chem., 2017, 7, 39–45 CrossRef.
- L. Zhou, C. Gao and W. Xu, Langmuir, 2010, 26, 11217–11225 CrossRef CAS.
- S. R. Chaurasia, A. R. Tiwari and B. M. Bhanage, J. Mol. Catal., 2019, 478 DOI:10.1016/j.mcat.2019.110565.
- M. Bagherzadeh, M. Bahjati and A. Mortazavi-Manesh, J. Organomet. Chem., 2019, 897, 200–206 CrossRef CAS.
- A. R. Hajipour, D. M. Hosini and F. Mohammadsaleh, New J. Chem., 2016, 40, 6939–6945 RSC.
- P. D. Stevens, G. Li, J. Fan, M. Yen and Y. Gao, Chem. Commun., 2005, 35, 4435–4437 RSC.
- M. N. Chen, L. P. Mo, Z. S. Cui and Z. H. Zhang, Curr. Opin. Green Sustain. Chem., 2019, 15, 27–37 CrossRef.
- R. S. Varma, ACS Sustainable Chem. Eng., 2016, 4, 5866–5878 CrossRef CAS.
- L. L. Chng, N. Erathodiyil and J. Y. Ying, Acc. Chem. Res., 2013, 46, 1825–1837 CrossRef CAS.
- S. Molaei, T. Tamoradi, M. Ghadermazi and A. Ghorbani-Choghamarani, Microporous Mesoporous Mater., 2018, 272, 241–250 CrossRef CAS.
- S. Lotfi, A. Nikseresht and N. Rahimi, Polyhedron, 2019, 173, 114148 CrossRef.
- C. Zhang, C. Li, J. Bai and H. Li, Catal. Lett., 2015, 145, 1764–1770 CrossRef CAS.
- F. Yakuphanoglu and M. Sekerci, J. Mol. Struct., 2005, 751, 200–203 CrossRef CAS.
- M. A. R. Da Silva, A. I. L. Ferreira, A. F. L. Santos, C. M. Ferreira, D. C. Barros, J. A. Reis, J. C. Costa, M. M. G. Calvinho, S. I. Rocha, S. P. Pinto and S. S. Freire, J. Chem. Thermodyn., 2010, 42, 371–379 CrossRef.
- A. A. Aly and K. M. El-Shaieb, Tetrahedron, 2004, 60, 3797–3802 CrossRef CAS.
- J. D. De León, S. Loera-Serna, T. A. Zepeda, D. Domínguez, B. Pawelec, A. M. Venezia and S. Fuentes-Moyado, Fuel, 2020, 266, 117031 CrossRef.
- E. Lagerspets, K. Lagerblom, E. Heliövaara, O. M. Hiltunen, K. Moslova, M. Nieger and T. Repo, J. Mol. Catal., 2019, 468, 75–79 CrossRef CAS.
- K. Patel, S. Kapoor, D. P. Dave and T. Mukherjee, J. Chem. Sci., 2005, 117, 311–316 CrossRef CAS.
- J. Guo, C. Lin, C. Jiang and P. Zhang, Appl. Surf. Sci., 2019, 475, 237–255 CrossRef CAS.
- P. Singh, N. Mahadevaiah, S. K. Parida and M. S. Hegde, J. Chem. Sci., 2011, 123, 577–592 CrossRef CAS.
- Y. Chen, B. He, A. Qin and B. Z. Tang, Sci. China: Chem., 2019, 62, 1017–1022 CrossRef CAS.
- S. T. Aziz and R. U. Islam, Catal. Lett., 2018, 148, 205–213 CrossRef CAS.
- M. Ghavami, M. Koohi and M. Z. Kassaee, J. Chem. Sci., 2013, 125, 1347–1357 CrossRef CAS.
- M. B. Gawande, A. Goswami, F. X. Felpin, T. Asefa, X. Huang, R. Silva, X. Zou, R. Zboril and R. S. Varma, Chem. Rev., 2016, 116, 3722–3811 CrossRef CAS.
- T. Tamoradi, N. Moeini, M. Ghadermazi and A. Ghorbani-Choghamarani, Polyhedron, 2018, 153, 104–109 CrossRef CAS.
- S. Zolfagharinia, E. Kolvari, N. Koukabi and M. M. Hosseini, J. Chem. Sci., 2017, 129, 1411–1421 CrossRef CAS.
- A. Ghorbani-Choghamarani, Z. Darvishnejad and B. Tahmasbi, Inorg. Chim. Acta, 2015, 435, 223–231 CrossRef CAS.
- Q. Liu, X. Zhao, F. Xu and G. Li, Tetrahedron Lett., 2020, 61, 151492 CrossRef CAS.
- Y. Dou, X. Huang, H. Wang, L. Yang, H. Li, B. Yuan and G. Yang, Green Chem., 2017, 19, 2491–2495 RSC.
- H. Zhang, M. Zhou, L. Xiong, Z. He, T. Wang, Y. Xu and K. Huang, Microporous Mesoporous Mater., 2018, 255, 103–109 CrossRef CAS.
- N. Noori, M. Nikoorazm and A. Ghorbani-Choghamarani, Microporous Mesoporous Mater., 2016, 234, 166–175 CrossRef CAS.
- T. Tamoradi, M. Ghadermazi and A. Ghorbani-Choghamarani, Catal. Lett., 2019, 149, 2645–2646 CrossRef CAS.
- Z. Chen, C. Wu, Z. Zhang, W. Wu, X. Wang and Z. Yu, Chin. Chem. Lett., 2018, 29, 1601–1608 CrossRef CAS.
- M. Bagherzadeh, M. Hosseini and A. Mortazavi-Manesh, Inorg. Chem. Commun., 2019, 107, 107495 CrossRef CAS.
- M. G. Kermanshah and K. Bahrami, RSC Adv., 2019, 9, 36103–36112 RSC.
- J. Tang, P. Yao, L. Wang, H. Bian, M. Luo and F. Huang, RSC Adv., 2018, 8, 40720–40730 RSC.
- R. Hasanpour, F. Feizpour, M. Jafarpour and A. Rezaeifard, New J. Chem., 2018, 42, 7383–7391 RSC.
- Y. Zhou, B. Xue, C. Wu, S. Chen, H. Liu, T. Jiu, Z. Li and Y. Zhao, Chem. Commun., 2019, 55, 13570–13573 RSC.
- R. H. Wu, M. Guo, M. X. Yu and L. G. Zhu, Dalton Trans., 2017, 46, 14348–14355 RSC.
- G. Saikia, K. Ahmed, C. Rajkhowa, M. Sharma, H. Talukdar and N. S. Islam, New J. Chem., 2019, 43, 17251–17266 RSC.
- R. Limvorapitux, H. Chen, M. L. Mendonca, M. Liu, R. Q. Snurr and S. T. Nguyen, Catal. Sci. Technol., 2019, 9, 327–335 RSC.
- M. Radko, A. Kowalczyk, P. Mikrut, S. Witkowski, W. Mozgawa, W. Macyk and L. Chmielarz, RSC Adv., 2020, 10, 4023–4031 RSC.
- S. Hou, N. Chen, P. Zhang and S. Dai, Green Chem., 2019, 21(455), 1455–1460 RSC.
- I. Malaescu, A. Lungu, C. N. Marin, P. Vlazan, P. Sfirloaga and G. M. Turi, Ceram. Int., 2016, 42, 16744–16748 CrossRef CAS.
- S. Golchinvafa, S. M. Masoudpanah and M. Jazirehpour, J. Alloys Compd., 2019, 809, 151746 CrossRef CAS.
- T. Tamoradi, A. Ghorbani-Choghamarani and M. Ghadermazi, New J. Chem., 2017, 41, 11714–11721 RSC.
- D. Karthickraja, S. Karthi, G. A. Kumar, D. K. Sardar, G. C. Dannangoda, K. S. Martirosyan and E. K. Girija, New J. Chem., 2019, 43, 13584–13593 RSC.
- B. L. Li, H. C. Hu, L. P. Mo and Z. H. Zhang, RSC Adv., 2014, 4, 12929–12943 RSC.
- R. Mozafari and F. Heidarizadeh, Polyhedron, 2019, 162, 263–276 CrossRef CAS.
- M. Nasrollahzadeh, Y. Bayat, D. Habibi and S. Moshaee, Tetrahedron Lett., 2009, 50, 4435–4438 CrossRef CAS.
- T. Tamoradi, M. Ghadermazi, A. Ghorbani-Choghamarani and S. Molaei, Res. Chem. Intermed., 2018, 44, 4259 CrossRef CAS.
- A. Ghorbani-Choghamarani, B. Tahmasbi, F. Arghand and S. Faryadi, RSC Adv., 2015, 5, 92174–92183 RSC.
- T. Tamoradi, M. Ghadermazi and A. Ghorbani-Choghamarani, Appl. Organomet. Chem., 2018, 32, e3974, DOI:10.1002/aoc.3974.
- G. Azadi, Z. Taherinia, A. Naghipour and A. Ghorbani-Choghamarani, J. Sulfur Chem., 2017, 38, 303–313 CrossRef CAS.
- E. Rafiee, M. Joshaghani and P. Ghaderi-Shekhi Abadi, J. Saudi Chem. Soc., 2017, 21, 599–609 CrossRef CAS.
- A. M. Al-Ajlouni, T. M. Daiafla and M. El-Khateeb, J. Mol. Catal. A: Chem., 2007, 275, 139–147 CrossRef CAS.
- X. Ku, H. Huang, H. Jiang and H. Liu, J. Comb. Chem., 2009, 11, 338–340 CrossRef.
- V. S. Pilyugin, S. L. Kuznetsova, Y. E. Sapozhnikov, G. E. Chikisheva, G. V. Kiseleva, T. P. Vorob'eva, E. V. Klimakova, N. A. Sapozhnikova, R. D. Davletov and Z. B. Galeeva, Russ. J. Gen. Chem., 2008, 78, 446–450 CrossRef CAS.
- A. Ghorbani-Choghamarani, Z. Moradi and G. Azadi, J. Sulfur Chem., 2017, 39, 237–251 CrossRef.
- S. G. Saremi, H. Keypour, M. Noroozi and H. Veisi, RSC Adv., 2018, 8, 3889–3898 RSC.
- S. H. Hosseini, M. Tavakolizadeh, N. Zohreh and R. Soleyman, Appl. Organomet. Chem., 2018, 32, e3953 CrossRef.
- S. Ghiami, M. A. Nasseri, A. Allahresani and M. Kazemnejadi, React. Kinet., Mech. Catal., 2019, 126, 383–398 CrossRef CAS.
- P. Sharma and A. P. Singh, Catal. Today, 2012, 198, 184–188 CrossRef CAS.
- J. Y. Niu, R. Wan, P. He, Z. Liu, X. Ma, P. Ma, V. Singh, C. Zhang, J. Niu and J. Wang, Chem.–Eur. J., 2020, 26, 8760–8766 CrossRef.
- H. Vardhan, G. Verma, S. Ramani, A. Nafady, A. M. Al-Enizi, Y. Pan, Z. Yang, H. Yang and S. Ma, ACS Appl. Mater. Interfaces, 2018, 11, 3070–3079 CrossRef.
- H. J. Xu, X. Y. Zhao, J. Deng, Y. Fu and Y. S. Feng, Tetrahedron Lett., 2009, 50, 434–437 CrossRef CAS.
- H. J. Yoon, J. W. Choi, H. Kang, T. Kang, S. M. Lee, B. H. Jun and Y. S. Lee, Synlett, 2010, 16, 2518–2522 Search PubMed.
- N. Panda, A. K. Jena and S. Mohapatra, Appl. Catal., A, 2012, 433, 258–264 CrossRef.
- S. Pakvojoud, M. Hatefi Ardakani, S. Saeednia and E. Heydari-Bafrooei, J. Sulfur Chem., 2020, 41, 561–580 CrossRef CAS.
- B. Zeynizadeh and N. Iranpoor, J. Chin. Chem. Soc., 2003, 50, 849–852 CrossRef CAS.
- A. Chatterjee and V. R. Jensen, ACS Catal., 2017, 7, 2543–2547 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra01029h |
| This journal is © The Royal Society of Chemistry 2021 |