DOI:
10.1039/D1RA00994J
(Paper)
RSC Adv., 2021,
11, 15017-15029
Retracted Article: Design and development of highly sensitive PEDOT-PSS/AuNP hybrid nanocomposite-based sensor towards room temperature detection of greenhouse methane gas at ppb level
Received
5th February 2021
, Accepted 11th April 2021
First published on 22nd April 2021
Abstract
Herein, we present fabrication of a novel methane sensor based on poly (3,4-ethylenedioxythiophene:poly (styrene sulfonic acid)) (p-PEDOT-PSS) and gold nanoparticles (AuNPs) treated with dimethyl sulfoxide (DMSO) and Zonyl using a spin coating technique. The nanocomposite films were further post treated with H2SO4 to improve the charge transport mechanism. The structural and morphological features of the composites were analyzed through scanning electronic microscopy, transmission electron microscopy, Fourier transform infra-red spectroscopy, UV-Vis spectroscopy and thermogravimetric analysis. Treatment with organic solvents and post treatment of H2SO4 significantly enhances the conductivity of the composite to 1800 S cm−1. The fabricated sensor shows an excellent sensing response, fast response and recovery time along with acceptable selectivity towards methane gas at ppb concentrations. Due to a simple fabrication technique, excellent conductivity, superior sensing performance and improved mechanical properties, the sensor fabricated in this study could potentially be used to detect greenhouse methane gas at low concentrations.
1. Introduction
Ever increasing demand for urbanization and industrialization in developed/developing countries lead to releasing harmful volatile organic compounds (VOCs) such as methane, NOx, H2S, SOx and COx etc. into the environment contributing to the increase in atmospheric concentration of greenhouse gases at a faster rate. Hence, there is an urgent need to detect these greenhouse gases at low concentrations to have a safe and healthy environment for living beings.1 Among the various greenhouse gases, the most prominent ones are carbon dioxide, nitrous oxide, methane and fluorocarbons, which contribute to global climate change. Methane being one of the important gases contributing to the greenhouse gas effect, its increasing concentration in the atmosphere posed a serious threat towards developing accurate sensing methods.2–8 Methane's concentration was growing in the atmosphere at a rate of 0.5 ppb per year until the year 2000. The current statistics indicates that it has increased by 12.5 ppb in 2014 and reached about 1872 ppb by July 2020. Even though the average concentrations of CH4 in the atmosphere has reached 1800+ ppb, methane emission from agricultural sources (greenhouse) and indoor quality of air has methane content at low concentrations (<1000 ppm). Hence, they need to be detected at low concentrations to minimize the adverse effects on human health. Apart from that, methane (CH4) is known to be highly flammable and its monitoring becomes extremely important in terms of safety considerations. Moreover, it becomes dangerously explosive when the concentration of CH4 in environment reaches about 5–15%.9,10 Also, the other important consequence of CH4 arises from natural gas leakage through pipelines that can lead to severe consequences if not identified and detected at low concentrations. Despite its negative impacts on the environment, natural gas is interesting for its abundance and clean burning process, and therefore, will continue to be used widely in the future researches.
In recent years, semiconducting metal oxide-based materials have been extensively used as chemiresistive sensors for monitoring environmental methane which can sense CH4 due to change in their electrical or optical properties.7,11–17 The main drawback of these semiconductor-based metal oxide sensors is being operated at high temperature and mechanically brittle. Hence, there is an urgent need to develop sensors to detect flammable gases such as methane gas in low concentrations at room temperature. Apart from that, the development in the field of organic electronics demands the sensing materials to be mechanically flexible and wearable to meet modern technological needs. In the recent past efforts are being made to fabricate such sensing devices that are low cost, better sensing abilities and highly stable.16 Conducting polymers have emerged as potential materials as chemiresistive sensors at room temperature with excellent sensing features such as improved sensitivities with faster response time. Advantages of using these conducting polymers as chemiresistive sensors is their excellent mechanical flexibility that allows facile fabrication of flexible and wearable sensors. Unlike conventional polymers that are being used as dielectrics and insulators, the presence of alternating single and double bonds on conducting polymer backbone leads to extended π-conjugation which supports for charge transport.18,19 Among the most used conjugated polymers poly (3,4-ethylenedioxythiophene)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly (styrene sulfonate) (PEDOT-PSS) (a mixture of two ionomers), is a commercially available, conductive polyelectrolyte complex with superior properties as an aqueous dispersion.20 Due to its excellent conductivity, optical transparency and processability in printing electronics, it has been extensively investigated for optoelectronic applications and fabrication of various sensors.20 The conductivity and sensitivity of commercially available PEDOT-PSS aqueous dispersions are low, these properties can be enhanced significantly by secondary doping of PEDOT-PSS using high boiling point, organic polar solvents.21–28 In the recent past, many researchers have demonstrated an effective strategy to improve the conductivity and sensitivity of PEDOT-PSS thin films by adding small quantities of polar solvents such as DMF, ethylene glycol, sorbitol, DMSO and glycerol.23–26 Other efficient strategy to improve the performance of PEDOT-PSS in organic electronics is to form hybrid composites of PEDOT-PSS using nanofillers.20 Highly efficient PEDOT-PSS based hybrid nanocomposites have gained considerable attention for the fabrication of various chemiresistive sensors that can be operated at room temperature.20,24,26 In the recent past, AuNPs, possessing high chemical stability, facile synthesis, surface functionalization, biocompatibility and tunable optical and electronic properties, have led to many promising applications in the field of sensor technology.29 The aspect ratio of AuNPs nanofillers, their functionalization and formation of interfacial region can play an important role in tuning the optical, electrical and sensing performance of PEDOT-PSS polymer.30
poly (styrene sulfonate) (PEDOT-PSS) (a mixture of two ionomers), is a commercially available, conductive polyelectrolyte complex with superior properties as an aqueous dispersion.20 Due to its excellent conductivity, optical transparency and processability in printing electronics, it has been extensively investigated for optoelectronic applications and fabrication of various sensors.20 The conductivity and sensitivity of commercially available PEDOT-PSS aqueous dispersions are low, these properties can be enhanced significantly by secondary doping of PEDOT-PSS using high boiling point, organic polar solvents.21–28 In the recent past, many researchers have demonstrated an effective strategy to improve the conductivity and sensitivity of PEDOT-PSS thin films by adding small quantities of polar solvents such as DMF, ethylene glycol, sorbitol, DMSO and glycerol.23–26 Other efficient strategy to improve the performance of PEDOT-PSS in organic electronics is to form hybrid composites of PEDOT-PSS using nanofillers.20 Highly efficient PEDOT-PSS based hybrid nanocomposites have gained considerable attention for the fabrication of various chemiresistive sensors that can be operated at room temperature.20,24,26 In the recent past, AuNPs, possessing high chemical stability, facile synthesis, surface functionalization, biocompatibility and tunable optical and electronic properties, have led to many promising applications in the field of sensor technology.29 The aspect ratio of AuNPs nanofillers, their functionalization and formation of interfacial region can play an important role in tuning the optical, electrical and sensing performance of PEDOT-PSS polymer.30
Even though considerable attention is being paid in the recent past towards development of methane gas sensors based on polymer composites, but the reports on room temperature detection of methane at ppb concentrations using chemiresistive sensors are not yet reported. Apart from that, due to the advent in flexible and wearable electronics, there is a growing need to develop robust and flexible methane gas sensors with ppb detection limits. In this study, we have developed a low cost, flexible, ultra-sensitive polymer nanocomposite film towards detection of methane gas at low concentration (at ppb level). For this purpose, we have demonstrated a novel strategy to improve the sensing performance and mechanical properties of chemiresistive sensors based on PEDOT-PSS/AuNPs composite with small quantities of additives like DMSO and Zonyl. The novel chemiresistive sensor fabricated using the combination of these materials resulted into excellent sensitivity towards methane at ppb level, short response/recovery time, long term stability and excellent selectivity along with improved mechanical properties.
2. Materials and methods
Conductive PEDOT-PSS (aqueous dispersion 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 wt%), dimethyl sulfoxide (DMSO: Reagent Plus, 99% pure), Zonyl, (FSO fluorosurfactant), AuNPs (average diameter ∼50 nm), PVDF (polyvinylidene fluoride) substrates, sulphuric acid (H2SO4: Reagent Plus 99.5% pure) methanol (99.9% pure), ethanol (99.9% pure), were purchased from Sigma-Aldrich (India), all the chemicals and reagents were used as received.
8 wt%), dimethyl sulfoxide (DMSO: Reagent Plus, 99% pure), Zonyl, (FSO fluorosurfactant), AuNPs (average diameter ∼50 nm), PVDF (polyvinylidene fluoride) substrates, sulphuric acid (H2SO4: Reagent Plus 99.5% pure) methanol (99.9% pure), ethanol (99.9% pure), were purchased from Sigma-Aldrich (India), all the chemicals and reagents were used as received.
2.1 Preparation of PEDOT-PSS/DMSO/Zonyl–Au nanocomposite solution
Conductive PEDOT-PSS (aqueous dispersion) as received was first sonicated for 30 minutes to get a uniform dispersion. This PEDOT-PSS dispersion (90 wt%) was mixed with solutions containing 5 wt% each of DMSO and Zonyl which is further sonicated for 2 hours to obtain uniform dispersions of these 3 compounds. Addition of DMSO primarily helps to improve the conductivity of PEDOT-PSS, while Zonyl facilitates excellent film forming ability for PEDOT-PSS and contributes towards improved mechanical properties. 5 wt% of AuNPs were dispersed into ethanol solution and sonicated for 2 hours followed by stirring for 1 hour to achieve better dispersion of Au nanoparticles. The dispersion containing Au nanoparticles was mixed with PEDOT-PSS/DMSO/Zonyl and further sonicated for 2 hours to achieve homogeneous dispersion and the nanocomposite was referred as PEDOT-PSS/DMSO/Zonyl–Au. This nanocomposite solution was further used to prepare the sensing layer over flexible PVDF substrates.
2.2 Fabrication of flexible composite methane sensor
Highly flexible PVDF substrates were cut into square sheets of dimensions 3 × 3 cm2 which are UV treated washed with DI water and ethanol to improve their hydrophobicity. The nanocomposite dispersion of PEDOT-PSS/DMSO/Zonyl–Au was spin coated at 1000 rpm for 45 seconds (which results into films of average thickness 52 ± 3 μm as determined using surface profiler) onto pre-cleaned PVDF substrates to form the sensing layer for methane gas. This sensing film on PVDF substrate was vacuum dried at 60 °C for 5 hours washed with DI water and soaked into H2SO4 bath for 2 minutes and further dried for 3 hours to obtain highly conductive composite film and is labeled as PDZAu nanocomposite for further discussions. We have also spin coated bare PEDOT-PSS and PEDOT-PSS/DMSO (labeled as PD) films onto PVDF substrate for comparative studies. Finally, gold (Au) electrodes arrays were deposited via thermal deposition technique by placing mask aligner and copper wires were connected through the Au-electrodes for electrical and sensor characteristic measurements (as indicated in schematic representation of Fig. 1).
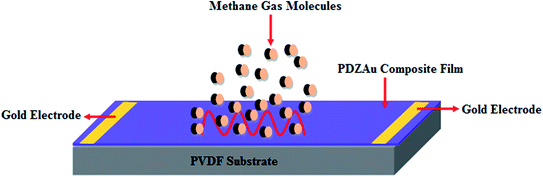 |
| | Fig. 1 Schematic representation of Sensor device fabrication. | |
2.3 Materials characterization
The surface morphologies of the samples were recorded using Zeiss Ultra-60 (Japan) scanning electron microscope. TEM image of the composite film was obtained using JEM-2100 (China) transmission electron microscope. Various functional groups present in the samples were analyzed by Thermo-Nicolet 6700 FTIR (Japan) spectrophotometer. The thermal stability of the samples in terms of TGA analysis was performed using a NETSCH STA-409PC thermal analyzer. The optical characterizations in terms of absorption spectra of the samples were investigated in UV-Vis region using an UV-Vis spectrometer (Analytikjena SPECORD S-600). Room temperature conductivity of the films was studied by 2 probe method using Keithley 6487 picoammeter/voltmeter. The Methane gas sensing performance of bare PEDOT-PSS, PD and PDZAu nanocomposite sensors were performed at room temperature using a sealed borosilicate glass chamber of volume 250 cm3. Target gas sources were mixed with the flux of synthetic air 2 l min−1, at different flow rate ratios and concentrations using mass flow control meters. The sensing performance of the films was analyzed through I–V parameters using custom data acquisition tool LabView graphic interface. A schematic view of laboratory designed gas sensing setup used to investigate the sensing performance using different test gases is represented in Fig. 2.
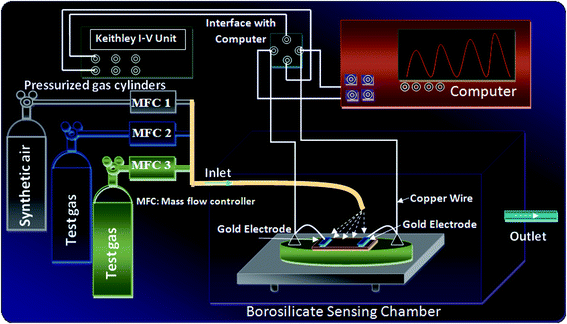 |
| | Fig. 2 Schematic experimental setup used for gas sensing studies. | |
3. Results and discussions
3.1 SEM and TEM analysis
The SEM micrographs of pristine PEDOT-PSS, AuNPs and PDZAu (PEDOT-PSS:DMSO:Zonyl:AuNPs) nanocomposite are shown in Fig. 3(a–c). Fig. 3(a) shows the SEM micrograph of pure PEDOT-PSS which reveals the formation of uniform and well-connected continuous film. Fig. 3(b) shows the SEM image of AuNPs, which reveals formation of spherical clusters having an average size of around ∼50 nm. The SEM micrograph of PDZAu nanocomposite (Fig. 3c) indicates that AuNPs are homogenously dispersed in the polymer matrix without the formation of aggregations and agglomerations. The inclusion of AuNPs resulted into micro-pores in the composite sample. The interconnected conducting network of polymer chains mediated via AuNPs plays a crucial role in enhancing the electrical properties as well sensing behavior of the nanocomposite. We have stabilized the concentration of AuNPs to 5 wt% since the higher concentration leads to agglomeration of AuNPs and affecting the quality of the film. The presence of interfacial region between PEDOT-PSS and AuNPs was proved by TEM image shown in Fig. 3(d). The TEM image shows contrast between dense PEDOT-PSS chains that can be located around spherical AuNPs. The bonding between AuNPs and sulphur atoms of PEDOT is attributed to be the reason behind the formation of dense interface that could lead to optimization of structural and electrical properties of the composites.30
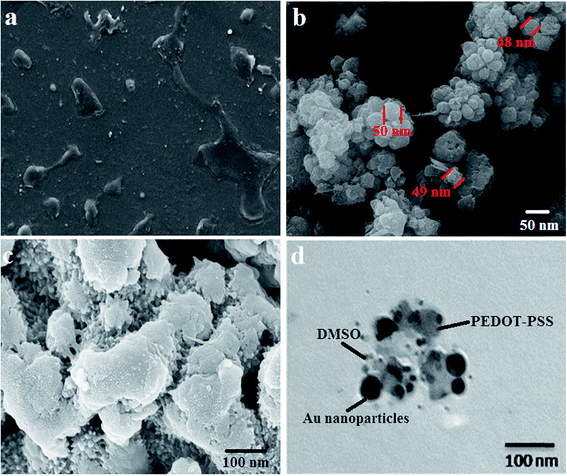 |
| | Fig. 3 SEM micrograph of (a) PEDOT-PSS (b) AuNPs (c) PDZAu nanocomposite (d) TEM image of PDZAu nanocomposite. | |
3.2 FTIR analysis
FTIR spectroscopy was employed for the determination of the functional groups of PEDOT-PSS, PD (PEDOT-PSS:DMSO) and PDZAu composite film. FT-IR spectra of pristine PEDOT-PSS, PD and PDZAu composites are depicted in Fig. 4(a). The prominent peaks in FT-IR spectra of PEDOT-PSS at 3445, 1645 and 1380 cm−1 associated with stretching vibration of –NH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– and –S
C– and –S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively and the prominent stretching at 1130 cm−1, 810 cm−1 are associated with the characteristic bands of PEDOT-PSS complex formation.23,31,32 The spectrum of PD shows vibration frequencies at about 2244 and 1641 cm−1 associated hydroxyl functional groups and vibrational modes of C
O, respectively and the prominent stretching at 1130 cm−1, 810 cm−1 are associated with the characteristic bands of PEDOT-PSS complex formation.23,31,32 The spectrum of PD shows vibration frequencies at about 2244 and 1641 cm−1 associated hydroxyl functional groups and vibrational modes of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds respectively. The FTIR spectra of the PDZAu nanocomposite indicate retention of major characteristic peaks corresponds to both PEDOT-PSS and AuNPs. The vibration at 1038 and 577 cm−1 correspond to C–N bonding and Au–O band, respectively.33 Some of the characteristic peaks of PEDOT-PSS and AuNPs either disappear or undergo slight red shift in the composite spectra due to the delocalization of electrons from aromatic ring, proving a strong presence of π–π* interaction between AuNPs and PEDOT-PSS. The loss of molecular ordering due to strong interactions among different phases further confirms the possible red shift in the spectra. Thus, the FTIR results confirm the well formation of PDZAu nanocomposites.
C double bonds respectively. The FTIR spectra of the PDZAu nanocomposite indicate retention of major characteristic peaks corresponds to both PEDOT-PSS and AuNPs. The vibration at 1038 and 577 cm−1 correspond to C–N bonding and Au–O band, respectively.33 Some of the characteristic peaks of PEDOT-PSS and AuNPs either disappear or undergo slight red shift in the composite spectra due to the delocalization of electrons from aromatic ring, proving a strong presence of π–π* interaction between AuNPs and PEDOT-PSS. The loss of molecular ordering due to strong interactions among different phases further confirms the possible red shift in the spectra. Thus, the FTIR results confirm the well formation of PDZAu nanocomposites.
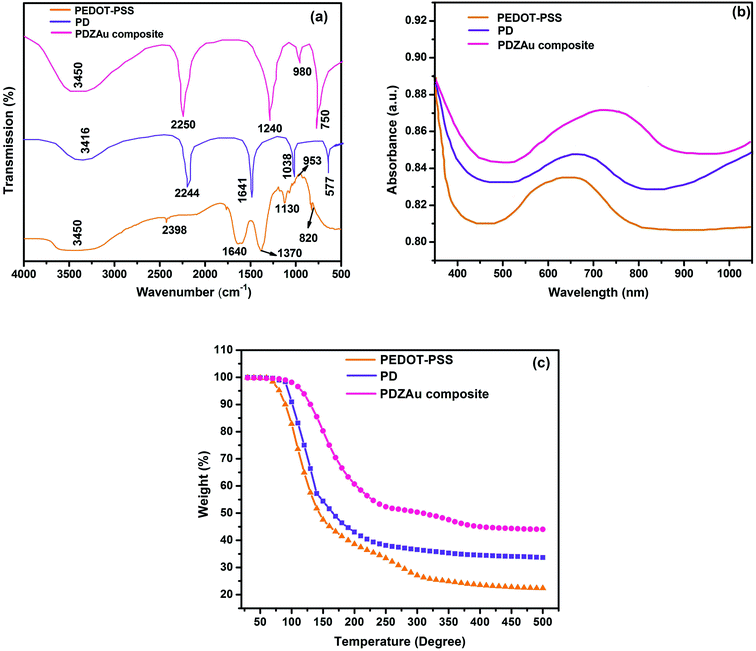 |
| | Fig. 4 (a) FTIR spectra (b) UV-Visible spectra and (c) TGA thermograms of PEDOT-PSS, AuNPs, PDZAu nanocomposite. | |
3.3 UV-Vis spectroscopy
Additional evidence for the successful formation of composite structure is deduced from UV-Vis spectroscopy. The absorption spectra of PEDOT-PSS, PD and PDZAu nanocomposite films, in the wavelength domain from 350 to 1050 nm, are presented in Fig. 4(b). We notice that the absorption peak (λmax) of pristine PEDOT-PSS, which is around 650 nm, is shifted towards high wavelength (red shift), with the addition of DMSO to PEDOT- PSS. The absorption peak of PDZAu composite films is observed around 740 nm, with a red shift by almost 100 nm in comparison with pure PEDOT-PSS. The red shifts in the absorption spectra are mainly due to the changes in conjugation length of the polymer chains with the addition of DMSO and AuNPs. It was speculated that the change in absorption peak intensity of the composite spectra results from increased π–π interaction among various components of the hybrid system. Such π–π interactions results in delocalization of electrons that may take part in conduction as well improved sensing performance of the hybrid composite. Moreover, we have chosen low filling concentrations of the AuNPs (5 wt%) to maintain the optimum transparency of the polymer, which is evident from the UV-Vis spectra of the PDZAu nanocomposite. Hence, the nanocomposite formation strategy adopted in this investigation indeed does not affect the average transparency of the conductive PEDOT-PSS polymer.
3.4 TGA analysis
Fig. 4(c) represents the thermograms of PEDOT-PSS, PD and PDZAu nanocomposites. The thermograms of all elaborated structures show a three-step weight loss; slight decomposition region, fast decomposition region and residual decomposition region. The first decomposition up to 100 °C is mainly due to the evaporation of H2O molecules from the films. The small weight loss in first stage indicates that very little water is present in the samples. For PEDOT-PSS thin films, the fast decomposition occurs in the second stage (100–300 °C) due to the chemical degradation and oxidizing decomposition of the skeletal PEDOT-PSS backbone chains.32 In the third region of the thermogram of PEDOT-PSS which corresponds to the residual decomposition zone, occurs beyond 300 °C, giving an overall weight loss of nearly ∼80%. The PD thin films exhibit gradual weight loss between 100 °C to 200 °C due to loss of DMSO solvent in the host PEDOT-PSS.34 It exhibits an overall weight loss of about 65% at 500 °C. In PDZAu composites thermogram, the second region (fast decomposition) starts around 200 °C. The overall weight loss (residual decomposition) observed for these nanocomposites in third region which starts from nearly 375 °C and a constant weight loss of 55% was achieved at 500 °C. The enhanced thermal stability of nanocomposite is mainly attributed to the strong binding between AuNPs and PEDOT-PSS via DMSO and Zonyl. This binding between PEDOT-PSS and AuNPs restricts the mobility of polymer chains when bound with nanofillers which acts as a physical barrier for polymer network. We conclude that the PDZAu nanocomposite thin film is thermally more stable than PEDOT:PSS polymer and PD films. The TGA studies also confirm that, the composite samples are highly stable to be used as room temperature methane gas sensors.
3.5 Electrical conductivity at room temperature
The conductivity of the films (PEDOT-PSS, PD and PDZAu composite) at room temperature (27 °C) was evaluated using the relation| |
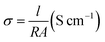 | (1) |
where R is the resistance, l is the separation between two electrodes and A is the vertical cross-sectional area of the films between two gold electrodes.
The electrical conductivity of PEDOT-PSS films depends on the phase segregated morphology, removal of insulating PSS and oxidation of PEDOT etc. Hence, doping treatment with organic solvents is an effective strategy towards improving the conductivity of the PEDOT-PSS films due to screening effects27,35–37 as well as improving the film forming ability resulting from high cohesion among the molecules.37 In our previous work we have demonstrated an efficient strategy to improve the conductivity of PEDOT-PSS via secondary doping of DMSO,38 such a strategy is expected to increase the free charge carrier concentrations in the PEDOT-PSS matrix. The room temperature conductivity of PEDOT-PSS, PD and PDZAu nanocomposites is shown in Fig. 5(a). The conductivity of bare PEDOT-PSS increases from 2 S cm−1 to 1895 S cm−1 for PDZAu composite after post treatment with strong acid. The conductivity of PDZAu nanocomposite film enhances due the formation of excellent conductive networks, wherein the charge carrier can easily hop between favorable sites. When the AuNPs are incorporated into PEDOT-PSS the charge trapping centers will be minimized, as well it leads to formation of interfacial regions. Formation of large interfacial regions at the interface between PEDOT-PSS and AuNPs leads to increased conductivity in PDZAu nanocomposite. The presence of such interfacial regions between PEDOT-PSS and AuNPs was confirmed by TEM image of Fig. 3(d). Furthermore, the effect of AuNPs presence in polymer matrix followed by post treatment with strong acid is expected to decrease energy gap and increase the charge carrier tunneling in the polymer matrix.
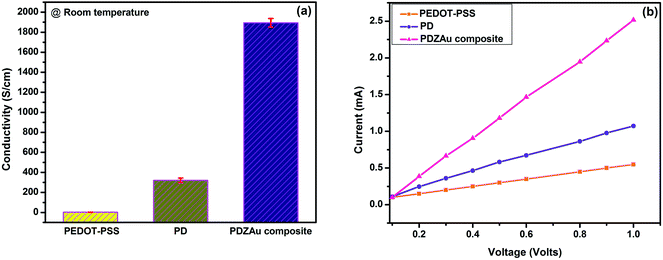 |
| | Fig. 5 (a) Conductivity and (b) current–voltage (I–V) characteristic of PEDOT-PSS, PD and PDZAu nanocomposite. | |
The current–voltage (I–V) characteristics of PEDOT-PSS, PD and PDZAu nanocomposites are illustrated in Fig. 5(b). I–V characteristics of both pristine and composite film are nearly linear (Ohmic-nature) during forward biasing upto 1 V. Amongst all the prepared samples, PDZAu nanocomposite shows higher current under the forward bias condition indicating an improved conductivity in these systems compared to pure PEDOT-PSS and PD. The nearly linear behavior of the films indicates an excellent adherence of the sensing film with the gold electrodes and superior work-function compatibility between the electrodes and sensing film.
3.6 Band gap energy
The optical band energy with different doping states for PEDOT-PSS and PDZAu nanocomposites were calculated using UV-Vis spectroscopy. For this purpose, the powders of different samples prepared in this work were dissolved in DI water and nylon filters were used to filter them. DI water was used as a reference indicator. Inbuilt software was used to determine the UV-adsorption of all the samples. The optical band gap energy was calculated using Tauc equation.39where (α) is the adsorption coefficient, (hν) is the photon energy, (A) is a constant (Eg) is the band gap energy (eV). The value of (n) was determined from the slope of ln(αhν) vs. ln(hν − Eg) plot. The value of n is used to determine the nature of material either direct band gap (n = 0.5) or indirect band gap (n = 2). The adsorption coefficient (α) was calculated using the Beer–Lamberts equation.39where (Ab) is the absorbance and (I) is the sample path length. Finally, the energy band gap can be calculated from the linear extrapolation of the plot between (αhν)2 on y-axis and hν on x-axis.
The band gap energies were calculated for all the samples used in the present investigation and are tabulated in Table 1. The data on energy band gap values (Table 1) indicates that the energy band of pure PEDOT-PSS significantly decreases for PD and PDZAu nanocomposites. This supports for the enhanced conductivity for PD and PDZAu nanocomposites. The presence of dopants such as DMSO, AuNPs and post treatment with H2SO4 significantly enhances the charge carrier concentration in the PEDOT-PSS matrix as a consequence the band gap energy decreases. The average value of (n) in Tauc eqn (2) for all the samples varies between 0.476 to 0.492, which indicates that the samples used in present investigation behaves as direct band gap materials.
Table 1 Optical band gap energies of different samples investigated in the present study using eqn (2) and (3)
| Sample |
HOMO (eV) |
LUMO (eV) |
Bang gap energy (Eg) (eV) |
Value of exponential (n) |
| AuNPs |
5.1 |
— |
Zero |
— |
| Pure PEDOT-PSS |
5.3 |
2.2 |
3.1 |
0.476–0.492, all the samples behave as direct band gap materials |
| PEDOT-PSS doped with DMSO (PD) |
5.3 |
2.94 |
2.36 |
| PEDOT-PSS with AuNPs |
5.1 |
3.81 |
1.29 |
| PEDOT-PSS with DMSO, Zonyl, AuNPs post treated with H2SO4 (PDZAu nanocomposite) |
5.1 |
4.48 |
0.62 |
3.7 Methane gas sensing performance
The gas sensing behavior of pristine PEDOT-PSS, PD and PDZAu nanocomposite thin films were analyzed for various concentrations of methane gas. The change in electrical resistance of the samples was determined at ambient temperature (27 °C) at a constant voltage of 1 V. The variation of resistance with different concentrations of methane gas is shown in the Fig. 6(a). The change in resistance of all prepared samples is nearly linearly for varying methane gas concentrations. The maximum change in resistance was observed in case of PDZAu nanocomposite when compared to pristine PEDOT-PSS and PD films. The presence of AuNPs in PEDOT-PSS strongly enhances the electric fields that are generated locally in their surface regions owing to localized surface plasmon resonance (LSRP).30 Generation of such local fields in the polymer matrix can tend to increase the resistance of the composite in the presence of methane gas. Furthermore, the increased resistance of PDZAu nanocomposite is due to the swelling effect upon exposure to methane gas. When the polymer thin films were exposed to gas species, the volume of the thin film get increases which leads to enhancement in the resistance, consequently the conductive networks via the sample get disrupted.24 The change in resistance of PDZAu nanocomposite may also be due to the formation of heterojunction between PEDOT-PSS and AuNPs with potential barrier at the interface, which influence the adsorption and diffusion of methane gas molecules. Methane has highly symmetrical and non-polar molecules with excellent enthalpy of formation between C–H bonds, which increases its stability in the gaseous phase. This makes it highly difficult to detect in the gaseous phase of green house in comparison to other gases. The sensing of methane gas molecules involves two steps, in the first step the environmental oxygen is adsorbed onto grain boundaries of sensor film. This adsorbed oxygen captures the electrons from conduction band of the sensing material and transform into chemisorbed oxygen. This chemisorbed oxygen forms a space charge depletion layer at the interface between PEDOT-PSS and AuNPs thereby substantially increasing the resistance.39 The room temperature reaction mechanism involved in the formation of chemisorbed oxygen at the interface between distinct phases of a sensing material can be understood through following mechanisms40
| O2(gas-phase) → O2(adsorbed) |
| O2(adsorbed) + e− → O2−(adsorbed), (for T < 100 °C) |
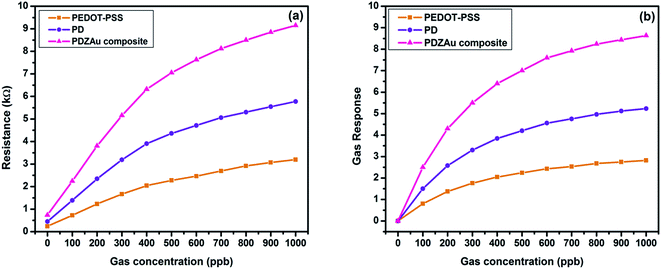 |
| | Fig. 6 Variation of (a) resistance and (b) sensor response for different concentrations of methane gas for PEDOT-PSS, PD and PDZAu nanocomposite. | |
During the second step of reaction, the greenhouse methane gas molecules interact with the oxygen ions (chemisorbed) to produce CO2 and H2O. This reaction produces excess electrons and pumps these extra electrons into conduction band thereby decreasing the thickness of depletion layer and reduces the resistance of the sensing film. This reaction can be explained as follows.41
| CH4(gas-phase) + 4O2−(adsorbed) → CO2(gas-phase) + 2H2O + 8e− |
The variation of gas sensitivity (response) for methane gas concentrations (20–1000 ppb) in pristine PEDOT-PSS, PD and PDZAu nanocomposite at room temperature (27 °C) is illustrated in the Fig. 6(b). The PDZAu nanocomposite shows an improved sensing response of 8.6 in comparison to pristine PEDOT-PSS which is about 2.5. The highest sensitivity in composite film is mainly due to the drop in resistance for PDZAu nanocomposite against PEDOT:PSS in the presence of greenhouse methane gas. Due to the addition of DMSO and gold nanoparticles into PEDOT:PSS, the highly clustered morphology is formed which helps the analyte gas molecules to interact with polymer matrix. The chemiresistive sensor fabricated in this study shows excellent gas response of ∼8.6 (760% sensitivity) at 1000 ppb concentration of methane gas. The sensors fabricated in the present study demonstrates excellent features towards methane gas detection in low concentrations at room temperatures in comparison to recent studies42–52 as summarized in Table 2. To the best of our knowledge this is the first study reported with such a high performance for room temperature detection of greenhouse methane gas at ppb level.
Table 2 Comparison between our work and recently published research (2017–2020) on methane gas sensorsa
| Materials used |
Operating temperature/substrate |
Methane gas concentration |
Sensing response |
Response/recovery time |
Reference |
| *NF- non flexible substrate, F-flexible substrate. |
| α-Fe2−xCuxO3 nanoparticles |
RT/NF |
1000 ppm |
1.03 |
Data not available |
4 |
| NiO/ZnO nanosheets |
340 °C/NF |
1000 ppm |
34.2% |
7/33 s |
5 |
| SnO2/WO3nanoplates |
110 °C/NF |
500 ppm |
2.85 |
Data not available |
7 |
| SnO2/Pt/MWCNTs |
RT/NF |
100 ppm |
28.25% |
176/763 s |
40 |
| SnO2@rGO-PANI |
RT/NF |
100 ppm |
26.1% |
Data not available |
42 |
| Li+/CNTs |
RT/NF |
500 ppm |
14.5% |
Data not available |
43 |
| NiO/In2O3 nanocomposites |
120 °C/NF |
500 ppm |
13% |
Data not available |
44 |
| α-Fe2−xCuxO3 |
RT/NF |
2000 ppm |
50% |
Data not available |
45 |
| Graphene–AgNPs |
RT/NF |
2000 ppm |
12% |
Data not available |
46 |
| NiO:Al thin films |
RT/NF |
100 ppm |
58% |
1373/95 s |
47 |
| TiO2 nanorods |
RT/NF |
60 ppm |
6068 |
60/100 s |
48 |
| Pd-doped SnO2/rGO |
RT/NF |
800–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm 000 ppm |
0.5–10% |
5/7 min |
49 |
| g-C3N4/ZnO |
320 °C/NF |
1000 ppm |
11.9% |
15/28 s |
50 |
| ZnO decoration with Zn2SnO4 |
250 °C/NF |
400 ppm |
81.38% |
10/30 s |
51 |
| Pd modified ZnO nano sheets |
200 °C/NF |
100 ppm |
8.65 |
Data not available |
52 |
| PEDOT-PSS:DMSO:Zonyl:AuNPs treated with H2SO4 |
RT/F |
20–1000 ppb |
8.6 @ 1000 ppb (∼760%) |
22/43 s @ 800 ppb |
Present work |
One of the important parameters to characterize the sensor performance is its response and recovery time. The performance of nanocomposite sensor for methane exposure (ON–OFF cycles) at 800 ppb indicates excellent repeatability as shown in Fig. 7(a). The response and recovery time calculated from the dynamic response plot of Fig. 7(a) indicates a very short response time of 22 seconds and a recovery time of 43 seconds for the composite at 800 ppb concentrations. Comparison between the response and recovery times for PEDOT-PSS, PD and PDZAu nanocomposite at 800 ppb of methane gas is shown in Fig. 7(b). The results obtained from response and recovery tests indicate that the fabricated sensors have fast response and recovery times.
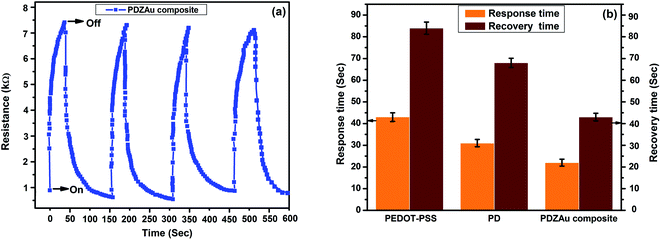 |
| | Fig. 7 (a) Dynamic response of PDZAu nanocomposite at 800 ppb of methane gas (b) response and recovery time for PEDOT-PSS, PD and PDZAu nanocomposite at 800 ppb of methane gas. | |
3.8 Hysteresis
The gas sensing hysteresis in terms of adsorption and desorption for each sample was experimentally determined upto 800 ppb of methane gas concentration. The pertinent hysteresis plots for PEDOT-PSS, PD and PDZAu nanocomposite are represented in Fig. 8(a–c). Among the prepared samples the PDZAu nanocomposite shows lowest hysteresis loss in comparison to pure PEDOT-PSS and PD thin films. The smaller hysteresis loss in PDZAu nanocomposite is mainly due to well dispersed DMSO and AuNPs in PEDOT-PSS matrix. The increased pi-conjugation in the composite system not only enhances the conductivity and sensitivity but also supports for faster absorption and desorption of methane gas molecules resulting into smaller hysteresis loss. These results also support for the superior sensing performance of the fabricated sensor based on PDZAu nanocomposite.
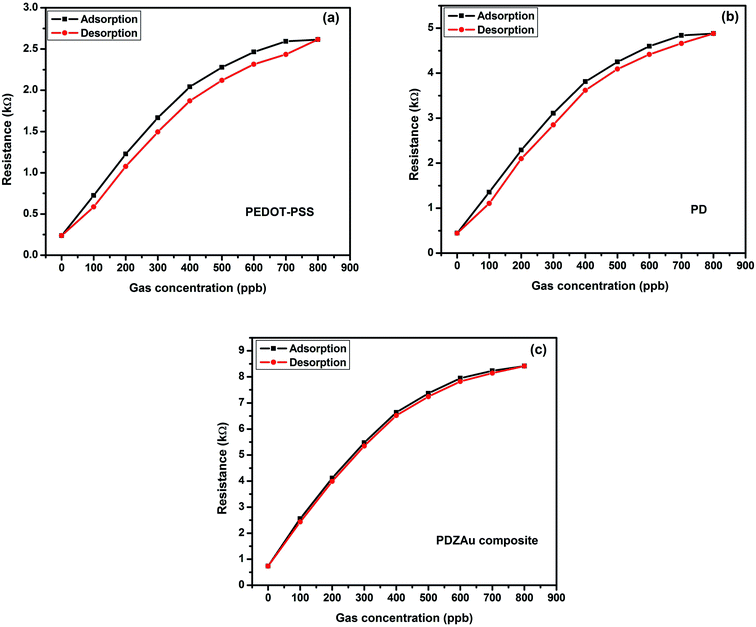 |
| | Fig. 8 Methane gas absorption–desorption (hysteresis) curves for (a) PEDOT-PSS, (b) PD and (c) PDZAu nanocomposite at 800 ppb. | |
3.9 Selectivity and stability tests
The selectivity test for PDZAu nanocomposite was carried out at ambient temperature (27 °C) at 800 ppb concentration of different test gases is shown in the Fig. 9(a). The selectivity test reveals the fact that, the PDZAu nanocomposite sensor displays excellent selectivity upon exposure of methane gas in comparison to other tested gases such as carbon dioxide, nitrous oxide, ethane and carbon monoxide respectively. This behavior could be expected due to interaction of PDZAu nanocomposites in the presence of different gas analytes. Even though both methane and ethane belong to the family of hydrocarbons, the improved performance in case of methane could be expected due to difference between C–H activations of lower (methane) and higher hydrocarbons (ethane) on the sensing surface. The ethane decomposition on sensing surface is more complex that could lead to series of partially dehydrogenated intermediates involving C–C bond breaking. Further indepth understanding of sensing behavior of these composites for different hydrocarbons are to be investigated. The selectivity studies confirm that PDZAu nanocomposite material is a superior candidate to fabricate methane sensors at room temperature (27 °C).
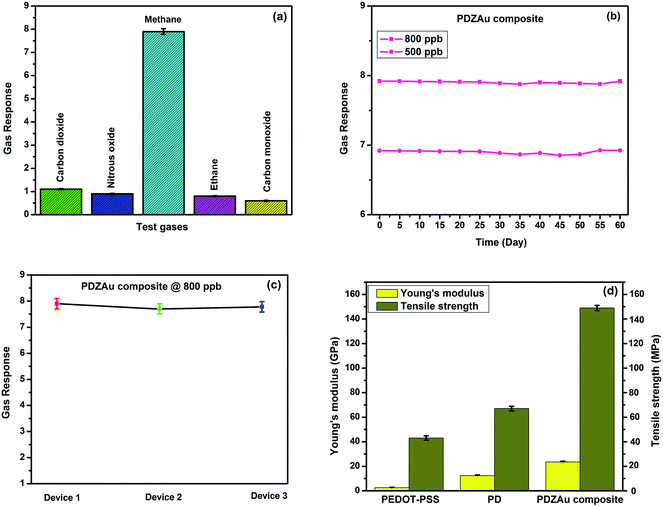 |
| | Fig. 9 (a) Selectivity studies of PDZAu nanocomposite sensor towards various tests gases at 800 ppb (b) long term stability of PDZAu nanocomposite sensor at 500 & 800 ppb of methane gas (c) sensing performance of 3 different PDZAu composites (d) Young's modulus and Tensile strength of PEDOT-PSS, PD and PDZAu nanocomposite. | |
The long-term sensor stability tests for PDZAu nanocomposite were carried out at 500 ppb and 800 ppb exposure of methane gas over a period of 60 days and are depicted in Fig. 9(b). The sensing response of PDZAu nanocomposite was analyzed at two different concentrations such as 500 and 800 ppb of methane gas. After analyzing the sensing response at these concentrations, the methane inlet is turned off and the samples were removed from the test chamber after they attain the baseline resistance. These samples were preserved in an air-tight vacuum desiccator till next measurement and same is repeated for a period of 60 days. The PDZAu nanocomposite demonstrates excellent stability for the period of 60 days without any loss in sensitivity. These results show that PDZAu nanocomposite can be a potential candidate to fabricate sensor towards detection of greenhouse methane gas at room temperature. Further we have tested three different devices fabricated using these PDZAu nanocomposites. It is interesting to see from Fig. 9(c) that, all the different sensor devices fabricated using these nanocomposite shows almost similar response at 800 ppb of methane gas.
3.10 Mechanical properties of the composite film
Mechanical properties of the pure PEDOT-PSS, PD and PDZAu nanocomposite in terms of Young's modulus and Tensile strength studied at room temperature (27 °C) are depicted in Fig. 9(d). Young's modulus and Tensile strength for PDZAu nanocomposite demonstrates improved mechanical properties due to Zonyl treatment and AuNPs addition in PEDOT-PSS. It is interesting to see that Young's modulus increases from 2.6 ± 0.1 GPa for PEDOT-PSS to 12.34 ± 0.1 GPa for PD and finally attains the value 23.45 ± 0.1 GPa for PDZAu nanocomposite. Similarly, the tensile strength of the PEDOT-PSS varies from 43 ± 2.2 MPa (pristine sample) to 67 ± 2.2 MPa (for PD) and finally reaches a value 149 ± 2.2 MPa (PDZAu nanocomposite). The initial increase in both Young's modulus and tensile strength of PEDOT-PSS:DMSO is due to formation of hybrid cross linking between PEDOT-PSS and DMSO phases that facilitates improved mechanical properties.6 Further, the addition of AuNPs and Zonyl in the composite improves the cohesion of molecules and forms strong hydrogen bonded network with PEDOT-PSS and DMSO. All these factors contribute towards excellent mechanical properties of the composite in comparison to bare PEDOT-PSS. The improved mechanical properties of the composite indicate that this material could potentially be used to fabricate mechanically robust methane sensors in flexible and wearable electronic devices.
4. Conclusions
We have demonstrated a simple strategy to fabricate chemiresistive sensors based on conductive PEDOT-PSS composites formed with combination of DMSO, Zonyl and AuNPs along with post treatment of strong acid (H2SO4) towards room temperature detection of greenhouse methane gas at ppb level. The morphological and structural features studied through various analytical techniques suggest a successful formation of composite structure. The post treatment of composite with H2SO4 significantly reduces the band gap energy and enhances the conductivity of PDZAu nanocomposite. The sensitive layer of PDZAu nanocomposite treated with H2SO4 was spin coated onto flexible PVDF substrate and employed towards detection of greenhouse methane gas (20–1000 ppb) at room temperature. The PDZAu nanocomposite demonstrates an excellent response of ∼8.6 (at 1000 ppb) with a response/recovery time of 22 s/43 s (@ 800 ppb) respectively. The improved sensing response and better selectivity towards methane shows the significance of these composites as low power consumption gas sensors at room temperature. The improved sensing behavior of the composite could be attributed to the formation of conductive network, synergetic π–π interactions among different phases of hybrid system that facilitates the delocalization of electrons. Apart from that, the post treatment of H2SO4 facilitates enhanced electric charge transport due to depletion of insulating PSS layers. The presence of DMSO, Zonyl and AuNPs significantly contributes towards improved Young's modulus and tensile strength of the composite due to better cohesion of molecules. In view of ease of fabrication, enhanced conductivity, excellent sensitivity, short response/recovery time and improved mechanical properties, the composite investigated in the present study could be used as flexible room temperature chemiresistive sensor for the real time monitoring of greenhouse methane gas at ppb level.
Author contributions
Authors SK, AP and NB contributed towards development of idea, design of materials, sensor studies, manuscript preparation. Authors acknowledge the scientific discussions and thoughts provided by Y.Mishra of Kiel-University Germany towards sensor studies. Authors AL, SAG and CP contributed towards materials characterization and discussions for manuscript preparation. Authors SK, AP contributed towards data finalization, review and editing of final manuscript draft and submission.
Conflicts of interest
Authors listed in the manuscript certify that they have NO affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Acknowledgements
The authors would like to acknowledge financial support for this work, from the Deanship of Scientific research (DSR), University of Tabuk, Tabuk, Saudi Arabia, under Grant No. S-1440-0267.
References
- Q. Zhou, L. Xu, A. Umar, W. Chen, R. J. S. Kumar and A. B. Chemical, Pt nanoparticles decorated SnO2 nano needles for efficient CO gas sensing applications, Sens. Actuators, B, 2018, 256, 656–664 CrossRef CAS.
- K. C. Lam, B. L. Huang and S. Q. Shi, Room-temperature methane gas sensing properties based on in situ reduced graphene oxide incorporated with tin dioxide, J. Mater. Chem., 2017, 5(22), 11131–11142 RSC.
- L. P. Yang, Z. Wang, X. Y. Zhou, X. F. Wu, N. Han and Y. F. Chen, Synthesis of Pd loaded mesoporous SnO2 hollow spheres for highly sensitive and stable methane gas sensor, RSC Adv., 2018, 8(43), 24268–24275 RSC.
- H. F. Liu, T. J. Peng, H. J. Sun, R. S. Xie and G. H. Ma, Room temperature methane sensing properties of α-Fe2-xCuxO3 nanoparticles, RSC Adv., 2017, 7(19), 11414–11419 RSC.
- S. Zhang, Y. Li, G. Sun, Bo Zhang, Y. Wang, J. Cao and Z. Zhang, Synthesis of NiO-decorated ZnO porous nanosheets with improved CH4 sensing performance, Appl. Surf. Sci., 2019, 497, 143811 CrossRef CAS.
- Z. P. Tshabalala, H. C. Swart and D. E. Motaung, Fabrication of TiO2 nanofibers based sensors for enhanced CH4 performance induced by notable surface area and acid treatment, Vacuum, 2021, 187, 110102 CrossRef CAS.
- D. Xue, Y. Wang, J. Cao, G. Sun and Z. J. T. Zhang, Improving methane gas sensing performance of flower-like SnO2 decorated by WO3 nanoplates, Talanta, 2019, 199, 603–611 CrossRef CAS PubMed.
- P. Tang, Q. J. Zhu, Z. X. Wu and D. Ma, Methane activation: the past and further, Energy Environ. Sci., 2014, 7(8), 2580–2591 RSC.
- J. Kamieniak, E. P. Randviir and C. E. Banks, The latest developments in the analytical sensing of methane, TrAC, Trends Anal. Chem., 2015, 73, 146 CrossRef CAS.
- C. Sun, P. R. Ohodnicki and E. M. Stewart, Chemical Sensing Strategies for Real-time Monitoring of Transformer Oil: A Review, IEEE Sens. J., 2017, 17, 5786 CAS.
- D. Xue, P. Wang, Z. Zhang, Y. J. S. Wang and A. B. Chemical, Enhanced methane sensing property of flower-like SnO2 doped by Pt nanoparticles: a combined experimental and first-principle study, Sens. Actuators, B, 2019, 126710 CrossRef CAS.
- S. Navazani, A. Shokuhfar, M. Hassanisadi, A. Di Carlo, N. Y. Nia and A. Agresti, A Pd-Pt decorated SnO2-rGO nano hybrid for high-performance resistive sensing of methane, J. Taiwan Inst. Chem. Eng., 2019, 95, 438–451 CrossRef CAS.
- N. M. Shaalan, D. Hamad, A. Aljaafari, A. Y. Abdel-Latief and M. A. Abdel-Rahim, Preparation and characterization of developed CuxSn1− xO2 nanocomposite and its promising methane gas sensing properties, Sensors, 2019, 19(10), 2257 CrossRef CAS PubMed.
- D. Zhang, N. Yin and B. Xia, Facile fabrication of ZnO nanocrystalline-modified graphene hybrid nanocomposite toward methane gas sensing application, J. Mater. Sci.: Mater. Electron., 2015, 26, 5937–5945 CrossRef CAS.
- L. Li, G. Li and Y. Yuan, Mesoporous PdO/Pt/Al2O3 film produced by reverse-micro-emulsion and its application for methane microsensor, RSC Adv., 2015, 5, 4586–4591 RSC.
- H. Tao, J. T. Culp, Ki-J. Kim, J. Devkota, C. Sun and P. R. Ohodnicki, State-of-the-art of methane sensing materials: A review and perspectives, Trends Anal. Chem., 2020, 125, 115820 CrossRef.
- N. Shaalan, M. Rashad, A. Moharram and M. Abdel-Rahim, Promising methane gas sensor synthesized by microwave-assisted Co3O4 nanoparticles, Mater. Sci. Semicond. Process., 2016, 46, 1 CrossRef CAS.
- H. Bai and G. Shi, Gas Sensors Based on Conducting Polymers, Sensors, 2007, 7, 267–307 CrossRef CAS.
- I. Fratoddi, I. Venditti, C. Cametti and M. V. Russo, Chemiresistive polyaniline-based gas sensors: A mini review, Sens. Actuators, B, 2015, 220, 534 CrossRef CAS.
- Y. Wen and J. Xu, Scientific Importance of Water-Processable PEDOT–PSS and Preparation, Challenge and New Application in Sensors of its Film Electrode: A Review, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 1121–1150 CrossRef CAS.
- Y. C. Wong, B. C. Ang and A. S. M. A. Haseeb, Aainaa Aqilah Baharuddin, and Yew Hoong Wong, Conducting Polymers as Chemiresistive Gas Sensing Materials: A Review, J. Electrochem. Soc., 2020, 167, 037503 CrossRef CAS.
- G. Huseynova, Y. H. Kim, J.-H. Lee and J. Lee, Rising advancements in the application of PEDOT-PSS as a prosperous transparent and flexible electrode material for solution-processed organic electronics, J. Inf. Disp., 2020, 21(No. 2), 71–91 CrossRef CAS.
- K. Syed, A. Pasha, N. Badi, M. Lakshmi and Y. K. Mishra, High performance flexible supercapacitors based on secondary doped PEDOT–PSS–graphene nanocomposite films for large area solid state devices, RSC Adv., 2020, 10, 10526 RSC.
- A. Pasha, K. Syed, F. Ahmed Khan and N. Dhananjaya, Fabrication of gas sensor device using poly (3, 4-ethylenedioxythiophene)-poly (styrenesulfonate)-doped reduced graphene oxide organic thin films for detection of ammonia gas at room temperature, Iran. Polym. J., 2019, 28, 183–192 CrossRef CAS.
- A. Pasha, K. Syed, A. Omar, O. A. Hartomy, M. Lakshmi and K. G. Manjunatha, Highly sensitive ethylene glycol-doped PEDOT– PSS organic thin films for LPG sensing, RSC Adv., 2018, 8, 18074 RSC.
- K. Syed, A. Pasha, N. Badi, M. Lakshmi, S. A. Al-Ghamdi, A. Hatem and AL-Aoh, PVA Treated PEDOT-PSS: TiO2 Nanocomposite Based High-Performance Sensors Towards Detection of Relative Humidity and Soil Moisture Content for Agricultural Applications, J. Polym. Environ., 2021, 29, 612–623 CrossRef.
- V. Laure, Kayser and Darren J. Lipomi, Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT-PSS, Adv. Mater., 2019, 31, 1806133 CrossRef PubMed.
- E. Dauzon, A. E. Mansour and M. R. Niazi, Rahim Munir, Detlef-M. Smilgies, Xavier Sallenave, Cedric Plesse, Fabrice Goubard, Aram Amassian, Conducting and Stretchable PEDOT-PSS Electrodes: Role of Additives on Self-Assembly, Morphology, and Transport, ACS Appl. Mater. Interfaces, 2019, 11, 19 CrossRef PubMed.
- G. Korotcenkov, V. Brinzari and B. K. Cho, Conductometric gas sensors based on metal oxides modified with gold nanoparticles: a review, Microchim. Acta, 2016, 183, 1033–1054 CrossRef CAS.
- R. Khalil, S. Homaeigohar, H. Dietrich and M. Elbahri, A shape tailored gold-conductive polymer nanocomposite as a transparent electrode with extraordinary insensitivity to volatile organic compounds (VOCs), Sci. Rep., 2016, 6, 33895 CrossRef CAS PubMed.
- H. Shi, C. C. Liu, J. K. Xu, H. J. Song, B. Y. Lu, F. X. Jiang, W. Q. Zhou, G. Zhang and Q. L. Jiang, Facile Fabrication of PEDOT-PSS/Polythiophenes Bilayered Nanofilms on Pure Organic Electrodes and Their Thermoelectric Performance, ACS Appl. Mater. Interfaces, 2013, 5, 12811 CrossRef CAS PubMed.
- P. Singh, A. Raman and N. Kumar, Spectroscopic and Simulation Analysis of Facile PEDOT-PSS Layer Deposition-Silicon for Pervoskite Solar Cell, Silicon, 2020, 12, 1769–1777 CrossRef CAS.
- N. Abdel-Raouf, N. M. Al-Enazi and I. B. M. Ibraheem, Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity, Arabian, J. Chem., 2017, 10, S3029–S3039 CrossRef CAS.
- Y.-K. Ren, S.-D. Liu, B. Duan, Ya-F. Xu, Z.-Q. Li, Y. Huang, L.-H. Hu, J. Zhu and S.-Y. Dai, Controllable intermediates by molecular self-assembly for optimizing the fabrication of large-grain perovskite films via one-step spin-coating, J. Alloys Compd., 2017, 705, 205–210 CrossRef CAS.
- V. Hebbar, R. F. Bhajantri, H. B. Ravikumar and S. Ningaraju, Role of free volumes in conducting properties of GO and rGO filled PVAPEDOT:PSS composite free standing films: A positron annihilation lifetime study, J. Phys. Chem. Solids, 2019, 126, 242–256 CrossRef CAS.
- X. Fan, W. Nie, H. Tsai, N. Wang, H. Huang, Y. Cheng, R. Wen, L. Ma, F. Yan and Y. Xia, PEDOT-PSS for Flexible and Stretchable Electronics: Modifications, Strategies, and Applications, Adv. Sci., 2019, 6, 1900813 CrossRef CAS PubMed.
- C. W. Ouyang, C.-W. Chu, F.-C. Chen, Q. Xu and Y. Yang, High-conductivity poly (3, 4-ethylenedioxythiophene): poly (styrene sulfonate) film and its application in polymer optoelectronic devices, Adv. Funct. Mater., 2005, 15, 203 CrossRef.
- A. Pasha, A. S. Roy, M. V. Murugendrappa and O. A. Al-Hartomy, Syed Khasim, Conductivity and dielectric properties of PEDOT-PSS doped DMSO nano composite thin films, J. Mater. Sci.: Mater. Electron., 2016, 27, 8332–8339 CrossRef CAS.
- P. Sakunpongpitiporn, K. Phasuksom, N. Paradee and A. Sirivat, Facile synthesis of highly conductive PEDOT: PSS via surfactant templates, RSC Adv., 2019, 9, 6363 RSC.
- S. Navazani, M. Hassanisadi, M. M Eskandari and Z. Talaei, Design and evaluation of SnO2-Pt/MWCNTs hybrid system as room temperature-methane sensor, Synth. Met., 2020, 260, 116267 CrossRef CAS.
- L. Yao, Y. Li, R. Yan, Y. Yang, R. Zhao, L. Su, Y. Kong, D. Ma, Y. Chen and Y. Wang, Construction of novel Pd-SnO2 composite nanoporous structure as a high-response sensor for methane gas, J. Alloys Compd., 2020, 826, 154063 CrossRef CAS.
- S. Navazani, A. Shokuhfar, M. Hassanisadi, A. Di Carlo and N. Shahcheraghi, Fabrication and characterization of a sensitive, room temperature methane sensor based on SnO2 at reduced graphene oxide-polyaniline ternary nano hybrid, Mater. Sci. Semicond. Process., 2018, 88, 139–147 CrossRef CAS.
- X. Chen, Z. Huang, L. Jia, C. Wu, Z. Wang and Y. Cui, Methane gas sensing behavior of lithium ion doped carbon nanotubes sensor, Vacuum, 2018, 154, 120–128 CrossRef CAS.
- Y. Wang, M. Yao, R. Guan, Z. Zhang and J. Cao, Enhanced methane sensing performance of NiO decorated In2O3 nanospheres composites at low temperature, J. Alloys Compd., 2021, 854, 157169 CrossRef CAS.
- H. Liu, T. Peng, H. Sun, R. Xiea and G. Ma, Room temperature methane sensing properties of α-Fe2-x CuxO3 nanoparticles, RSC Adv., 2017, 7, 11414 RSC.
- R. Ghanbari, S. Rosa, M. H. Sheikhi, M. M. Golshan and Z. Karami Horastani, Graphene Decorated with Silver Nanoparticles as a Low-Temperature Methane Gas Sensor, ACS Appl. Mater. Interfaces, 2019, 11(24), 21795–21806 CrossRef CAS PubMed.
- E. Gagaoudakis, G. Michail, D. Katerinopoulou, K. Moschovis, E. Iliopoulos, G. Kiriakidis and V. B. E. Aperathitis, Transparent p-type NiO:Al thin films as room temperature hydrogen and methane gas sensors, Mater. Sci. Semicond. Process., 2020, 109, 104922 CrossRef CAS.
- Z. P. Tshabalala, K. Shingangea, B. P. Dhonge, O. M. Ntwaeaborwa, G. H. Mhlongo and D. E. Motaung, Fabrication of ultra-high sensitive and selective CH4 room temperature gas sensing of TiO2 nanorods: Detailed study on the annealing temperature, Sens. Actuators, B, 2017, 238, 402–419 CrossRef CAS.
- Sh. Nasresfahani, M. H. Sheikhi, M. Tohidi and A. Zarifkar, Methane gas sensing properties of Pd-doped SnO2/reduced graphene oxide synthesized by a facile hydrothermal route, Mater. Res. Bull., 2017, 89, 161–169 CrossRef CAS.
- X. Li, Y. Li, G. Sun, Na Luo, Bo Zhang and Z. Zhang, Synthesis of a Flower-Like g-C3N4/ZnO Hierarchical Structure with Improved CH4 Sensing Properties, Nanomaterials, 2019, 9, 724 CrossRef CAS PubMed.
- X. Li, Y. Lia, G. Sun, Bo Zhang, Y. Wang and Z. Zhang, Enhanced CH4 sensitivity of porous nanosheets-assembled ZnO microflower by decoration with Zn2SnO4, Sens. Actuators, B, 2020, 304, 127374 CrossRef CAS.
- Y. Wang, X. Meng, M. Yao, G. Sun and Z. Zhang, Enhanced CH4 sensing properties of Pd modified ZnO nanosheets, Ceram. Int., 2019, 45, 13150–13157 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2021 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *ab,
Apsar Pashac,
Nacer Badiab,
Adnen Ltaiefa,
S. A. Al-Ghamdiab and
Chellasamy Panneerselvamd
*ab,
Apsar Pashac,
Nacer Badiab,
Adnen Ltaiefa,
S. A. Al-Ghamdiab and
Chellasamy Panneerselvamd
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly (styrene sulfonate) (PEDOT-PSS) (a mixture of two ionomers), is a commercially available, conductive polyelectrolyte complex with superior properties as an aqueous dispersion.20 Due to its excellent conductivity, optical transparency and processability in printing electronics, it has been extensively investigated for optoelectronic applications and fabrication of various sensors.20 The conductivity and sensitivity of commercially available PEDOT-PSS aqueous dispersions are low, these properties can be enhanced significantly by secondary doping of PEDOT-PSS using high boiling point, organic polar solvents.21–28 In the recent past, many researchers have demonstrated an effective strategy to improve the conductivity and sensitivity of PEDOT-PSS thin films by adding small quantities of polar solvents such as DMF, ethylene glycol, sorbitol, DMSO and glycerol.23–26 Other efficient strategy to improve the performance of PEDOT-PSS in organic electronics is to form hybrid composites of PEDOT-PSS using nanofillers.20 Highly efficient PEDOT-PSS based hybrid nanocomposites have gained considerable attention for the fabrication of various chemiresistive sensors that can be operated at room temperature.20,24,26 In the recent past, AuNPs, possessing high chemical stability, facile synthesis, surface functionalization, biocompatibility and tunable optical and electronic properties, have led to many promising applications in the field of sensor technology.29 The aspect ratio of AuNPs nanofillers, their functionalization and formation of interfacial region can play an important role in tuning the optical, electrical and sensing performance of PEDOT-PSS polymer.30
poly (styrene sulfonate) (PEDOT-PSS) (a mixture of two ionomers), is a commercially available, conductive polyelectrolyte complex with superior properties as an aqueous dispersion.20 Due to its excellent conductivity, optical transparency and processability in printing electronics, it has been extensively investigated for optoelectronic applications and fabrication of various sensors.20 The conductivity and sensitivity of commercially available PEDOT-PSS aqueous dispersions are low, these properties can be enhanced significantly by secondary doping of PEDOT-PSS using high boiling point, organic polar solvents.21–28 In the recent past, many researchers have demonstrated an effective strategy to improve the conductivity and sensitivity of PEDOT-PSS thin films by adding small quantities of polar solvents such as DMF, ethylene glycol, sorbitol, DMSO and glycerol.23–26 Other efficient strategy to improve the performance of PEDOT-PSS in organic electronics is to form hybrid composites of PEDOT-PSS using nanofillers.20 Highly efficient PEDOT-PSS based hybrid nanocomposites have gained considerable attention for the fabrication of various chemiresistive sensors that can be operated at room temperature.20,24,26 In the recent past, AuNPs, possessing high chemical stability, facile synthesis, surface functionalization, biocompatibility and tunable optical and electronic properties, have led to many promising applications in the field of sensor technology.29 The aspect ratio of AuNPs nanofillers, their functionalization and formation of interfacial region can play an important role in tuning the optical, electrical and sensing performance of PEDOT-PSS polymer.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 wt%), dimethyl sulfoxide (DMSO: Reagent Plus, 99% pure), Zonyl, (FSO fluorosurfactant), AuNPs (average diameter ∼50 nm), PVDF (polyvinylidene fluoride) substrates, sulphuric acid (H2SO4: Reagent Plus 99.5% pure) methanol (99.9% pure), ethanol (99.9% pure), were purchased from Sigma-Aldrich (India), all the chemicals and reagents were used as received.
8 wt%), dimethyl sulfoxide (DMSO: Reagent Plus, 99% pure), Zonyl, (FSO fluorosurfactant), AuNPs (average diameter ∼50 nm), PVDF (polyvinylidene fluoride) substrates, sulphuric acid (H2SO4: Reagent Plus 99.5% pure) methanol (99.9% pure), ethanol (99.9% pure), were purchased from Sigma-Aldrich (India), all the chemicals and reagents were used as received.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– and –S
C– and –S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively and the prominent stretching at 1130 cm−1, 810 cm−1 are associated with the characteristic bands of PEDOT-PSS complex formation.23,31,32 The spectrum of PD shows vibration frequencies at about 2244 and 1641 cm−1 associated hydroxyl functional groups and vibrational modes of C
O, respectively and the prominent stretching at 1130 cm−1, 810 cm−1 are associated with the characteristic bands of PEDOT-PSS complex formation.23,31,32 The spectrum of PD shows vibration frequencies at about 2244 and 1641 cm−1 associated hydroxyl functional groups and vibrational modes of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds respectively. The FTIR spectra of the PDZAu nanocomposite indicate retention of major characteristic peaks corresponds to both PEDOT-PSS and AuNPs. The vibration at 1038 and 577 cm−1 correspond to C–N bonding and Au–O band, respectively.33 Some of the characteristic peaks of PEDOT-PSS and AuNPs either disappear or undergo slight red shift in the composite spectra due to the delocalization of electrons from aromatic ring, proving a strong presence of π–π* interaction between AuNPs and PEDOT-PSS. The loss of molecular ordering due to strong interactions among different phases further confirms the possible red shift in the spectra. Thus, the FTIR results confirm the well formation of PDZAu nanocomposites.
C double bonds respectively. The FTIR spectra of the PDZAu nanocomposite indicate retention of major characteristic peaks corresponds to both PEDOT-PSS and AuNPs. The vibration at 1038 and 577 cm−1 correspond to C–N bonding and Au–O band, respectively.33 Some of the characteristic peaks of PEDOT-PSS and AuNPs either disappear or undergo slight red shift in the composite spectra due to the delocalization of electrons from aromatic ring, proving a strong presence of π–π* interaction between AuNPs and PEDOT-PSS. The loss of molecular ordering due to strong interactions among different phases further confirms the possible red shift in the spectra. Thus, the FTIR results confirm the well formation of PDZAu nanocomposites.

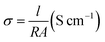


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm
000 ppm




