Open Access Article
Open Access Article2-Amino-3,5-dicarbonitrile-6-sulfanylpyridines: synthesis and multiple biological activity – a review
Nail S. Akhmadiev,
Vnira R. Akhmetova * and
Askhat G. Ibragimov
* and
Askhat G. Ibragimov
Institute of Petrochemistry and Catalysis, Russian Academy of Science, 141 Prospekt Octyabrya, 450075 Ufa, Russian Federation. E-mail: vnirara@mail.ru; Fax: +7 3472 842750; Tel: +7 3472 842750
First published on 23rd March 2021
Abstract
This review integrates the published data of the last decade (from 2010 to 2020) on the synthesis of the 2-amino-3,5-dicarbonitrile-6-sulfanylpyridine scaffold, the derivatives of which are widely used in the synthesis of biologically active compounds. Currently, no systematic accounts of synthetic routes towards this class of heterocyclic compounds can be found in the literature. The present-day trends in the catalytic synthesis of 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines are considered using pseudo-four-component reaction (pseudo-4CR) by condensation of malononitrile molecules with thiols and aldehydes, and alternative three-component (3CR) condensations of malononitrile with 2-arylidenemalononitrile and S-nucleophiles.
1. Introduction
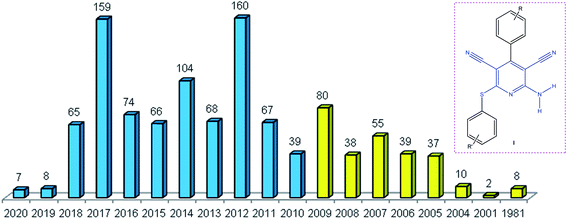
The pyridine skeleton is a structural part of numerous natural alkaloids, metal complexes, and organic compounds,1 including drug molecules.2 A method for the design of highly functionalized pyridine compounds is based on the condensation involving malononitrile, aldehydes, and thiols. The attraction of this method is the simple introduction of accessible reagents giving a pyridine ring with various functional groups, which can be used to perform further transformations.3 Previously, these reactions were considered in the context of classical multicomponent transformations and were included as single examples in some relevant reviews.4Therefore, in this review we give a systematic account of original approaches developed in the last decade to the synthesis of 2-amino-3,5-dicarbonitrile-6-sulfanylpyridine scaffold, derivatives of which are highly functionalized heterocyclic compounds with a potential biological activity. The synthesis is based on two approaches to the target products, first, cyclocondensation of two malononitrile molecules with aromatic aldehydes and thiols (pseudo-4CR) and, second, three-component cyclocondensation of malononitrile with 2-arylidenemalononitrile and thiols (3CR). Analysis of published data on the synthesis of 2-amino-6-sulfanylpyridine-3,5-dicarbonitrile derivatives I performed using the SciFinder®5 database demonstrated that the year of 2012 was the most effective period for this subject (Fig. 1).
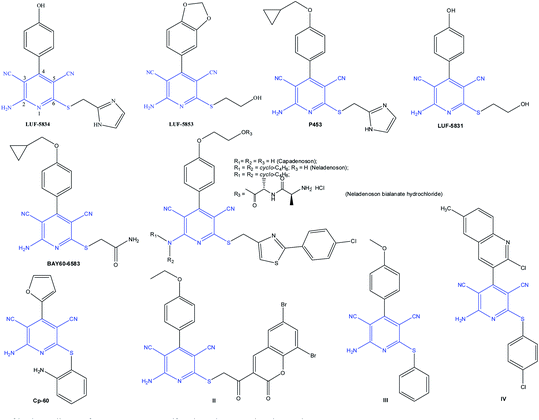
The library of synthesized 2-amino-6-sulfanylpyridine-3,5-dicarbonitriles with various substituents at the C-2, C-4, or C-6 positions of the pyridine scaffold shows unique therapeutic properties. For example, non-nucleoside agonists for the treatment of cardiovascular diseases were proposed on the basis of substituted pyridines. There are quite a few non-ribose compounds possessing low nanomolar activity and improved selectivity towards adenosine receptors (ARs) of A1, A2A, and A2B subtypes; this subject is addressed in a number of reviews.6 Fig. 2 depicts the structural diversity of such molecules, in particular, LUF5853, a partial hA1AR agonist, with the ligand – receptor binding affinity Ki hA1 of 11 ± 2 nM;7 LUF5834, a partial adenosine A2B receptor agonist (EC50 hA2B of 12 ± 2 nM);8 P453, a strong hA2B receptor agonist (EC50 hA2B of 9.5 ± 0.9 nM);9 BAY60-6583, an adenosine A2B receptor agonist (EC50 = 3 nM);6 and LUF-5831, an adenosine A1 receptor agonist (Ki = 144 nM).10 Also, noteworthy is the therapeutic agent capadenoson (completed Phase II clinical trials), which is a highly efficient selective partial adenosine A1 receptor agonist (A1AR) (EC50 of 0.1 nM), and adenosine A2B receptor agonist (EC50 of 8.94 ± 0.33 nM),11 developed by Bayer pharmaceutical company for the use in atrial fibrillation and stable angina patients. Previously, capadenoson was shown to decrease the electrically induced tachycardia in rats by 45%.12 Neladenoson bialanate hydrochloride (phase II clinical trials) was used as a water-soluble partial A1 receptor agonist for oral administration in patients with chronic cardiac insufficiency.11,13
 | ||
| Fig. 2 Skeletal diversity of biologically significant 2-amino-6-sulfanylpyridine-3,5-dicarbonitrile structures. | ||
2-Amino-6-sulfanylpyridine-3,5-dicarbonitrile Cp-60 inhibits accumulation of PrPSc in scrapie-infected mouse neuroblastoma cells ScN2a (IC50 18.0 ± 1.5 mM).14 The molecule of II exhibits inhibitory activity in vitro against HIV-1 integrase (IC50 = 4 μM).15
In addition, polyfunctional pyridines with structure I exhibit anticorrosion properties. According to electrochemical impedance spectroscopy, potentiodynamic polarization, and weight loss measurements, the studied pyridines (the substituent Ar contains –H, -OMe, or –NO2 in the C-4 position) behave as mixed-type corrosion inhibitors in 1 M HCl; the lead compound is 2-amino-4-(4-methoxyphenyl)-6-(phenylsulfanyl)pyridine-3,5-dicarbonitrile III with inhibition efficiency of 97.6% when present in 1.22 mmol L−1 concentration.16
Antimicrobial activity was found for a series of new pentasubstituted pyridine derivatives bearing a quinoline moiety in the C-4 position of the pyridine ring. Among them, compound IV exhibited activities against Escherichia coli (MIC = 62.5 μg mL−1), Bacillus subtilis (MIC = 200 μg mL−1), Clostridium tetani (MIC = 250 μg mL−1), and Salmonella typhi (MIC = 100 μg mL−1), the activities being higher than or equal to those of ampicillin used as the reference substance.17
2. One-pot synthesis of 2-amino-6-sulfanylpyridine-3,5-dicarbonitrile scaffold
The catalytic synthesis of 2-amino-6-sulfanylpyridine-3,5-dicarbonitrile 4 with spectroscopic evidence for the structures of products was performed for the first time in 1981 by S. Kambe and co-workers according to one-pot 3CR protocol (Scheme 1). The target product 4 was prepared in two ways: by the reaction of 2-arylidenemalononitrile 1 with thiol 2 (pathway I) and by the reaction of thiol 2 with malononitrile 3 (pathway II), which resulted in the formation of intermediate imines A and B. Triethylamine was used as the catalyst; reaction proceeded in ethanol and gave pyridines in 17% to 49% yields depending on the nature of Ar substituents in the starting compound 1.18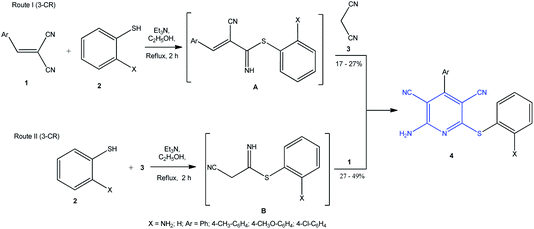 | ||
| Scheme 1 Two approaches to one-pot of the synthesis of 2-amino-6-sulfanylpyridine-3,5-dicarbonitrile derivatives 4 via 3-CR. | ||
The following catalysts were proposed earlier for the synthesis of pyridines 4 and their analogues using the pseudo-four-component reaction (pseudo-4CR) of malononitrile, aldehydes, and thiols: Et3N,19 diazabicycloundecene (DBU),20 1,4-diazabicyclo[2.2.2]octane (DABCO),21 1-butyl-3-methylimidazolium hydroxide ([bmim]OH) ionic liquid,22 KF·Al2O3,23 tetrabutylammonium hydroxide (TBAH) or piperidine,24 nano-SiO2,25 piperidine/MW,26 ZnCl2/MW,27 and KF–Al2O3/MW.28 Highly functionalized bis-pyridines 8 were prepared using bis-isothiuronium salt 6 or 1,2-ethanedithiol 7 as thiolating agents (Scheme 2).19,29
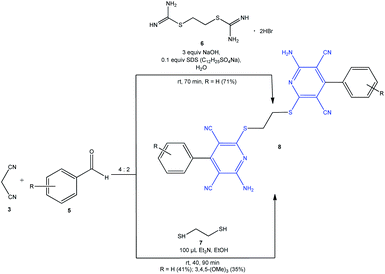 | ||
| Scheme 2 One-pot synthesis of highly functionalized bis-pyridines 8 by using different thiolating agents 6 and 7. | ||
Meanwhile, most of the cited methods suffer from number of drawbacks such as low yields of target products, long time and drastic conditions of the synthesis, and high catalyst toxicity or complex catalyst preparation procedure.
Over recent years, considerable progress has been made in the catalysis of this reaction, which increases the product yields or allows conducting the reactions under mild conditions. The most recent achievements in the synthesis of 2-amino-6-sulfanylpyridine-3,5-dicarbonitriles 4 by condensation of two moles of malononitrile 3, thiols 2, and aldehydes 5 (pseudo-4CR, Scheme 3) are summarized in Table 1, which gives 60 examples of target compounds of type 4 with indicated conditions of synthesis, yields of products, and practical applications of the products.
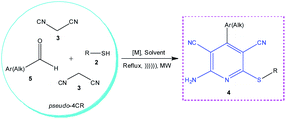 | ||
| Scheme 3 Construction of 2-amino-3,5-dicarbonitrile-6-sulfanylpyridine scaffold 4 by pseudo-4CR with the participation of 2 moles of malononitrile, 1 mol of aldehydes and 1 mol of thiols. | ||
| No. | Catalyst [M], mol% or mol eq. | Solvent | Temperature, °C | Reaction time, min | Yield 4, % | Activity | Substitutes R or Ar/Alk | Reference |
|---|---|---|---|---|---|---|---|---|
| a No information about the frequency and power of the device.b Bacillus subtilis, Clostridium tetani, Streptococcus Pneumonia, Escherichia coli, Salmonella typhi, Vibrio cholera, Aspergillus Fumigates, Candida albicans.c A549 (adenocarcinomic human), MCF-7 (breast cancer cell), MDA-MB-231 (human breast cancer), HBE (human bronchial epithelial).d Staphylococcus aureus, Staphylococcus epidermidis, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa; MCF-7 (adenocarcinoma), SNB-19 (glioblastoma), HCT-116 (colon colorectal carcinoma), HSF (human foreskin fibroblast).e Micrococcus luteus, Staphylococcus aureus, Escherichia coli, Klebsiella pneumonia. | ||||||||
| Organocatalysts | ||||||||
| 1 | Et3N 3 drops on 1 mmol 5, nano-sized MgO 50 mg on 1 mmol 5 | C2H5OH | rt | 180–420 | 44–50 | — | R = Ph | 3 |
| 50 | 60–300 | 65–75 | Ar = Ph; 4-Cl-C6H4; 4-OMe-C6H4; 4-Me-C6H4 | |||||
| 2 | Et3N 6 drops on 1 mmol 5 | C2H5OH | Reflux | 300 | 45–72 | Inhibitor | R = Ph | 30 |
| α-Glucosidase | Ar = Ph; 3-NO2-C6H4; 4-C6H5-C6H4; 2-Me-C6H4; 3-Py; 2-Cl-C6H4; 2-F-C6H4; 4-Cl-C6H4; 4-OH-C6H4; 3-OH-C6H4; 3-OH-4-OMe-C6H3; 2-Cl-3-OMe-C6H3; 3-OMe-4-OH-C6H3; 3-OMe-4-F-C6H3; 3-OMe-4-OH-5-I-C6H2; 3-OMe-4-Br-5-OMe-C6H2; 2-Br-4-OMe-5-OMe-C6H2; 3-Br-4-OMe-5-OMe-C6H2; 2-OMe-3-OMe-4-OMe-C6H2; 2-OMe-3-OMe-4-OMe-C6H2; 3,4,5-(OMe)3-C6H2; 2-OMe-4-OMe-C6H3; 4,5-(OMe)2-C6H3; 3,5-(OMe)2-C6H3; 1-Nh; 2-Nh; 3-C6H5CH2O-4-OMe-C6H4; 4-C6H5CH2O-C6H4 | |||||||
| 3 | Diethylamine 20 mol% | C2H5OH | rt | 240–360 | 67–82 | R = C2H4OH; Ph; Bn; 2-NH2-C6H4; 4-Cl-C6H4; 4-Me-C6H4; 4-OMe-C6H4; 4-OMe-CH2C6H4 | 31 | |
| Ar = Ph; 3,4-(OMe)2-C6H3; 4-Br-C6H4; 4-Cl-C6H4; 4-OMe-C6H4; 3-NO2-C6H4; 4-Me-C6H4; 4-OH-C6H4; 2-thienyl; 4-CN-C6H4; 4-(CH3)2CH-C6H4; cyclo-3,4-(OCH2O)-C6H3; 2-furyl; 2,6-(CH3)2-C6H3; 2,6-(Cl)2-C6H3 | ||||||||
| 4 | Deep eutectic solvent (DES) (choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1 urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)), 0.5 mL on 1 mmol 5 2)), 0.5 mL on 1 mmol 5 |
DES | 60 | 80–240 | 60–82 | R = Ph; 4-Me-C6H4; 4-Br-C6H4 | 32 | |
| Alk/Ar = n-C7H15; Ph; 4-Cl-C6H4; 3-NO2-C6H4; 3-Br-C6H4; 2-thienyl; 2-furyl; 4-Me-C6H4; 4-Br-C6H4; 3-OMe-C6H4; 4-OMe-C6H4; 1-Nh | ||||||||
| 5 | — | Water–choline hydroxide (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
Reflux | 15–50 | 85–94 | R = Ph | 33 | |
| Ar = Ph; 4-OMe-C6H4; 4-NO2-C6H4; 2-furyl; 4-Cl-C6H4; 4-Br-C6H4; 4-Me-C6H4; 4-OH-C6H4; 2-thienyl; 3-NO2-C6H4; 2-NO2-C6H4; 2-Nh | ||||||||
| 6 | Baker's yeast, 1 g on 9.4 mmol 5 | C2H5OH | rt | 40 | 82–93 | — | R = Ph | 34 |
| Ar = Ph; 4-Br-C6H4; 4-F-C6H4; 4-Cl-C6H4; 4-OMe-C6H4; 4-OH-C6H4; 2-NO2-C6H4; 4-N(Me)2-C6H4; 3,4-(OMe)2-C6H3; 4-Me-C6H4 | ||||||||
| 7 | Water extract of banana | C2H5OH | 65 | 10–45 | 80–90 | — | R = Ph; 4-Cl-C6H4; n-C4H9; n-C8H17 | 35 |
| Alk/Ar = Ph; 3-OH-C6H4; 4-OH-C6H4; 4-OMe-C6H4; 4-Me-C6H4; 3-Br-C6H4; 4-Cl-C6H4; 2-Cl-C6H4; 4-F-C6H4; 4-NO2-C6H4; 4-Py; 2-thienyl; 2-Nh | ||||||||
| 8 | Tetra-n-butylammonium fluoride (1.0 mol L−1 in THF), 10 mol% | H2O | 80 | 45–630 | 62–96 | R = Ph; 2-NH2-C6H4 | 36 | |
| Alk/Ar = Me; Et; Ph; 4-Cl-C6H4; 4-F-C6H4; 4-OMe-C6H4; 4-OH-C6H4; 4-NO2-C6H4; 4-Me-C6H4; 4-OH-3-Me-C6H3; 3,4-(OMe)2-C6H3; 2-furyl; 2-thienyl; piperonyl | ||||||||
| 9 | o-Iodoxybenzoic acid, 10 mol% | H2O | 70 | 90–150 | 69–83 | R = Ph; 2-Br-C6H4; 2,4,6-Me3-C6H2; 2-Me-C6H4; 4-Cl-C6H4 | 37 | |
| Ar = Ph; 4-Me-C6H4; 4-OMe-C6H4; 3,4-(OMe)2-C6H3; 4-NO2-C6H4; 4-Br-C6H4; 4-Cl-C6H4; 2,6-(Cl)2-C6H4; 3,4-Me2-C6H3 | ||||||||
| 10 | Diethylamine, 20 mol% → Dess–Martin periodinane (DMP) (1 mmol) | C2H5OH → DMF | rt | 1.5–2.5 | 90–96 | R = Et; n-Bu; C6H11; Ph; 4-Br-C6H4; 4-Cl-C6H4; 4-OMe-C6H4; 4-Me-C6H4 | 38 | |
| 5–10 | Ar = 2,6-(Me)2-C6H3; 2,6-(OMe)2-C6H3; 2,6-Cl2-C6H3; 2,6-F2-C6H3 | |||||||
| 11 | Piperidine | C2H5OH, CH3CN | Reflux | 180–1440 | 5–86 | R = Ph; 4-Cl-C6H4 | 39 | |
| MWa | 90 | 0.5–60 | 6–97 | Alk/Ar = t-Bu; Ph; 4-F-C6H4; 4-Cl-C6H4; 2-thienyl; 2,6-(Cl)2-C6H3; 2,6-(F)2-C6H3; 2-Cl-6-F-C6H3; 2-F-6-CF3- C6H3 | ||||
| 12 | Piperidine, 0.03 mL on 5 mmol 5 | C2H5OH | Reflux | 180 | 57–86 | Antimicrobial activityb |  |
17 |
| 13 | Imidazole, 0.2 mmol on 1 mmol 5 | C2H5OH | Reflux | 30–120 | 81–92 | — | R = C6H11; Ph; 2-Me-C6H4 | 40 |
| Alk/Ar = C6H11; Ph; 4-OMe-C6H4; 4-CN-C6H4; 2-Nh; 4-Cl-C6H4 | ||||||||
| 14 | L-Arginine, 20 mol% | H2O | Reflux | 30–90 | 81–96 | R = C6H11; Ph; Bn; 2-NH2-C6H4; 2-CH3-C6H4 | 41 | |
| Alk/Ar = C6H11; Ph; 2-Nh; 4-Br-C6H4; 4-Cl-C6H4; 4-Me-C6H4; 4-OMe-C6H4; 3-NO2-C6H4; 2,6-(OMe)2-C6H3; 3,4-(OMe)2-C6H3; 2,6-(Cl)2-C6H3 | ||||||||
| 15 | N,N′-Di(1H-tetraazol-5-yl)-6H,12H-5,11-ethanedibenzo[b,f][1,5]diazocine-3,9-dicarboxamide, 5 mol% | EtOH | Reflux | Antitumor activityc | R = Ph; C2H4OH; 4-Me-C6H4; 4-Cl-C6H4 | 42 | ||
| Ar = Ph; 4-Me-C6H4; 4-Cl-C6H4; 4-OMe-C6H4; 4-NO2-C6H4; Pr; quinoline; 4-CN-C6H4; 4-Br-C6H4; 2-Me-2-furil; 2-Me-2-thienyl; 2-CH3-Pr; 2-Br-Pr | ||||||||
| 16 | Choline methoxide, 5–10 mol% | H2O–C2H5OH (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
50–60 | 20–40 | — | — | R = Ph | 43 |
| Ar = Ph; 4-NO2-C6H4; 4-Cl-C6H4; 4-Me-C6H4; 4-OMe-C6H4; 4-OH-C6H4; 4-Br-C6H4 | ||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Nanomaterial-based catalysts | ||||||||
| 17 | Nano-CaO, 0.01 g on 1 mmol 5 | H2O–C2H5OH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
50 | 80–150 | 70–92 | — | R = Ph; 4-Me-C6H4 | 44 |
| Ar = Ph; 4-Br-C6H4; 4-Cl-C6H4; 4-OMe-C6H4; 3-NO2-C6H4; 4-NO2-C6H4; 3-Me-C6H4; 4-Me-C6H4; 4-CN-C6H4; 3-OH-C6H4; 4-OH-C6H4 | ||||||||
| 18 | SnO nanoparticles, 6 mol% | C2H5OH (abs.) | 60 | 54–142 | 79–92 | — | R = Ph, 4-Me-C6H4; 4-OMe-CH2C6H4 | 45 |
| Ar = Ph, 4-OMe-CH2C6H4; 3-NO2-C6H4; 4-NO2-C6H4; 4-Cl-C6H4; 3-CH3-C6H4; 4-OH-C6H4; 4-Br-C6H4 | ||||||||
| 19 | CuI nanoparticles, 10 mol% | C2H5OH | 60 | 85–200 | 70–94 | — | R = C2H4OH; Ph; 4-Me-C6H4; 4-OMe-C6H4 | 46 |
| Alk/Ar = CH3; n-C4H9; Ph, 3-Me-C6H4; 4-Me-C6H4; 3-OH-C6H4; 4-OH-C6H4; 4-OMe-C6H4; 3-NO2-C6H4; 4-NO2-C6H4; 4-Br-C6H4; 4-Cl-C6H4; 4-SMe-C6H4; 4-CN-C6H4 | ||||||||
| 20 | ZnO nanoparticles, 0.015 g on 1 mmol 5, 20 mol% | C2H5OH | 50 | 80–150 | 75–94 | R = Ph; 4-Me-C6H4 | 47 | |
| Ar = Ph; 3-Me-C6H4; 4-Me-C6H4; 3-OH-C6H4; 4-OH-C6H4; 4-OMe-C6H4; 3-NO2-C6H4; 4-NO2-C6H4; 4-Cl-C6H4; 4-Br-C6H4; 4-CN-C6H4 | ||||||||
| 21 | Nanocrystalline MgO (NAP-MgO), 0.1 g on 1 mmol 5 | C2H5OH | 50 | 120–540 | 41–69 | R = C6H11; Ph; Bn; 4-Me-C6H4; 4-Cl-C6H4; 2-furyl | 48 | |
| Ar = Ph; 4-OMe-C6H4; 4-Me-C6H4; 4-NO2-C6H4; 4-OH-C6H4; 4-Cl-C6H4; 4-HOOC-C6H4; 2-furyl; cyclo-3,4-(OCH2O)-C6H3 | ||||||||
| 22 | Heterogeneous nanocatalyst Cu(II)/L-His@Fe3O4 | H2O | 80 | 60 | 86–95 | R = Ph | 49 | |
| Ar = Ph; 4-Cl-C6H4; 4-Me-C6H4; 4-Br-C6H4; 4-OMe-C6H4; 4-OH-C6H4; 4-F-C6H4; 3,4-(OMe)2-C6H3; 4-NO2-C6H4 | ||||||||
| 23 | Nano-TiO2, 5 mol% 0.06 g on 1 mmol 5 | C2H5OH | Reflux | 14–27 | 89–97 | R = 4-Me-C6H4 | 50 | |
| Ar = Ph; 3-Cl-C6H4; 4-Cl-C6H4; 3-Br-C6H4; 4-Br-C6H4; 3-NO2-C6H4; 4-NO2-C6H4; 4-OMe-C6H4 | ||||||||
| Nano-TiO2, 5 mol% | C2H5OH | rt | 60 | 81–87 | R = 4-Me-C6H4 | 51 | ||
| Ar = Ph; 3-Cl-C6H4; 4-Cl-C6H4; 3-Br-C6H4; 4-Br-C6H4; 3-NO2-C6H4; 4-NO2-C6H4; 4-OMe-C6H4 | ||||||||
| 24 | 1,4-Dinitropyrazine-1,4-diium trinitromethanide {[1,4-pyrazine-NO2][C(NO2)3]2} nanostructured molten salt (NMS), 2 mol% | Solvent free reaction conditions | rt | 20–40 | 83–93 |  |
52 | |
| 25 | Covalently bonded sulfonic acid nano magnetic graphene oxide (Fe3O4@GO-Pr-SO3H), 0.06 g on 1 mmol 5 | EtOH | Reflux | 19–27 | 89–95 | R = Ph; 4-Me-C6H4 | 53 | |
| Ar = Ph; 3-Cl-C6H4; 3-Br-C6H4; 4-Br-C6H4; 4-Cl-C6H4; 4-NO2-C6H4; 3-NO2-C6H4; 4-NO2-C6H4; 4-OMe-C6H4; 2-furil; 2-thienyl | ||||||||
| 26 | CoII(macrocyclic Schiff base ligand containing 1,4-diazepane) immobilized on Fe3O4 nanoparticles (Fe3O4@CoII), 0.02 g on 1 mmol 5 | — | 100 | 11–25 | 90–98 | — | R = Ph | 54 |
| Ar = Ph; 3-Py; 4-N(Me)2-C6H4; 3-NO2-C6H4; 4-NO2-C6H4; 4-OH-C6H4; 2-thienyl; 4-F-C6H4 | ||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Bronsted and Lewis acids and basic catalysis | ||||||||
| 27 | NH4OH, 12 mol% | MеOH (abs.) | rt | 360 | 75–90 | — | R = Ph | 55 |
| Ar = Ph; 4-Cl-C6H4; 2-Cl-C6H4; 4-OMe-C6H4; 2-NO2-C6H4 | ||||||||
| 28 | NH4OH, 12 mol% | MеOH (abs.) | rt | 360 | 60–90 | R = Ph | 56 | |
| Ar = Ph; 4-OH-C6H4; 4-OMe-C6H4; 2-OMe-C6H4; 3,4-(OMe)2-C6H3; 2-NO2-C6H4 | ||||||||
| 29 | H3BO3, 15 mol%, CTAB, 10 mol% | H2O | 80 | 25–50 | 79–92 | R = Ph; 2-NH2-C6H4 | 57 | |
| H3BO3, 15 mol%, CTAB, 10 mol%, )))))) (35 kHz, 200 W) | 8–15 | 83–74 | Ar = Ph; 4-Cl-C6H4; 4-F-C6H4; 4-OMe-C6H4; 4-HO-C6H4; 4-NO2-C6H4; 4-Me-C6H4; 4-HO-3-OMe-C6H3; 3,4-(OMe)2-C6H3; piperonyl; 2-furyl; 2-thienyl | |||||
| 30 | H3BO3, 15 mol%, CTAB, 10 mol%, ))))))a | H2O | 80 | — | — | Adsorption and anti-corrosion activity | 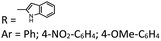 |
58 |
| 31 | Phosphotungstic acid, 2 mol%, cetrimonium bromide, 10 mol% | H2O | 80 | 30–50 | 70–93 | R = Ph, 4-NH2-C6H4 | 59 | |
| Ar = Ph, 4-Cl-C6H4; 4-OH-C6H4; 4-NO2-C6H4; 4-Me-C6H4; 4-OMe-C6H4; 4-CHO-C6H4; 4-OH-3-OMe-C6H3 | ||||||||
| 32 | KOH, 10 mol% | C2H5OH | rt | 60 | 25–40 | Antibacterial and antineoplastic activitiesd | 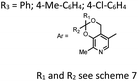 |
60 |
| 33 | KOH, 10 mol% | C2H5OH | rt | 30–90 | 71–90 | R = C6H11; Ph; Bn; 2-NH2-C6H4; 2-Me-C6H4 | 61 | |
| Alk/Ar = C6H11; Ph; Bn; 4-OMe-C6H4; 3-Cl-C6H4; 4-Cl-C6H4; 4-Me-C6H4; 4-Br-C6H4; 4-SMe-C6H4; 3-NO2-C6H4; β-C10H7; 4-CN-C6H4 | ||||||||
| 34 | NaOH, 1 mol eq., )))))) (40 kHz, 250 W) | C2H5OH | rt | 90–120 | 90–96 | R = Bn | 62 | |
| Ar = Ph; 4-OMe-C6H4; 4-Br-C6H4; 4-OH-C6H4; 4-N(Me)2-C6H4 | ||||||||
| 35 | NaCl, 15 mol% | H2O | Reflux | 2–180 | 18–90 | R = Ph | 63 | |
| NaCl, 15 mol%, ))))))a | Reflux | 20–35 | 22–92 | Alk/Ar = CH3; n-Pr; Ph, 4-Cl-C6H4; 4-NO2-C6H4; 4-OMe-C6H4; piperonyl; 4-OH-3-OMe-C6H3; 3,4-(OMe)2-C6H3; 2-thienyl; 2-furyl; 4-OH-C6H4; 4-Me-C6H4 | ||||
| 36 | K2CO3, 20 mol%, KMnO4 1.1 mol eq. | H2O–C2H5OH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Reflux | 45–180 | 60–90 | R = C2H4OH; Ph; 4-Cl-C6H4; 4-Me-C6H4; 4-NH2-C6H4 | 64 | |
| Alk/Ar = CH3(CH2)6; 4-OMe-C6H4; 3,4-(OMe)2-C6H3; 3,4,5-(OMe)3-C6H2; 3-OH-C6H4; 4-OH-C6H4; 2,6-(Cl)2-C6H4; 4-Cl-C6H4; 4-Br-C6H4; 4-F-C6H4; 4-CN-C6H4; 3-NO2-C6H4; 2-thienyl; 3-Py | ||||||||
| 37 | K2CO3, 10 mol%, | PEG-400 | 40 | 1–60 | 82–92 | R = Ph; 4-Br-C6H4; 4-OMe-C6H4; 2-NH2-C6H4 | 65 | |
| Alk/Ar = Ph; 4-Me-C6H4; 4-OMe-C6H4; 4-Cl-C6H4; 4-Br-C6H4; 3-NO2-C6H4; 4-NO2-C6H4; 2-thienyl; 2-furyl; 3-HO-C6H4; 4-OH-C6H4 | ||||||||
| 38 | K2CO3, 1 mol eq., grinding in a pestle | Solvent free reaction conditions | rt | 20–35 | 82–92 | Antibacterial activitye | R = 2-mercaptopyridine | 66 |
| Ar = Ph; 3,4-F2-C6H3; 4-F-C6H4; 4-Br-C6H4; 4-OMe-C6H4; 3-OH-C6H4; 4-NO2-C6H4; 3,4,5-(OMe)3-C6H2; 4-Py | ||||||||
| 39 | NaHCO3, 10 mol% | H2O–C2H5OH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
110 | 8 | 87–93 | R = 2-NH2-C6H4 | 67 | |
| Ar = Ph; 4-Me-C6H4; 4-F-C6H4; 4-OMe-C6H4; 4-Cl-C6H4; 3,4-(OMe)2-C6H3; 2,4-Cl2-C6H4; 3-Cl-C6H4; 2-Cl-C6H4; 4-COOH-C6H4; 2-HO-C6H4; 4-HO-C6H4; 2,5-(OMe)2-C6H3; 1-Nh; 4-N(Me)2-C6H4; 3-indolyl; hydrocinnamyl; 4-Br-C6H4; cinnamamyl; 9-anthracyl | ||||||||
| 40 | 10% aqueous suspension of aluminum oxide | H2O | rt | 50–100 | 79–90 | R = Ph; 2-NH2-C6H4 | 68 | |
| Ar = Ph; 4-Cl-C6H4; 4-F-C6H4; 4-OMe-C6H4; 4-OH-C6H4; 2-NO2-C6H4; 4-NO2-C6H4; 3,4-(OMe)2-C6H3; piperonyl; 2-furyl; 2-thieny; 4-OH-3-OMe-C6H3 | ||||||||
| 41 | Sc(OTf)2, 5 mol% | C2H5OH | Reflux | 120 | 65–85 | R = Ph; 4-NH2-C6H4; 4-Br-C6H4 | 69 | |
| Ar = Ph; 3-Br-C6H4; 4-F-C6H4; 4-Br-C6H4; 4-Cl-C6H4; 4-OMe-C6H4; 3,4-(OMe)2-C6H3; 2-Cl-6-F-C6H3; 2-OMe-3-Br-C6H3; 2-Cl-6-Cl-C6H3; 2-F-6-F-C6H3 | ||||||||
| 42 | CH3COONa, 12 mol%, MW (280 W) | MeOH (abs.) | — | 3–12 | 62–92 | R = Ph; C2H4OH | 70 | |
| Ar = Ph; 4-Cl-C6H4; 2-OMe-C6H4; 4-OMe-C6H4; 2-NO2-C6H4; 4-NO2-C6H4; 4-OH-C6H4; 4-OH-3-OMe-C6H3; 3-OH-4-OMe-C6H3; CH2-CH2-C6H3; 3,4-(OMe)2-C6H3; 3,4,5-(OMe)3-C6H2; 2-furyl | ||||||||
| 43 | C6H5COONa, 10 mol% | PEG-400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
50 → 70 | 90–110 | 82–88 | R = Ph | 71 | |
| Ar = Ph; 4-OMe-C6H4; 4-Cl-C6H4; 3-NO2-C6H4; 3-OH-C6H4; 4-OH-C6H4; 4-Me-C6H4; 4-Br-C6H4 | ||||||||
| 44 | Cs2CO3, 5 mol% and tetra-n-butylammonium bromide, 5 mol% | CH3OH | rt | 180 | 85–92 | R = Ph; 4-Me-C6H4; 4-OMe-C6H4 | 72 | |
| Ar = Ph; 4-Me-C6H4; 4-F-C6H4; 4-Cl-C6H4; 4-NO2-C6H4; 3-OMe-4-OH-C6H3; 3,4-(OMe)2-C6H3; 4-OMe-C6H4; 2-furyl; 2-thienyl; 4-OH-C6H4 | ||||||||
| 45 | Zn(II) or Cd(II) metal–organic frameworks, 2 mol% | Solvent free reaction conditions | 100 | 30–60 | 61–88 | — | R = Ph; 4-Cl-C6H4; 4-Me-C6H4; 4-OMe-C6H4; | 73 |
| Ar = Ph, 4-F-C6H4; 4-Cl-C6H4; 4-OMe-C6H4; 2-NO2-C6H4; 4-Me-C6H4; 2-thienyl; n-C5H11 | ||||||||
| 46 | ZrOCl2·8H2O/NaNH2, 20 mol%, ))))))a | [Bmim]BF4 | rt | 5–20 | 90–98 | R = Ph; C6F5; 4-Br-C6H4; 2-Nh | 74 | |
| Ar = Ph; 2-NO2-C6H4; 2,4-(NO2)2-C6H3; 4-Me-C6H4; 4-Br-C6H4; 4-F-C6H4; 4-CF3-C6H4; 2-Nh; 2-furyl | ||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Heterogeneous catalysts | ||||||||
| 47 | Functionalized organosilane with spherical mesoporous silica nanoparticles with grafted piperidine, 20 mg on 1 mmol 5 | H2O | Reflux | 180–360 | 76–95 | R = Ph, 3-Cl-C6H4; 2-Nh; 4-Me-C6H4 | 75 | |
| Ar = 4-OMe-C6H4; 4-Cl-C6H4; 4-Br-C6H4; 4-CN-C6H4; 4-Py; 4-N(Me)2-C6H4 | ||||||||
| 48 | Propylphosphonium hydrogen carbonate ionic liquid supported on nano-silica (PPHC–nSiO2) (0.7 mol%) | Solvent free reaction conditions | 50 | 20–33 | 80–95 | — | 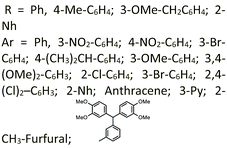 |
76 |
| 49 | Silica-bonded N-propyldiethylenetriamine, 0.1 g on 1 mmol 5 | C2H5OH | rt | 30–45 | 75–90 | — | R = 2-NH2-C6H4; 4-Me-C6H4 | 77 |
| Ar = Ph; 4-Br-C6H4; 3-Cl-C6H4; 4-Cl-C6H4; 4-OMe-C6H4; 4-Me-C6H4; 4-OEt-C6H4; 3-NO2-C6H4; 4-NO2-C6H4; 4-CN-C6H4 | ||||||||
| 50 | 2-Hydroxyethylammonium sulphonate immobilized on γ-Fe2O3 nanoparticles (γ-Fe2O3-2-HEAS), 0.08 g on 1 mmol 5 | Solvent free reaction conditions | 50 | 5–20 | 79–91 | R = n-Bu Ph; 4-Cl-C6H4; 4-Me-C6H4; 4-OMe-C6H4 | 78 | |
| Alk/Ar = Me; Ph; 4-Me-C6H4; 4-Cl-C6H4; 2-Nh; 3-Py; 3-C6H4(CH2)2 | ||||||||
| 51 | 2-Hydroxyethylammonium acetate immobilized on Fe2O3 nanoparticles (Fe3O4-2-HEAA), 1 mol%, 0.016 g on 1 mmol 5 | Solvent free reaction conditions | 70 | 5–15 | 80–90 | R = n-Bu; Ph; 4-Cl-C6H4; 4-Me-C6H4; 4-OMe-C6H4 | 79 | |
| Alk/Ar = Me; Ph; 4-Cl-C6H4; 4-Me-C6H4; 2-Nh; 3-Py; 3-C6H4(CH2)2 | ||||||||
| 52 | Molecular sieves (MS 4A), 200 mg on 1 mmol 5, )))))) (35 kHz, 200 W) | H2O | Reflux | 40–120 | 78–91 | R = Ph; 2-NH2-C6H4 | 80 | |
| 30–60 | 81–90 | Ar = Ph; 4-Cl-C6H4; 4-F-C6H4; 4-NO2-C6H4; 4-OH-C6H4; 4-Me-C6H4; 3,4-(OMe)2-C6H3; piperonyl; 2-furyl; 2-thienyl; 4-OMe-C6H4; 3-OMe-4-OH-C6H3 | ||||||
| 53 | Na2SiO2 5 mol% | C2H5OH | rt | 60 | 78–82 | R = 4-Me-C6H4 | 81 | |
| Ar = Ph; 3-Cl-C6H4; 4-Cl-C6H4; 4-Br-C6H4; 3-NO2-C6H4; 4-NO2-C6H4; 4-OMe-C6H4 | ||||||||
| 54 | Graphene oxide–TiO2 (GO–TiO2), 20 mg on 1 mmol 5 | H2O | rt | 60–120 | 81–89 | R = Ph | 82 | |
| Ar = Ph; 4-Br-C6H4; 2-OH-C6H4; 4-OMe-C6H4; 4-CHO-C6H4; 2-NO2-C6H4; 2-OH-5-Br-C6H3; 2,3-(OH)2-C6H3; 4-CF3-C6H4 | ||||||||
| 55 | Ceramic glass, 20 mg on 1 mmol 5 | H2O | Reflux | 120 | 76–95 | R = Ph; 3-Cl-C6H4; 4-Cl-C6H4; 4-Br-C6H4; 2-Nh | 83 | |
| Ar = Ph; 4-OMe-C6H4; 2-Br-C6H4; 3-Br-C6H4; 4-Br-C6H4; 4-Me-C6H4; 4-OMe-C6H4; 4-Cl-C6H4; 3,4-(OMe)2-C6H3; 4-Py | ||||||||
| 56 | Dolomite limestone, 5.0 mass%, )))))) (35 kHz, 160/640 W) | H2O–C2H5OH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
45–50 | 30–45 | 90–98 | R = 2-Py; Ph | 84 | |
| Ar = Ph; 3-OH-C6H4; 4-OMe-C6H4; 3,4,5-(OMe)3-C6H2; 4-NO2-C6H4; 4-F-C6H4; 3-Br-C6H4; 4-Br-C6H4; 3,4-(F)2-C6H3; 2-Py; 4-Me-C6H4; 4-Cl-C6H4 | ||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Ionic liquids | ||||||||
| 57 | [Bmim]Br, 1.2 mmol | — | 120 | 4–12 | 75–86 | R = Ph | 85 | |
| Ar = Ph; 3-Br-C6H4; 4-Br-C6H4; 4-Cl-C6H4; 4-OH-C6H4; 4-OMe-C6H4; 4-Me-C6H4 | ||||||||
| 58 | 1-(2-Aminoethyl)pyridinium hydroxide, 1.0 mmol | H2O–C2H5OH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
rt | 30–60 | 76–89 | R = Ph; 2-NH2-C6H4 | 86 | |
| Ar = Ph; 4-OMe-C6H4; 4-Me-C6H4; 4-OH-C6H4; 4-Cl-C6H4; 4-F-C6H4; 3-Br-C6H4; 3-OMe-4-OH-C6H3; 4-NO2-C6H4 | ||||||||
| 59 | — | [Bmim]BF4 | 50 | 20–30 | 78–89 | R = Ph; 2-NH2-C6H4 | 87 | |
| Ar = Ph; 3-Br-C6H4; 4-Cl-C6H4; 4-F-C6H4; 4-NO2-C6H4; 4-OH-C6H4; 4-Me-C6H4; 4-OMe-C6H4 | ||||||||
| 60 | 2-Hydroxyethylammonium acetate, 0.5 mL on 1 mmol 5 | H2O | rt | 5 | 70–96 | — | R = Ph; n-Bu, 4-OMe-C6H4; 4-Me-C6H4; 4-Cl-C6H4 | 88 |
| Ar = Me, Ph, Bn, 2-Nh, 3-Py, 4-Cl-C6H4; 4-Me-C6H4; 4-CHO-C6H4 | ||||||||
Analysis of the results of the last decade presented in the Table 1 indicates that methods for the synthesis of 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines are developing towards green chemistry principles: the use and regeneration of catalysts, including nanocomposites (examples 17–26), Bronsted and Lewis acids and bases (examples 27–46), and heterogeneous catalysts (examples 47–56, Table 1); the use of green solvents (altogether 14 examples of using water), in particular, together with ionic liquids (examples 57–60); physicochemical treatment (microwave and ultrasonic irradiation) together with catalysts (altogether 8 examples). The catalytic activation is still the major trend (60 examples, Table 1). A particular place belongs to organocatalysts taken in minor quantities, which illustrates a metal-free strategy (examples 1–16, Table 1).
In most of the synthesized 2-amino-6-sulfanylpyridine-3,5-dicarbonitriles, ethanol, water, or their mixtures are proposed as solvents. The use of an ionic liquid together with a catalyst and ultrasonic irradiation (ZrOCl2·8H2O/NaNH2, ultrasonic irradiation )))))), [bmim]BF4) at room temperature induces a synergistic effect, giving substituted pyridines in more than 90% yields within 5 minutes (example 46, Table 1).74 A fairly promising is the use of a deep eutectic solvent (DES) (choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1
urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)) as a green reaction medium and a catalyst (example 4, Table 1).32 An additional advantage of using DES is the possibility of reuse (three cycles without the loss of activity) with a simple recovery procedure.
2)) as a green reaction medium and a catalyst (example 4, Table 1).32 An additional advantage of using DES is the possibility of reuse (three cycles without the loss of activity) with a simple recovery procedure.
Bayat and co-workers used nitroketene dithioacetal 9 as the S-nucleophile (CH3S−) in the pseudo-4-CR to prepare the desired pyridines 4 in 55%–76% yields (Scheme 4). A drawback of the method is the formation of an equimolar amount of 2-(nitromethylene)imidazolidine 10 by-product formed in the condensation.89
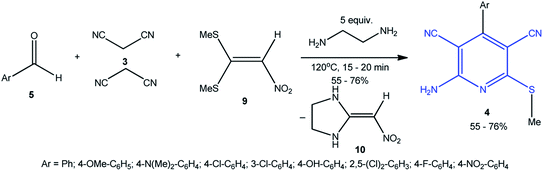 | ||
| Scheme 4 Synthesis of 2-amino-6-(methylsulfanyl)pyridine-3,5-dicarbonitriles 4 using nitroketenedithioacetal 9 as S-nucleophile. | ||
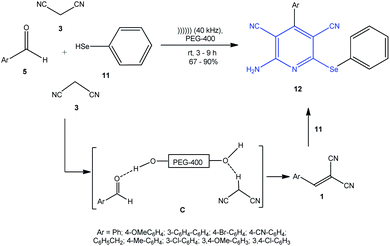
2-(Phenylseleno)pyridines 12, selenium analogues of sulfanylpyridines 4, were synthesized from malonodinitrile 3, aldehydes 5, and PhSeH 11 in polyethylene glycol (PEG-400) as the solvent under ultrasonic irradiation (Scheme 5). The authors assumed that PEG-400 is favorable for in situ formation of arylmethylenemalononitriles 1.90
3. Design, synthesis of biologically active compounds with 2-amino-6-sulfanylpyridine-3,5-dicarbonitrile scaffold
Grigor'ev and co-workers91 developed an original approach for the synthesis of privileged scaffolds, 4-acyl-2-amino-3,5-dicarbonitrile-6-sulfanylpyridines 14, by heterocyclization of potassium 2-acyl-1,1,3,3-tetracyanopropenides 13 with thiols 2 in superbasic medium, DMSO-Na or DMSO-NaH,92 in which the target products were formed in more than 60% yields (Scheme 6).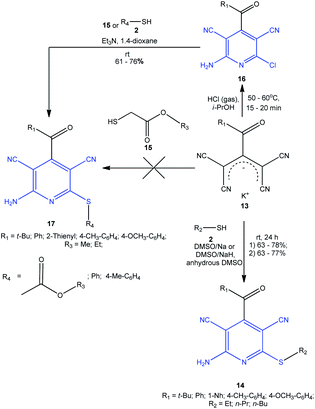 | ||
| Scheme 6 Synthesis of 4-acyl-2-amino-3,5-dicarbonitrile-6-sulfanylpyridines 14, 17 based on potassium 2-acyl-1,1,3,3-tetracyanopropenides 13. | ||
In the case where thioglycolic acid esters 15 were used as the starting reactants, it was impossible to isolate the target pyridines 17. However, the synthesis of compounds 17 from 2-chloropyridines 16 follows the SNAr mechanism and proceeds under milder conditions, involving thioglycolates 15 and arylthiols 2.93
The mentioned research group continued these studied by the synthesis of a combinatorial series of functionalized 2-amino-6-sulfanylpyridine-3,5-dicarbonitriles with a pyridoxine moiety 21 (Scheme 7).60 The proposed one-pot synthesis is based on the pseudo-4CR of pyridoxine derivative 20, two moles of malononitrile 3, and thiols 2 in the presence of 10 mol% KOH, giving the target pyridine-3,5-dicarbonitriles 21 in more than 25% yield. For increasing the solubility and enhancing the antimicrobial activity, the resulting sulfanylpyridines were regioselectively converted to quaternary salts 22 and 23. The compounds exhibited pronounced antimicrobial activity against Staphylococcus aureus (MIC = 2 μg mL−1), Staphylococcus epidermidis (MIC = 1 μg mL−1), and Bacillus subtilis (MIC = 1 μg mL−1), which exceeded the activity of reference samples (myramistin, benzalkonium chloride). The activity of compounds depends on their lipophilicity and decreases in the series R1, R2 = octyl > pentyl > ethyl.
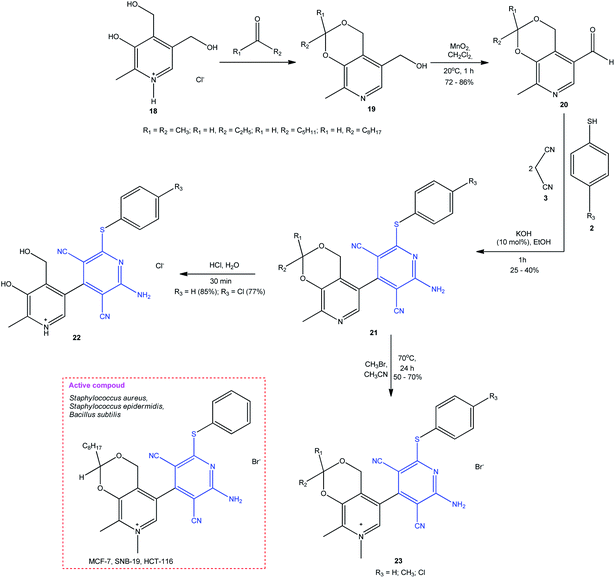 | ||
| Scheme 7 Design of 2-amino-6-sulfanylpyridine-3,5-dicarbonitriles 23 with pyridoxine moiety exhibiting antimicrobial and antineoplastic activity. | ||
Some of compounds 19 had a cytotoxic activity against some types of tumor cells: MCF-7 (IC50 = 2.8 μM) (human breast cancer cell line), SNB-19 (IC50 = 5.1 μM) (glioblastoma cell line), and HCT-116 (IC50 = 2.8 μM) (human colon cancer cell line), being inferior to the activity of doxorubicin used as the ref. 60 The authors also noted that these compounds do not show selectivity to the HSF normal cells (human foreskin fibroblasts), e.g., for the lead compound, IC50 = 2.8 μM, which indirectly attests to poor selectivity of their action and toxicity in experiments in vivo.
In order to enhance the biological activity of target sulfanylpyridines, the aldehyde or thiol component was modified by introducing the pharmacophore groups. As an example, consider the synthesis of pyridine 29 from amino acid 24 (Scheme 8).94 Primary screening for the in vitro antimicrobial activity revealed the highest activity (MIC = 15.625 μg mL−1) against Escherichia coli, Pseudomonas aeruginosa, Candida albicans, and Aspergillus niger for compounds with phenyl and 3-chlorophenyl substituents at the sulfur atom in pyridine 29.
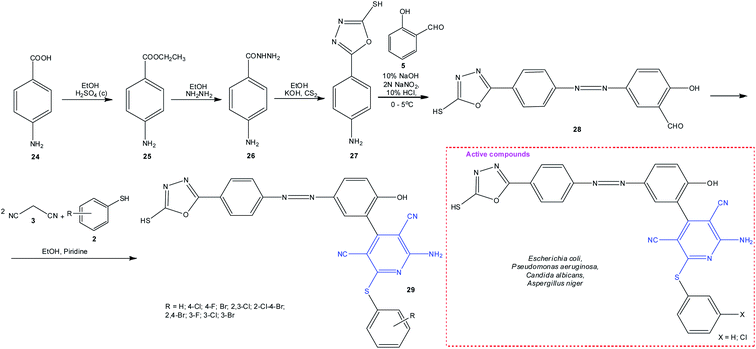 | ||
| Scheme 8 Synthesis of 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines 29 containing 1,3,4-oxadiazole moiety exhibiting antimicrobial activity. | ||
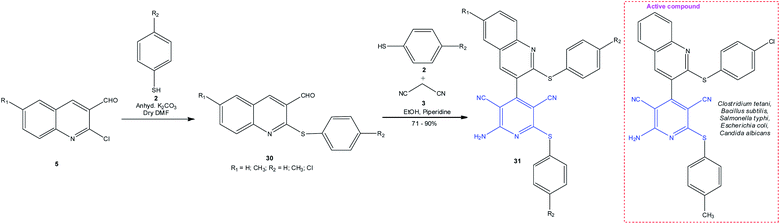
A proposed route towards antimicrobial agents includes the synthesis of hybrid structures 31 containing 2-(ArS)-amino-3,5-dicyanopyridine and 2-(ArS)-quinoline moieties (Scheme 9).95 For this purpose, 3-formyl-2-phenylsulfanylquinoline 30, obtained by the reaction of 2-chloro-3-formylquinoline 5 with thiols 2, was used as the aldehyde component in pseudo-4-CR. The resulting compounds 31 possessed clear-cut antibacterial and fungicidal activities in vitro against the Streptococcus pneumoniae, Bacillus subtilis, Clostridium tetani, Escherichia coli, Salmonella typhimurium, Vibrio cholera, Aspergillus fumigatus, and Candida albicans strains.
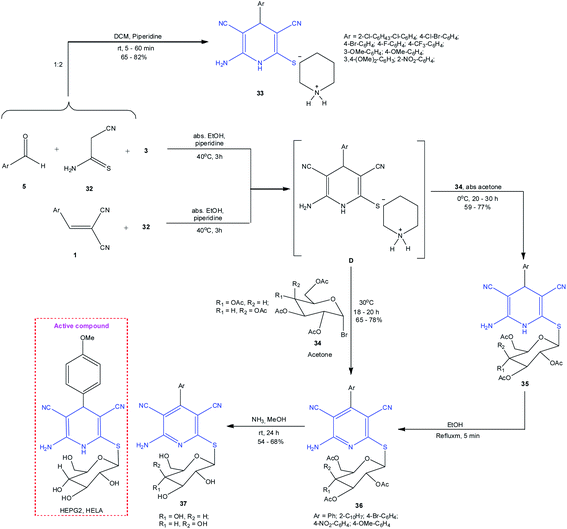
A recently proposed method96 for the synthesis of piperidinium salts 33 is based on the 3-CR of cyanothioacetamide 32 with malononitrile 3 and aromatic aldehydes 5 in the presence of piperidine. With the goal to prepare pyridine cytostatic agents, Abbas and co-workers performed a four-step synthesis of a number of new 3,5-dicyanopyridine thioglycosides 37.97 The obtained piperidinium salts of dihydropyridinethiones D were treated, without isolation, with 2,3,4,6-tetra-O-acetyl-α-D-gluco- and galactopyranosyl bromides 34 to give the H-form of product 35 (Scheme 10). The subsequent aromatization and acetate deprotection resulted in the formation of 3,5-dicyanopyridine thioglycosides 37 in more than 50% yields. The in vivo anticancer activities against HEPG2 (human hepatocellular carcinoma cells) and HELA cell lines were an order of magnitude higher for the derivatives with glycopyranosyl moieties than for the corresponding acetyl derivatives.
In 2016, Soumya and co-workers synthesized polycyclic hybrid peptidomimetic 43 (Scheme 11) bearing three pharmacophore moieties by linking the pyridine ring to the coumarin chromophore via a triazole linker. The authors implemented pseudo-4CR using 4- propynyloxybenzaldehyde 5, acetyl chloride 38, and 3-bromopropanenitrile 40 followed by copper(I)-catalyzed [3 + 2]azide–alkyne cycloaddition (CuAAC). Triazide 42 was prepared by a two-step procedure from coumarin 39, benzaldehyde 6, and 3-bromopropionic acid 40. The intermediate brominated derivative 41 was easily transformed into triazide 42 on treatment with NaN3. An additional screening of molecule 43 revealed the activity against the human breast carcinoma cells (MCF-7) with IC50 = 40 μM mL−1.98
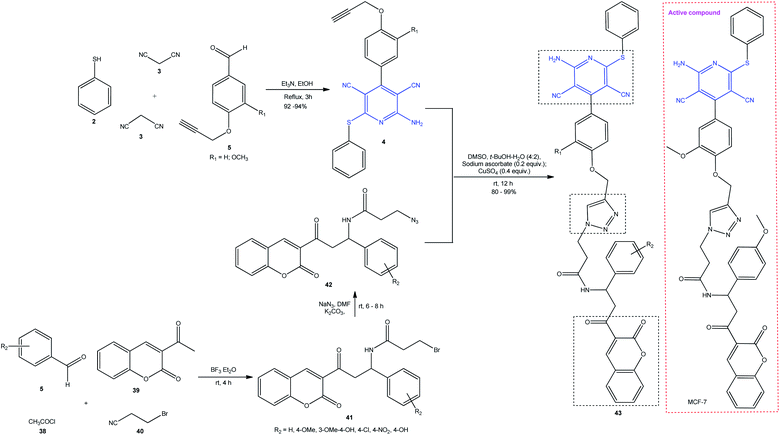 | ||
| Scheme 11 Synthesis of polycyclic hybrid peptidomimetics 43 with pyridine, coumarin, and triazole pharmacophore moieties. | ||
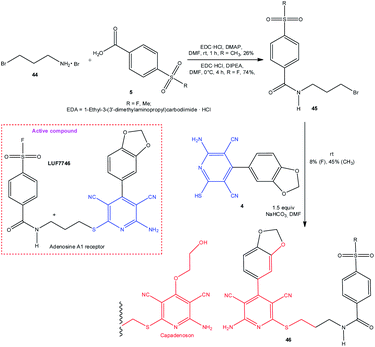
Recently, a method was proposed for the preparation of functionalized 3,5-dicyanopyridines 46, a structural analogue of capadenoson (Scheme 12).99 Fluorine-containing compound 46 (LUF7746) was fount to be a partial adenosine A1 receptor agonist with E50 = 61 ± 1% (hA1AR).
Catarzi and co-workers developed a method for the synthesis of a series of new pyridines 50, which were studied for the structure–activity relationship with respect to adenosine receptors.100 This approach is based on the transformation of the thiophenyl group in pyridines 4 into a mercapto group on treatment with Na2S followed by hydrolysis to thiol 48. The subsequent alkylation of 2-mercaptopyridine 48 with 2-(chloromethyl)-1H-imidazole or methyl chloroacetate 52 in the presence of sodium hydrogen carbonate at room temperature afforded target pyridine 51 (Scheme 13). It was shown that the sulfanyl-1H-imidazol-2-yl moiety in the C-6 position of the resulting molecule affects the activity of adenosine receptor agonists. The highest activity towards the hA2B receptor was found for 2-amino-6-[(1H-imidazol-2-ylmethyl)sulfanyl]-4-[4-(prop-2-en-1-yloxy)phenyl]pyridine-3,5-dicarbonitrile in a low nanomolar concentration range (EC50 = 27 ± 21 nM).
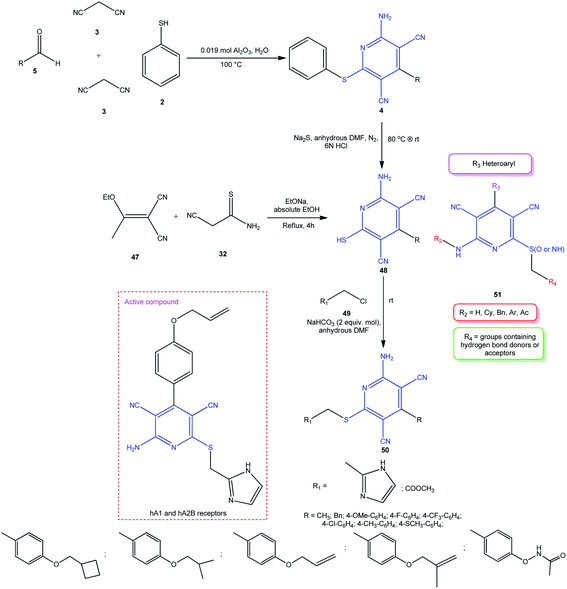 | ||
| Scheme 13 Multistage synthesis of imidazolyl- and acetylpyridines 50 exhibiting the activity of adenosine receptor agonists. | ||
The subsequent studies of this group aimed at the introduction of various substituents in the pyridine scaffold 51 demonstrated good possibilities for enhancing the biological effect (Scheme 13).101
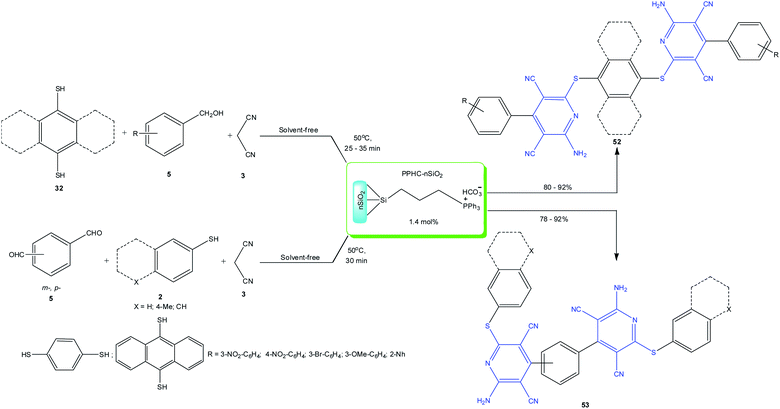
A method was proposed for the synthesis of polycyclic compounds 52 and 53 (Scheme 14) in 80%–92% yields by the reaction of malononitrile with dialdehydes/dithiols and an ionic liquid, propylphosphonium hydrogen carbonate, supported on nanosilica (PPHC–nSiO2), which served as a heterogeneous catalyst.76 A drawback of the proposed method is the three-stage preparation procedure of the PPHC–nSiO2 catalyst and that the ionic liquid contains phosphonium compounds, which is not quite consistent with green chemistry principles, as noted in the literature.102
4. Conclusions
The analysis of publications devoted to the chemistry and biological activity of 2-amino-6-sulfanylpyridine-3,5-dicarbonitriles indicates the continued interest of synthetic chemists in the last decade. Latest data summary in this review show the further development of the catalytic multicomponent reactions of malononitrile, aldehydes, and thiols (selenols) for the synthesis of new pharmaceutical agents based on the 2-amino-3,5-dicarbonitrile-6-sulfanylpyridine framework. Today, cluster of these compounds has been obtained with a yield of more than 70% using available and effective catalysts based on triethylamine, inorganic bases or boric acid, as well as Lewis acids, with most of which are realized in combination with ultrasonic irradiation. Attention is also drawn to innovative approaches using nanocatalysts, ionic liquids and catalysis with ceramic glass, eutectic mixture “choline chloride-urea”, baker's yeast, allowing to obtain target pyridines in 80–98% yields. Another innovative segment is the expansion of the range of thiolating agents; in addition to thiols, dithioacetals and isothiuronium salts have been proposed. In our opinion, new discoveries await chemical researchers and pharmacists in the field of cyano-substituted seleno-pyridines.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work was financially partly supported by the Russian Science Foundation (project no. 19-73-00070) and within the framework of the State Assignment AAAA-A19-119022290010-9.References
- (a) S. X. Lin, M. A. Curtis and J. Sperry, Bioorg. Med. Chem., 2020, 28, 115820, DOI:10.1016/j.bmc.2020.115820; (b) M. N. Zafar, A. H. Atif, M. F. Nazar, S. H. Sumrra, Gul-E-Saba and R. Paracha, Russ. J. Coord. Chem., 2016, 42, 1–18, DOI:10.1134/S1070328416010097; (c) L. Yet, Six-membered ring systems: pyridine and benzo derivatives, in Progress in Heterocyclic Chemistry, ed. G. W. Gribble and J. A. Joule, Elsevier, 2020, vol. 31, p. 431 Search PubMed.
- M. Baumann and I. R. Baxendale, Beilstein J. Org. Chem., 2013, 9, 2265–2319, DOI:10.3762/bjoc.9.265.
- F. Alinaghizadeh, M. Zahedifar, M. Seifi and H. Sheibani, J. Braz. Chem. Soc., 2016, 27, 663–669, DOI:10.5935/0103-5053.20150309.
- (a) G. M. Ziarani, Z. Kheilkordi and P. Gholamzadeh, Mol. Diversity, 2020, 24, 771–820, DOI:10.1007/s11030-019-09964-1; (b) M. Driowya, A. Saber, H. Marzag, L. Demange, R. Benhida and K. Bougrin, Molecules, 2016, 21, 492, DOI:10.3390/molecules21040492; (c) Y. Gu, Green Chem., 2012, 14, 2091–2128, 10.1039/C2GC35635J; (d) C. Allais, J.-M. Grassot, J. Rodriguez and T. Constantieux, Chem. Rev., 2014, 114, 10829–10868, DOI:10.1021/cr500099b.
- SciFinder – chemical abstracts service, https://scifinder-n.cas.org, accessed on September 2020 Search PubMed.
- (a) D. D. Ben, C. Lambertucci, M. Buccioni, A. M. Navia, G. Marucci, A. Spinaci and R. Volpini, Pharmaceuticals, 2019, 12, 150, DOI:10.3390/ph12040150; (b) K. A. Jacobson, D. K. Tosh, S. Jain and Z.-G. Gao, Front. Cell. Neurosci., 2019, 13, 124, DOI:10.3389/fncel.2019.00124; (c) P. G. Baraldi, M. A. Tabrizi, F. Fruttarolo, R. Romagnoli and D. Preti, Purinergic Signalling, 2008, 4, 287–303, DOI:10.1007/s11302-008-9097-z.
- L. C. W. Chang, J. K. von Frijtag Drabbe Künzel, T. Mulder-Krieger, R. F. Spanjersberg, S. F. Roerink, G. van den Hout, M. W. Beukers, J. Brussee and A. P. IJzerman, J. Med. Chem., 2005, 48, 2045–2053, DOI:10.1021/jm049597+.
- M. W. Beukers, L. C. W. Chang, J. K. von Frijtag Drabbe Künzel, T. Mulder-Krieger, R. F. Spanjersberg, J. Brussee and A. P. IJzerman, J. Med. Chem., 2004, 47, 3707–3709, DOI:10.1021/jm049947s.
- M. Betti, D. Catarzi, F. Varano, M. Falsini, K. Varani, F. Vincenzi, D. B. Diego, C. Lambertucci and V. Colotta, Eur. J. Med. Chem., 2018, 150, 127–139, DOI:10.1016/j.ejmech.2018.02.081.
- L. H. Heitman, T. Mulder-Krieger, R. F. Spanjersberg, J. K. von Frijtag Drabbe Künzel, A. Dalpiaz and A. P. Ijzerman, Br. J. Pharmacol., 2006, 147, 533–541, DOI:10.1038/sj.bjp.0706655.
- (a) D. Meibom, B. Albrecht-Küpper, N. Diedrichs, W. Hübsch, R. Kast, T. Krämer, U. Krenz, H.-G. Lerchen, J. Mittendorf, P. G. Nell, F. Süssmeier, A. Vakalopoulos and K. Zimmermann, ChemMedChem, 2017, 12, 728–737, DOI:10.1002/cmdc.201700151; (b) J.-A. Baltos, E. A. Vecchio, M. A. Harris, C. X. Qin, R. H. Ritchie, A. Christopoulos, P. J. White and L. T. May, Biochem. Pharmacol., 2017, 135, 79–89, DOI:10.1016/j.bcp.2017.03.014.
- L. Bott-Flügel, A. Bernshausen, H. Schneider, P. Luppa, K. Zimmermann, B. Albrecht-Küpper, R. Kast, K.-L. Laugwitz, H. Ehmke, A. Knorr and M. Seyfarth, PLoS One, 2011, 6, 18048, DOI:10.1371/journal.pone.0018048.
- (a) A. A. Voors, H.-D. Düngen, M. Senni, S. Nodari, P. Agostoni, P. Ponikowski, J. J. Bax, J. Butler, R. J. Kim, B. Dorhout, W. Dinh and M. Gheorghiade, J. Clin. Pharmacol., 2017, 57, 440, DOI:10.1002/jcph.828; (b) M. C. Erber, J. Anlahr, J. Nicolai and M. Pfeffer, Parenteral pharmaceutical composition comprising neladenoson bialanate, WO Pat., 2019180072A1, 2019 Search PubMed.
- (a) M. L. Barreca, N. Iraci, S. Biggi, V. Cecchetti and E. Biasini, Pathogens, 2018, 7, 27, DOI:10.3390/pathogens7010027; (b) K. Guo, R. Mutter, W. Heal, T. R. K. Reddy, H. Cope, S. Pratt, M. J. Thompson and B. Chen, Eur. J. Med. Chem., 2008, 43, 93–106, DOI:10.1016/j.ejmech.2007.02.018.
- J. Deng, T. Sanchez, L. Q. Al-Mawsawi, R. Dayam, R. A. Yunes, A. Garofalo, M. B. Bolger and N. Neamati, Bioorg. Med. Chem., 2007, 15, 4985–5002, DOI:10.1016/j.bmc.2007.04.041.
- Sudheer and M. A. Quraishi, Ind. Eng. Chem. Res., 2014, 53, 2851–2859, DOI:10.1021/ie401633y.
- J. A. Makawana, M. P. Patel and R. G. Patel, Med. Chem. Res., 2012, 21, 616–623, DOI:10.1007/s00044-011-9568-6.
- S. Kambe, K. Saito, A. Sakurai and H. Midorikawa, Synthesis, 1981, 531–533, DOI:10.1055/s-1981-29513.
- N. M. Evdokimov, A. S. Kireev, A. A. Yakovenko, M. Yu. Antipin, I. V. Magedov and A. Kornienko, J. Org. Chem., 2007, 72, 3443–3453, DOI:10.1021/jo070114u.
- R. Mamgain, R. Singh and D. S. Rawat, J. Heterocycl. Chem., 2009, 46, 69–73, DOI:10.1002/jhet.32.
- N. M. Evdokimov, I. V. Magedov, A. S. Kireev and A. Kornienko, Org. Lett., 2006, 8, 899–902, DOI:10.1021/ol052994+.
- B. C. Ranu, R. Jana and S. Sowmiah, J. Org. Chem., 2007, 3, 3152–3154, DOI:10.1021/jo070015g.
- B. Das, B. Ravikanth, A. S. Kumar and B. S. Kanth, J. Heterocycl. Chem., 2009, 46, 1208–1212, DOI:10.1002/jhet.206.
- K. Guo, M. J. Thompson and B. Chen, J. Org. Chem., 2009, 74, 6999–7006, DOI:10.1021/jo901232b.
- S. Banerjee and G. Sereda, Tetrahedron Lett., 2009, 50, 6959–6962, DOI:10.1016/j.tetlet.2009.09.137.
- K. Guo, M. J. Thompson, T. R. K. Reddy, R. Mutter and B. Chen, Tetrahedron, 2007, 63, 5300–5311, DOI:10.1016/j.tet.2007.03.139.
- M. Sridhar, B. C. Ramanaiah, C. Narsaiah, B. Mahesh, M. Kumaraswamy, K. K. R. Mallu, V. M. Ankathi and P. S. Rao, Tetrahedron Lett., 2009, 50, 3897–3900, DOI:10.1016/j.tetlet.2009.04.051.
- K. N. Singh and S. K. Singh, ARKIVOC, 2009, XIII, 153–160, DOI:10.3998/ark.5550190.0010.d13.
- Z.-q. Wang, Z.-m. Ge, T.-m. Cheng and R.-t. Li, Synlett, 2009, 12, 2020–2022, DOI:10.1055/s-0029-1217529.
- M. Ali, K. M. Khan, M. Mahdavi, A. Jabbar, S. Shamim, U. Salar, M. Taha, S. Perveen, B. Larijani and M. A. Faramarzi, Bioorg. Chem., 2020, 100, 103879, DOI:10.1016/j.bioorg.2020.103879.
- U. V. Desai, M. A. Kulkarni, K. S. Pandit, A. M. Kulkarni and P. P. Wadgaonkar, Green Chem. Lett. Rev., 2014, 7, 228–235, DOI:10.1080/17518253.2014.925144.
- N. Azizi and M. S. Haghayegh, ChemistrySelect, 2017, 2, 8870–8873, DOI:10.1002/slct.201701682.
- A. U. Khandebharad, S. R. Sarda, M. N. Farooqui, M. A. K. Pathan and B. R. Agrawal, Polycyclic Aromat. Compd., 2020, 40, 832–839, DOI:10.1080/10406638.2018.1485713.
- A. S. Chavan, A. S. Kharat, M. R. Bhosle and R. A. Mane, Synth. Commun., 2017, 47, 1777–1782, DOI:10.1080/00397911.2017.1350982.
- A. Allahi and B. Akhlaghinia, Phosphorus, Sulfur Silicon Relat. Elem., 2020, 196, 328–336, DOI:10.1080/10426507.2020.1835905.
- P. V. Shinde, B. B. Shingate and M. S. Shingare, Chin. J. Chem., 2011, 29, 1049–1054, DOI:10.1002/cjoc.201190178.
- S. Takale, J. Patil, V. Padalkar, R. Pisal and A. Chaskar, J. Braz. Chem. Soc., 2012, 23, 966–969, DOI:10.1590/S0103-50532012000500024.
- R. V. Kupwade, S. S. Khot, M. A. Kulkarni, U. V. Desai and P. P. Wadgaonkar, RSC Adv., 2017, 7, 38877–38883, 10.1039/C7RA07738F.
- Z. Fang, F. Zhang, B. H. Zou, W. He, H. Zhong, D. Ji and K. Guo, Adv. Mater. Res., 2013, 749, 293–298, DOI:10.4028/www.scientific.net/AMR.749.293.
- M. N. Khan, S. Pal, S. Karamthulla and L. H. Choudhury, RSC Adv., 2014, 4, 3732–3741, 10.1039/C3RA45252B.
- M. A. Aziz and M. Farooqui, World J. Pharm. Res., 2016, 5, 1255–1267 CAS.
- W. Hui, X. Jiangbiao, Z. Peng, W. Yu and Y. Rui, Polysubstituted pyridine derivative and the preparation method and application thereof, CN Pat., 108727259 A, 2018 Search PubMed.
- Y. Caibo, Z. Xin, W. Shenghua, G. Tao, H. Huihui, Y. Cuiping and C. Zhaolian, Method for preparing 2-amino-4-phenyl-6-(phenylthio)-3,5-dicyanopyridine derivative with green catalysis, CN Pat., 104649967 B, 2016 Search PubMed.
- J. Safaei-Ghomi, M. A. Ghasemzadeh and M. Mehrabi, Sci. Iran., Trans. C, 2013, 20, 549–554, DOI:10.1016/j.scient.2012.12.037.
- J. Safaei-Ghomi, H. Shahbazi-Alavi and E. Heidari-Baghbahadorani, RSC Adv., 2014, 4, 50668–50677, 10.1039/C4RA04769A.
- J. Safaei-Ghomi and M. A. Ghasemzadeh, J. Sulfur Chem., 2013, 34, 233–241, DOI:10.1080/17415993.2012.728220.
- J. Safaei-Ghomi and M. A. Ghasemzadeh, Acta Chim. Slov., 2012, 59, 697–702 CAS.
- M. L. Kantam, K. Mahendar and S. J. Bhargava, J. Chem. Sci., 2010, 122, 63–69, DOI:10.1007/s12039-010-0007-x.
- M. Norouzi, A. Ghorbani-Choghamarani and M. Nikoorazm, RSC Adv., 2016, 6, 92387–92401, 10.1039/C6RA19776K.
- S. J. Shams-Najafi, M. Gholizadeh and A. J. Ahmadpour, J. Chin. Chem. Soc., 2019, 66, 1531–1536, DOI:10.1002/jccs.201900041.
- B. Baghernejad, Bull. Chem. Soc. Ethiop., 2014, 28, 149–153, DOI:10.4314/bcse.v28i1.18.
- M. A. Zolfigol, M. Safaiee, B. Ebrahimghasri, S. Baghery, S. Alaie, M. Kiafar, A. Taherpour, Y. Bayat and A. Asgari, J. Iran. Chem. Soc., 2017, 14, 1839–1852, DOI:10.1007/s13738-017-1123-z.
- A. Nakhaei and H. Nakhaei, Heterocycl. Lett., 2018, 8, 27–33 CAS.
- H. Ebrahimiasl, D. Azarifar, J. Rakhtshah, H. Keypour and M. Mahmoudabadi, Appl. Organomet. Chem., 2020, 34, 5769, DOI:10.1002/aoc.5769.
- G. M. Nazeruddin, Y. I. Shaikh and A. A. Shaikh, RJPBCS, 2014, 5, 1773–1779 Search PubMed.
- Y. I. Shaikh, A. A. Shaikh and G. M. Nazeruddin, J. Chem. Pharm. Res., 2012, 4, 4953–4956 CAS.
- P. V. Shinde, S. S. Sonar, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2010, 51, 1309–1312, DOI:10.1016/j.tetlet.2009.12.146.
- C. Verma, M. A. Quraishi, H. Lgaz, L. O. Olasunkanmi, E.-S. M. Sherif, R. Salghi and E. E. Ebenso, J. Mol. Liq., 2019, 283, 491–506, DOI:10.1016/j.molliq.2019.03.105.
- M. S. Su, X. J. Ji, B. B. Zhao, M. Tian and J. J. Ma, J. Chem. Soc. Pak., 2015, 37, 1130–1134 CAS.
- A. A. Grigor’ev, N. V. Shtyrlin, R. R. Gabbasova, M. I. Zeldi, D. Yu. Grishaev, O. I. Gnezdilov, K. V. Balakin, O. E. Nasakin and Yu. G. Shtyrlin, Synth. Commun., 2018, 48, 2288–2304, DOI:10.1080/00397911.2018.1501487.
- Md. N. Khan, S. Pal, T. Parvin and L. H. Choudhury, RSC Adv., 2012, 2, 12305–12314, 10.1039/C2RA21385K.
- R. Pagadala, S. Maddila and S. B. Jonnalagadda, Ultrason. Sonochem., 2014, 21, 472–477, DOI:10.1016/j.ultsonch.2013.08.024.
- J. B. Gujar, M. A. Chaudhari, D. S. Kawade and M. S. Shingare, Tetrahedron Lett., 2014, 55, 6939–6942, DOI:10.1016/j.tetlet.2014.10.125.
- S. Mishra and R. Ghosh, Synth. Commun., 2012, 42, 2229–2244, DOI:10.1080/00397911.2011.555284.
- M. Kidwai and R. Chauhan, J. Iran. Chem. Soc., 2014, 11, 1005–1013, DOI:10.1007/s13738-013-0368-4.
- L. S. Reddy, T. R. Reddy, R. B. Mohan, A. Mahesh, Y. Lingappa and N. C. G. Reddy, Chem. Pharm. Bull., 2013, 61, 1114–1120, DOI:10.1248/cpb.c13-00412.
- N. M. Panchani and H. S. Joshi, Chem. Biol. Interface, 2016, 6, 92–98 CAS.
- P. V. Shinde, B. B. Shingate and M. S. Shingare, Bull. Korean Chem. Soc., 2011, 32, 459–462, DOI:10.5012/bkcs.2011.32.2.459.
- S. S. Kottawar, S. A. Siddiqui and S. R. Bhusare, Heterocycl. Commun., 2012, 18, 249–252, DOI:10.1515/hc-2012-0103.
- M. S. Pandharpatte, H. A. Osman, A.-B. M. Osman and G. M. Nazeruddin, Chem. Sci. Trans., 2017, 6, 1–7, DOI:10.7598/cst2017.1277.
- A. Ahad and M. Farooqui, Int. J. Chem. Sci., 2016, 14, 1789–1796 Search PubMed.
- V. T. Kamble, S. T. Atkore, P. M. Pisal, M. Sadaf and R. V. Thakre, Quarterly Journal of Iranian Chemical Communication, 2016, 4, 186–196 CAS.
- M. Thimmaiah, P. Li, S. Regati, B. Chen and J. Cong-Gui Zhao, Tetrahedron Lett., 2012, 53, 4870–4872, DOI:10.1016/j.tetlet.2012.06.139.
- M. R. P. Heravi and F. Fakhr, Tetrahedron Lett., 2011, 52, 6779–6782, DOI:10.1016/j.tetlet.2011.10.031.
- S. Ray, P. Das, A. Bhaumik, M. Pramanik and C. Mukhopadhyay, Recoverable Recyclable Catal., 2014, 1, 34–57, DOI:10.2478/recat-2014-0001.
- F. Rahmani, I. Mohammadpoor-Baltork, A. R. Khosropour, M. Moghadam, S. Tangestaninejad and V. Mirkhani, RSC Adv., 2015, 5, 39978–39991, 10.1039/C5RA03569D.
- K. Niknam and A. R. Hosseini, Org. Chem. Res., 2017, 3, 16–24, DOI:10.22036/ORG.CHEM..2017.41100.
- S. Sobhani and M. Honarmand, Appl. Catal., A, 2013, 467, 456–462, DOI:10.1016/j.apcata.2013.08.006.
- S. Sobhani, F. Nasseri and F. Zarifi, J. Iran. Chem. Soc., 2018, 15, 2721–2732, DOI:10.1007/s13738-018-1460-6.
- P. V. Shinde, V. B. Labade, B. B. Shingate and M. S. Shingare, J. Mol. Catal. A: Chem., 2011, 336, 100–105, DOI:10.1016/j.molcata.2011.01.005.
- M. M. Heravi, M. Khorshidi, Y. Sh. Beheshtia and B. Baghernejad, Bull. Korean Chem. Soc., 2010, 31, 1343–1344, DOI:10.5012/bkcs.2010.31.5.1343.
- S. Kumari, A. Shekhar and D. D. Pathak, New J. Chem., 2016, 40, 5053–5060, 10.1039/C5NJ03380B.
- P. Manna and P. K. Maiti, Tetrahedron Lett., 2015, 6, 5094–5098, DOI:10.1016/j.tetlet.2015.07.038.
- K. Godugu, V. Divya Sri Yadala, M. K. M. Pinjari, T. R. Gundala, L. R. Sanapareddy and C. G. R. Nallagondu, Beilstein J. Org. Chem., 2020, 16, 1881–1900, DOI:10.3762/bjoc.16.156.
- A. Davoodnia, P. Attar, H. Eshghi, A. Morsali, N. Tavakoli-Hoseini and A. Tavakoli-Nishaburi, Asian J. Chem., 2011, 23, 1273–1275 CAS.
- D. Hao, Z. Yun-lei, S. Xiao-peng, Y. Jin-ming and F. Dong, Res. Chem. Intermed., 2014, 40, 587–594, DOI:10.1007/s11164-012-0984-0.
- T. Jinjin and G. Hongyun, Chin. J. Org. Chem., 2012, 32, 193–196, DOI:10.6023/cjoc1105133.
- S. Sobhani and M. Honarmand, C. R. Chim., 2013, 16, 279–286, DOI:10.1016/j.crci.2012.10.011.
- M. Bayat, M. Saboni and B. Notash, J. Heterocycl. Chem., 2018, 55, 313–317, DOI:10.1002/jhet.3051.
- (a) M. N. Khan, S. Karamthulla, L. H. Choudhury and Md. S. H. Faizi, RSC Adv., 2015, 5, 22168–22172, 10.1039/C5RA02403J; (b) J. Soni, N. Sahiba, A. Sethiya and S. Agarwal, J. Mol. Liq., 2020, 315, 113766, DOI:10.1016/j.molliq.2020.113766.
- A. A. Grigor'ev, S. V. Karpov, Y. S. Kayukov, M. Yu. Belikov and O. E. Nasakin, Tetrahedron Lett., 2015, 56, 6279–6281, DOI:10.1016/j.tetlet.2015.09.130.
- V. Vashchenko, L. Kutulya and A. Krivoshey, Synthesis, 2007, 2125–2134, DOI:10.1002/chin.200747094.
- Ya. S. Kayukov, S. V. Karpov, O. V. Kayukova and A. A. Grigor’ev, Russ. J. Org. Chem., 2020, 56, 1313–1316, DOI:10.1134/S1070428020070283.
- Y. O. Bhola and Y. T. Naliapara, World Sci. News, 2019, 117, 221–227 CAS.
- M. B. Kanani and M. P. Patel, Med. Chem. Res., 2013, 22, 2912–2920, DOI:10.1007/s00044-012-0292-7.
- F. S. Hosseini, M. Bayat and M. Masoumi, J. Sulfur Chem., 2019, 40, 65–74, DOI:10.1080/17415993.2018.1523412.
- H.-A. S. Abbas, W. A. El Sayed and N. M. Fathy, Eur. J. Med. Chem., 2010, 45, 973–982, DOI:10.1016/j.ejmech.2009.11.039.
- T. V. Soumya, C. M. Ajmal and D. Bahulayan, Bioorg. Med. Chem. Lett., 2017, 27, 450–455, DOI:10.1016/j.bmcl.2016.12.044.
- X. Yang, M. A. Dilweg, D. Osemwengie, L. Burggraaff, D. van der Es, L. H. Heitman and A. P. IJzerman, Biochem. Pharmacol., 2020, 180, 114144, DOI:10.1016/j.bcp.2020.114144.
- D. Catarzi, F. Varano, K. Varani, F. Vincenzi, S. Pasquini, D. D. Ben, R. Volpini and V. Colotta, Pharmaceuticals, 2019, 12, 159, DOI:10.3390/ph12040159.
- M. Betti, D. Catarzi, F. Varano, M. Falsini, K. Varani, F. Vincenzi, S. Pasquini, L. di Cesare Mannelli, C. Ghelardini, E. Lucarini, D. D. Ben, A. Spinaci, G. Bartolucci, M. Menicatti and V. Colotta, J. Med. Chem., 2019, 62, 6894–6912, DOI:10.1021/acs.jmedchem.9b00106.
- G. Cevasco and C. Chiappe, Green Chem., 2014, 16, 2375–2385, 10.1039/C3GC42096E.
| This journal is © The Royal Society of Chemistry 2021 |