Open Access Article
Open Access ArticleClass II biocompatible E-Shell 300 3D printing material causes severe developmental toxicity in Danio rerio embryos and reduced cell proliferation in vitro – implications for 3D printed microfluidics
Zuzana Nejedlá,
David Poustka,
Regina Herma,
Michaela Liegertová,
Marcel Štofik ,
Jiří Smejkal,
Václav Šícha,
Pavel Kaule and
Jan Malý
,
Jiří Smejkal,
Václav Šícha,
Pavel Kaule and
Jan Malý *
*
Department of Biology, Faculty of Science, Jan Evangelista Purkinje University, České mládeže 8, 400 96, Usti nad Labem, Czech Republic. E-mail: jan.maly@ujep.cz; Fax: +420 475 283 622; Tel: +420 475 283 622
First published on 4th May 2021
Abstract
Additive manufacturing is a new technology that represents a highly promising, cheap, and efficient solution for the production of various tools in the biomedicine field. In our study, the toxicity of the commercially available E-Shell 300 series photopolymer, which is used in the manufacture of hearing aids and other implants and which could be potentially exploited in microfluidic device fabrication, was tested using in vivo and in vitro biological models. We examined B14 cell proliferation in direct contact with the three-dimensional (3D)-printed material as well as in water extracts to evaluate in vitro cytotoxicity. Similarly, in vivo tests were performed using an OECD-standardized fish embryo acute toxicity (FET) test on Danio rerio embryos in direct contact with the material and in extracts as well. Despite E-Shell 300 3D-printed material being declared as class-IIa biocompatible, in the case of direct contact with both biological models, the results demonstrated a considerable negative impact on cell proliferation and severe developmental toxicity. In this study, up to 84% reduced cell proliferation in vitro and 79% mortality of in vivo models were observed. In contrast, a negligible toxic influence of E-Shell 300 water extracts was present. Four different post-processing treatments to reduce the toxicity were also tested. We observed that post-printing treatment of 3D-printed material in 96% ethanol can reduce embryonic mortality in the FET test by 71% and also completely eliminate negative effects on cell proliferation. We analyzed leachates from the polymeric structures by mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy, and we discovered the presence of surfactant residues. In summary, our results indicate the importance of biocompatibility testing of the 3D printing photopolymer material in direct contact with the given biological model. On the other hand, the possibility of eliminating toxic effects by an appropriate post-processing strategy opens the door for broader applications of E-Shell 300 photopolymers in the development of complex microfluidic devices for various biological applications.
1. Introduction
Three-dimensional (3D) printing is a fast, affordable production technology. In addition to rapid prototyping, this approach also provides creative solutions for a variety of applications in biomedicine, biomaterials, and microfluidics. The usability of 3D printing ranges from the manufacture of prosthetic replacements1 and surgical instruments2 to various applications in dentistry,3 medical imaging,4 and tissue engineering.5 3D printing also offers effective solutions for cell culture systems that might incorporate perfusion technology, microfluidics,6 and specialized tools for cell cultivation.3D printing, also referred to as additive manufacturing, is a digital fabrication technology that originated from the layer-by-layer fabrication of 3D structures directly from computer-aided design (CAD) drawing.7 A wide range of materials and 3D printing approaches are currently in use, as are, for example, binder jetting, fused deposition modeling (FDM), selective laser sintering (SLS), electron beam melting (EBM), and selective heat sintering (SHS). Recently, stereolithography (SLA) and digital light processing (DLP) technologies were widely studied for the manufacturing of biocompatible microfluidics for cell manipulations and cultivations due to the possibility of fast and highly precise production of complex devices with favorable optical, physicochemical, and mechanical properties. Both technologies are based on the polymerization of photo-reactive polymers by using a laser or conventional ultraviolet light source (UV).8 The products' final properties are influenced by the type of photo-reactive polymers, photo-initiators, dyes, pigments, surfactants, UV absorbers, and many other components of the complex liquid precursor material. The essential parameters for photopolymerization are the light source wavelength, exposure time, and power supply amount.9
The use of 3D printing technology for the fabrication of microfluidic devices for cultured cells or various in vivo animal model cultivations was already demonstrated. The key components of these devices—the micro-channels—which provide the simulation of real conditions for living organisms, can be fabricated by 3D printing with high accuracy. With two-photon polymerization-based 3D printers, it is even possible to attain a resolution down to 100 nanometers.10 As an example, 3D-printed devices were developed for the toxicity screening of chemicals using embryos of model fish Danio rerio.11,12 Cell culturing platforms have also been created. Tan et al. demonstrated culturing of fibroblast cells on a platform fabricated from PLGA using E-jet printing.13 Because commonly used 2D cell in vitro cultures do not accurately imitate conditions in living organisms due to altered signaling between the cells, the cultivation of so-called 3D cell cultures (e.g., organoids or spheroids in 3D-printed platforms) is currently in focus.14
A wide range of materials has already been tested for the 3D printing of microfluidic systems. However, it is clear that the selection of the appropriate material is crucial to obtain results unaffected by the device itself. When designing the microfluidic devices for the cultivation of various biological systems (e.g., cell cultures, organs-on-a-chip, model organisms), tools for medical use or for tissue engineering applications, a number of additional factors must be taken into account when selecting printing materials, such as biocompatibility, bioactivity, or biodegradability. Numerous systems and materials have been adapted from other fields, where the biological response is not an issue. Physical properties (strength, transparency, durability, stability, etc.) of the discussed material naturally cannot be ignored. However, a thorough understanding of the biocompatibility of all components as well as used fabrication processes is crucial for their possible biological application. This approach should enable the long-term maintenance of tissue and cell cultures without affecting cell viability or eliciting an immune response in some specific applications.
In this context, several possible sources of 3D-printed material's toxicity for the cells and vertebrate organisms were already described, including the releasement of residues, unpolymerized monomers, and chemical additives.15–18 The toxicity was manifested as, for example, reduced cell viability,17 an inflammatory response,19 or an infection.20 A number of recent studies show that even after thorough treatment of printed structures according to the manufacturer's instructions, the release of some potentially toxic photopolymer components may occur (photoinitiator, monomer, shorter polymer chains, and several recognized additives).21–23 The main release mechanisms of these substances are either material degradation and erosion or extraction (leaching) with a solvent.24 Even at optimum photopolymerization conditions, the conversion of monomers to polymers is insufficient and reaches only approximately 55–60% of total polymerization in the most efficient systems.25 The leaching of unreacted components from the 3D-printed object could be influenced by its geometry.21 As an example, the negative impact of E-Shell 200 and E-Shell 300 photopolymer on the early development of bovine embryos was documented26 despite a declared biocompatibility according to ISO 10993 (category IIa). In another study, the biocompatibility of four tested commercial photopolymers (VisiJetCrystal EX200, Watershed 11122XC, Fototec SLA 7150 Clear, and ABS plus P-430) was evaluated by a FET test. The embryonic development of Danio rerio was monitored, and even if the manufacturer instructions were followed, all of the above-mentioned photopolymers were highly toxic, resulting in substantial embryo mortality rates.15 The published results have shown that polymer extract was highly toxic in all tested photopolymers for the entire range of indicator organisms (invertebrates and vertebrates).17
Several studies have, therefore, been aimed at reducing the toxicity of these materials through various physical treatments and suggestions for so-called post-processing procedures.19 Post-printing treatment to reduce toxicity is based both on physical and chemical processes. As an example, Inoue and Ikuta27 reported that heat treatment over 225 °C could remove cytotoxic substances. Other approaches include washing in a variety of solvents—most commonly in isopropyl alcohol (IPA)—followed by UV treatment and drying. This procedure is based on rinsing off harmful resin residues from the surface of the fabricated objects (particularly monomers and photoinitiators) under UV radiation.21,28,29 In the case of some polymers, a reduction of the toxic effects was achieved by soaking the structures in solvents. For example, Fototec 7150 photopolymer showed improved biocompatibility after soaking in 99% ethanol.15 Dental prostheses and orthodontic appliances made of commercial resins are commonly soaked in water for up to 24 hours, during which time unwanted components are extracted, thus reducing their side effects.30 Another method is based on various coating procedures of the polymeric structures in order to prevent the release of toxic substances into the aquatic environment. In this way, the toxic effects of E-Shell 300 and HTM140 photopolymer structures were suppressed with parylene-C5 coating.31
Here, we present a biocompatibility study of commercially available E-Shell 300 photopolymer as a promising photopolymer for DLP 3D printing of microfluidic devices for biological applications. This photopolymer is a potential candidate for 3D cell and tissue culture cultivation tool design (e.g., organoids, spheroids, organ-on-a-chip) and for culturing of in vivo model organisms (Danio rerio embryos) in microfluidic format due to its declared biocompatibility (class IIa according to ISO 10993) and favorable optical, mechanical, and physicochemical properties. Because in such proposed applications, the biological objects are in direct physical contact with the polymeric surface of a functional device, we have focused our attention on in vitro and in vivo toxicity assays to compare the toxicity effects on biological models with the assays performed in leachates. We discuss the substantial disparities obtained by both types of assays and propose a solution for elimination of the observed high toxicity of E-Shell 300. Our results could contribute to the future successful employment of this polymer in the fabrication of microfluidic devices for a wide range of biological applications.
2. Materials and methods
2.1 3D printing
CAD 3D models created in software Fusion 360 (Autodesk) were printed from the photopolymer E-Shell 300 series (EnvisionTEC Inc.) via a DLP process on a 3D printer Perfactory 4 LED (EnvisionTEC Inc.) with the height of one step being 100 μm. According to the manufacturer's recommendations, printed objects were washed in isopropyl alcohol (propan-2-ol), sonicated for 2 × 5 minutes (Shesto), and then exposed to a varying dose of UV light (10/50/100/250 J cm−2) from both sides under a photolithography lamp (mercury light source, λ = 350–450 nm; Newport), the spectral distribution and maxima of which are comparable to the Otoflash G171 (Puretone Ltd) unit mentioned in the manufacturer's instructions for use. As a final step, some samples were placed into distilled water or 96% ethanol bath for 2 × 24 hours while being magnetically stirred to wash out any toxic residues.2.2 In vitro cytotoxicity assays
First experimental setup. 2 ml of medium containing 5 × 105 B14 cells per ml was added to each well of the 12-well polystyrene cultivation plate (Sigma). Subsequently, each of the printed and post-cured specimens was placed in the wells in a way to avoid direct contact with the well's surface. Cultivation plates were then placed into the incubator. After 24 hours, 50% of the cell culture samples were stained and observed. In the remaining samples, the medium was exchanged with 2 ml of fresh DMEM, and the cultivation proceeded for a total of 48 hours before staining.
Second experimental setup. 0.5 ml of medium containing 5 × 105 B14 cells per ml was added into each of the printed and post-cured E-Shell 300 containers. These containers were placed into the incubator. After 24 hours, 50% of the cell culture samples were stained and observed. In the remaining samples, the medium was exchanged with 0.5 ml of fresh DMEM, and the cultivation proceeded for a total of 48 hours before staining.
2.3 Mass spectrometry
Molecular cation masses [M + H] of substances extracted from bulk polymer material were analyzed using an LCQ Fleet ion trap mass spectrometer with an ESI probe (Thermo Scientific Inc). Twenty-five μl of ethanol or water leachate was mixed with 1 ml of acetonitrile. Twenty-five μl of diluted samples were injected using a Finnigan Surveyor Autosampler Plus into a 150 μl min−1 stream of MS grade acetonitrile and further into the ESI probe. Voltages of 4.97 kV in the ion source, 95.00 V on the tube lens, and 25.99 V on the capillary were used. Samples were introduced into the probe at a capillary temperature of 275 °C and nitrogen flow of 50.0 p.d.u. sheath gas, 3.0 p.d.u. aux gas, and 2.0 p.d.u. sweep gas. Cation peaks were measured.2.4 Nuclear magnetic resonance spectroscopy
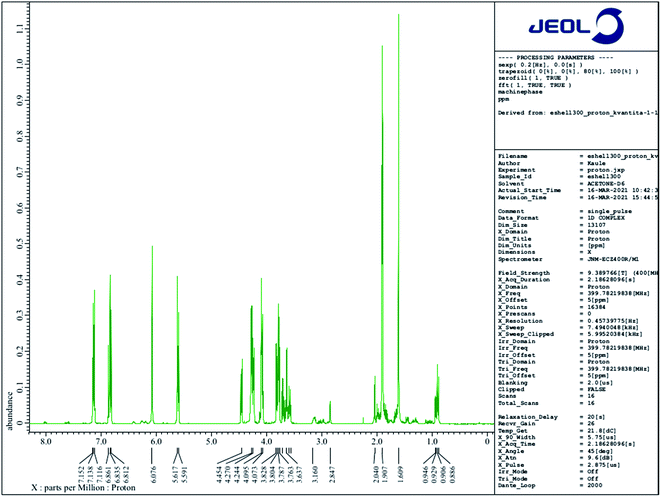
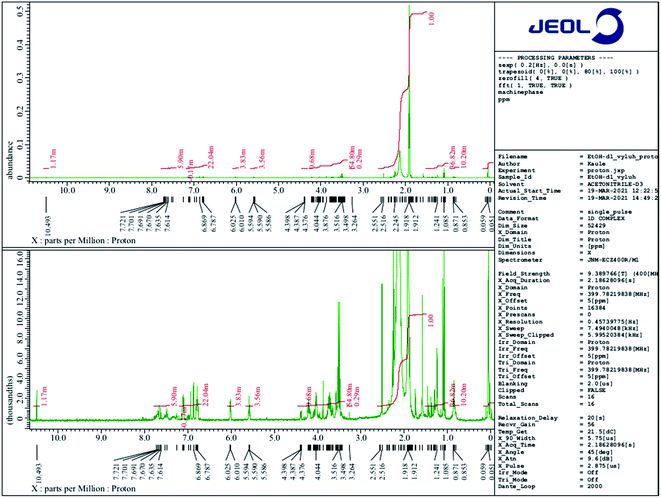
1H NMR spectra were measured and obtained using NMR spectrometer JEOL 400 MHz JNM-ECZ400R/M1 based on the superconducting magnet JASTEC 400/54/JJYH/W (9.39 T, 54 mm standard bore), equipped with an automatic tuning and matching system of nuclei falling into the range of high sensitivity ROYALPROBE HFX.2.5 In vivo assays
In addition to eukaryotic cells as an in vitro model, a vertebrate model, specifically Danio rerio, was selected for in vivo bioassays (standardized FET tests) according to OECD guidelines for the testing of chemicals.33 This model has become increasingly popular for rapid in vivo toxicity testing and developmental assays. The aquatic vertebrate Danio serves as an excellent and convenient model for the toxicity analysis of water-soluble substances. With the high-genetic-homology toothier vertebrates and mammals, the high-throughput genetic and chemical screenings are becoming a powerful tool for in vivo toxicity evaluation.34–37Type A sample. According to a published protocol,39 extracts of the tested samples were prepared. A porous 3D-printed block with a surface area of 7 cm2 was added into a glass flask with 10 ml of aerated E3 medium. Each block was leached in darkness for 72 hours at room temperature (23 ± 1 °C) under continuous magnetic stirring at approximately 240 rpm. The resultant extract was considered as a 100% concentrate. Prior to the experiment, each cultivation microplate was saturated by overnight incubation with the corresponding extract to saturate the well surface in a nonspecific manner (eliminates fluctuations in testing concentrations due to chemicals in the medium binding to the well surface).
Type B sample. The 24-well plate was printed and treated as described in Section 2.1. Each well was filled with 200 μl of the E3 medium and served as an incubator for one embryo. The development and health state of each embryo was observed directly in each plate with a stereomicroscope for a total of 96 hours.
3. Results and discussion
3.1 3D printing
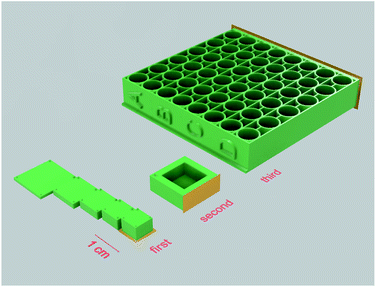
Several sets of objects, each targeting a different possible factor that could impact cell proliferation, were modeled and printed out of photopolymer E-Shell 300. The first set of experiments was designed to assess the effect of varying thickness of the specimen used. As the manufacturer mentions in the instructions for use of the E 300 polymer, it is possible that with increasing thickness of the printed specimens, post-curing may not be sufficient, leading to an accumulation of unpolymerized material in the central area of the printed models. For testing this hypothesis, rectangular objects with a constant volume of 200 mm3 (but of varying thickness and other proportions) were prepared (Fig. 2 – first set) and subsequently tested for the effect of potentially toxic agents leaking from the unpolymerized material into the cultivation media on the adherent living cells. All samples from this set of experiments were post-cured by a UV light dose of 250 J cm−2 from both sides.The second set of specimens was prepared for the purpose of cultivating living cells in direct contact with the photopolymer surface. A simple rectangular container with a bottom side thickness of 1 mm was modeled (Fig. 2 – second set). This set aimed at two variables, namely the dose of UV light used for post-curing procedures and the possible extraction of toxic agents by soaking the specimens in distilled water or ethanol. In the first case, samples were exposed to an increasing dose of UV light 0/10/100 J cm−2 from both sides. Because the 100 J cm−2 dose already takes a relatively long time and the risk of damaging the material by UV light increases, for testing the extraction possibilities, a middle dose of 50 J cm−2 was used; subsequently, the specimens were either left untreated or soaked in distilled water or ethanol.
The third set of specimens was designed for FET tests and based on similar dimensions as commercially available 96-well cell culture plates and post-cured by a dose of UV light of 50 J cm−2 from each side (Fig. 2 – third set).
3.2 In vitro cytotoxicity assays
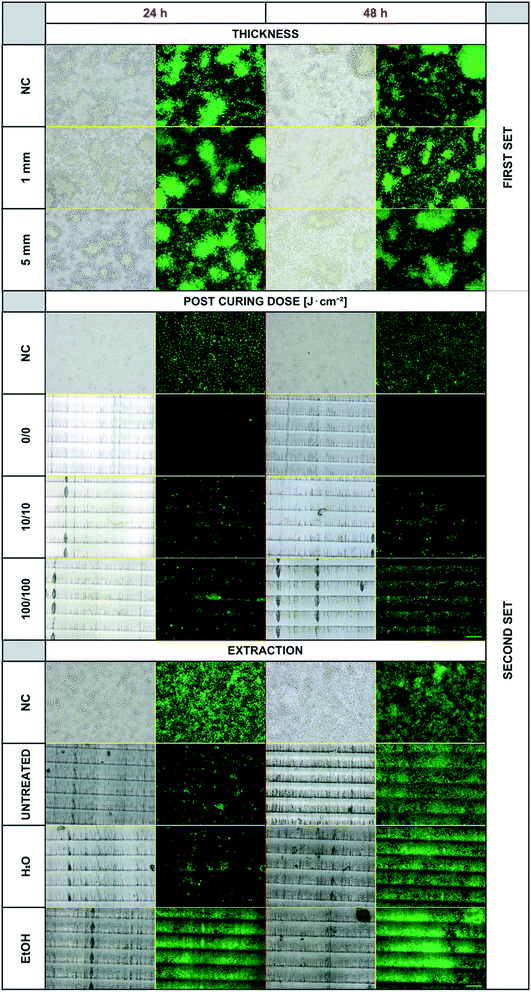
Experiments were first performed with the first set of 3D-printed specimens. Even though a negative effect of the photopolymer's presence in the cell culturing medium on the cell viability was expected (particularly in the case of thicker objects), we did not observe any inhibition of cell proliferation or any differences in dead cell numbers in any of the samples. The results for the two examples of specimens' thickness (1 and 5 mm) compared to the negative controls are presented in Fig. 3 – upper panel. There are no noticeable differences in the proliferated cell counts in any of these experiments. The dose of UV light 250 J cm−2 used for post-curing seems to be sufficient for thorough polymerization of all the specimens independently of the thickness used. In this experimental setup, the cells were not forced to proliferate and grow directly on the photopolymer surface. Therefore, the experiment setup was changed by involving the second set of objects and lowering the post-curing UV dose (because a high dose of 250 J cm−2 and long exposure to UV light caused changes in the photopolymer's optical properties).Fig. 3 – middle panel shows the results of B14 cell cultivation using the second set of 3D-printed objects. These experiments confirmed that the cultured cells show growth inhibition if they are forced to grow on the surface of the photopolymer. This finding indicates the more complex release kinetics of the photopolymer's metabolically active agents. Secondly, the crucial requirement of post-curing was verified. Without any additional exposure to UV light after printing, almost no cells proliferated on the model's surface during the first 24 hours, and no living cells were observed after 48 hours of incubation. The situation improved with post-curing by using a relatively small dose of 10 J cm−2 from both sides, and the difference was even more noticeable for the dose of 100 J cm−2, particularly after 48 hours of incubation.
According to the manufacturer's instructions for the use of E-Shell 300, 2000–4000 flashes in regard to the shape of the printed specimen are recommended. The Otoflash G171 operates at 10 flashes per second, which equals 200–400 seconds in semicontinuous UV light from two lamps with a total power of 200 W. For the comparison, in our case, 10 J cm−2 corresponds to 145 seconds and 100 J cm−2 to 1450 seconds of continuous UV light from a 1000 W power lamp of adequate wavelength distribution. Visible horizontal lines in the images are individual layers (steps) from a 3D printer and a result of the chosen printing orientation. The fact that the cells prefer specific areas of varying surface morphology is an interesting phenomenon, which will be further studied.
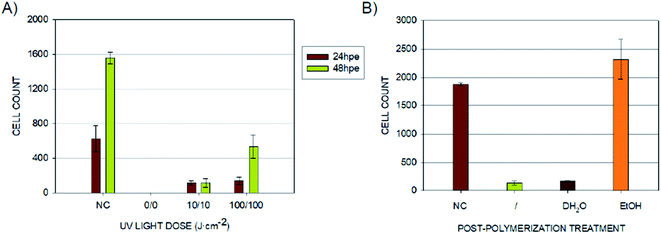
Because Carve et al. were able to significantly reduce the toxicity of the photopolymer Fototec 7150 by treating it with 99% ethanol and in the case of some polymeric materials, even distilled water was demonstrated to serve the same purpose,21 we followed a similar approach with E-Shell 300. Bathing the printed specimens for 2 × 24 hours in distilled water did not provide any improvement when compared to untreated samples. On the other hand, using a 96% ethanol bath dramatically increased the proliferation of B14 cells within the first 24 hours (Fig. 3 and 5).
These results show that, particularly in the case of the direct contact between the living cells and the photopolymer, E-Shell 300 can cause substantial cell proliferation inhibition in the first 24 hours of cultivation if the correct post-treatment after 3D printing itself is not performed; this finding corresponds well with other works published in this area.15,21,24,30 The dose of UV light used for post-curing of 3D-printed objects seems to play a key role in the ability of living cells to proliferate on such surfaces. Insufficient doses could potentially result in a leakage of toxic residues into the cultivation media or on the object surface, potentially then prohibiting the cells from proliferating and growing on the surface. The presented results further indicate that the mentioned residues can be pre-washed away by treating the printed specimens in concentrated ethanol, which was demonstrated to improve cell proliferation significantly (Fig. 4B). Interestingly, the leakage of the potentially toxic compounds from the 3D-printed objects to the leachates was not sufficient to inhibit the B14 cell proliferation when used as media for conventional cultivation well-plates. This fact should be considered when designing an appropriate format for the valid cytotoxicity test for 3D-printed materials, which are supposed to come in direct contact with living cells and tissues.
3.3 FET test
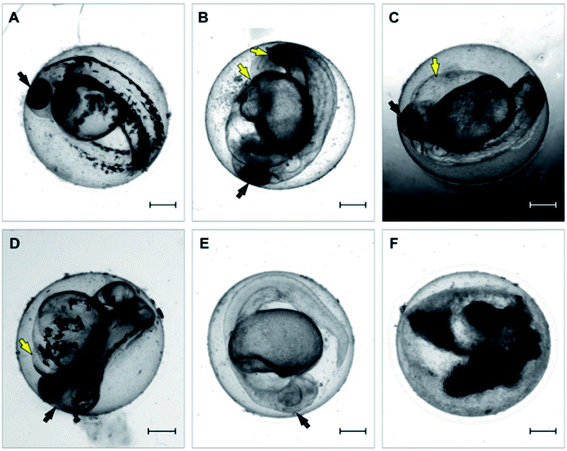
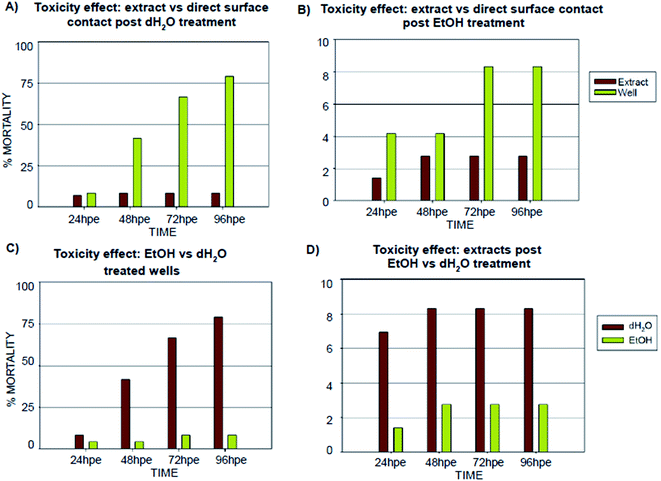
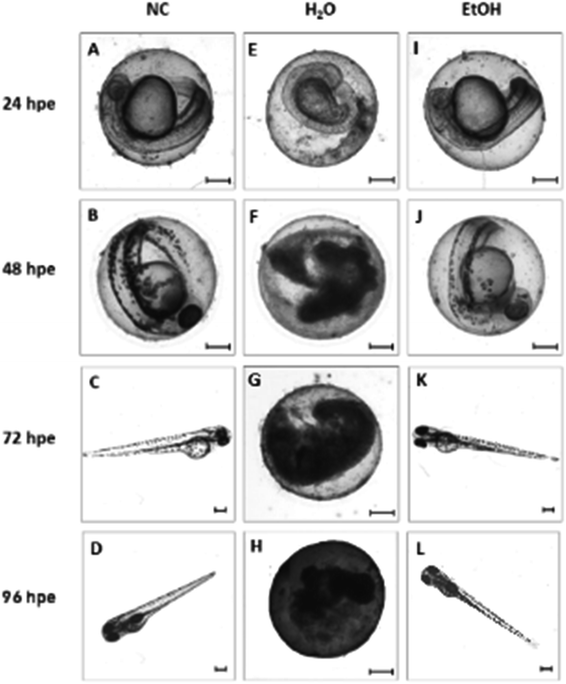
Inspired by the results of the in vitro experiments showing remarkable differences in cell growth between the use of extracts (leachates) when compared to direct contact with 3D-printed material, we followed a similar experimental setup with Danio rerio embryos, with the aim of determining whether this effect would be similar in vivo. The results obtained from embryo cultivations in extracts (leachates) and from cultivation experiments where the direct contact between embryo and the specimen surface was enabled are summarized in Fig. 5 and 6.According to the in vitro experiments, we observed similar differences in embryo viability between the two types of samples (leachate/extract vs. direct surface contact). Whereas the tests of extracts (type A sample) demonstrated a mortality level comparable to the negative controls in all of the extracts, the direct contact of embryos with the tested material (type B sample) resulted in enhanced developmental toxicity. Up to 79% of embryo mortality was observed at 96 hpe (Fig. 5A). The observed mortality endpoints differed substantially between these two sample types. The extracts caused mainly growth retardation (50% of overall morphological effects), whereas direct contact with the material surface caused severe malformations or embryo coagulation. These findings support the idea of a toxic effect on the early developmental stage, particularly gastrulation. The impact of a teratogenic effect causing malformations is high depending on the embryonic stage. The most critical and most sensitive phase of development is around 24 hpf, at the time of organogenesis.42 At this stage, a very rapid differentiation occurs, and due to the considerable degree of cell proliferation, cells are more susceptible to teratogenic factors, and coagulation and structural malformations occur. It should be noted here that plastic polymers are not typically regarded as toxic, and their eventual toxic effect is attributed to the release of residues, chemical additives, or unpolymerized particles. Current toxicity tests of plastic polymers are commonly based on testing of the extracts only.43,44 The ZET™ Medical Device Polymer Biocompatibility Screen Test developed at Microtest Laboratories, which also uses Danio rerio embryos as a model system, is designed by medical device manufacturers and bio-material researchers. ISO guideline 10993 supports the ZET™ test for biocompatibility screening and is based solely on polymer extracts.45 Our results bring attention to reconsideration of whether objective information on biocompatibility is obtained by such tests where no direct contact of the material with the organism is tested. Unfortunately, the lack of similar studies does not allow a comparison of results by the previously described methods of toxicity testing.
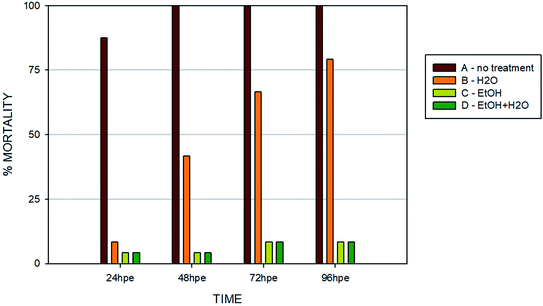
Another set of experiments was performed to evaluate the effect of different post-curing treatments of the polymer on embryos in direct contact with the surface (type B sample) (Fig. 7). Four different extracts were tested: (i) no treatment after polymerization; (ii) leaching in dH2O; (iii) leaching in 96% EtOH; (iv) leaching in EtOH + dH2O. The printing process and UV light dose was the same for all of the specimens (50 J cm−2). Similarly with the experiments performed in vitro, we observed that biocompatibility can be increased by appropriate methods of post-curing treatment.
Without additional treatment, the mortality reaches 100% as early as at 48 hpe (Fig. 7). Such “raw” printed material is extremely harmful to correct embryonic development. The post-curing leaching in the dH2O has some effect in reducing the toxicity but is still not sufficient to eliminate it completely (mortality reached 79% at 96 hpe). On the contrary, we observed a significant reduction of toxicity simply by rinsing the printed specimens in 96% ethanol, thus reducing the mortality to approx. 8.5% at 96 hpe. Similar values were obtained for the combination of subsequent rinsing in 96% ethanol and dH2O (also 8.3% of mortality); however, we observed a difference in the success of hatching from the chorion. Whereas leaching in ethanol resulted in successful hatching of 82% of surviving embryos, additional rinsing with water increased the hatching success to 100%. According to the literature, it is possible that the ethanol residues delay hatching.46 Although the effect of alcohol is often associated with embryonic pericardial edema,47 this endpoint was not observed in this experimental setup, most likely due to thorough drying to remove residual alcohol before use of the specimen. As shown in Fig. 6B and D, 96% ethanol-treated samples in direct contact with embryos showed a similarly low mortality (up to 48 hpe) as compared to the extracts (type A sample). Leaches obtained from ethanol-treated specimens were also less toxic (mortality approx. 2.8% at 96 hpe) than leaches prepared from dH2O-treated samples (mortality 8.2% at 96 hpe).
The results from in vitro and in vivo assays involving various post-polymerization treatments compared well. With regard to in vitro cultures, it can be noted that the release of toxicants from the photopolymer occurs within a relatively short time period (first 24 hours). There was almost no visible proliferation of cells incubated on non-treated specimens during the first 24 hours, which corresponds to the results from in vivo assays, where the mortality in 24 hpe reached almost 88%. However, these early-released toxicants were most likely metabolized or otherwise removed by the cells because at 48 hours post-exposure, proliferation was fully restored. This is a trend that cannot be observed in in vivo tests because disruption of the equilibrium in the early stages of embryonic development results in lethality. On the contrary, the development of fish embryos cultivated in the printed specimens treated by dH2O was comparable to the negative control after the first 24 hours. However, significant developmental defects were observed after 48 hours of incubation.
3.4 Identifying toxic residues
Based on a material safety data sheet (MSDS) provided by the manufacturer, four substances contained in E-Shell 300 have been classified as hazardous: (i) acrylic resin as a base material, (ii) tetrahydrofurfuryl methacrylate (THFMA), for which the LC50 value tested on Pimephales promelas reaches 34.7 mg l−1, on rat 4000 mg kg−1; (ii) urethane dimethacrylate (UDMA), for which the LC50 value was set at 10.1 mg l−1 (Danio rerio) and >2000 mg kg−1 (rat), and (iii) monoacylphosphine oxide-based photoinitiator diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide (TPO), for which the LC50 tested on Daphnia is 3.53 mg l−1 and on rat >5000 mg kg−1. However, E-Shell 300 and other resins for SLA may comprise more than 20 substances.21,28,49 We decided to conduct several qualitative chemical analyses of extracted solutions because the repeated 24 hour extraction of the UV-hardened polymer helped us to overcome the problem with the high cytotoxicity of the 3D-printed product.Based on 1H NMR and in accordance with data collected from ESI MS of ethanol leachate (see further) we conclude that Triton™ X-100 carboxylate and Triton™ X-100 terminal alkene group modified surfactants could be leaching from the cured polymer objects and causing observed issues. A study concerning ecotoxicity and estrogeno-mimetic activity of the alkylphenol polyethylene glycols was published in 1994.40 The synthesis and characterization of various carboxylic and other Triton derivatives as endocrine disruptors identical to some of Triton derivatives which occur after UV irradiation was published in Japanese in 2007.48 The UV decomposition products of Triton X-100 were recently recognized more toxic for aquatic organisms (Daphnia magna, Aliivibrio fischeri) than initial Triton.49
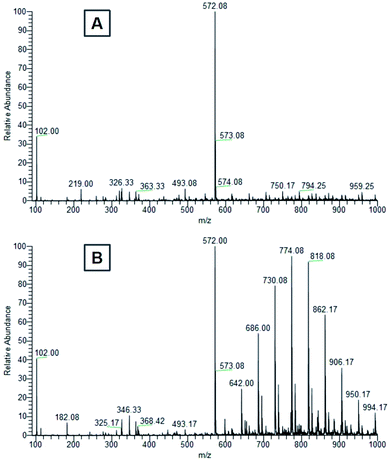 | ||
| Fig. 10 Mass spectra of (A) distilled water and (B) ethanol leachates from the first 24 hours of cured E-Shell 300 objects extraction. | ||
The peak of similar position and intensity was also present in ethanol leachate (Fig. 10B). Moreover, it was accompanied by a set of characteristic repetition peaks (m/z ∼ 44 Da) of some polyethylene glycol derivative indicating the presence of other Triton derivatives. We identified these peaks as one-time double bond terminally alkene decorated derivatives of Triton X-100 mixture. Obtained peaks correspond nicely both with masses of the most probable products (Table 1) calculated using Thermo Scientific Xcalibur software as well as with the shape of the mass spectrum.51 It is not clear to us, why the Triton X-100 containing seven ethylene glycol units prefer to react under UV to terminal carboxylic acid (soluble in water, also in ethanol) and other derivatives containing more than seven units reach in the degradation elimination of the terminal –OH group to alkene.
| Chem. formula | Calculated mass | Detected in ethanol | Detected in water |
|---|---|---|---|
| (–CH2CH2O–)7(–COOH) | 572.73 | 572.00; 573.08 | 572.00; 573.08 |
(–CH2CH2O–)8(–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) CH2) |
598.41 | 598.00 | — |
(–CH2CH2O–)9(–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) CH2) |
642.43 | 642.00 | — |
(–CH2CH2O–)10(–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) CH2) |
686.46 | 686.00 | — |
(–CH2CH2O–)11(–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) CH2) |
730.49 | 730.08 | — |
(–CH2CH2O–)12(–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) CH2) |
774.51 | 774.08 | — |
(–CH2CH2O–)13(–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) CH2) |
818.54 | 818.04 | — |
(–CH2CH2O–)14(–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) CH2) |
862.56 | 862.17 | — |
(–CH2CH2O–)15(–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) CH2) |
906.59 | 906.17 | — |
(–CH2CH2O–)16(–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) CH2) |
950.62 | 950.17 | — |
(–CH2CH2O–)17(–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) CH2) |
994.64 | 994.17 | — |
Levels of these toxic residues were significantly reduced especially by ethanol as a selective extraction solvent, and the purified material was finally identified to be more friendly/compatible with both cultivated cells and Danio rerio after completion of this very easy procedure. Mass spectra (Fig. 10) document that water is for the extraction of more cytotoxic Triton derivatives not as effective as ethanol. Even though one significant Triton peak was detected in the water leachate, higher molecular mass variants were not present. On the other hand, all variants of Triton in a measured range were able to be extracted from the cured polymer into ethanol. Hence, most of the harmful surfactant could be extracted by ethanol, whereas a limited amount remained present in the distilled water. These findings correspond well with toxicity experiments and explain why ethanol treatment was so effective at reducing the photopolymer's negative effects on our biological models, why the water treatment helped only partially and also why water extracts themselves were not particularly harmful to the biological models.
According to our results, the approach to cytotoxicity testing of the material plays a crucial role in a potential outcome. The data obtained from in vitro assays, where the test sample was a water extract of the tested polymer, are significantly different from the results obtained from incubation of the cells in direct contact with the surface of the material. This finding raises the question whether the emerging industrially-produced materials for 3D printing are properly tested. The photopolymer E-Shell 300 is a material commonly used for fabrication of medical devices (hearing aid shells and otoplastics) mainly due to the declared class IIa biocompatibility according to ISO 10993.52
Our results are also in accordance with the growing evidence of relatively high toxicity risks of 3D-printed materials and show the importance of correctly testing their toxicity. Ngan et al.38 tested the biocompatibility of the photopolymer MED610 and, similarly to us, presented a comparison of in vitro and in vivo testing. The primary myoblasts as a cell culture model were used. In addition to 2D cultures, 3D cell structures cultivated in hydrogel were also analyzed. The cell culture was derived from the limbs of the rat C57BL/6, which also served as an in vivo model. Post-printing treatment based on leaching with Milli-Q water, isopropyl alcohol, or 80% EtOH was studied. The manufacturer's recommended procedure and its adapted version, involving sonication as the current leaching process, were also compared.53 The results showed markedly poorer proliferation of cells exposed to MED610 cleaned according to the manufacturer's protocol, in contrast to cells exposed to MED610 cleaned with a sonication protocol. In vivo assays were based on the implantation of 3D-printed discs under the skin of rats. Implants treated according to the manufacturer caused significantly more FBGCs (foreign-body giant cells) surrounding the implantation site.54 Alifui-Segbaya and George published the results of a biocompatibility test of the related material E-Shell 450 also by using a FET test according to the OECD.55 Similarly to us, they observed a mortality reduction of 65% for the alcohol-treated sample versus the untreated one (analogous to our dH2O treatment). In addition, they report reduced pigmentation when the sample was treated with ethanol. We also observed this trend in our test, but reduced pigmentation was not considered an endpoint in our assay. Ferraz et al. published toxicity tests performed with five different polymers (E-Shell 200, E-Shell 300, PIC100, polystyrene, and PDMS) using an early bovine embryo development test. Both E-Shells (200 and 300) showed strong inhibition of embryonic development, which was in correlation with increasing amounts of diethyl phthalate and polyethylene glycol as determined by mass spectrometry. All tested polymers, except polyester, were shown to induce estrogen receptor transactivation.26 Macdonald et al. performed a toxicity study of Fototec 7150 photopolymer on Danio rerio embryos. Similarly to our results, he observed a reduction of mortality by more than 80% as compared to a control sample (unwashed polymer) when the photopolymer material was washed in 99% ethanol.15
As discussed above, our results fit well with the recent observations of relatively high in vitro and in vivo toxicity of 3D-printed materials and contribute to the identification of their potential risks. Our study also suggests a simple solution to overcome the potential toxicity by appropriate post-printing treatments. Our work highlighted the significant discrepancy in data obtained by various approaches to toxicity testing (extracts vs. direct surface contact), which should be considered when developing, for example, 3D-printed microfluidic devices for biomedicine applications.
4. Conclusions
The toxicity of the commercially available E-Shell 300 photopolymer, which is commonly used in the manufacture of hearing aids and other implants and which could also be potentially exploited in the fabrication of microfluidic devices, was tested using in vivo and in vitro biological models. We studied B14 cell proliferation in direct contact with the 3D-printed material as well as in water extracts to evaluate the cytotoxicity in vitro. Similarly, in vivo tests were performed using an OECD-standardized fish embryo acute toxicity (FET) test on Danio rerio embryos in direct contact with the surface of the material and in extracts as well. In the case of direct contact with both biological models, the results showed a considerable negative influence on cell proliferation (≈16% cell number as compared to control sample in first 24 hours of cultivation). Post-print leaching of the photopolymer in 96% ethanol increased the cell proliferation to values comparable to the negative control. A toxicity effect on cells was negligible as compared to control samples in the case of cultivation in leachates from 3D-printed objects/specimens. In vivo toxicity tests matched the in vitro results in most aspects. FET tests on Danio rerio demonstrated the considerable importance of testing the polymer in direct contact with the model organism. Whereas tests of leachates caused a mortality of lower than 10%, direct contact with the surface of the tested material led to a 79% mortality at 96 hpe. The post-print leaching of photopolymer in 96% ethanol reduced the mortality by more than 71% at 96 hpe. These results were further explained using mass spectrometry of ethanol and water leachates, demonstrating the presence of industrial surfactant. Our results agree with the growing evidence of the relatively high toxicity risks of 3D-printed materials and show the importance of carrying out appropriate toxicity assessments. The discovered source of material's toxicity differs from the harmful components described in the E-Shell 300 safety data sheet. Nevertheless, our findings also illustrate that the E-Shell 300 photopolymer could be successfully applied in the fabrication of microfluidic devices for biomedical applications if an appropriate post-printing treatment of the 3D-printed material is used.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the assistance provided by the Research Infrastructure NanoEnviCz (Project No. LM 2018124) supported by the Ministry of Education, Youth and Sports of the Czech Republic and the European Union – European Structural and Investments Funds in the frame of the Operational Programme Research Development and Education, the ERDF/ESF project “UniQSurf – Centre of Biointerfaces and Hybrid Functional Materials” (No. CZ.02.1.01/0.0/0.0/17_048/0007411), the project no. TJ01000077 of the Technology Agency of the Czech Republic, the project no. UJEP-IGA-TC-2019-53-01-2; UJEP-SGS-2018-53-005-3 and UJEP-SGS-2020-53-006-3.References
- T. Matsuda and M. Mizutani, Liquid acrylate-endcapped biodegradable poly(epsilon-caprolactone-co-trimethylene carbonate). II. Computer-aided stereolithographic microarchitectural surface photoconstructs, J. Biomed. Mater. Res., 2002, 62, 395–403, DOI:10.1002/jbm.10295.
- J. Y. Wong and A. C. Pfahnl, 3D Printing of Surgical Instruments for Long-Duration Space Missions, Aviat., Space Environ. Med., 2014, 85, 758–763, DOI:10.3357/ASEM.3898.2014.
- R. L. Boyd, R. Miller and V. Vlaskalic, The Invisalign system in adult orthodontics: mild crowding and space closure cases, J. Clin. Orthod., 2000, 203–212 Search PubMed.
- T. M. Binder, D. Moertl, G. Mundigler, G. Rehak, M. Franke, G. Delle-Karth, W. Mohl, H. Baumgartner and G. Maurer, Stereolithographic biomodeling to create tangible hard copies of cardiac structures from echocardiographic data, J. Am. Coll. Cardiol., 2000, 35, 230–237, DOI:10.1016/S0735-1097(99)00498-2.
- K. Murphy, S. Dorfman, R. J. Law and V. A. Le, Devices, systems, and methods for the fabrication of tissue utilizing UV cross-linking, US Pat., US10201964B2, 2019 Search PubMed.
- N. Bhattacharjee, A. Urrios, S. Kang and A. Folch, The upcoming 3D-printing revolution in microfluidics, Lab Chip, 2016, 16, 1720–1742, 10.1039/C6LC00163G.
- S. A. M. Tofail, E. P. Koumoulos, A. Bandyopadhyay, S. Bose, L. O'Donoghue and C. Charitidis, Additive manufacturing: scientific and technological challenges, market uptake and opportunities, Mater. Today, 2018, 21, 22–37, DOI:10.1016/j.mattod.2017.07.001.
- Z.-X. Low, Y. T. Chua, B. M. Ray, D. Mattia, I. S. Metcalfe and D. A. Patterson, Perspective on 3D printing of separation membranes and comparison to related unconventional fabrication techniques, J. Membr. Sci., 2017, 523, 596–613, DOI:10.1016/j.memsci.2016.10.006.
- A. Muller and S. Karevska, How 3D Printing Technology Could Change World Trade, in EY's Glob. 3D Print. Rep. 2016, 2016, https://www.ey.com/Publication/vwLUAssets/ey-global-3d-printing-report-2016-fullreport/$FILE/ey-global-3d-printing-report-2016-full-report.pdf Search PubMed.
- B. H. Cumpston, S. P. Ananthavel, S. Barlow, D. L. Dyer, J. E. Ehrlich, L. L. Erskine, A. A. Heikal, S. M. Kuebler, I.-Y. S. Lee, D. McCord-Maughon, J. Qin, H. Röckel, M. Rumi, X.-L. Wu, S. R. Marder and J. W. Perry, Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication, Nature, 1999, 398, 51–54, DOI:10.1038/17989.
- A. T. Monstad-Rios, C. J. Watson and R. Y. Kwon, ScreenCube: A 3D Printed System for Rapid and Cost-Effective Chemical Screening in Adult Zebrafish, Zebrafish, 2018, 15, 1–8, DOI:10.1089/zeb.2017.1488.
- J. N. Wittbrodt, U. Liebel and J. Gehrig, Generation of orientation tools for automated zebrafish screening assays using desktop 3D printing, BMC Biotechnol., 2014, 14, 36, DOI:10.1186/1472-6750-14-36.
- Z. Tan, T. Liu, J. Zhong, Y. Yang and W. Tan, Control of cell growth on 3D-printed cell culture platforms for tissue engineering, J. Biomed. Mater. Res., Part A, 2017, 105, 3281–3292, DOI:10.1002/jbm.a.36188.
- L. J. Y. Ong, A. Islam, R. DasGupta, N. G. Iyer, H. L. Leo and Y.-C. Toh, A 3D printed microfluidic perfusion device for multicellular spheroid cultures, Biofabrication, 2017, 9, 045005, DOI:10.1088/1758-5090/aa8858.
- N. P. Macdonald, F. Zhu, C. J. Hall, J. Reboud, P. S. Crosier, E. E. Patton, D. Wlodkowic and J. M. Cooper, Assessment of biocompatibility of 3D printed photopolymers using zebrafish embryo toxicity assays, Lab Chip, 2016, 16, 291–297, 10.1039/C5LC01374G.
- S. M. Oskui, G. Diamante, C. Liao, W. Shi, J. Gan, D. Schlenk and W. H. Grover, Assessing and Reducing the Toxicity of 3D-Printed Parts, Environ. Sci. Technol. Lett., 2016, 3, 1–6, DOI:10.1021/acs.estlett.5b00249.
- F. Zhu, T. Friedrich, D. Nugegoda, J. Kaslin and D. Wlodkowic, Assessment of the biocompatibility of three-dimensional-printed polymers using multispecies toxicity tests, Biomicrofluidics, 2015, 9, 061103, DOI:10.1063/1.4939031.
- E. Schmelzer, P. Over, B. Gridelli and J. C. Gerlach, Response of Primary Human Bone Marrow Mesenchymal Stromal Cells and Dermal Keratinocytes to Thermal Printer Materials In Vitro, J. Med. Biol. Eng., 2016, 36, 153–167, DOI:10.1007/s40846-016-0118-z.
- V. K. Popov, A. V. Evseev, A. L. Ivanov, V. V. Roginski, A. I. Volozhin and S. M. Howdle, Laser stereolithography and supercritical fluid processing for custom-designed implant fabrication, J. Mater. Sci.: Mater. Med., 2004, 15, 123–128, DOI:10.1023/B:JMSM.0000011812.08185.2a.
- P. S. D'Urso, D. J. Effeney, W. J. Earwaker, T. M. Barker, M. J. Redmond, R. G. Thompson and F. H. Tomlinson, Custom cranioplasty using stereolithography and acrylic, Br. J. Plast. Surg., 2000, 53, 200–204, DOI:10.1054/bjps.1999.3268.
- M. Carve and D. Wlodkowic, 3D-Printed Chips: Compatibility of Additive Manufacturing Photopolymeric Substrata with Biological Applications, Micromachines, 2018, 9, 91, DOI:10.3390/mi9020091.
- F. Zhu, J. Skommer, N. P. Macdonald, T. Friedrich, J. Kaslin and D. Wlodkowic, Three-dimensional printed millifluidic devices for zebrafish embryo tests, Biomicrofluidics, 2015, 9, 046502, DOI:10.1063/1.4927379.
- D. Lithner, Å. Larsson and G. Dave, Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition, Sci. Total Environ., 2011, 409, 3309–3324, DOI:10.1016/j.scitotenv.2011.04.038.
- W. Geurtsen, Biocompatibility of Resin-Modified Filling Materials, Crit. Rev. Oral Biol. Med., 2000, 11, 333–355, DOI:10.1177/10454411000110030401.
- J. L. Ferracane and J. R. Condon, Post-cure heat treatments for composites: properties and fractography, Dent. Mater., 1992, 8, 290–295, DOI:10.1016/0109-5641(92)90102-I.
- M. de Almeida Monteiro Melo Ferraz, H. H. W. Henning, P. Ferreira da Costa, J. Malda, S. Le Gac, F. Bray, M. B. M. van Duursen, J. F. Brouwers, C. H. A. van de Lest, I. Bertijn, L. Kraneburg, P. L. A. M. Vos, T. A. E. Stout and B. M. Gadella, Potential Health and Environmental Risks of Three-Dimensional Engineered Polymers, Environ. Sci. Technol. Lett., 2018, 5, 80–85, DOI:10.1021/acs.estlett.7b00495.
- Y. Inoue and K. Ikuta, Detoxification of the Photocurable Polymer by Heat Treatment for Microstereolithography, Procedia CIRP, 2013, 5, 115–118, DOI:10.1016/j.procir.2013.01.023.
- H. Liu and C. He, Additive use in photopolymer resin for 3D printing to enhance the appearance of printed parts, US Pat., US9574039B1, 2017, http://www.freepatentsonline.com/9574039.html Search PubMed.
- B. S. Jiemin, C. Kennedy and A. Lickhus, Three-dimensional fabricating material systems for producing dental products, WO/2014/078537, 2014.
- G. Schmalz and D. Arenholt-Bindslev, Biocompatibility of dental materials, Springer, Berlin, 2009 Search PubMed.
- S. van den Driesche, F. Lucklum, F. Bunge and M. Vellekoop, 3D Printing Solutions for Microfluidic Chip-To-World Connections, Micromachines, 2018, 9, 71, DOI:10.3390/mi9020071.
- W. S. Rasband, ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA, 1997–2016 Search PubMed.
- OECD, Test No. 236: Fish Embryo Acute Toxicity (FET) Test, OECD, 2013, DOI:10.1787/9789264203709-en.
- R. Nagel, DarT: the embryo test with the zebrafish Danio rerio--a general model in ecotoxicology and toxicology, ALTEX, 2002, 19(suppl 1), 38–48 Search PubMed.
- S. Padilla and S. Glaberman, The zebrafish (Danio rerio) model in toxicity testing, in Introd. Interdiscip. Toxicol., Elsevier, 2020, pp. 525–532, DOI:10.1016/B978-0-12-813602-7.00037-5.
- Z. Dang, L. T. M. van der Ven and A. S. Kienhuis, Fish embryo toxicity test, threshold approach, and moribund as approaches to implement 3R principles to the acute fish toxicity test, Chemosphere, 2017, 186, 677–685, DOI:10.1016/j.chemosphere.2017.08.047.
- M. Entzeroth, H. Flotow and P. Condron, Overview of High-Throughput Screening, Curr. Protoc. Pharmacol., 2009, 44(9) DOI:10.1002/0471141755.ph0904s44.
- R. Sever, et al., E3 medium (for zebrafish embryos), Cold Spring Harb. Protoc., 2011, 2011(10) DOI:10.1101/pdb.rec066449.
- F. Zhu, Development of lab-on-a-chip devices for automated zebrafish embryo bioassay, Doctor of Philosophy (PhD), RMIT University, 2016, http://researchbank.rmit.edu.au/view/rmit:161664.
- R. White, S. Jobling, S. A. Hoare, J. P. Sumpter and M. G. Parker, Environmentally persistent alkylphenolic compounds are estrogenic, Endocrinology, 1994, 135(1), 175–182, DOI:10.1210/endo.135.1.8013351.
- SigmaPlot 10.0, Systat Software, Inc., San Jose, California, U.S., www.systsoftware.com Search PubMed.
- A. D. Dayan, General and Applied Toxicology, 2nd edition, Occup. Environ. Med., 2000, 57, 431d–4431, DOI:10.1136/oem.57.6.431d.
- D. Lithner, I. Nordensvan and G. Dave, Comparative acute toxicity of leachates from plastic products made of polypropylene, polyethylene, PVC, acrylonitrile–butadiene–styrene, and epoxy to Daphnia magna, Environ. Sci. Pollut. Res., 2012, 19, 1763–1772, DOI:10.1007/s11356-011-0663-5.
- S. Bejgarn, M. MacLeod, C. Bogdal and M. Breitholtz, Toxicity of leachate from weathering plastics: an exploratory screening study with Nitocra spinipes, Chemosphere, 2015, 132, 114–119, DOI:10.1016/j.chemosphere.2015.03.010.
- B. Michaels, A New Biocompatibility Test: Zebrafish Embryo Toxicity Testing, Med. Device Diagn. Ind., 2014, https://www.mddionline.com/new-biocompatibility-test-zebrafish-embryo-toxicity-testing Search PubMed.
- A. H. Lutte, K. M. Capiotti, N. L. G. da Silva, C. S. d. O. da Silva, L. W. Kist, M. R. Bogo and R. S. D. Silva, Contributions from extracellular sources of adenosine to the ethanol toxicity in zebrafish larvae, Reprod. Toxicol., 2015, 53, 82–91, DOI:10.1016/j.reprotox.2015.04.001.
- M. J. Reimers, A. R. Flockton and R. L. Tanguay, Ethanol- and acetaldehyde-mediated developmental toxicity in zebrafish, Neurotoxicol. Teratol., 2004, 26, 769–781, DOI:10.1016/j.ntt.2004.06.012.
- T. Toyokawa, et al., Synthesis of chemicals related to environmental endocrine disruptors, Kyushu Kyoritsu Daigaku Kogakubu Kenkyu Hokoku, 2007, 2007(31), 1–8 Search PubMed.
- E. H. Jho, S. H. Yun, P. Thapa and J.-W. Nam, Changes in the aquatic ecotoxicological effects of Triton X-100 after UV photodegradation, Environ. Sci. Pollut. Res., 2021, 28(9), 11224–11232, DOI:10.1007/s11356-020-11362-2.
- B. O. Keller, J. Sui, A. B. Young and R. M. Whittal, Interferences and contaminants encountered in modern mass spectrometry, Anal. Chim. Acta, 2008, 627(1), 71–81, DOI:10.1016/j.aca.2008.04.043.
- LCO Fleet Tune plus and Qual Browser, Thermo Fisher Scientific, Inc., 1998.
- International Organization for Standardization, Biological evaluation of medical devices — Part 18: Chemical characterization of medical device materials within a risk management process, 2020, https://www.iso.org/standard/64750.html Search PubMed.
- A. L. Coats, J. P. Harrison, J. S. Hay and M. J. Ramos, Stereolithography resins and methods, US Pat., 7211368, 2007 Search PubMed.
- C. G. Y. Ngan, C. D. O'Connell, R. Blanchard, M. Boyd-Moss, R. J. Williams, J. Bourke, A. Quigley, P. McKelvie, R. M. I. Kapsa and P. F. M. Choong, Optimising the biocompatibility of 3D printed photopolymer constructs in vitro and in vivo, Biomed. Mater., 2019, 14, 035007, DOI:10.1088/1748-605X/ab09c4.
- F. Alifui-Segbaya and R. George, Biocompatibility of 3D-Printed Methacrylate for Hearing Devices, Inventions, 2018, 3, 52, DOI:10.3390/inventions3030052.
| This journal is © The Royal Society of Chemistry 2021 |