Open Access Article
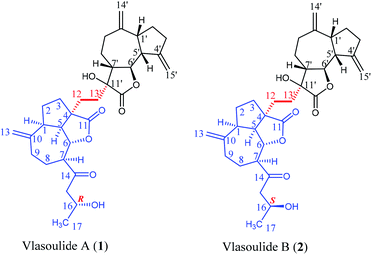
Open Access ArticleVlasoulides A and B, a pair of neuroprotective C32 dimeric sesquiterpenes with a hexacyclic 5/7/5/5/(5)/7 carbon skeleton from the roots of Vladimiria souliei†
Zhi-li Wu ab,
Qun Wangb,
Lu Fub,
Ri-xin Sub,
Ze-shi Sunb and
Hui-liang Li*ab
ab,
Qun Wangb,
Lu Fub,
Ri-xin Sub,
Ze-shi Sunb and
Hui-liang Li*ab
aSchool of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing, Jiangsu 211198, P. R. China. E-mail: faranli@hotmail.com
bSchool of Pharmacy, Second Military Medical University, Shanghai 200433, P. R. China
First published on 3rd February 2021
Abstract
Vlasoulides A and B (1 and 2), a pair of epimeric C32 sesquiterpene lactone dimers, featuring a 5/7/5/5/(5)/7 ring system were isolated from the roots of Vladimiria souliei. Their chemical structures were determined by comprehensive analysis of spectroscopic data, including HRESIMS and 1D and 2D NMR spectroscopic data. Their absolute configurations were established by Mosher's method and ECD experiments. Furthermore, biological studies showed that compound 1 showed prominent neuroprotective effects against glutamate-induced neurotoxicity in PC-12 cells, with EC50 values of 13.54 ± 0.33 μM, while, the EC50 value of compound 2 is greater than 30 μM.
Introduction
The large genus Vladimiria belonging to the family Asteraceae comprises about 12 species. Most of them are widely distributed in East Asia, especially in the Sichuan Province, China.1,2 The roots are usually applied in traditional Chinese medicine to treat a number of diseases, showing antitumor, analgesic, anti-inflammatory, gastric ulcer resistant and antibacterial activities.3–6 Furthermore, according to previous phytochemical studies, they were found to contain many sesquiterpenes, steroids, phenylpropanoids, flavonoids, and triterpenes.7–9 Aiming to search for new and rare SLDs with unique skeletons and novel bioactivities from this genus Vladimiria, we investigated the chemical constituents of the roots of Vladimiria souliei. Over the past few years, some rare sesquiterpene lactone dimers (SLDs), with influence on NO production and the activation of NF-κB pathway,10–14 were isolated from the title plant. At the same time, we also have separated some rare dimeric sesquiterpenes exhibiting potential neuroprotection activity from Vladimiria souliei in our previous study.15,16 Therefore, as a continuing investigation on this plant, two C32 sesquiterpene dimers, vlasoulides A and B (1 and 2), were isolated from the same plant. Herein, we describe the isolation, structural elucidation and neuroprotective activities of two new compounds (Fig. 1).Experimental
General experimental procedure
Column chromatography (CC): silica gel H (10–40 μm; Marine Chemical Factory, Qingdao, P. R. China); MCI gel CHP-20P; Sephadex LH-20 (Pharmacia Fine Chemicals, Piscataway, NJ, USA); RP-C18 gel (40–63 μm; Daiso, Co, Japan) were used for column chromatography. TLC: silica gel plates, visualization by spraying with 10% H2SO4 in EtOH and dragendorff's reagent. Semi-preparative HPLC: Agilent 1260 series with a Zorbax SB-C18 (5 μM, 9.4 mm × 25 cm) column. Melting point: X-4B apparatus and was uncorrected. IR spectra: Bruker Vector 22 (KBr pellets). Optical rotation: Autopol VI (serial no. 90079, manufactured by Rudolph Research Analytical, Hackettstown, NJ). UV spectra were obtained by the DAD detector of HLPC (Agilent 1260). NMR Spectra: Bruker Ascend-500 spectrometer (500 MHz); δ in ppm with SiMe4 as internal standard. MS: Agilent MSD-Trap-XCT (for ESI) and Q-Tof micro mass spectrometer (for HR-ESI).Plant material
The whole plant of Vladimiria souliei was collected at Dajin, Sichuan Province of China, in October, 2019, and authenticated by Prof. Bao-Kang Huang of Second Medical Military University. Currently A voucher specimen (No. 20191001) is deposited in School of Pharmacy, Second Military Medical University.Extraction and isolation
The dried and chipped roots of V. souliei (50.0 kg) were extracted by maceration with 95% ethanol overnight at room temperature (3 × 60 L). After remove of solvent, the ethanol extract (5.60 kg) was partitioned between water and petroleum ether (PE)/ethyl acetate (EtOAc), successively, to give PE, EtOAc and water extracts. EtOAc extract (1.15 kg) was segmented by MCI column chromatography (MeOH/H2O, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 to 100
70 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to give 8 fractions (Fr. 1–8). Fraction 5 (52.5 g) was further isolated by ODS column chromatography (MeOH/H2O, 30
0) to give 8 fractions (Fr. 1–8). Fraction 5 (52.5 g) was further isolated by ODS column chromatography (MeOH/H2O, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 to 90
70 to 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to obtain 8 subfractions (Fr. 5.1–5.8). Subfraction 5.4 (5.5 g) was further purified by Sephadex LH-20 column chromatography (PE
10) to obtain 8 subfractions (Fr. 5.1–5.8). Subfraction 5.4 (5.5 g) was further purified by Sephadex LH-20 column chromatography (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc
EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 10
MeOH, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 6 subfractions (Fr. 5.5.1–5.5.6). Subfraction 5.5.4 (252 mg) was purified by semi-preparative RP-C18 HPLC (CH3CN/H2O, 70
1) to give 6 subfractions (Fr. 5.5.1–5.5.6). Subfraction 5.5.4 (252 mg) was purified by semi-preparative RP-C18 HPLC (CH3CN/H2O, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to produce compounds 1 (7.2 mg) and 2 (6.5 mg). Above all, compounds 1 (7.2 mg) and 2 (6.5 mg) were obtained.
30) to produce compounds 1 (7.2 mg) and 2 (6.5 mg). Above all, compounds 1 (7.2 mg) and 2 (6.5 mg) were obtained.
Compound characterization of 1 and 2
(R)-and (S)-MTPA esters of compounds 1 and 2
To each compounds 1 and 2 (each 1.5 mg) in pyridine-d5 (130 μL) was separately added (R)-(−)-MTPA (5 μL) and (S)-(+)-MTPA (5 μL) at room temperature, followed by stirring at 40 °C for 8 h, and each reaction mixture was transferred into a 1.7 mm NMR tube.Neuroprotection assay
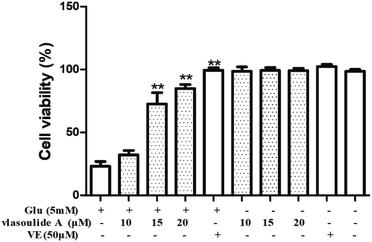
The PC-12 cells were purchased from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco's modified eagle's medium (DMEM) containing 10% FBS supplemented with 100 μg mL−1 penicillin and 100 μg mL−1 streptomycin in a humidified atmosphere containing 5% CO2 at 37 °C. Compounds 1 and 2 and vitamin E were dissolved in dimethyl sulfoxide (DMSO) and freshly prepared each time before use. Then, the PC-12 cells were seeded in 96-well culture plates at 8 × 103 cells per mL at 37 °C for 12 h. Then the cells were incubated with glutamate for an additional 24 h and the drugs (10, 15, 20 μM, respectively) were pretreated for 1 h before treated with glutamate. Cell viability was determined by the CCK8 assay, after treatment, 10 μL of CCK8 were added to each well and incubated at 37 °C for 4 h. The optical density (OD) was spectrophotometrically measured at 450 nm (CCK8) using a microplate reader, respectively (BioTek Instruments, Inc.).Results and discussion
Vlasoulide A (1) was isolated as white power, possessing the molecular formula C32H42O7 determined by positive HRESIMS m/z 561.2828 ([M + Na]+, calcd 561.2823), indicating 12 degrees of unsaturation.Comprehensive analysis of the 1H and 13C NMR spectra of 1, 32 carbon signals of the 13C NMR spectrum were classified by HSQC spectrum as one CH3, fourteen CH2 (including three sp2 carbons), nine CH (two oxygenated ones as well as one hydroxy methine), and eight quaternary carbons (assigned as two carbonyl groups, two sp3 and six sp2 carbons respectively) (ESI, Tables S1†). Therefore, through a detailed analysis of 1D NMR spectra of 1 indicated that the compound 1 should be a dimeric sesquiterpene lactone.
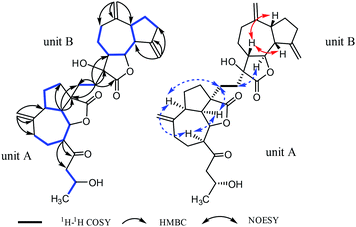
The planar structure of 1 was determined by the analysis of 1H–1H COSY and HMBC spectra. Two proton-bearing structural fragments: H-5/H-6/H-7/H2-8/H2-9 and H2-15/H-16/H3-17 were observed by analyzing the 1H–1H COSY spectra, together with the HMBC correlations of H2-13/C-1, C-9 and C-10; H-5/C-3 and C-12; H-7/C-14 and C-15 as depicted with arrows from H to C. The above conjectured that the unit A should be deduced as a ring-opening guaianolide moiety. Furthermore, a five-membered ring newly generated fused with the seven-membered ring and the lactone ring in unit A at C-1, C-4 and C-5 which revealed by the 1H–1H COSY correlations of H2-3/H2-2/H-1/H-5 and the HMBC cross-peaks from H-5 (δH 2.78) to C-3 and C-4 (Fig. 2). Meanwhile, the structure of unit B was further determined to be a guaianolide moiety, confirmed by 1H–1H COSY correlations of H2-3′/H2-2′/H-1′/H-5′/H-6′/H-7′/H2-8′/H2-9′ as well as the HMBC correlations of H2-14′/C-1′, C-9′ and C-10′; H2-15′/C-3′, C-4′ and C-5′; H2-13′/C-7′, C-11′ and C-12′ (Fig. 2). Finally, the units A and B were linked directly via a C–C bond between C-13′ and C-12 according to the 1H–1H COSY correlations of H2-13′/H2-12, combine with the HMBC correlations of H2-13′ (δH 1.81, 1.69, 1H, respectively)/C-12 (δC 30.5), C-4 (δC 54.3), C-7′ (δC 48.7), C-11′ (δC 76.7) and C-12′ (δC 176.8). Thus, the planar structure of 1 was determined as shown in Fig. 1.
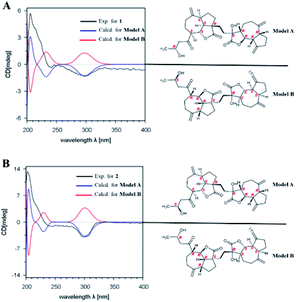
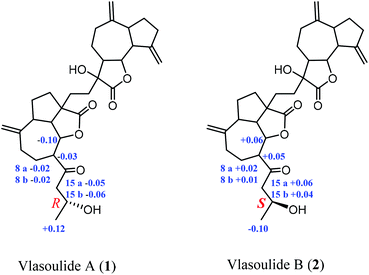
The relative configuration of 1 was established by the NOESY spectrum. H-1, H-5, H-7 and H-1′, H-5′, H-7′ in units A and B were on the same face and assigned as α-orientation and β-oriented in 1 based on the similar NOESY correlations of H-1/H-5/H-7 and H-1′/H-5′/H-7′. And, the H-6/H-7 and H-6′/H-7′ were in the trans-form due to the large coupling constant between H-6/H-7 (J = 9.8 Hz) and H-6′/H-7′ (J = 9.5 Hz) (Fig. 2). In addition, the relative configuration of C-11′ and C-4 were resolved by the NOESY correlations of H-6′/H2-13′ and H2-12/H-1/H-5 (Fig. 2), exhibiting that the CH2-13′ and CH2-12 were α-oriented. At the same time, by comparing the calculated and experimental ECD spectra, we found that the experimental ECD spectrum of compound 1 (Fig. 3A) was in good accordance with the calculated curve for the (1S,4R,5S,6S,7S,16R,1′R,5′R,6′R,7′R,11′S) stereoisomer. Additionally, the observed ΔδH(S–R) values of the (S)- and (R)-MTPA esters established the absolute configuration of C-16 in 1 as R17 (Fig. 4), which was consistent with the ECD results. Thus, the structure and absolute configuration of the vlasoulide A (1) was fully defined as shown in Fig. 1.
Interestingly, vlasoulide B (2), a 16-epimer of 1, was also an optically active white power. HRESIMS data at m/z 561.2829 ([M + Na]+, calcd 561.2823) of 2 implied that its molecular formula was C32H42O7, same as 1. The 1H and 13C NMR data of 2 resembled those of compound 1 (ESI, Tables S1†), suggesting the presence of ring-opening guaianolide and guaianolide units. The analysis of its 2D NMR data (1H–1H COSY and HMBC) revealed that the two compounds had the same 2D structure. Furthermore, inspecting the differences between 1 and 2 on NMR data we found the most obvious change was the C-15 and H2-15, the chemical shift of C-15 was down field shifted from δC 50.5 in 1 to δC 51.6 in 2, meanwhile, an obvious up field shifted of H2-15 from δH 2.67, 2.66 in 1 to δH 2.63, 2.62 in the 1H NMR was observe. Thus, these two new compounds represent a pair of stereoisomers possessing opposite configuration at C-16, which was identical to the ΔδH(S–R) results (Fig. 4). At the same time, the experimental ECD spectrum of 2 also fits well with the calculated spectrum of (1S,4R,5S,6S,7S,16S,1′R,5′R,6′R,7′R,11′S) 2 (Fig. 3B). Therefore, the structure of vlasoulide B (2) was established as shown in Fig. 1.
All the isolated compounds were evaluated for their neuroprotective effects against glutamate-induced neurotoxicity in PC-12 cells using CCK8 assay. Compound 1 showed prominent neuroprotective activity against glutamate-induced neurotoxicity in PC-12 cells, with EC50 value of 13.54 ± 0.33 μM. While, the EC50 values of the compound 2 is greater than 30 μM. Meanwhile, the neuroprotective effects were dose dependent and there were no significant adverse effects on the growth of PC-12 cells with compound 1 solo treatment as shown in Fig. 5. Collectively, these results suggest that compound 1 showed significant neuroprotective activity against glutamate-induced neurotoxicity in PC-12 cells at concentration of 20 μM.
Conclusions
In conclusion, vlasoulides A and B (1 and 2), two rare and original C32 sesquiterpenoid lactone dimers comprising of two sesquiterpene lactone units, have been isolated and elucidated for the first time from Vladimiria souliei. Moreover, compound 1 showed neuroprotective activity against glutamate-induced neurotoxicity in PC-12 cells, with EC50 value of 13.54 ± 0.33 μM. These results will supply scientific foundation for the scientific research of this plant, as well as might be greatly useful for studying on neuroprotection.Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
The work was supported by National Major Science and Technology Projects of China (2017ZX09301012-004, 2018ZX09711001-003-015), Professor of Chang Jiang Scholars Program, Shanghai Engineering Research Center for the Preparation of Bioactive Natural Products (16DZ2280200), the Scientific Foundation of Shanghai China (16401901600, 17401901700), the National Key Research and Development Program of China (2017YFC1700200).References
- Editorial commission of Traditional Chinese Medicine, State Administration of Traditional Chinese Medicine, Traditional Chinese Medicine, Shanghai Science & Technology Press, 1999, vol. 7, pp. 815–817 Search PubMed.
- J. J. Chen, D. Q. Fei, S. G. Chen and K. Gao, J. Nat. Prod., 2007, 18, 547–550 Search PubMed.
- J. J. Chen, H. B. Wei, Y. Z. Xu, J. Zeng and K. Gao, Planta Med., 2013, 79, 1470–1473 CrossRef CAS.
- Q. H. Wu, C. M. Liu, Y. J. Chen and K. Gao, Helv. Chim. Acta, 2006, 89, 915–922 CrossRef CAS.
- J. Xu, X. J. Zhao, Y. Q. Guo, W. B. Hou and S. Z. Zhang, Chin. Chem. Lett., 2009, 20, 1472–1474 CrossRef CAS.
- C. L. Li and J. Sheng, Adv. Mater. Res., 2013, 634, 901–904 Search PubMed.
- Z. G. Wang, X. R. Lan, Y. Y. Xiao and Y. Zhang, Chin. Pharm., 2006, 17, 303–304 CAS.
- J. J. Chen, W. Bai, F. R. Gobu, C. H. Wu, J. Zeng and M. Sun, J. Asian Nat. Prod. Res., 2015, 17, 1–8 CrossRef CAS.
- J. Xu, P. Zhang, Z. J. Ma, Y. Q. Guo, X. J. Zhao and K. Wei, Phytochem. Lett., 2009, 2, 204–206 CrossRef CAS.
- Y. X. Yang, S. Gao and H. L. Li, RSC Adv., 2015, 7, 31–40 Search PubMed.
- R. X. Tan, J. Jakupovic, F. Bohlmann and Z. J. Jia Schuster, Phytochemistry, 1990, 29, 1209–1212 CrossRef CAS.
- L. P. Chen, G. Z. Wu, J. P. Zhang and H. L. Li, Sci. Rep., 2017, 43837 CrossRef.
- Z. L. Wu, J. X. Wang, L. P. Chen, H. L. Li and W. D. Zhang, Fitoterapia, 2018, 125, 117–122 CrossRef CAS.
- H. Wei, G. Ma, Y. Peng, C. He and P. Xiao, Chemical Constituents of the Roots of Dolomiaea souliei, Chem. Nat. Compd., 2014, 37, 1249–1253 Search PubMed.
- Z. L. Wu, Q. Wang, H. Y. Dong, H. L. Li and W. D. Zhang, Fitoterapia, 2018, 128, 192–197 CrossRef CAS.
- Z. L. Wu, Q. Wang, J. X. Wang, X. K. Xu, Y. H. Shen, H. L. Li and W. D. Zhang, Org. Lett., 2018, 20, 7567–7570 CrossRef CAS.
- H. Zhang, L. T. Xu and D. M. Ren, et al., Chin. Chem. Lett., 2020, 31, 1259–1262 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra00075f |
| This journal is © The Royal Society of Chemistry 2021 |