Open Access Article
Open Access ArticleEnhanced gas separation and mechanical properties of fluorene-based thermal rearrangement copolymers
Jianhua Zhanga,
Yunhua Lu *a,
Guoyong Xiaoa,
Mengjie Houb,
Lin Lib and
Tonghua Wang*b
*a,
Guoyong Xiaoa,
Mengjie Houb,
Lin Lib and
Tonghua Wang*b
aSchool of Chemical Engineering, University of Science and Technology Liaoning, Anshan, Liaoning 114051, P. R. China. E-mail: lee.lyh@163.com; Fax: +86 412 5216702; Tel: +86 412 5929952
bSchool of Chemical Engineering, Dalian University of Technology, Dalian, Liaoning 116024, P. R. China. E-mail: wangth@dlut.edu.cn
First published on 7th April 2021
Abstract
A series of thermal rearrangement (TR) copolymer membranes were prepared by the copolymerization of 9,9-bis(3-amino-4-hydroxyphenoxyphenyl) fluorene (BAHPPF), 9,9-bis(3-amino-4-hydroxyphenyl)fluorene (BAHPF) and 2,2′-bis(3,4′-dicarboxyphenyl)hexafluoropropane dianhydride (6FDA), followed by thermal imidization and further thermal rearrangement. The effects of molar ratio of diamines on the structure and properties of copolymer membranes were studied. The copolymer precursors CP-4:6 and CP-5:5 exhibited excellent mechanical properties. The mechanical properties of precursor membranes rapidly decreased with the increase of thermal treatment temperatures, but the tensile strength of TRCP-4:6 still reached 21.2 MPa. In general, the gas permeabilities of TR copolymers increased with the increase of BAHPF content. Comparatively, TRCP-3:7 and TRCP-4:6 showed higher gas permeabilities, coupled with high O2/N2 and CO2/CH4 selectivities. Especially, the H2, CO2, O2, N2 and CH4 permeabilities of TRCP-4:6 reached 244.4, 269.0, 46.8, 5.20 and 4.60 Barrers respectively, and the selectivities for CO2/CH4 and O2/N2 were 58.48 and 9.00, which exceeded the 2008 upper bound. Therefore, these TR copolymer membranes are expected to be one of the candidate materials for gas separation applications.
1. Introduction
Membrane separation has many advantages, such as simple process, high efficiency, saving energy consumption, low cost and space-efficient, and has exhibited broad application prospects, including hydrogen purification, CO2 capture in natural gas, gas dehumidification, organic mixed gas separation and recovery.1–3 At present, the commercial gas separation membranes are mainly polymeric membranes, involving cellulose acetate (CA), polyphenylene ether (PEO), polyimide (PI), etc.4–6 These separation membranes rely on a close packed structure and appropriate chain rigidity to form tiny gas transmission channels, so they usually display high separation selectivities but low gas permeabilities. According to the Robeson's 1991 and 2008 gas separation upper limits, most of the commercial membranes operate under the upper limit, meaning a low work efficiency.7–9 Therefore, the development of high-performance membrane materials with high gas permeabilities and selectivities is urgently needed.In recent years, thermal rearrangement (TR) polymers have attracted the attention of researchers due to their excellent gas separation performance.10–15 TR polymers are mostly based on hydroxy-containing polyimide (HPI) as precursors. After proper thermal treatment, HPI first forms a carboxy–benzoxazole intermediate, and then decarboxylates to generate a heteroaromatic benzoxazole structure.16–18 In TR polymers, the rotation and movement of benzoxazole ring and phenylene group are relatively difficult, which enhances the rigidity of polymer chain and gives the polymers high free volume fraction.19–22 It is reported that the bimodal microporous structure of TR polymers is in the range of 0.3–0.4 Å and 0.7–0.9 Å.23–26 The unique hourglass structure allows the TR polymer to have both high gas permeabilities and selectivities simultaneously.27–30 However, the trade-off relationship between gas permeabilities and selectivities still exists, so for some gas pairs, such as O2/N2 and CO2/CH4, the separation performance of TR polymers needs to be further improved to meet the practical applications. Therefore, many HPI precursors with novel chemical structures have been designed to improve the separation performance of membrane materials.31–36
As we have known that the rigid and bulky structures easily destroy the regular arrangement of molecular chains, reduce the interaction between molecular chains, and increase the free volume fraction, leading to high gas permeabilities and moderate selectivities.37–42 For example, Fahd et al. introduced the triptycene structures into the main chain of TR polymer, so the permeability of O2 was 311 Barrer, and the O2/N2 selectivity was 5.4, which was close to the 2008 upper bound.43 In addition, the rigid and large volume fluorene groups are helpful to hinder the entanglement and rotation of molecular chain, and increase the spacing between the molecular chains, resulting in high permeabilities of TR membranes.44–46 Generally, TR polymers derived from rigid macromolecular chains exhibit excellent gas separation properties, but they often require higher rearrangement temperatures and show poor mechanical properties. On the contrary, TR polymers based on flexible chain skeletons show lower rearranged temperatures and higher gas selectivities, but the gas permeabilities are relatively weakened to some extent.45,46 Hence, adjusting the rigidity of macromolecular chain by copolymerization is an effective way to obtain TR membranes with excellent comprehensive properties.
Our research group found that the TR polymers derived from 9,9-bis(3-amino-4-hydroxyphenyl)fluorene (BAHPF) displayed poor mechanical properties, film-formation and high thermal rearrangement temperatures, while the TR polymers based on 9,9-bis[4-(4-amino-2-hydroxyphenoxy)phenyl]fluorene (BAHPPF) exhibited good film-formation and mechanical properties, but low gas permeabilities.45,46 It is expected that the TR membranes exhibit excellent separation performance and moderate mechanical properties to meet the practical applications. Therefore, in this study, a series of TR copolymer membranes were prepared by the copolymerization of BAHPF, BAHPPF and 2,2′-bis(3,4′-dicarboxyphenyl)hexafluoropropane dianhydride (6FDA), followed by thermal imidization and further rearrangement. The effects of molar ratio of diamines on the structure, mechanical, thermal properties and gas separation performance of copolymer membranes were investigated by FTIR, XRD, XPS, DSC, DMA, tensile testing and gas permeabilities measurement. The final gas separation properties were easily adjusted by copolymerization ratio, and the mechanical properties of the TR membranes were also kept as much as possible. Thus, the fluorene-containing TR copolymer membranes will be potential candidate materials in the field of gas separation application.
2. Experimental
2.1 Materials
9,9-Bis(3-amino-4-hydroxyphenyl)fluorene (BAHPF) and 9,9-bis[4-(4-amino-2-hydroxyphenoxy)phenyl]fluorene (BAHPPF) were synthesized according to our reported method.45,46 2,2′-bis(3,4′-dicarboxyphenyl)hexafluoropropane dianhydride (6FDA) was obtained from Chinatech (Tianjin) Chemical Co., Ltd, dried at 180 °C for 24 hours in a vacuum oven before use. N,N-Dimethylacetamide (DMAc) was purchased from Sinopharm Chemical Reagent Co., Ltd (China) without any treatment.2.2 Preparation of copolymer precursors
Firstly, BAHPF and BAHPPF were copolymerized with 6FDA in varying molar ratios to form the poly(amic acid) (PAA) solution, followed by thermal imidization to obtain copolymer precursors, as shown in Scheme 1. Taking the synthesis of CP-5:5 as an example: BAHPF (0.005 mol, 1.9023 g), BAHPPF (0.005 mol, 2.8232 g) and DMAc (55.3 mL) were added to a two-neck 250 mL flask equipped with a mechanical stirrer in ice water bath. After the diamines were completely dissolved, 6FDA (0.01 mol, 4.4424 g) was introduced and continuously stirred for 12 hours to form a homogeneous PAA solution with a concentration of 15 wt%. Then, the PAA solution was evenly spread on a clean glass plate with a glass rod, and kept on a constant temperature platform at 40 °C for 24 hours to slowly evaporate the most solvent. In a far-infrared oven, the sample was further heated at 80 °C for 1 hour, 150 °C for 1 hour and 250 °C for 0.5 hour. After natural cooling, the sample was immersed in hot water at 60 °C for at least 30 minutes to remove the membrane from the glass plate, and the obtained membrane was further dried in a vacuum oven at 100 °C for 24 hours. The copolymer precursor (CP) was called as CP-5:5. According to the same procedure, other copolymer membranes CP-9:1, CP-8:2, CP-7:3, CP-6:4, CP-4:6, CP-3:7 and CP-2:8, were also prepared. Here, the ratio of two numbers represents the molar ratio of BAHPPF to BAHPF.2.3 Preparation of TR copolymer membranes
The above membranes were cut into 2 cm × 2 cm square samples and sandwiched between two graphite plates. Then, the samples were placed into a tube furnace and heated to 450 °C at a heating rate of 3 °C min−1 and kept for 1 hour in nitrogen atmosphere (200 mL min−1). Finally, the furnace was naturally cooled down to room temperature. Here, the obtained membranes were named as TRCP-9:1, TRCP-8:2, TRCP-7:3, TRCP-6:4, TRCP-5:5, TRCP-4:6, TRCP-3:7 and TRCP-2:8, respectively.2.4 Characterization
The mechanical properties of copolymer membranes were tested by HY0350 tensile testing machine (Shanghai Hengyi Precision Instrument Co., Ltd, China) with a tensile rate of 10 mm min−1. The sample size was 8 cm × 1 cm, and the gauge distance was 5 cm. At least three samples were cut from each membrane, and the average value of mechanical properties was calculated. The dynamic mechanical properties of copolymer samples were studied by Dynamic thermomechanical analyzer DMA 8000 (PerkinElmer, USA) from 50 to 400 °C at a heating rate of 10 °C min−1 with 1 Hz in N2 atmosphere (80 mL min−1). Glass transition temperatures (Tg) of TR samples were measured by a PerkinElmer Instruments DSC 4000 from 50 to 400 °C with a heating rate of 20 °C min−1 in nitrogen atmosphere (19.8 mL min−1). The second scanning curves were used to estimate the Tg value. The weight losses of copolymer samples were recorded by Pyris 1 TGA thermogravimetric analyzer (PerkinElmer, UAS) at a heating rate of 10 °C min−1 and a nitrogen purge of 50 mL min−1 in the range of 50–700 °C.The Fourier transform infrared (FTIR) measurements were conducted on a Nicolet IS 10 spectrometer (USA) from 4000 to 500 cm−1. Wide-angle X-ray diffraction (WAXD) was performed on X′ Pert Powder X-ray diffractometer (PANalytical, Almelo, Netherlands) from 5° to 70° with copper Kα radiation (operating at 40 kV and 40 mA) to show the changes in intersegmental properties of these samples. The average d-spacing values were calculated according to Bragg's law using eqn (1):
nλ = 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (1) |
The traditional constant pressure-variable volume method was used to characterize the gas permeabilities of the membranes with the high purity gas (>99.99%), including H2, O2, N2, CO2 and CH4. The test temperature was 30 °C, and the pressure was 0.01 MPa. The calculation of gas permeability followed eqn (2).
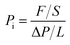 | (2) |
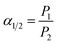 | (3) |
3. Results and discussion
3.1 Synthesis and characterization of copolymer precursors
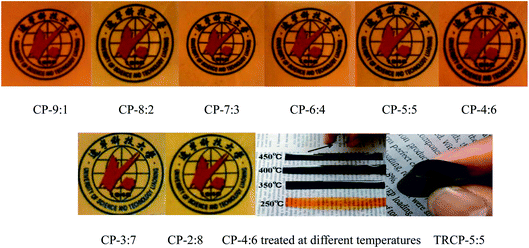
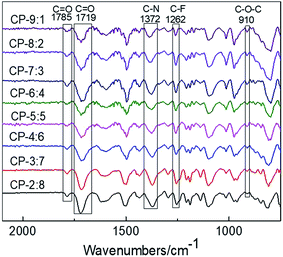
The images of eight kinds of copolymer precursors and CP-4:6 treated at 350, 400 and 450 °C are shown in Fig. 1. These precursor membranes exhibited yellow or brown and transparent, and the color of precursor membranes turned to black after thermal rearrangement at 450 °C for 1 hour. All obtained TR copolymer membranes could be bent to some extent, especially TRCP-4:6 and TRCP-5:5, indicating that these TR copolymers exhibited excellent flexibility.The infrared spectra of copolymer precursors are shown in Fig. 2. It can be seen that all of curves show the characteristic peaks of the imide ring at 1785 and 1719 cm−1, which corresponds to symmetrical stretching vibration and bending vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the imide group, respectively. The peak at 1372 cm−1 is attributed to the stretching vibration of C–N bond in imide ring, and the peak of ether bond is located at 910 cm−1. Furthermore, the characteristic peak of C–F bond in 6FDA appears at 1262 cm−1. These characteristic peaks indicate that these precursors have mostly undergone the imidization reaction.
O in the imide group, respectively. The peak at 1372 cm−1 is attributed to the stretching vibration of C–N bond in imide ring, and the peak of ether bond is located at 910 cm−1. Furthermore, the characteristic peak of C–F bond in 6FDA appears at 1262 cm−1. These characteristic peaks indicate that these precursors have mostly undergone the imidization reaction.
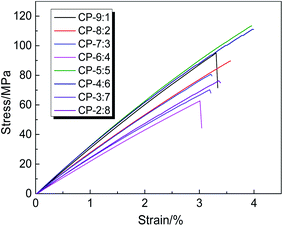
The stress–strain curves of copolymer precursor membranes are given in Fig. 3, and the corresponding mechanical properties data are listed in Table 1. According to the curves, these membranes show elastic deformation and fracture stages, but no obvious yield zone. The elongations at break of all samples are less than 4.0%, indicating the obvious rigidity characteristic of the macromolecular chain. Despite the presence of flexible ether bonds, the rigid and bulky fluorene groups effectively hinder the movement or rotation of molecular chains, and reduce the interaction between molecular chains, resulting in poor toughness. Moreover, the copolymerization is obviously affected by activity of diamines, so the molecular weight and its distribution of copolymers should be diverse, resulting in some irregular change of mechanical properties. Especially, when the molar ratio of BAHPPF to BAHPF was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, the intact membrane was not obtained due to high rigidity, large volume structure and low reactivity of BAHPF. According to the experimental results, the tensile strength, Young's modulus and elongation at break of CP-5:5 and CP-4:6 were better than other samples, and the tensile strength reached 113.4 and 111.1 MPa, the elastic modulus attained 3.3 and 3.1 GPa, and the elongation at break achieved 4.0%, respectively. The results show that the appropriate copolymerization ratio of BAHPPF to BAHPF effectively improves the mechanical properties of precursor membrane materials to meet the further thermal rearrangement.
9, the intact membrane was not obtained due to high rigidity, large volume structure and low reactivity of BAHPF. According to the experimental results, the tensile strength, Young's modulus and elongation at break of CP-5:5 and CP-4:6 were better than other samples, and the tensile strength reached 113.4 and 111.1 MPa, the elastic modulus attained 3.3 and 3.1 GPa, and the elongation at break achieved 4.0%, respectively. The results show that the appropriate copolymerization ratio of BAHPPF to BAHPF effectively improves the mechanical properties of precursor membrane materials to meet the further thermal rearrangement.
| Samples | Average thickness (mm) | Tga (°C) | TTRb (°C) | Tensile strength (MPa) | Young's modulus (GPa) | Elongation at break (%) |
|---|---|---|---|---|---|---|
a Tg: the glass transition temperature was estimated by Tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ–T curves measured by DMA.b TTR: the temperature at the maximum rate of thermally induced rearrangement was obtained by DTG–T curve. δ–T curves measured by DMA.b TTR: the temperature at the maximum rate of thermally induced rearrangement was obtained by DTG–T curve. |
||||||
| CP-9:1 | 0.044 | 345 | 419 | 95.1 ± 3.0 | 2.9 ± 0.2 | 3.3 ± 0.2 |
| CP-8:2 | 0.049 | 361 | 419 | 89.7 ± 4.0 | 2.8 ± 0.1 | 3.6 ± 0.1 |
| CP-7:3 | 0.053 | 366 | 418 | 80.8 ± 2.0 | 2.7 ± 0.1 | 3.2 ± 0.1 |
| CP-6:4 | 0.050 | 389 | 424 | 62.7 ± 4.0 | 2.2 ± 0.1 | 3.0 ± 0.3 |
| CP-5:5 | 0.059 | 344 | 425 | 113.4 ± 2.0 | 3.3 ± 0.2 | 4.0 ± 0.1 |
| CP-4:6 | 0.077 | 347 | 425 | 111.1 ± 3.0 | 3.1 ± 0.2 | 4.0 ± 0.1 |
| CP-3:7 | 0.067 | 362 | 423 | 70.3 ± 3.0 | 2.4 ± 0.3 | 3.2 ± 0.2 |
| CP-2:8 | 0.050 | 366 | 424 | 76.4 ± 4.0 | 2.4 ± 0.1 | 3.4 ± 0.1 |
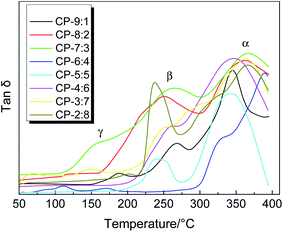
Dynamic mechanical analyzer DMA 8000 can not only accurately measure the glass transition temperature (Tg) of polymers, but also explore other thermal relaxation behaviors during the test. The Tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ–T curves of precursor samples are shown in Fig. 4, which mainly include two or three obvious relaxation stages. The γ relaxation below 200 °C probably corresponds to some sub-glass relaxation related to local mobility due to the existence of ether linkage. The β relaxation below 300 °C is largely caused by further thermal imidization. The biggest α relaxation is ascribed to the transitions from glassy state to rubber state of the molecular chain. The temperature in this stage, corresponding to the peak of the loss factor (Tan
δ–T curves of precursor samples are shown in Fig. 4, which mainly include two or three obvious relaxation stages. The γ relaxation below 200 °C probably corresponds to some sub-glass relaxation related to local mobility due to the existence of ether linkage. The β relaxation below 300 °C is largely caused by further thermal imidization. The biggest α relaxation is ascribed to the transitions from glassy state to rubber state of the molecular chain. The temperature in this stage, corresponding to the peak of the loss factor (Tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ), is commonly defined as the Tg. As shown in Table 1, the Tg values of these precursors are mainly concentrated at 337 to 389 °C, indicating that these precursors have excellent thermal properties. Although there are many flexible ether bonds in the polymer backbone, the bulky fluorenyl group provides larger steric hindrance for the movement and rotation of molecular chain and reduces the interaction between molecular chains, resulting in excellent thermal properties. In general, the copolymers with more flexible ether linkages, irregular arrangement and lower molecular weight possibly exhibit lower Tg values, such as CP-9:1, CP-5:5 and CP-6:4. Due to the relatively fast heating rate of 10 °C min−1 during the DMA testing, the obtained Tg values are higher, but the effect of molar ratio of diamines on the thermal properties is obviously displayed.
δ), is commonly defined as the Tg. As shown in Table 1, the Tg values of these precursors are mainly concentrated at 337 to 389 °C, indicating that these precursors have excellent thermal properties. Although there are many flexible ether bonds in the polymer backbone, the bulky fluorenyl group provides larger steric hindrance for the movement and rotation of molecular chain and reduces the interaction between molecular chains, resulting in excellent thermal properties. In general, the copolymers with more flexible ether linkages, irregular arrangement and lower molecular weight possibly exhibit lower Tg values, such as CP-9:1, CP-5:5 and CP-6:4. Due to the relatively fast heating rate of 10 °C min−1 during the DMA testing, the obtained Tg values are higher, but the effect of molar ratio of diamines on the thermal properties is obviously displayed.
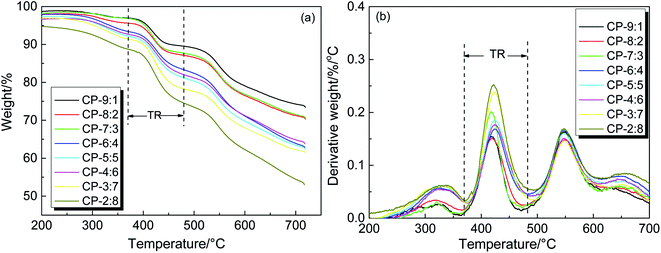
Thermogravimetric analysis was used to study the thermal stability and thermal rearrangement process of copolymer precursor samples. The TGA–T and DTG–T curves are shown in Fig. 5. It can be seen that the process of weight loss are roughly divided into three stages. The first slight mass loss between 250 °C and 370 °C is mainly caused by the volatilization of residual solvent and further imidization. The second prominent weight loss in the range of 370–480 °C is related to the release of CO2 when the hydroxyl-containing copolymer precursor undergoes thermal rearrangement to form PBO. The thermal rearrangement temperature (TTR) is defined as the temperature at the maximum conversion rate, which is listed in Table 1. The TTR values of precursors are mainly around 420 °C, and the precursors with more ether bonds exhibit a relatively low TTR, indicating that the rearrangement is closely related to the ability of segment movement.10 Furthermore, we previously reported that the TTR of HPEI (BAHPPF-6FDA) was 371 °C, while in this study the TTR values of all copolymers were higher than that of HPEI, indicating that the introduction of BAHPF significantly increased the TTR because of the rigid and bulky fluorene structure.46 The third obvious weight loss appears when the temperature is higher than 480 °C, which is attributed to the decomposition of polymer backbone. In this stage, the maximum mass loss rate of copolymers is mainly concentrated at about 550 °C, which indicates that these copolymers exhibit good thermal stabilities. According to the above analysis, the final thermal treatment temperature of the precursor samples was set at 450 °C for 1 hour, in order to complete most of the rearranged conversion and avoid the thermal decomposition.
3.2 Synthesis and characterization of TR copolymer membranes
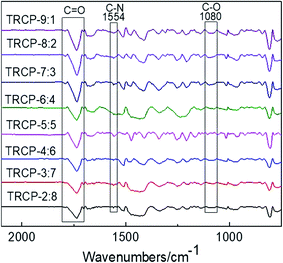
The chemical structures of TR membranes obtained at 450 °C are characterized by FTIR and presented in Fig. 6. Compared with the copolymers without rearrangement, the asymmetric and symmetric stretching vibration absorption peaks corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond at 1785 cm−1 and 1719 cm−1 are obviously weakened, and the characteristic absorption peaks of benzoxazole (N
O bond at 1785 cm−1 and 1719 cm−1 are obviously weakened, and the characteristic absorption peaks of benzoxazole (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O) appear at 1554 cm−1 and 1080 cm−1 respectively, indicating that the imide ring undergoes rearrangement reaction to form benzoxazole structures.
C–O) appear at 1554 cm−1 and 1080 cm−1 respectively, indicating that the imide ring undergoes rearrangement reaction to form benzoxazole structures.
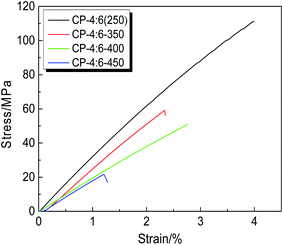
The mechanical properties of TR polymers are extremely important for their future industrial applications. In order to investigate the change of mechanical properties induced by thermal treatment, the CP-4:6 was selected and thermally treated at 350 °C, 400 °C and 450 °C for 1 hour, respectively. The stress–strain curves and corresponding mechanical properties are shown in Fig. 7 and Table 2, and the images of these membranes are given in Fig. 1. It is obvious that the color of the samples has changed from brown to black after thermal treatment, but these membranes still remain good flexibility. With the increase of thermal treatment temperature, the mechanical properties decreased sharply. Compared with the copolymer precursor membrane (250 °C), the tensile strength, Young's modulus and elongation at break of TR membrane (450 °C) decreased by 81%, 42% and 70%, respectively. Although the formation of benzoxazole structure was conducive to maintaining the rigidity of the main chain, a large number of micropores were produced due to the release of CO2 during thermal rearrangement. In addition, a little decomposition of the backbone may occur, reducing the ability of the main chain to resist damage. It is reported that the mechanical properties of TR membranes were improved by introducing non-TR PI segments to meet the needs of testing and practical application, but the permeabilities of membrane materials had to be sacrificed.38 In this study, CP-4:6–450 i.e. TRCP-4:6 still retained better mechanical properties by incorporating flexible ether linkages, and achieved higher gas permeabilities simultaneously. However, compared with spiroTR-PBO-BP-450 containing spiro ring structures, the mechanical data of TRCP-4:6-450 were lower.40 The reason might be that the precursor of spiroTR-PBO-BP-450 was prepared by azeotropic imidization, leading to high molecular weight of 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 to enhance the mechanical properties of TR polymers.
000 g mol−1 to enhance the mechanical properties of TR polymers.
| Samples | Tensile strength (MPa) | Young's modulus (GPa) | Elongation at break (%) | References |
|---|---|---|---|---|
| CP-4:6 (250) | 111.1 ± 3.0 | 3.1 ± 0.2 | 4.0 ± 0.1 | This work |
| CP-4:6-350 | 59.2 ± 4.0 | 2.1 ± 0.1 | 2.3 ± 0.2 | This work |
| CP-4:6-400 | 50.9 ± 3.0 | 2.0 ± 0.2 | 2.7 ± 0.1 | This work |
| CP-4:6-450 | 21.2 ± 2.0 | 1.8 ± 0.1 | 1.2 ± 0.3 | This work |
| TPI-PBO-0.5-450 | 16 ± 3 | 2.27 ± 0.26 | 0.7 ± 0.1 | 38 |
| TPI-PBO-0.75-450 | 13 ± 4 | 2.46 ± 0.40 | 0.6 ± 0.2 | 38 |
| SpiroTR-PBO-BP-450 | 94.4 | — | 14.9 | 40 |
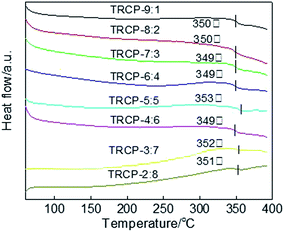
The DSC curves of TR copolymers are shown in Fig. 8. Each DSC curve exhibits only one Tg, indicating that these polymers are random copolymers. Because the rigid fluorenyl and benzoxazole structure limit the movement of molecular segments, the Tg values of TR copolymers mainly center at about 350 °C. These membrane materials with excellent thermal properties could be suitable for high temperature environments.
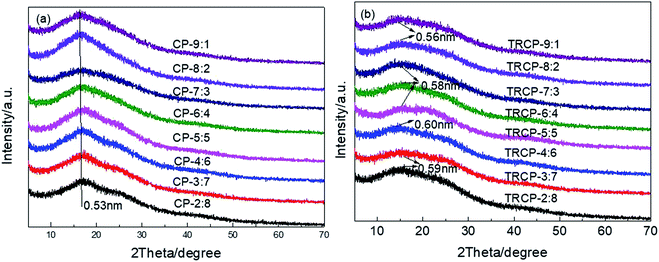
XRD was used to characterize the microstructure of the precursors and TR membranes. As shown in Fig. 9, all precursors and TR copolymers exhibit no sharp diffraction peak, only a broad peak, which indicates that these polymers are primarily amorphous structures. The presence of rigid and bulky fluorenyl and –CF3 groups effectively limits the regular packing of the macromolecular chain and increases the distance between molecular chains. According to the Bragg equation, the d-spacing values of copolymer precursors are mostly concentrated at 0.53 nm. After the thermal rearrangement, the amorphous characteristics are more obvious, and the d-spacing values of TR copolymers are significantly increased, which is ascribed to the formation of rigid polybenzoxazole backbone and the release of small molecular gas CO2. By comparison, TRCP-4:6 showed the maximum d-spacing value of 0.60 nm, which could provide larger transmission channels for the small molecule gases.
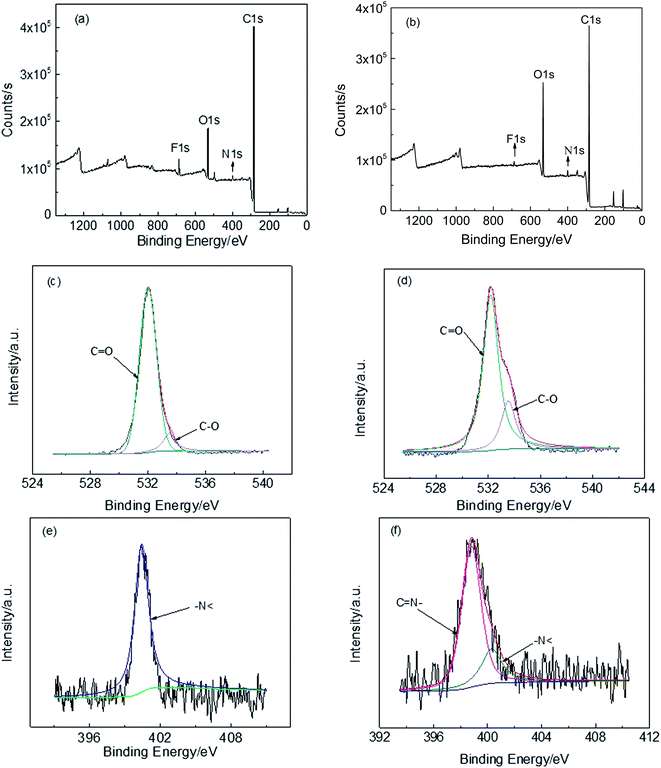
The XPS analysis was utilized to study the change of chemical structure between precursors and TR copolymers. As shown in Fig. 10(a) and (b), the general XPS spectra give a valuable insight of CP-4:6 and TRCP-4:6 samples, indicating the presence of C, N, O and F elements. The fitting curves of O and N suggest that the benzoxazole structures have been formed from copolymer precursors containing hydroxyl groups. In Fig. 10(c) and (d), there are two fitting peaks at 533.5 eV and 532.0 eV, which are assigned to C–O of ether linkage and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in imide ring. Compared with Fig. 10(c), the proportion of fitting peak at 533.5 eV (C–O) in Fig. 10(d) obviously increased due to the partial conversion from C
O in imide ring. Compared with Fig. 10(c), the proportion of fitting peak at 533.5 eV (C–O) in Fig. 10(d) obviously increased due to the partial conversion from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in imide ring to C–O of benzoxazole structure. Fig. 10(e) shows only one fitting peak at 400.4 eV, corresponding to –N< of imide ring, while there are two fitting peaks at 399.0 eV (C
O in imide ring to C–O of benzoxazole structure. Fig. 10(e) shows only one fitting peak at 400.4 eV, corresponding to –N< of imide ring, while there are two fitting peaks at 399.0 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– of benzoxazole ring) and 400.4 eV (–N< of imide ring) in Fig. 10(f). Based on the area of two fitting peaks, the proportion of benzoxazole rings is about 76%, indicating that the thermal conversion was approximately completed by 76%.
N– of benzoxazole ring) and 400.4 eV (–N< of imide ring) in Fig. 10(f). Based on the area of two fitting peaks, the proportion of benzoxazole rings is about 76%, indicating that the thermal conversion was approximately completed by 76%.
3.3 Gas separation performance
Based on the same dianhydride 6FDA, the molar ratio of two diamines has affected the mechanical and thermal properties of copolymer membranes, which may influence the gas permeabilities of the resultant TR membranes. In order to evaluate the gas separation performance of TR copolymers, five kinds of high purity gases including H2, N2, O2, CH4 and CO2 were used to test the gas permeation properties of these membrane materials. The gas permeabilities and ideal selectivities are summarized in Table 3.| Samples | Permeabilitiesa/Barrerb | Ideal selectivitiesc | |||||
|---|---|---|---|---|---|---|---|
| H2 | CO2 | O2 | N2 | CH4 | O2/N2 | CO2/CH4 | |
| a All permeation results were obtained at 30 °C and 0.01 MPa.b 1 Barrer = 10−10 cm(STP)3 cm cm−2 s−1 cmHg−1 = 3.35 × 10−16 mol m m−2 s−1 Pa−1.c Permeability ratio of two gases. | |||||||
| TRCP-9:1 | 135.5 | 119.9 | 20.5 | 6.05 | — | 3.39 | — |
| TRCP-8:2 | 143.1 | 124.8 | 22.9 | 5.29 | — | 4.33 | — |
| TRCP-7:3 | 180.5 | 155.4 | 24.8 | 5.20 | — | 4.77 | — |
| TRCP-6:4 | 189.9 | 163.0 | 30.0 | 8.40 | 3.62 | 3.57 | 45.03 |
| TRCP-5:5 | 213.8 | 196.4 | 39.5 | 5.69 | 4.21 | 6.94 | 46.65 |
| TRCP-4:6 | 244.4 | 269.0 | 46.8 | 5.20 | 4.60 | 9.00 | 58.48 |
| TRCP-3:7 | 247.1 | 241.3 | 41.8 | 9.84 | 4.10 | 4.25 | 58.85 |
| TRCP-2:8 | 214.0 | 201.3 | 34.4 | 7.14 | 4.15 | 4.82 | 48.51 |
| TR-PEBO-450-1 (ref. 47) | 95.3 | 41.4 | 10.0 | 1.89 | 1.45 | 5.3 | 28.6 |
| TR-PBOI-c-420 (ref. 48) | — | 61.9 | 14.3 | 3.2 | 1.48 | 4.47 | 41.8 |
| TR-APAF-TP49 | — | 111 | 28.7 | 5.72 | 3.12 | 5.0 | 36 |
| O-PIM-PBO-1 (ref. 39) | 245 | 257 | 47 | 11 | 10 | 4.3 | 26 |
| spiroTR-PBO-6F50 | 429 | 675 | 120 | 30 | 34 | 3.9 | 20 |
| SPDA-SBF-PBO51 | 775 | 1280 | 225 | 61.6 | 84.8 | 3.7 | 15.1 |
As shown in Table 3, the gas permeabilities of TR membranes, except for TRCP-4:6, mainly follow the order: P (H2) >P (CO2) > P (O2) >P (N2) >P (CH4), which is just opposite to the order of the dynamic diameter of gas molecules: H2 (0.289 nm) < CO2 (0.33 nm) < O2 (0.346 nm) < N2 (0.364 nm) < CH4 (0.38 nm). Nevertheless, for the TRCP-4:6 membrane, the permeability of H2 is less than that of CO2, which is similar to the result of polymers of intrinsic microporosity (PIM) reported in the literature.39 The TRCP-4:6 probably forms a large amount of free volume, which is beneficial to diffusion of small molecules in the polymer. Generally, the gas permeabilities of TR membranes increased with the BAHPF content increasing. Especially, TRCP-4:6 and TRCP-3:7 exhibited better gas permeabilities than other TR copolymers, while the selectivity of CO2/CH4 was over 58, indicating high gas permeabilities coupled with high selectivities. It is interesting that the gas permeabilities and selectivities are improved at the same time, and there is no trade-off relationship. Besides, it was found that the TR copolymers with more flexible ether bonds showed relatively lower gas permeabilities, which was probably attributed to the close accumulation of molecular chains, reducing the channels for gas molecules to pass through the polymer. Among them, the H2, CO2, O2, N2 and CH4 permeabilities of TRCP-4:6 membrane reached 244.4, 269.0, 46.8, 5.20 and 4.60 Barrers respectively, coupled with the O2/N2 of 9.00 and CO2/CH4 of 58.48. Compared with some reported TR polymers in Table 3, TRCP-4:6 exhibited excellent gas permeabilities and ideal selectivities, simultaneously. The results show that the excellent gas permeabilities coupled with ideal selectivities could be attained by effectively adjusting copolymerization ratio. The reasonable collocation of rigid structures and flexible linkages optimizes the shape of the “hourglass” to form effective gas permeation and separation channels. Additionally, the XRD results were largely consistent with the gas separation results. Therefore, these TR copolymers, especially TRCP-4:6, show higher gas permeabilities and selectivities, as well as better mechanical and thermal properties, displaying excellent comprehensive performance.
In order to further evaluate the gas separation performance of membrane materials, Robeson summarized the hypothetical upper limit of the relationship between gas permeability and selectivity for valuable gas pairs, and proposed the 1991, 2008 and 2015 “upper limit”.9 The increase of the upper limit indicates that the separation efficiency of membrane materials has been improved significantly with the efforts of researchers and engineers, including the molecular structure design of new polymer materials and the effective development of mixed matrix membrane materials. As shown in Fig. 11(a), the five kinds of fluorene-based TR copolymer membranes exceed or approach the 2008 trade-off line for CO2/CH4 separation. Especially, TRCP-3:7 and TRCP-4:6 have exceeded the 2008 upper bound, showing high separation efficiency. According to Fig. 11(b), the separation performance of TRCP-4:6 for O2/N2 is very close to the 2015 upper bound, implying better gas separation behavior. Therefore, the proper copolymerization of BAHPF and BAHPPF not only improves the gas permeation flux, but also enhances the separation selectivities. These TR membranes are expected to be applied to the field of gas separation in the future.
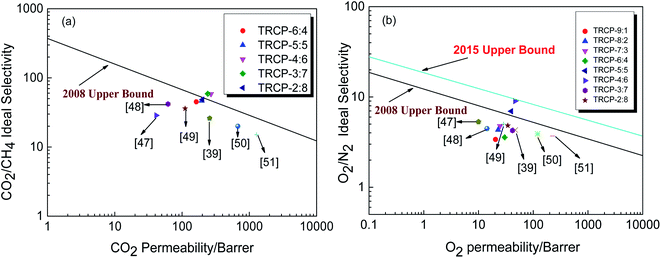 | ||
| Fig. 11 Relationship between gas permeability and selectivity of TR membranes (a) CO2 vs. CO2/CH4; (b) O2 vs. O2/N2. | ||
4. Conclusions
A series of TR copolymer membranes were prepared by the copolymerization of 9,9-bis(3-amino-4-hydroxyphenoxyphenyl)fluorene (BAHPPF), 9,9-bis(3-amino-4-hydroxyphenyl)fluorene (BAHPF) and 2,2′-bis(3,4′-dicarboxyphenyl)hexafluoropropane dianhydride (6FDA), followed by thermal imidization and thermal rearrangement reactions. The copolymers as precursor showed excellent mechanical and thermal properties, especially CP-4:6 and CP-3:7. After thermal rearrangement at 450 °C, the TRCP-4:6 still remained better mechanical properties, including tensile strength of 21.2 MPa, Young's modulus of 1.8 GPa and breaking elongation of 1.2%. In general, the gas permeabilities of resulting TR membranes increased with the increase of BAHPF content. Especially, the permeabilities of H2, CO2, O2, N2 and CH4 for TRCP-4:6 reached 244.4, 269.0, 46.8, 5.20 and 4.60 Barrers respectively, and the CO2/CH4 selectivities of 58.48 and O2/N2 selectivities of 9.00 exceeded the 2008 upper bound. More importantly, by adjusting the molar ratio of diamines, the TR copolymers successfully attained better gas permeabilities, coupled with higher selectivities simultaneously, which broke away from the conventional trade-off rules. Therefore, the TR copolymer membranes exhibit outstanding mechanical, thermal and gas separation properties, which are expected to be applied in the field of gas separation in the future.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant number: 21878033), and University of Science and Technology Liaoning Talent Project Grants (Grant number: 601011507-17).References
- J. H. Lee, J. Lee, H. J. Jo, J. G. Seong, J. S. Kim, W. H. Lee, J. Moon, D. Lee, W. J. Oh, J. G. Yeo and Y. M. Lee, J. Membr. Sci., 2017, 539, 412–420 CrossRef CAS.
- Y. Liu, Z. Liu, A. Morisato, N. Bhuwania, D. Chinn and W. J. Koros, J. Membr. Sci., 2020, 601, 117910 CrossRef CAS.
- H. Borjigin, Q. Liu, W. Zhang, K. Gaines, J. S. Riffle, D. R. Paul, B. D. Freeman and J. E. McGrath, Polymer, 2015, 75, 199–210 CrossRef CAS.
- H. Sanaeepur, A. E. Amooghin, S. Bandehali, A. Moghadassi and B. V. Bruggen, Prog. Polym. Sci., 2019, 91, 80–125 CrossRef CAS.
- Y. Xie, T. T. Wang, X. H. Liu, K. Zou and W. Q. Deng, Nat. Commun., 2013, 4, 1960–1966 CrossRef PubMed.
- C. Zhang, P. Li and B. Cao, J. Membr. Sci., 2017, 528, 206–216 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 1991, 62, 165–185 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- R. Swaidan, B. Ghanem and I. Pinnau, ACS Macro Lett., 2015, 4, 947–951 CrossRef CAS.
- R. Guo, D. F. Sanders, Z. P. Smith, B. D. Freeman, D. R. Paul and J. E. McGrath, J. Mater. Chem. A, 2013, 1, 6063–6072 RSC.
- S. Kim, J. Hou, Y. Wang, R. Ou, G. P. Simon, J. G. Seong, Y. M. Lee and H. Wang, J. Mater. Chem. A, 2018, 6, 7668–7674 RSC.
- A. E. Amooghin, S. Mashhadikhan, H. Sanaeepur, A. Moghadassi, T. Matsuura and S. Ramakrishna, Prog. Mater. Sci., 2018, 102, 222–295 CrossRef.
- Y. M. Xu, N. L. Le, J. Zuo and T. S. Chung, J. Membr. Sci., 2016, 499, 317–325 CrossRef CAS.
- N. Du, H. B. Park, M. M. Dal-Cin and M. D. Guiver, Energy Environ. Sci., 2012, 5, 7306–7322 RSC.
- H. Wang, D. R. Paul and T.-S. Chung, Polymer, 2013, 54, 2324–2334 CrossRef CAS.
- G. Tullos and L. Mathias, Polymer, 1999, 40, 3463–3468 CrossRef CAS.
- G. L. Diego and L. Dmitri, Polym. Bull., 2002, 48, 261–269 CrossRef.
- H. B. Park, S. H. Han, C. H. Jung, Y. M. Lee and A. J. Hill, J. Membr. Sci., 2009, 359, 11–24 CrossRef.
- S. H. Han, N. Misdan, S. Kim, C. M. Doherty, A. J. Hill and Y. M. Lee, Macromolecules, 2010, 43, 7657–7667 CrossRef CAS.
- C. Y. Soo, H. J. Jo, Y. M. Lee, J. R. Quay and M. K. Murphy, J. Membr. Sci., 2013, 444, 365–377 CrossRef CAS.
- C. A. Scholes, C. P. Ribeiro, S. E. Kentish and B. D. Freeman, Sep. Purif. Technol., 2014, 124, 134–140 CrossRef CAS.
- H. B. Park, C. H. Jung, Y. M. Lee, A. J. Hill, S. J. Pas, S. T. Mudie, E. Van Wagner, B. D. Freeman and D. J. Cookson, Science, 2007, 318, 254–258 CrossRef CAS PubMed.
- S. Kim and Y. M. Lee, Prog. Polym. Sci., 2015, 43, 1–32 CrossRef CAS.
- Y. Jiang, F. T. Willmore, D. Sanders, Z. P. Smith, C. P. Ribeiro, C. M. Doherty, A. Thornton, A. J. Hill, B. D. Freeman and I. C. Sanchez, Polymer, 2011, 52, 2244–2254 CrossRef CAS.
- A. K. Pate and N. K. Acharya, Int. J. Hydrogen Energy, 2020, 45, 18685–18692 CrossRef.
- K. A. Stevens, Z. P. Smith, K. L. Gleason, M. Galizia, D. R. Paul and B. D. Freeman, J. Membr. Sci., 2017, 533, 75–83 CrossRef CAS.
- M. Galizia, K. A. Stevens, D. R. Paul and B. D. Freeman, J. Membr. Sci., 2017, 537, 83–92 CrossRef CAS.
- A. Brunetti, M. Cersosimo, J. S. Kim, G. Dong, E. Fontananova, Y. M. Lee, E. Drioli and G. Barbieri, Int. J. Greenh. Gas. Con., 2017, 61, 16–26 CrossRef CAS.
- X. Jiang, X. Xiao, J. Dong, X. Xu, X. Zhao and Q. Zhang, J. Membr. Sci., 2018, 564, 605–616 CrossRef CAS.
- C. A. Scholes and B. D. Freeman, J. Membr. Sci., 2018, 563, 676–683 CrossRef CAS.
- W. Liu and W. Xie, Ind. Eng. Chem. Res., 2013, 53, 871–879 CrossRef.
- M. Calle, H. J. Jo, C. M. Doherty, A. J. Hill and Y. M. Lee, Macromolecules, 2015, 48, 2603–2613 CrossRef CAS.
- X. Hu, W. H. Lee, J. Zhao, J. S. Kim, Z. Wang, J. Yan, Y. Zhuang and Y. M. Lee, J. Membr. Sci., 2020, 604, 118053 CrossRef CAS.
- C. Aguilar-Lugo, C. Álvarez, Y. M. Lee, J. G. de la Campa and Á. E. Lozano, Macromolecules, 2018, 51, 1605–1619 CrossRef CAS.
- J. I. Choi, C. H. Jung, S. H. Han, H. B. Park and Y. M. Lee, J. Membr. Sci., 2009, 349, 358–368 CrossRef.
- X. Ma, R. Swaidan, B. Teng, H. Tan, O. Salinas, E. Litwiller, Y. Han and I. Pinnau, Carbon, 2013, 62, 88–96 CrossRef CAS.
- S. M. Meckler, J. E. Bachman, B. P. Robertson, C. Zhu, J. R. Long and B. A. Helms, Angew. Chem., Int. Ed., 2018, 57, 4912–4916 CrossRef CAS PubMed.
- S. Luo, Q. Zhang, T. K. Bear, T. E. Curtis, R. K. Curtis, C. M. Doherty, A. J. Hill and R. Guo, J. Membr. Sci., 2018, 551, 305–314 CrossRef CAS.
- H. Shamsipur, B. A. Dawood, P. M. Budd, P. Bernardo, G. Clarizia and J. C. Jansen, Macromolecules, 2014, 47, 5595–5606 CrossRef CAS.
- Q. Liu, D. R. Paul and B. D. Freeman, Polymer, 2016, 82, 378–391 CrossRef CAS.
- A. Tena, S. Rangou, S. Shishatskiy, V. Filiz and V. Abetz, Sci. Adv., 2016, 2, e1501859 CrossRef PubMed.
- S. Kim, K. T. Woo, J. M. Lee, J. R. Quay, M. K. Murphy and Y. M. Lee, J. Membr. Sci., 2014, 453, 556–565 CrossRef CAS.
- F. Alghunaimi, B. Ghanem, Y. Wang, O. Salinas, N. Alaslai and I. Pinnau, Polymer, 2017, 121, 9–16 CrossRef CAS.
- Y. F. Yeong, H. Wang, K. P. Pramoda and T. S. Chung, J. Membr. Sci., 2012, 397, 51–65 CrossRef.
- Y. Lu, J. Hao, L. Li, J. Song, G. Xiao, H. Zhao, Z. Hu and T. Wang, React. Funct. Polym., 2017, 119, 134–144 CrossRef CAS.
- Y. Lu, J. Zhang, G. Xiao, L. Li, M. Hou, J. Hu and T. Wang, RSC Adv., 2020, 10, 17461–17472 RSC.
- M. Calle and Y. M. Lee, Macromolecules, 2011, 44, 1156–1165 CrossRef CAS.
- F. Gan, J. Dong, X. Xu, M. Li, X. Zhao and Q. Zhang, Polymer, 2019, 185, 121945 CrossRef CAS.
- B. Díez, P. Cuadrado, Á. Marcos-Fernández, J. G. de la Campa, A. T. Tena, P. Prádanos, L. Palacio, Y. M. Lee, C. Alvarez, Á. E. Lozano and A. Hernández, React. Funct. Polym., 2018, 127, 38–47 CrossRef.
- S. Li, H. J. Jo, S. H. Han, C. H. Park, S. Kim, P. M. Budd and Y. M. Lee, J. Membr. Sci., 2013, 434, 137–147 CrossRef CAS.
- X. Ma, O. Salinas, E. Litwiller and I. Pinnau, Polym. Chem., 2014, 5, 6914–6922 RSC.
| This journal is © The Royal Society of Chemistry 2021 |