Open Access Article
Open Access ArticleImpact of exogenous metal ions on peri-implant bone metabolism: a review
Wei Chenab,
Wen-qing Zhuab and
Jing Qiu *ab
*ab
aDepartment of Oral Implantology, Affiliated Hospital of Stomatology, Nanjing Medical University, Nanjing, 210029, PR China. E-mail: qiujing@njmu.edu.cn; Tel: +86 25 69593085
bJiangsu Key Laboratory of Oral Disease, Nanjing Medical University, Nanjing, 210029, PR China
First published on 7th April 2021
Abstract
The development of effective methods to promote the osseointegration of dental implants by surface modification is an area of intense research in dental materials science. Exogenous metal ions present in the implant and surface modifications are closely related to the bone metabolism around the implant. In the complex oral microenvironment, the release of metal ions caused by continuous corrosion of dental implants has an unfavorable impact on the surrounding tissue, and then affects osseointegration, leading to bad results such as loosening and falling off in the late stage of the implant. Besides, these ions can even be distributed in distant tissues and organs. Currently, surface modification techniques are being developed that involve different processing technologies including the introduction of exogenous metal ions with different properties onto the surface of implants to improve performance. However, most metal elements have some level of biological toxicity and can only be used within a safe concentration range to exert the optimum biological effects on recipients. In this paper, we review the adverse effects of metal ions on osseointegration and highlight the emerging applications for metal elements in improving the performance of dental implants.
1. Introduction
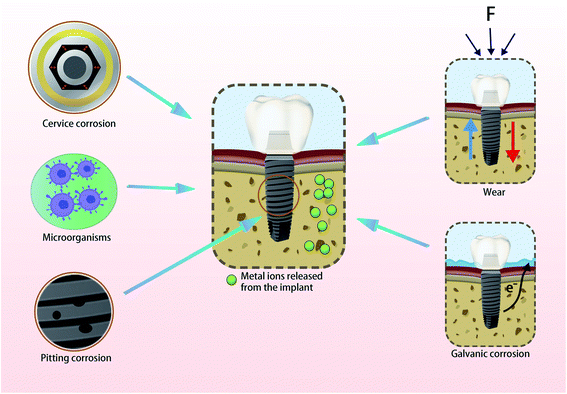
Dental implant materials are biologically safe, resistant to corrosion and have excellent mechanical properties for rapid re-passivation in biological environments.1,2 However, changes in the bacterial flora and pH of the oral cavity have pronounced effects on the oral microenvironment.3–5 These changes make traditional cobalt–chromium alloy and titanium materials highly susceptible to corrosion resulting in many deficiencies in osseointegration and antibacterial properties. The release of exogenous metal ions caused by the long-term use of dental implant materials (Fig. 1) can also impact the surrounding tissues and affect osseointegration resulting in loosening and loss of implants.6,7 These ions may be distributed in distant tissues and organs in the body. The concentrations of individual metals and their locations in the body are summarized in Table 1.8–15| Reference | Implant alloys | Animal model and area of implant insertion | Localization of the metal particles | Method of detection | Concentrations |
|---|---|---|---|---|---|
| Puskar T. et al.8 | Co–Cr alloy | Human hip | Peri-implant tissue | Inductively coupled plasma mass spectrometry (ICP-MS) | Co: about 150 ng mL−1, Cr: about 1500 ng mL−1 |
| Sampson B. et al.9 | Co–Cr alloy | Human hip | Plasma and tissues | ICP-MS | Cr: 5–100 μg L−1 |
| Co: 5–300 μg L−1 | |||||
| Rubio J. C. et al.10 | Co–Cr alloy | Rat femur | Liver, kidneys, spleen, lungs | ICP-MS | Co: kidneys: about 0.5 ng mL−1 |
| Liver: about 0.15 ng mL−1 | |||||
| Lungs: about 0.15 ng mL−1 | |||||
| Spleen: about 0.12 ng mL−1 | |||||
| Cr: kidneys: about 0.5 ng mL−1 | |||||
| Liver: about 0.33 ng mL−1 | |||||
| Lungs: about 0.55 ng mL−1 | |||||
| Spleen: about 0.6 ng mL−1 | |||||
| He X. et al.11 | Ti dental implants | Human madible | Peri-implant bone tissue | Inductively coupled plasma optical emission spectrometry (ICP-OES) | 7064 ± 1932 μg kg−1-bone weight |
| Wennerberg et al.12 | Turned implant, blasted implant | New Zealand rabbit tibia | Peri-implant tissue | X-ray fluorescence spectroscopy (SRXRF) | Turned implant surface: 20–100 wt. ppm |
| Secondary ion mass spectrometry (SIMS) | Blasted implant surface: the highest titanium concentration reached about 100 wt. ppm | ||||
| Weingart D. et al.13 | Plasma-coated titanium screw implants | Beagle dogs mandible and maxilla | Lymph nodes, visceral organs | Flameless atomic absorption spectroscopy (FAAS) | Lymph nodes: 0.16–9.0 μg g−1 |
| Visceral organs: 0.01 and 0.21 μg g−1 | |||||
| Schliephake et al.14 | Titanium, machined | Minipig mandible | Lungs, liver and kidneys | FAAS | Kidneys: 2.92 ± 0.69 ng mg−1 |
| Liver: 11.5 ± 1.35 ng mg−1 | |||||
| Lungs: 135.7 ± 12.42 ng mg−1 | |||||
| Sarmiento-González A. et al.15 | Ti wire (purity 99.99%) | Rat femur | Liver, kidneys, spleen, lungs, heart | Double focusing inductively coupled plasma mass spectrometer (DF-ICP-MS) | Liver: 78.1 ± 9.8 ng g−1 dry tissue |
| Kidneys: 210 ± 76 ng g−1 dry tissue | |||||
| Spleen: 632 ± 177 ng g−1 dry tissue | |||||
| Lungs: 578 ± 189 ng g−1 dry tissue | |||||
| Heart: 160 ± 60 ng g−1 dry tissue |
The improvement of dental implant performance is an area of intense research in dental materials science. The surface modification of implants by metal ions can increase the antibacterial and corrosion resistance properties of implants and also accelerate the process of osseointegration.16 However, the specific roles and molecular mechanisms of exogenous metal ions in guiding bone growth on the surface of implants are yet to be systematically studied.
Various surface modification techniques can be used to incorporate metal ions into implants that can produce different biological effects. Based on the principles of introducing metal ions onto titanium surfaces, direct or indirect methods can be used. Direct methods modify the titanium surface to form oxide coatings containing metal elements and include approaches such as hydrothermal treatment, micro-arc oxidation and plasma immersion ion implantation (PIII). Indirect methods can be used to obtain apatite coatings with good biocompatibility by doping metal ions in hydroxyapatite (HA) coating on the titanium surface and include approaches such as plasma spraying, the sol–gel method, electrochemical deposition, selective laser melting and magnetron sputtering.17 The performances of different surface modification techniques used for the incorporation of metallic ions are summarized in Table 2.17–32 In this paper, we review the adverse effects of metal ions on osseointegration and highlight the emerging applications for metal elements in improving the performance of dental implants.
| Method | Definition | Advantages | Disadvantages |
|---|---|---|---|
| Hydrothermal treatment18,19 | Chemical reactions that take place in a liquid phase at high temperature | Simple; economical, effective; environmentally friendly; uniform thickness of the deposited layer | Some substances are heat-sensitive |
| PIII20,21 | A material engineering process by which ions are accelerated in an electrical field and impacted onto the surface of a substrate to change the surface chemistry | Suitable for processing implants with complex shapes | Technical complexity; high costs; high dissolution rate |
| Micro-arc oxidation22,23 | Arc discharge is used to enhance and activate the reaction on the anode, which produces a thick and relatively stable oxide film on the surface of the metal and its alloy | Multi-microporous structure can achieve long-term stable release of metal ions; corrosion-resistant | Technical complexity |
| Electrochemical deposition24,25 | The metal matrix is immersed in the aqueous solution containing the gold-plated ions, and the direct current is passed through to make the positive ions discharge on the surface of the cathode to obtain the metal film | Low dissolution rate; effective | Low fatigue strength, poor adhesion between coating and implant |
| Sol–gel method26,27 | The use of metal inorganic salts or metal alcohol salts in water or alcohol solvent hydrolysis or alcoholysis reaction, the formation of the sol through drying dehydration into gel, and then after heat treatment to obtain the product | Uniform layers; the preparation process is easy to control; low processing temperature | Fabrication steps; environmentally unfriendly; debonding of the coating layer |
| Plasma spraying17,28 | A kind of gas is ionized by non-transfer arc to form a high temperature plasma jet, and the powder is introduced into it, and the jet is accelerated and impinges on the surface of the substrate to form a coating | Effective, lower possibility of coating degradation, | Nonuniform coatings, poor adhesion between coating and implants; high dissolution rate; the preparation temperature is too high to make HA decomposition |
| Magnetron sputtering29,30 | It uses charged particles to bombard the target surface in vacuum, and the particles are deposited on the surface of the cold metal substrate to form a coating structure | Effective; high purity of layers; ability to coat implants with complex shapes; strong adhesion of films; dense and uniform coatings | Technical complexity; high costs; the subsequent heat treatment is needed to restore the crystalline state of the coating structure, and the high temperature treatment will destroy the HA lattice to some extent |
| Selective laser melting31,32 | This technique uses metal powder to be completely melted under the heat of a laser beam, then cooled and formed coatings | Good mechanical property; a high degree of processing freedom | The rough surface of titanium implants modified by SLM alone can promote bacterial adhesion and biofilm formation, so it is necessary to combine with other means to give the implant antibacterial property |
2. Adverse effects of metal ions on implant osseointegration and mechanisms
2.1 Cobalt and chromium ions
Co–Cr alloy is one of the commonly used implant materials which has excellent mechanical property and corrosion resistance. It has been used in dental prosthodontics since 1929. The Co–Cr alloy is biocompatible and has osteoconductive properties that promote rapid osteointegration.33 However, in the oral environment, the long-term use of Co–Cr alloy leads to the release of cobalt and chromium ions.8 Especially in the presence of inflammatory microenvironment, Liu et al. demonstrated the corrosion of Co–Cr alloy using electrochemical tests. The corrosion leads to the accumulation of cobalt and chromium ions around the bone which have an impact on the tissue.34 Reclaru et al. detected corrosion pits on Co–Cr alloy detected by scanning electron microscopy (SEM), which confirmed the existence of corrosion.35 Cobalt and chromium ions at concentrations of 5–100 μg L−1 and 5–300 μg L−1 respectively have been detected in the plasma and tissues of patients undergoing metal repair surgery.36Cobalt and chromium ions have been shown to regulate the expression of many genes involved in the production of cytokines, chemokines and other regulatory molecules that have a functional role on osteoblasts. Anika et al. found that a mixture of cobalt and chromium ions at 200 μg L−1 upregulated the gene expression of pro-osteolytic mediators (IL-6, IL-8, TNF-α, MCP-1, MMP1, TIMP1) and could act to inhibit the proliferation and to promote the death of osteoblasts. However, cobalt or chromium ions alone have little effect on osteoblasts.37 Studies have also determined the effect of cobalt and chromium ions on the expression of members of the TGF-β family which are essential regulators of osteoblast differentiation and maturation. Specifically, 50–250 μM of cobalt and chromium ions did not affect the proliferation of osteoblasts whilst the same concentrations of cobalt ions could significantly down-regulate the expression of all TGF-β family molecules. However, these changes did not affect the ability of osteoblasts to mineralize. In contrast, chromium ions had no significant effect on the expression of TGF-β family molecules but significantly inhibited the mineralization of osteoblasts. These observations may be related to the formation of complex hydrated isomers in the phosphate environment of cell culture processes resulting in insoluble chromium phosphate.38
Other studies have confirmed that cobalt ions have no side effects on the growth and development of osteoblasts whilst chromium ions may delay the regular intake processes in cells by binding to serum albumin and transferrin. This leads to the delayed expression of related genes and affects the mineralization ability of cells.39 However, it is not known how chromium ions enter cells and how they act to regulate cell differentiation and mineralization by affecting downstream transport molecules.
2.2 Titanium ions
Since the discovery of osseointegration by Branemark, titanium materials have been widely used in the field of dental implants. Although titanium materials have excellent biocompatibility, good corrosion resistance, strong mechanical properties and as an inert material, titanium can form a dense oxide film on the surface to protect the titanium surface.1 However, in the oral environment, due to the presence of the surrounding electrolyte fluid and the adhesion of oral microorganisms, the oxide film is corroded causing damage to the titanium surface structure.40 Yu Xiaoyu et al. detected the micromorphological changes on commercial pure titanium (cpTi) under an inflammatory environment. SEM and electrochemical tests showed decreased resistance to corrosion under this condition.41 Studies showed the increase of the corrosion rate and the damage of oxide film according to the electrochemical tests and SEM images in the presence of oral bacteria.42,43 Hanawa et al. studied the regeneration of the oxide film on titanium surfaces and predicted the release of titanium ions when the oxide film was destroyed.44 The release of titanium ions affects the integration of the titanium implant with the bone and can eventually cause the implant to fall off due to poor bone integration.45Current theories suggest that implant failure is caused by bone resorption around the implant due to the aseptic loosening of the implant–bone interface after bone destruction, dissolution and absorption. The failure rate of implants is around 20% and titanium ions are potentially related to aseptic loosening.46 Studies have shown that titanium ions accumulate locally during the implantation process and affect the behavior of the cells around implants, thus impacting osseointegration.47
Many research reports have focused on the release of titanium ions around implants. Ferguson et al. put forward the problem of titanium ions release around titanium implants. The concentration of titanium ions in adjacent tissues was around 20 times higher than that in normal tissues after about 5 months of dental implantation.48 Since then, the release of titanium from human implants after osseointegration has been studied. The concentration of titanium ions in the serum around the survived implants is 20.89 μmol L−1. However, around the failed implants, the local accumulation of titanium ions in serum is 83.56 μmol L−1.49 Ducheyne et al. changed the pore size of the titanium material and detected the release of titanium ions in bone tissue after 12 months of implantation. They showed that the enrichment of titanium reached 700 pg g−1 in the bone whilst there was no relationship between the concentration of aggregation and the roughness of the surface.50
Many in vitro experiments have shown that high concentrations of titanium ions have adverse effects on peri-implant cells including enhancing osteoclasts activity, inhibiting osteoblasts formation and activating inflammatory cells. Liao et al. reported that 10 mg L−1 of titanium ions could significantly inhibit the proliferation of osteoblasts, while 5 mg L−1 of titanium ions could down-regulate the expression of the OSN and OPN genes in osteoblasts and inhibit osteoblasts differentiation.51 Thompson et al. found that titanium ions could not inhibit the proliferation of bone marrow mesenchymal stem cells at low doses but could significantly interfere with the process of osteogenic differentiation and hinder the mineralization of cell matrix.52 Blumenthal et al. found that titanium ions could bind to the crystal surface of HA and destroy the growth site of the crystal, thereby reducing the formation of HA. So the local enrichment caused by titanium release affects the normal mineralization of the osteoid and can interfere with the ability of the implant–bone interface to repair. Therefore, once the implant is loosened it is difficult to stabilize through the process of bone repair.53
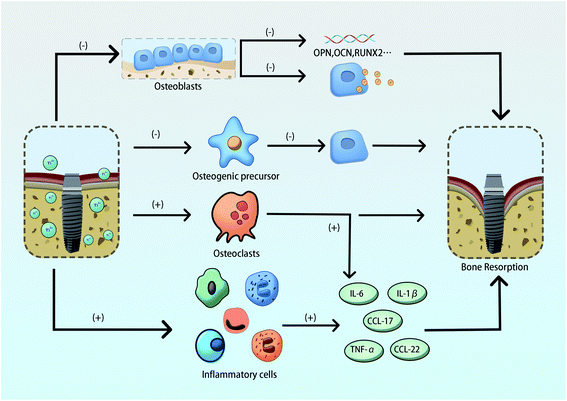
In addition to inhibiting the development of osteogenic precursor cells into osteoblasts, excessive titanium ions can also inhibit mineralization of the matrix and deposition of calcium salts to weaken the osteogenic ability of osteoblasts. They can also activate the formation of osteoclasts and inflammatory cells to produce inflammatory factors that accelerate bone resorption. Meng Bo et al. found that titanium ions of 100 ppm (μmol L−1) promoted the formation and activation of osteoclasts to increase bone resorption.54 Cadosch suggested that the accumulation of titanium ions may induce osteoclast synthesis and increase thymus and activation regulators (CCL17/TARC), as well as macrophage-derived chemokine (CCL22/MDC).55 Titanium ions induce bone resorption by accelerating the maturation of osteoclasts. Studies have shown that osteoclasts induced by titanium ions accounted for about 20% of the total osteoclasts.56 Other studies have reported that titanium ions can activate inflammatory cells and up-regulate the expression of a variety of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-1β, which can also enhance osteoclast differentiation and cause bone resorption.57,58
The pathological accumulation of titanium ions caused by various factors in the body stimulates an imbalance between osteogenesis and osteoclasts around implants. These changes can increase corrosion of the implant material to produce more ions and activate a positive feedback process. However, the role of titanium ions in aseptic loosening in causing bone destruction, dissolution and absorption at the implant–bone interface needs to be further studied.
Studies have confirmed that titanium ions may stimulate bone immunity and metabolism through direct and indirect pathways that impact osseointegration. It has been shown that titanium ions at 10 ppm can block the nuclear transfer level of YAP molecules by inhibiting the Hippo/YAP pathway in osteoblasts, thereby affecting the binding of downstream key molecules to inhibit the behavior of osteoblasts.7 Fritz et al. also found that the NF-κB inflammatory signaling pathway was activated in osteoblasts following titanium exposure causing the up-regulated expression of cytokines TNF-α, IL-6 and IL-8 that activate downstream inflammatory responses. The expression of these inflammatory factors may contribute to the occurrence of aseptic peripheral inflammation of the implant.59 Tang et al. showed that titanium ions can stimulate inflammation through crosstalk with the Hippo-YAP/NF-κB pathway.60 Besides, studies have shown that titanium ions can activate the RANK/RANKL pathway and promote osteoclast differentiation. Titanium ions may up-regulate the expression of RANKL and M-CSF in osteoblasts by activating inflammatory factors and also act on osteoclast precursor cells to further induce the formation of osteoclasts.61 In summary, it is undisputed that the presence of free titanium ions around the implant and the accumulation of abnormal titanium ions can induce the loss of surrounding bone through a highly complex mechanism (Fig. 2).
Developments in material science and processing technologies have driven the emergence of new dental implant materials. Apart from commercial pure titanium and Ti–6Al–4V, other titanium alloy implant materials have been developed. Ti–Zr alloy has improved corrosion resistance and mechanical properties compared to conventional materials.62 In addition, Ti–Ag, Ti–Cu and other titanium alloys have attracted attention due to their good mechanical and biological properties. However, more prospective trials and experimental studies in animal models are needed to provide an improved scientific theoretical basis for future clinical applications.63,64
3. Peri-implant bone remodeling of implants with metal ions incorporation and mechanisms
3.1 Magnesium ions
Magnesium is an essential trace element in the human body that plays a vital role in metabolic activities.65 50% of the total magnesium exists in bone in the human body.66 Magnesium can regulate the transport of potassium and calcium ions, maintain the structure and function of substances. Besides, it can catalyze the activation and inhibition of enzymes and regulate the process of the cell cycle, proliferation and differentiation. Magnesium is also involved in maintaining the stability of the genome as it is associated with the production of oxidative stress and carcinogenesis.67 Besides, Mg is a biodegradable element. However, the appropriate incorruption onto the titanium surface can promote the corrosion resistance.68The role of magnesium in bone formation has been extensively explored. Studies have shown that magnesium is an essential element for HA cations in bone tissue and abnormal concentrations of magnesium ions cause alterations in the structure of HA.69,70 More and more studies have shown that the effect of magnesium on bone metabolism was mainly reflected in the activation of osteoblasts and inhibition of osteoclasts to promote osteogenic development and maintain the strength and density of bones at a certain concentration.71,72 It has been proposed that 6–10 mM magnesium ions can improve osteogenic activity and differentiation, however, when the concentration of magnesium ions exceeded 18 mM, it had the opposite effects.73 Magnesium ions can increase the proliferation, differentiation and adhesion of pre-osteoblasts, while the excessively high concentrations can inhibit these processes and the mineralization of the extracellular matrix by competing with calcium ions for calcium ion channels. These processes interfere with the balance of intracellular and extracellular calcium ions.74 In animal models, studies have shown that new bones form faster around magnesium alloys after implantation.75
Based on the special osteogenic ability of magnesium, magnesium alloys and Mg-containing surface modification technology have gradually become the focus of research in the field of biomedicine. Magnesium ions have been immobilized onto titanium surfaces using different methods and improve the performance of implants. Mg was successfully immobilized onto a titanium surface by treating the titanium with 0.1 mol L−1 MgCl2 solution at 200 °C by hydrothermal treatment. The study found that the number of hydroxyapatite globular clusters on the surface of the specimens increased after hydrothermal treatment. These data suggested that the incorporation of magnesium ions under hydrothermal treatment could improve osseointegration without affecting the macroscopic morphology of implants.76 In another study, pure titanium was alkalized in a high concentration sodium hydroxide (NaOH) solution and then transferred to dilute magnesium chloride (MgCl2) solution for ions exchange, and finally heat treated. The 3D nano-reticular structure containing magnesium titanate was obtained on the surface of the pure titanium sample that could accelerate the adsorption of bovine serum albumin (BSA) on its surface. It was further demonstrated that the Mg-containing surface could promote the adhesion, proliferation and differentiation of MC3T3-E1 cells.77 Sul et al. described in their research that the micro-arc oxidation method could make magnesium ions dissolved in electrolyte and then enter the oxide film of titanium. The surface of magnesium titanate formed by micro-arc oxidation can release magnesium ions and combine with a large number of bone matrix proteins through electrostatic adsorption. This process acts to promote contact between the implant and the bone, thereby promoting biochemical bonding and enhancing the osseointegration the modified oxide film could accelerate the osseointegration.78
Although many studies have demonstrated that modified titanium implants incorporated with magnesium ions can accelerate osseointegration and improve the performance of implants, the mechanisms involved remain unclear. Magnesium has been associated with osteogenic signaling pathways and may in part explain the mechanism by which magnesium promotes new bone formation. Studies have shown that magnesium ions increase the expression of VEGF and type X collagen by up-regulating the transcription factors of the HIF family (HIF-1 and HIF-2) in bone marrow mesenchymal stem cells. These changes improve intraosseous vascular regeneration and osteogenic differentiation. Magnesium ions can also promote the expression of VEGF and type X collagen by combining with calcitonin to improve PGC-1α, and play an important role in the process of osteogenic differentiation.79 Studies have demonstrated that magnesium ions can regulate the activity of osteoclasts and inhibit the expression of NFATc1 by blocking the RANK/RANKL/NF-κB pathway, which is an important signaling axis of osteoclast differentiation. Changes in the expression of osteoclast-related genes and proteins (TRAP, CTR, MMP9) are inhibited and reduce osteoclast activity.80 The molecular mechanism of how magnesium ions promote osteogenic activation and inhibit osteoclasts require further investigation and will provide a strong theoretical for the future development of modified materials to improve the success of dental implants.
3.2 Silver ions
Silver ions have excellent antibacterial properties and do not enable the development of drug resistance.81 Silver ions have also been shown to have anti-inflammatory effects. In the early stages after oral implant implantation, the initial healing process is affected by the proinflammatory responses of inflammatory cells. In the later stages, inflammation around implants caused by bacteria and other microorganisms can impact the stability of osseointegration and retention of implants.82 Silver ions induce the differentiation of stem cells into osteoblasts.83 Therefore, the antibacterial, anti-inflammatory and osteogenic effects of silver ions may improve the performance of traditional implants by enhancing the healing ability of tissues around the implant and the formation of new bones.84Studies have investigated silver loading on the surface of titanium implants. Cao et al. used PIII to construct a silver coating on the surface of titanium that resulted in antibacterial properties.85 Marta et al. demonstrated improved corrosion resistance and antibacterial properties after silver doping on titanium dioxide nanotubes.86 Studies have also embedded silver ions onto the surface of pure titanium to construct a nano-scale silver-doped surface. The structure are very stable and the peripheral release of silver ions resulting in low cellular toxicity. This silver-containing titanium surface have improved biosafety and antibacterial ability properties.87
Other studies have found that the surface of titanium after implantation of silver ions can improve the early adhesion of bone marrow mesenchymal stem cells. This can also increase ALP activity, the level of mineralization and up-regulate osteogenic gene expression to improve osseointegration.88 After the electrodeposition of silver on the surface of anodized titanium nanotubes, it has been shown that titanium nanotubes affect inflammatory reactions in soft tissues to provide a favorable environment for implant osseointegration.89
The specific metabolic mechanisms of silver doping on implant surfaces remain to be fully determined. It has been hypothesized that the antibacterial property of modified surface nano-silver/titanium oxide is based on the synergistic interface effect of “Schottky contact”. The enrichment of electrons can induce an oxidation reaction and reduce colonization of the bacteria on the material surface, whilst cells on the surface do not have the electron-conducting structures and do not produce the oxidation reaction.90 Silver can be introduced into the titanium surface by PIII. This silver-incorporated structure can up-regulate integrin α5 expression in stem cells and activate osteogenic markers by cooperating with the MAPK/ERK signal axis to promote osseointegration.91 The mechanism of silver-containing titanium structures continues to be explored and will provide a molecular basis for reducing infection and enhancing osseointegration.
3.3 Zinc ions
Zinc is also an essential trace element in the human body that is widely involved in metabolic processes.92 Zinc also has a very high antioxidant capacity and plays an antibacterial role.93 Studies have reported the relationship between zinc and bone metabolism, confirming that zinc is an important cofactor for ALP and collagenase, and is involved in the differentiation and mineralization of osteoblasts.94 Zinc has been shown to regulate osteogenic activity and can enhance the healing effect of surgical implants for osteoporosis.95 Vivo experiments in rat models showed that the healing rate of peripheral bone is improved and the stability of osseointegration increased after the implantation of zinc-containing implants.96 It has been reported that zinc can promote the proliferation and differentiation of MC3T3-E1 cells at a dose of 50 μM.97Zinc ions are essential to the activity of the zinc finger transcription factors (vitamin D receptor and Osterix protein) that regulate bone metabolism and anti-inflammatory effects.98,99 At present, the incorporation of zinc onto the surface of titanium implants is also a trend of surface modification, and the release of zinc ions has a great impact on osseointegration. Experimental results have shown that zinc can be immobilized onto the surface of titanium by micro-arc oxidation and hydrothermal technology. When zinc ions are present at a concentration of 3.53 mg L−1 they can significantly promote the proliferation of MC3T3-E1 cells.100 When the atomic percentage of zinc ions immobilized in titanium surface coating is 13.54%, the proliferation of osteoblasts and the spreading morphology of pseudopodium is significantly improved along with the up-regulated activity of ALP.101 Zinc loading surfaces can enhance the activity of ALP and promote the expression of osteogenic related genes such as OCN and Runx2 in osteoblasts.102
Zn-containing titanium nanotubes show that the size of nanotubes can affect the release of zinc ions and further affect the osteogenic differentiation of MC3T3-E1 cells by regulating the function of macrophages.103 In the process of zinc ion deposition on the titanium surfaces, longer deposition times and higher zinc contents decrease of the levels E. coli and S. aureus. These data proved that Zn-containing titanium surfaces can inhibit the adhesion and growth of bacteria and reduce the inflammatory response around the implant.104 The mechanism of zinc in promoting osseointegration is through to involve the action of oxygen free radicals to remove the wound around the bone and create favorable conditions for new bone formation.105 Zhu et al. obtained a zinc-containing titanium surface by hydrothermal treatment and found increased corrosion resistance of this surface under oxidative stress.106 Also, Shao et al. detected improved biocompatibility and antibacterial properties of zinc-containing nanowires.107 Further studies are required to demonstrate the molecular mechanism of zinc ions in bone metabolism.
3.4 Calcium ions
Calcium is an essential signal transduction ion in cells that regulates the transcription of many genes and transcription factors to control biological functions. 99% of the calcium in the human body is deposited in the bones and teeth in the form of bone salts.108 The bone is the main site of calcium deposition. Bone calcium exists in the form of hydroxyapatite that is also a vital component of commercial bone substitute materials, scaffolds and coatings. It is an indisputable fact that a certain amount of calcium ions can promote osteogenesis.109 Studies indicated that calcium-containing titanium implants had better corrosion behaviors.110 Barradas et al. found that both tricalcium phosphate and hydroxyapatite can induce high expression levels of osteogenic differentiation markers in mesenchymal stem cells to promote bone formation.111Many approaches have aimed to improve the performance of implants by incorporating calcium onto the surface of titanium. Ming et al. fabricated calcium-containing micro–nano-titanium by introducing calcium ions onto the surface of SLA titanium implants using hydrothermal treatment. This increased surface hydrophilicity and promoted the pseudopodium spread of MC3T3-E1 cells. The study also found that the surface could slowly release 0.85 ppm calcium ions, which promoted the proliferation and up-regulated expression of osteogenic protein molecule (Runx2, OCN) of osteoblasts. It proved that the introduction of calcium ions could promote the osteogenic ability to a certain extent.112 Shao et al. prepared calcium-containing nanowires on the surface of SLA titanium by hydrothermal treatment by adjusting the concentration ratio of calcium hydroxide to hydrogen peroxide. It was found that when the concentration ratio of calcium hydroxide to hydrogen peroxide was 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the surface of calcium-containing nanowires had better osteoblasts compatibility. This calcium-containing surface promoted adhesion and subsequent proliferation and differentiation of osteoblasts and had the potential to improve osseointegration.113 Other studies deposited calcium on the surface of titanium by PIIID also improved osseointegration.114
1, the surface of calcium-containing nanowires had better osteoblasts compatibility. This calcium-containing surface promoted adhesion and subsequent proliferation and differentiation of osteoblasts and had the potential to improve osseointegration.113 Other studies deposited calcium on the surface of titanium by PIIID also improved osseointegration.114
These data show that the mechanism of calcium in bone development is complex. It has been suggested that calcium ions can participate in regulating the differentiation of osteoblasts through the Wnt signaling pathway.115 Also, calcium ions mainly monitor intracellular and extracellular calcium concentrations through the Ca channel protein and downstream related signaling molecules that are involved in regulating osteogenic behaviors.116 Further study is required to clarify the mechanism of calcium ions in bone metabolism that may guide the development and application of calcium-containing titanium modified materials.
3.5 Strontium ions
Strontium is a trace element that mostly exists in the skeletal system. A large number of studies have shown that strontium is closely related to bone metabolism and plays an important role in osteogenesis–osteoclast balance.117 Strontium is a homologous element of calcium. It can replace calcium to form strontium apatite and is often mixed with calcium in biomaterials.117,118 Wang prepared strontium ions doped with zeolite coatings on a titanium surface and found Sr ions were slowly released to promote corrosion resistance and bioactivity.119 Strontium has been immobilized onto surfaces using the hydrothermal method and has been shown to promote adhesion and proliferation in osteoblasts.120 2–25% of strontium can be immobilized onto titanium surfaces by micro-arc oxidation and increasing strontium content directly affects osteoblasts.121The mechanism of strontium action in bone metabolism is thought to be through the promotion of differentiation and mineralization mediated by the increased expression of ALP and bone sialic acid glycoprotein in osteoblasts.122 Strontium can also regulate differentiation through the RANK/RANKL axis of osteoclasts to inhibit bone resorption.123,124 Others have demonstrated that strontium can inhibit the expression of mRNA and osteoclast-specific gene expression (TRAP, cathepsin K, MMP9, NFATc1) by inhibiting RANKL-mediated NF-κB and Akt/NFATc1 by constructing a strontium-coated coating on titanium nanotubes. Thereby inhibiting the formation of osteoclasts and promoting bone healing.125 Further study of the mechanism of strontium on osseointegration can help to better define the properties of strontium doped titanium implants and inform the development of novel implant materials to improve osseointegration.
3.6 Other metal ions
Other trace elements such as manganese, copper and iron have some attractive physical and chemical properties. Coatings containing manganese can be prepared by micro-arc oxidation and PIIID, and show excellent corrosion resistance. It can also promote the secretion of collagen and mineralization of the extracellular matrix resulting in a better ability to promote bone differentiation.126,127 Iron ions immobilized onto titanium dioxide coatings can significantly promote the antibacterial activity and proliferation of MC3T3-E1 cells along with the production of pseudopods that are conducive to cell adhesion.128 The incorporation of copper on the surface of titanium suggests that low dose copper could promote the proliferation of bone marrow mesenchymal stem cells, up-regulate the expression of osteogenic related genes, and induce angiogenesis.1294. Application of metal elements in improving implant performance
The surface modification of most implants with metal ions is limited to certain elements that give excellent properties but these can be further optimized using several metals. In clinical applications, the osseointegration, antibacterial and anti-inflammatory properties of implants are the most critical factors. Optimum approaches to introduce metals onto the surface of implants and drive complementary advantages is an area of intense research interest.Studies have shown that zinc and silver co-containing titanium surface could form micro-galvanic couples, which could improve the corrosion resistance. Besides, antibacterial and osteogenic properties have been promoted due to the released Zn ions and the embedded Ag NPs.130 The incorporation of magnesium and silver reduces cytotoxicity to cells and provides better antibacterial and osteogenesis effects on the titanium surface.131 Also, the combination of zinc and magnesium can improve the osteoinductive ability of titanium substrates to accelerate the formation of blood vessels improve the antibacterial properties.132 The incorporation of silver and calcium induces the creation of “micro-batteries” on the surface of the titanium. The cathode of the “micro-battery” can undergo a hydrogen evolution reaction whilst the anode can undergo a corrosion reaction. The combination of the two can cause excessive oxidative stress around the bacteria to reduce the adverse effects.133 Future modification technologies should aim to incorporate multiple metal ions onto the implant surface to amplify the promotion of bone metabolism around the implant and improve overall performance.
5. Conclusions
Research developments have aimed to use surface treatment technologies to optimize the performance of implants. The new technology of introducing exogenous metal ions with different properties onto the surface of implants results in advantages including improved osseointegration, corrosion resistance, antibacterial and anti-inflammatory properties.However, metal elements have associated levels of biological toxicity. Titanium or cobalt–chromium alloy is widely used in implant materials and may release excessive titanium, cobalt or chromium ions causing damage to surrounding tissues and affect osseointegration. Only when metal elements are used at safe concentrations can the best biological effects be obtained to promote bone metabolism. At present, many studies on the modification of implant surface by adding metal elements just remain at the level of the phenomenon that the introduction of elements can promote bone formation and development. There are still blanks in the appropriate concentration of metal ions on the surface of the modified material, the actual concentration of exogenous metal ions around the modified implants applied to the in vivo experiment, and the mechanism of released metal ions. Future studies should focus on the relationship between the introduction dose of exogenous metal ions on the implant surface and bone metabolism around the implant. It is crucial to elucidate the molecular mechanism of the effect of exogenous metal ions on bone metabolism and to better understand the material-biological properties from the incorporation of metal elements at the molecular level. In this way, we can determine the optimum concentrations at which exogenous metal ions are doped in surface modifications to improve the performance of titanium implants in the clinic.
Author contributions
WC contributed to the conception, literature collection, organization and drafted the manuscript. WQZ contributed to the conception, literature collection and organization. JQ contributed to the conception, literature collection, organization in the present study and critically revised the manuscript. All authors agreed to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.Conflicts of interest
All authors declare that they have no conflicts of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Project Number: 81870799), the Jiangsu Provincial Key Research and Development Program (Project Number: BE2019728), the Jiangsu Provincial Medical Youth Talent (Project Number: QNRC2016850), the Nanjing Medical University-SUYAN Group Intelligent Innovation Research and Development Project (Project Number: NMU-SY201806), the Southeast University-Nanjing Medical University Cooperative Research Project (Project Number: 2242017K3DN14), and the Foundation of Priority Academic Program Development of Jiangsu Higher Education Institutions (Project Number: 2018-87).References
- Y. Oshida, E. B. Tuna, O. Aktören and K. Gençay, Int. J. Mol. Sci., 2010, 11, 1580–1678 CrossRef CAS PubMed.
- L. Ottria, D. Lauritano, M. Andreasi Bassi, A. Palmieri, V. Candotto, A. Tagliabue and L. Tettamanti, J. Biol. Regul. Homeostatic Agents, 2018, 32, 81–90 CAS.
- F. Yu, O. Addison and A. J. Davenport, Acta Biomater., 2015, 26, 355–365 CrossRef CAS PubMed.
- A. Revathi, A. D. Borrás, A. I. Muñoz, C. Richard and G. Manivasagam, Mater. Sci. Eng. C, 2017, 76, 1354–1368 CrossRef CAS PubMed.
- R. L. W. Messer, G. Tackas, J. Mickalonis, Y. Brown, J. B. Lewis and J. C. Wataha, J. Biomed. Mater. Res., Part B, 2009, 88, 474–481 CrossRef.
- K. Apaza-Bedoya, M. Tarce, C. A. M. Benfatti, B. Henriques, M. T. Mathew, W. Teughels and J. C. M. Souza, J. Periodontal Res., 2017, 52, 946–954 CrossRef CAS PubMed.
- W.-Q. Zhu, P.-P. Ming, J. Qiu, S.-Y. Shao, Y.-J. Yu, J.-X. Chen, J. Yang, L.-N. Xu, S.-M. Zhang and C.-B. Tang, J. Appl. Toxicol., 2018, 38, 824–833 CrossRef CAS PubMed.
- T. Puskar, D. Jevremovic, R. J. Williams, D. Eggbeer, D. Vukelic and I. Budak, Materials, 2014, 7, 6486–6501 CrossRef PubMed.
- B. Sampson and A. Hart, Ann. Clin. Biochem., 2012, 49, 118–131 CrossRef CAS PubMed.
- J. C. Rubio, M. C. Garcia-Alonso, C. Alonso, M. A. Alobera, C. Clemente, L. Munuera and M. L. Escudero, J. Mater. Sci.: Mater. Med., 2008, 19, 369–375 CrossRef CAS PubMed.
- X. He, F. X. Reichl, Y. Wang, B. Michalke, S. Milz, Y. Yang, P. Stolper, G. Lindemaier, M. Graw, R. Hickel and C. Högg, Dent. Mater., 2016, 32, 1042–1051 CrossRef CAS PubMed.
- A. Wennerberg, A. Ide-Ektessabi, S. Hatkamata, T. Sawase, C. Johansson, T. Albrektsson, A. Martinelli, U. Södervall and H. Odelius, Clin. Oral Implants Res., 2004, 15, 505–512 CrossRef PubMed.
- D. Weingart, S. Steinemann, W. Schilli, J. R. Strub, U. Hellerich, J. Assenmacher and J. Simpson, Int. J. Oral Surg., 1994, 23, 450–452 CrossRef CAS.
- H. Schliephake, G. Reiss, R. Urban, F. W. Neukam and S. Guckel, Int. J. Oral Maxillofac. Implants, 1993, 8, 502–511 CAS.
- A. Sarmiento-González, J. R. Encinar, J. M. Marchante-Gayón and A. Sanz-Medel, Anal. Bioanal. Chem., 2009, 393, 335–343 CrossRef PubMed.
- H. Chouirfa, H. Bouloussa, V. Migonney and C. Falentin-Daudré, Acta Biomater., 2019, 83, 37–54 CrossRef CAS PubMed.
- L. Le Guéhennec, A. Soueidan, P. Layrolle and Y. Amouriq, Dent. Mater., 2007, 23, 844–854 CrossRef PubMed.
- G. Lin, C. Zhou, M. Lin, A. Xu and F. He, Clin. Oral Implants Res., 2019, 30, 777–790 CrossRef PubMed.
- J. Vishnu, K. M. Vignesh, V. Gopal, C. Bartomeu Garcia, P. Hameed, G. Manivasagam and T. J. Webster, Nanomedicine, 2019, 20, 102016 CrossRef CAS PubMed.
- Y. Liang, J. Xu, J. Chen, M. Qi, X. Xie and M. Hu, Mol. Med. Rep., 2015, 11, 4225–4231 CrossRef CAS PubMed.
- J. Xu, G. Ding, J. Li, S. Yang, B. Fang, H. Sun and Y. Zhou, Appl. Surf. Sci., 2010, 256, 7540–7544 CrossRef CAS.
- Y. Li, W. Wang, H. Liu, J. Lei, J. Zhang, H. Zhou and M. Qi, Mater. Sci. Eng. C, 2018, 87, 90–103 CrossRef CAS PubMed.
- P. J. Hou, K. L. Ou, C. C. Wang, C. F. Huang, M. Ruslin, E. Sugiatno, T. S. Yang and H. H. Chou, J. Mech. Behav. Biomed. Mater., 2018, 79, 173–180 CrossRef CAS PubMed.
- M. Furko, Y. Jiang, T. A. Wilkins and C. Balázsi, Mater. Sci. Eng. C, 2016, 62, 249–259 CrossRef CAS PubMed.
- M. Lu, H. Chen, B. Yuan, Y. Zhou, L. Min, Z. Xiao, X. Zhu, C. Tu and X. Zhang, Int. J. Nanomed., 2020, 15, 6605–6618 CrossRef CAS PubMed.
- A. Jaafar, C. Hecker, P. Árki and Y. Joseph, Bioengineering, 2020, 7, 127 CrossRef CAS PubMed.
- M. Sharafipour, H. Oveisi and A. Meshkini, J. Biomed. Mater. Res., Part A, 2020, 108, 882–894 CrossRef CAS PubMed.
- P. Hameed, V. Gopal, S. Bjorklund, A. Ganvir, D. Sen, N. Markocsan and G. Manivasagam, Colloids Surf., B, 2019, 173, 806–815 CrossRef CAS PubMed.
- J. A. Jansen, J. G. Wolke, S. Swann, J. P. Van der Waerden and K. de Groot, Clin. Oral Implants Res., 1993, 4, 28–34 CrossRef CAS PubMed.
- A. I. Kozelskaya, E. N. Bolbasov, A. S. Golovkin, A. I. Mishanin, A. N. Viknianshchuk, E. V. Shesterikov, A. Ashrafov, V. A. Novikov, A. Y. Fedotkin, I. A. Khlusov and S. I. Tverdokhlebov, Materials, 2018, 11, 1949 CrossRef PubMed.
- X. Hu, R. Xu, X. Yu, J. Chen, S. Wan, J. Ouyang and F. Deng, Biomed. Mater. (Bristol, U. K.), 2018, 13, 045015 CrossRef PubMed.
- H. Xing, R. Li, Y. Wei, B. Ying, D. Li and Y. Qin, Front. Bioeng. Biotechnol., 2020, 8, 367 CrossRef PubMed.
- J. L. Gilbert, S. Sivan, Y. Liu, S. B. Kocagöz, C. M. Arnholt and S. M. Kurtz, J. Biomed. Mater. Res., Part A, 2015, 103, 211–223 CrossRef PubMed.
- Y. Liu and J. L. Gilbert, J. Biomed. Mater. Res., Part B, 2018, 106, 209–220 CrossRef CAS PubMed.
- L. Reclaru, H. Lüthy, P. Y. Eschler, A. Blatter and C. Susz, Biomaterials, 2005, 26, 4358–4365 CrossRef CAS PubMed.
- B. Scharf, C. C. Clement, V. Zolla, G. Perino, B. Yan, S. G. Elci, E. Purdue, S. Goldring, F. Macaluso, N. Cobelli, R. W. Vachet and L. Santambrogio, Sci. Rep., 2014, 4, 5729 CrossRef CAS PubMed.
- A. Jonitz-Heincke, J. Tillmann, A. Klinder, S. Krueger, J. P. Kretzer, P. J. Høl, A. C. Paulus and R. Bader, Materials, 2017, 10, 734 CrossRef PubMed.
- S. Drynda, A. Drynda, B. Feuerstein, J. Kekow, C. H. Lohmann and J. Bertrand, J. Biomed. Mater. Res., Part A, 2018, 106, 2105–2115 CrossRef CAS PubMed.
- C. Tkaczyk, O. L. Huk, F. Mwale, J. Antoniou, D. J. Zukor, A. Petit and M. Tabrizian, J. Biomed. Mater. Res., Part A, 2010, 94, 214–222 CrossRef PubMed.
- S. Noumbissi, A. Scarano and S. Gupta, Materials, 2019, 12, 368 CrossRef CAS PubMed.
- X. Y. Yu, W. Q. Zhu, W. Chen, W. Q. Chen, S. M. Zhang and J. Qiu, Mater. Sci. Eng. C, 2021, 119, 111610 CrossRef CAS PubMed.
- L.-n. Xu, X.-y. Yu, W.-q. Chen, S.-m. Zhang and J. Qiu, RSC Adv., 2020, 10, 8198–8206 RSC.
- S. M. Zhang, J. Qiu, F. Tian, X. K. Guo, F. Q. Zhang and Q. F. Huang, J. Mater. Sci.: Mater. Med., 2013, 24, 1229–1237 CrossRef CAS PubMed.
- T. Hanawa, K. Asami and K. Asaoka, J. Biomed. Mater. Res., 1998, 40, 530–538 CrossRef CAS.
- K. T. Kim, M. Y. Eo, T. T. H. Nguyen and S. M. Kim, Int. J. Implant Dent., 2019, 5, 10 CrossRef PubMed.
- T. Fretwurst, K. Nelson, D. P. Tarnow, H. L. Wang and W. V. Giannobile, J. Dent. Res., 2018, 97, 259–265 CrossRef CAS PubMed.
- T. Wachi, T. Shuto, Y. Shinohara, Y. Matono and S. Makihira, Toxicology, 2015, 327, 1–9 CrossRef CAS PubMed.
- A. B. Ferguson Jr, P. G. Laing and E. S. Hodge, J. Bone Jt. Surg., Am. Vol., 1960, 42, 77–90 CrossRef.
- J. J. Jacobs, A. K. Skipor, J. Black, R. m. Urban and J. O. Galante, J. Bone Jt. Surg., Am. Vol., 1991, 73, 1475–1486 CrossRef CAS.
- P. Ducheyne, G. Willems, M. Martens and J. Helsen, J. Biomed. Mater. Res., 1984, 18, 293–308 CrossRef CAS PubMed.
- H. Liao, T. Wurtz and J. Li, J. Biomed. Mater. Res., 1999, 47, 220–227 CrossRef CAS PubMed.
- G. J. Thompson and D. A. Puleo, Biomaterials, 1996, 17, 1949–1954 CrossRef CAS PubMed.
- N. C. Blumenthal and V. Cosma, J. Biomed. Mater. Res., 1989, 23, 13–22 CrossRef CAS PubMed.
- B. Meng, X. Yang, Y. Chen, J. Zhai and X. Liang, Mol. Med. Rep., 2010, 3, 1065–1069 CAS.
- D. Cadosch, O. P. Gautschi, E. Chan, H.-P. Simmen and L. Filgueira, J. Biomed. Mater. Res., Part A, 2010, 92, 475–483 Search PubMed.
- D. Cadosch, E. Chan, O. P. Gautschi, J. Meagher, R. Zellweger and L. Filgueira, J. Biomed. Mater. Res., Part A, 2009, 91, 29–36 CrossRef PubMed.
- R. Messous, B. Henriques, H. Bousbaa, F. S. Silva, W. Teughels and J. C. M. Souza, Clin. Oral Invest., 2021, 25, 1627–1640 CrossRef PubMed.
- X. Wang, Y. Li, Y. Feng, H. Cheng and D. Li, J. Periodontal Res., 2019, 54, 329–338 CrossRef CAS PubMed.
- E. A. Fritz, T. T. Glant, C. Vermes, J. J. Jacobs and K. A. Roebuck, J. Orthop. Res., 2002, 20, 490–498 CrossRef CAS PubMed.
- K. M. Tang, W. Chen, Z. H. Tang, X. Y. Yu, W. Q. Zhu, S. M. Zhang and J. Qiu, J. Appl. Toxicol., 2021, 41, 561–571 CrossRef CAS PubMed.
- S. Qiu, F. Zhao, X. Tang, F. Pei, H. Dong, L. Zhu and K. Guo, Mol. Cell. Biochem., 2015, 399, 131–141 CrossRef CAS PubMed.
- J. M. Cordeiro, L. P. Faverani, C. R. Grandini, E. C. Rangel, N. C. da Cruz, F. H. Nociti Junior, A. B. Almeida, F. B. Vicente, B. R. G. Morais, V. A. R. Barão and W. G. Assunção, Mater. Sci. Eng. C, 2018, 92, 849–861 CrossRef CAS PubMed.
- J. Hu, H. Li, X. Wang, L. Yang, M. Chen, R. Wang, G. Qin, D. F. Chen and E. Zhang, Mater. Sci. Eng. C, 2020, 115, 110921 CrossRef CAS PubMed.
- Z. Lei, H. Zhang, E. Zhang, J. You, X. Ma and X. Bai, Mater. Sci. Eng. C, 2018, 92, 121–131 CrossRef CAS PubMed.
- S. L. Volpe, Adv. Nutr., 2013, 4, 378s–383s CrossRef CAS PubMed.
- T. Okuma, Nutrition, 2001, 17, 679–680 CrossRef CAS.
- J. H. de Baaij, J. G. Hoenderop and R. J. Bindels, Physiol. Rev., 2015, 95, 1–46 CrossRef PubMed.
- A. Bigham, A. Saudi, M. Rafienia, S. Rahmati, H. Bakhtiyari, F. Salahshouri, M. Sattary and S. A. Hassanzadeh-Tabrizi, Mater. Sci. Eng. C, 2019, 96, 765–775 CrossRef CAS PubMed.
- A. Laskus and J. Kolmas, Int. J. Mol. Sci., 2017, 18, 2542 CrossRef PubMed.
- J. Zhang, L. Tang, H. Qi, Q. Zhao, Y. Liu and Y. Zhang, Adv. Healthcare Mater., 2019, 8, e1901030 CrossRef PubMed.
- L. Wu, F. Feyerabend, A. F. Schilling, R. Willumeit-Römer and B. J. C. Luthringer, Acta Biomater., 2015, 27, 294–304 CrossRef CAS PubMed.
- F. Mammoli, S. Castiglioni, S. Parenti, C. Cappadone, G. Farruggia, S. Iotti, P. Davalli, J. A. M. Maier, A. Grande and C. Frassineti, Int. J. Mol. Sci., 2019, 20, 385 CrossRef PubMed.
- J. Wang, X.-Y. Ma, Y.-F. Feng, Z.-S. Ma, T.-C. Ma, Y. Zhang, X. Li, L. Wang and W. Lei, Biol. Trace Elem. Res., 2017, 179, 284–293 CrossRef CAS PubMed.
- L. Zhang, C. Yang, J. Li, Y. Zhu and X. Zhang, Biochem. Biophys. Res. Commun., 2014, 450, 1390–1395 CrossRef CAS PubMed.
- Q. Zhao, L. Yi, L. Jiang, Y. Ma, H. Lin and J. Dong, Nanomedicine, 2019, 14, 1109–1133 CrossRef PubMed.
- X. Shi, M. Nakagawa, G. Kawachi, L. Xu and K. Ishikawa, J. Mater. Sci.: Mater. Med., 2012, 23, 1281–1290 CrossRef CAS PubMed.
- Y. Lai, Y. Li, H. Cao, J. Long, X. Wang, L. Li, C. Li, Q. Jia, B. Teng, T. Tang, J. Peng, D. Eglin, M. Alini, D. W. Grijpma, G. Richards and L. Qin, Biomaterials, 2019, 197, 207–219 CrossRef CAS PubMed.
- Y.-T. Sul, C. Johansson, E. Byon and T. Albrektsson, Biomaterials, 2005, 26, 6720–6730 CrossRef CAS PubMed.
- S. Yoshizawa, A. Brown, A. Barchowsky and C. Sfeir, Connect. Tissue Res., 2014, 55(suppl 1), 155–159 CrossRef CAS PubMed.
- M. Li, W. Wang, Y. Zhu, Y. Lu, P. Wan, K. Yang, Y. Zhang and C. Mao, Acta Biomater., 2018, 77, 365–379 CrossRef CAS PubMed.
- I. X. Yin, J. Zhang, I. S. Zhao, M. L. Mei, Q. Li and C. H. Chu, Int. J. Nanomed., 2020, 15, 2555–2562 CrossRef CAS PubMed.
- V. T. Noronha, A. J. Paula, G. Durán, A. Galembeck, K. Cogo-Müller, M. Franz-Montan and N. Durán, Dent. Mater., 2017, 33, 1110–1126 CrossRef CAS PubMed.
- S. Patil and N. Singh, Colloids Surf., B, 2019, 176, 150–155 CrossRef CAS PubMed.
- M. Ma, R. Wan, H. Gong, X. Lv, S. Chu, D. Li, H. Gu and C. Peng, J. Nanosci. Nanotechnol., 2019, 19, 3777–3791 CrossRef CAS PubMed.
- H. Cao, X. Liu, F. Meng and P. K. Chu, Biomaterials, 2011, 32, 693–705 CrossRef CAS PubMed.
- M. Nycz, K. Arkusz and D. G. Pijanowska, Nanomaterials, 2019, 9, 1072 CrossRef CAS PubMed.
- H. Cao, W. Zhang, F. Meng, J. Guo, D. Wang, S. Qian, X. Jiang, X. Liu and P. K. Chu, ACS Appl. Mater. Interfaces, 2017, 9, 5149–5157 CrossRef CAS PubMed.
- A. Mohandas, A. G. Krishnan, R. Biswas, D. Menon and M. B. Nair, Mater. Sci. Eng. C, 2017, 75, 115–124 CrossRef CAS PubMed.
- A. Cochis, S. Ferraris, R. Sorrentino, B. Azzimonti, C. Novara, F. Geobaldo, F. Truffa Giachet, C. Vineis, A. Varesano, A. Sayed Abdelgeliel, S. Spriano and L. Rimondini, J. Mater. Chem. B, 2017, 5, 8366–8377 RSC.
- J. Li, X. Liu, Y. Qiao, H. Zhu and C. Ding, Colloids Surf., B, 2014, 113, 134–145 CrossRef CAS PubMed.
- H. Cao, W. Zhang, F. Meng, J. Guo, D. Wang, S. Qian, X. Jiang, X. Liu and P. K. Chu, ACS Appl. Mater. Interfaces, 2017, 9, 5149–5157 CrossRef CAS PubMed.
- S. Choi, X. Liu and Z. Pan, Acta Pharmacol. Sin., 2018, 39, 1120–1132 CrossRef CAS.
- P. Petrini, C. R. Arciola, I. Pezzali, S. Bozzini, L. Montanaro, M. C. Tanzi, P. Speziale and L. Visai, Int. J. Artif. Organs, 2006, 29, 434–442 CrossRef CAS PubMed.
- K. Yusa, O. Yamamoto, M. Iino, H. Takano, M. Fukuda, Z. Qiao and T. Sugiyama, Arch. Oral Biol., 2016, 71, 162–169 CrossRef CAS PubMed.
- K. Yusa, O. Yamamoto, H. Takano, M. Fukuda and M. Iino, Sci. Rep., 2016, 6, 29462 CrossRef CAS PubMed.
- Y. Qiao, W. Zhang, P. Tian, F. Meng, H. Zhu, X. Jiang, X. Liu and P. K. Chu, Biomaterials, 2014, 35, 6882–6897 CrossRef CAS PubMed.
- D. Liang, M. Yang, B. Guo, J. Cao, L. Yang and X. Guo, Biol. Trace Elem. Res., 2012, 146, 340–348 CrossRef CAS PubMed.
- M. Jarosz, M. Olbert, G. Wyszogrodzka, K. Młyniec and T. Librowski, Inflammopharmacology, 2017, 25, 11–24 CrossRef CAS PubMed.
- K. Nakashima, X. Zhou, G. Kunkel, Z. Zhang, J. M. Deng, R. R. Behringer and B. de Crombrugghe, Cell, 2002, 108, 17–29 CrossRef CAS PubMed.
- A. Ito, K. Ojima, H. Naito, N. Ichinose and T. Tateishi, J. Biomed. Mater. Res., 2000, 50, 178–183 CrossRef CAS PubMed.
- X. Shen, Y. Hu, G. Xu, W. Chen, K. Xu, Q. Ran, P. Ma, Y. Zhang, J. Li and K. Cai, ACS Appl. Mater. Interfaces, 2014, 6, 16426–16440 CrossRef CAS PubMed.
- M. Yamaguchi, M. Goto, S. Uchiyama and T. Nakagawa, Mol. Cell. Biochem., 2008, 312, 157–166 CrossRef CAS PubMed.
- B. Chen, Y. You, A. Ma, Y. Song, J. Jiao, L. Song, E. Shi, X. Zhong, Y. Li and C. Li, Int. J. Nanomed., 2020, 15, 2095–2118 CrossRef CAS PubMed.
- G. Jin, H. Cao, Y. Qiao, F. Meng, H. Zhu and X. Liu, Colloids Surf., B, 2014, 117, 158–165 CrossRef CAS PubMed.
- M. Montazerolghaem, Y. Ning, H. Engqvist, M. Karlsson Ott, M. Tenje and G. Mestres, J. Mater. Sci.: Mater. Med., 2016, 27, 23 CrossRef PubMed.
- W. Q. Zhu, S. Y. Shao, L. N. Xu, W. Q. Chen, X. Y. Yu, K. M. Tang, Z. H. Tang, F. M. Zhang and J. Qiu, J. Nanobiotechnol., 2019, 17, 55 CrossRef PubMed.
- S.-y. Shao, J.-x. Chen, H.-y. Tang, P.-p. Ming, J. Yang, W.-q. Zhu, S.-m. Zhang and J. Qiu, Appl. Surf. Sci., 2020, 515, 146107 CrossRef CAS.
- K. D. Cashman, Br. J. Nutr., 2002, 87(suppl 2), S169–S177 CrossRef CAS.
- J.-W. Park, H.-K. Kim, Y.-J. Kim, C.-H. An and T. Hanawa, Clin. Oral Implants Res., 2009, 20, 684–690 CrossRef PubMed.
- A. R. Rafieerad, M. R. Ashra, R. Mahmoodian and A. R. Bushroa, Mater. Sci. Eng. C, 2015, 57, 397–413 CrossRef CAS PubMed.
- A. M. Barradas, V. Monticone, M. Hulsman, C. Danoux, H. Fernandes, Z. Tahmasebi Birgani, F. Barrère-de Groot, H. Yuan, M. Reinders, P. Habibovic, C. van Blitterswijk and J. de Boer, Integr. Biol., 2013, 5, 920–931 CrossRef CAS PubMed.
- P.-p. Ming, S.-y. Shao, J. Qiu, J. Yang, Y.-j. Yu, J.-x. Chen, W.-q. Zhu and C.-b. Tang, Appl. Surf. Sci., 2017, 416, 790–797 CrossRef CAS.
- S. Y. Shao, P. P. Ming, Q. Jing, Y. J. Yu and C. B. Tang, RSC Adv., 2017, 7, 6753–6761 RSC.
- M. F. Maitz, R. W. Poon, X. Y. Liu, M. T. Pham and P. K. Chu, Biomaterials, 2005, 26, 5465–5473 CrossRef CAS PubMed.
- J. Bolander, Y. C. Chai, L. Geris, J. Schrooten, D. Lambrechts, S. J. Roberts and F. P. Luyten, Biomaterials, 2016, 86, 106–118 CrossRef CAS.
- Y. Liu, R. Yang, X. Liu, Y. Zhou, C. Qu, T. Kikuiri, S. Wang, E. Zandi, J. Du, I. S. Ambudkar and S. Shi, Cell Stem Cell, 2014, 15, 66–78 CrossRef CAS PubMed.
- W. E. Cabrera, I. Schrooten, M. E. De Broe and P. C. D'Haese, J. Bone Miner. Res., 1999, 14, 661–668 CrossRef CAS PubMed.
- Y. Li, W. Wang, F. Yu, D. Wang, S. Guan, Y. Li and M. Qi, Mater. Sci. Eng. C, 2020, 109, 110610 CrossRef CAS.
- S. Wang, R. Li, D. Li, Z. Y. Zhang, G. Liu, H. Liang, Y. Qin, J. Yu and Y. Li, J. Mater. Chem. B, 2018, 6, 3254–3261 RSC.
- J. Wen, J. Li, H. Pan, W. Zhang, D. Zeng, L. Xu, Q. Wu, X. Zhang, X. Liu and X. Jiang, J. Mater. Chem. B, 2015, 3, 4790–4804 RSC.
- L. Mao, L. Xia, J. Chang, J. Liu, L. Jiang, C. Wu and B. Fang, Acta Biomater., 2017, 61, 217–232 CrossRef CAS PubMed.
- G.-X. Ni, B. Shu, G. Huang, W. W. Lu and H.-B. Pan, J. Biomed. Mater. Res., Part B, 2012, 100, 562–568 CrossRef PubMed.
- S. Zhu, X. Hu, Y. Tao, Z. Ping, L. Wang, J. Shi, X. Wu, W. Zhang, H. Yang, Z. Nie, Y. Xu, Z. Wang and D. Geng, Sci. Rep., 2016, 6, 36251 CrossRef CAS PubMed.
- D. Hu, K. Li, Y. Xie, H. Pan, J. Zhao, L. Huang and X. Zheng, J. Biomater. Appl., 2017, 31, 1135–1147 CrossRef CAS PubMed.
- B. Mi, W. Xiong, N. Xu, H. Guan, Z. Fang, H. Liao, Y. Zhang, B. Gao, X. Xiao, J. Fu and F. Li, Sci. Rep., 2017, 7, 2328 CrossRef.
- L. Yu, Y. Tian, Y. Qiao and X. Liu, Colloids Surf., B, 2017, 152, 376–384 CrossRef CAS PubMed.
- Q. M. Zhao, Y. Y. Sun, C. S. Wu, J. Yang, G. F. Bao and Z. M. Cui, Nanotoxicology, 2020, 14, 289–309 CrossRef CAS PubMed.
- Y. Tian, H. Cao, Y. Qiao, F. Meng and X. Liu, Acta Biomater., 2014, 10, 4505–4517 CrossRef CAS PubMed.
- L. Yu, G. Jin, L. Ouyang, D. Wang, Y. Qiao and X. Liu, J. Mater. Chem. B, 2016, 4, 1296–1309 RSC.
- G. Jin, H. Qin, H. Cao, S. Qian, Y. Zhao, X. Peng, X. Zhang, X. Liu and P. K. Chu, Biomaterials, 2014, 35, 7699–7713 CrossRef CAS PubMed.
- Y. Zhao, H. Cao, H. Qin, T. Cheng, S. Qian, M. Cheng, X. Peng, J. Wang, Y. Zhang, G. Jin, X. Zhang, X. Liu and P. K. Chu, ACS Appl. Mater. Interfaces, 2015, 7, 17826–17836 CrossRef CAS PubMed.
- Y. Yu, G. Jin, Y. Xue, D. Wang, X. Liu and J. Sun, Acta Biomater., 2017, 49, 590–603 CrossRef CAS PubMed.
- H. Cao, K. Tang and X. Liu, Mater. Horiz., 2018, 5, 264–267 RSC.
| This journal is © The Royal Society of Chemistry 2021 |