Open Access Article
Open Access ArticleDetermination of naphazoline HCl, pheniramine maleate and their official impurities in eye drops and biological fluid rabbit aqueous humor by a validated LC-DAD method†
Khadiga M. Kelaniab,
Maha A. Hegazy a,
Amal M. Hassanb and
Mahmoud A. Tantawy
a,
Amal M. Hassanb and
Mahmoud A. Tantawy *ac
*ac
aAnalytical Chemistry Department, Faculty of Pharmacy, Cairo University, Kasr el Aini Street, Cairo, 11562, Egypt. E-mail: mahmoud.eltantawy@pharma.cu.edu.eg; matantawy@hotmail.com
bAnalytical Chemistry Department, Faculty of Pharmacy, Modern University for Technology and Information, El-hadaba El-Wosta, Mokatam, 5th district, Cairo, Egypt
cChemistry Department, Faculty of Pharmacy, October 6 University, 6 October City, Giza, Egypt
First published on 10th February 2021
Abstract
A simple RP-HPLC-DAD method was developed and validated, as per the ICH guidelines, for simultaneous determination of naphazoline HCl (NPZ) & pheniramine maleate (PHN) along with three of their official impurities. Chromatographic separation was performed on a hypersil ODS column (5 mm, 250–4.6 mm i.d.) with isocratic elution using phosphate buffer pH 6.0: acetonitrile (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, v/v) as mobile phase, at a flow rate of 1.0 mL min−1 and UV detection at 260.0 nm. The developed method was found to be linear over the concentration ranges of 5.00–45.00 μg mL−1 for NPZ and NPZ impurity B and 10.00–110.00 μg mL−1, 10–70 μg mL−1 and 10–120 μg mL−1 for PHN, and PHN impurity A and B, respectively, with correlation coefficient values <0.999 for the five cited compounds. The method was confirmed to be accurate, robust and precise with RSD >2.0%. LOD and LOQ values for the five cited compounds were calculated. Moreover, the method was also validated in rabbit aqueous humor as per the US food and drug administration (FDA) bioanalytical validation guidelines. Finally, the proposed method was applied for the analysis of the two drugs along with their impurities in dosage form and spiked aqueous humor samples.
30, v/v) as mobile phase, at a flow rate of 1.0 mL min−1 and UV detection at 260.0 nm. The developed method was found to be linear over the concentration ranges of 5.00–45.00 μg mL−1 for NPZ and NPZ impurity B and 10.00–110.00 μg mL−1, 10–70 μg mL−1 and 10–120 μg mL−1 for PHN, and PHN impurity A and B, respectively, with correlation coefficient values <0.999 for the five cited compounds. The method was confirmed to be accurate, robust and precise with RSD >2.0%. LOD and LOQ values for the five cited compounds were calculated. Moreover, the method was also validated in rabbit aqueous humor as per the US food and drug administration (FDA) bioanalytical validation guidelines. Finally, the proposed method was applied for the analysis of the two drugs along with their impurities in dosage form and spiked aqueous humor samples.
1 Introduction
With growing pharmaceutical industries, and development of modern analytical techniques, more concern is given by the pharmacopoeias and regulatory authorities to drug purity and impurity detection. Drug purity is an essential factor for ensuring drug safety and quality.1 Moreover, some of the impurities may contribute to the drug side effects, and thus development of an analytical method for their determination is a must.2According to the world allergy organization, allergic conjunctivitis is considered as a broad group of allergic conditions including; inflammation of conjunctiva, itching, edema and increased lacrimation.3 In high frequency, allergic rhinitis and asthma may also occur.4 Generally, ocular allergies affect about 5–22% of the population.5 There are different types of mast cell stabilizer and antihistaminic drugs used to control those allergies.6 In addition, decongestant drugs play an important role through reducing swelling and edema of conjunctiva.7
Naphazoline HCl (NPZ), used as a decongestant drug, has a sympathomimetic effect on α adrenergic receptors. It acts on those receptors in the arterioles of the conjunctiva, resulting in reducing swelling and edema.8 It is chemically designated as [2-(naphthalen-1-ylmethyl)-4, 5-dihydro-1H-imidazole; HCl]. It is an official drug in the US (USP)9 and British (BP)10 pharmacopeias where its assay was performed by HPLC methods. Moreover, BP reports four official impurities; A, B, C and D. Literature review revealed that NPZ has been determined either in bulk powder or in presence with other drugs using several techniques including; spectrophotometry,11–20 HPLC,21–32 TLC33 and capillary electrophoresis.34–40
Pheniramine maleate (PHN) is an antihistaminic drug with anticholinergic properties. It binds to histamine H1 receptors, leading to inhibiting phospholipase A2, reducing cyclic GMP levels, and decreasing histamine. PHN is commonly found in eye drops, which are used for treatment of allergic conjunctivitis.41 Chemically, it is [(Z)-but-2-enedioic acid; N,N-dimethyl-3-phenyl-3-pyridin-2-ylpropan-1-amine]. HPLC methods are used for its assay in USP and BP. Two impurities (A and B) were specified in BP for PHN.9,10 On the other hand, it has been determined in its bulk powder or in the presence of other drugs using several methods, such as spectrophotometric,42,43 HPLC,30–32,44–51 capillary electrophoretic40,52 and titrimetric.53
NPZ and PHN have been co-formulated in eye drops for treatment of eye inflammation and allergic conjunctivitis. This combination has found to be more effective than using each drug separately.54 Reviewing the previous literature reveals that NPZ and PHN have been simultaneously determined by HPLC30–32 and capillary electrophoresis.40 However, there is no reported analytical method dealing with their determination along with their official impurities. Therefore, the aim of this work was to analyze NPZ and PHN along with three selected impurities, namely; NPZ impurity B, PHN impurity A and B, using a validated RP-HPLC-DAD method. The method was then applied for the determination of the five compounds (Fig. 1) in their quinary mixture, eye drops and spiked rabbit aqueous humor (for the purpose of determining their extraction efficiency). In this study, animal models were preferable, as they offer more flexibility in obtaining sufficient amounts of ocular fluid without causing a tissue damage. Rabbits were the animals of choice since they are small, easy to handle, low cost, and have a similar eye size to humans.55
2 Experimental
2.1. Instruments
The HPLC-DAD system consisted of pump with different flow rates (model Waters Alliance 2695) equipped with a photodiode array detector (DAD) and a 100 mL injection loop. A hypersil ODS column (5 mm, 250–4.6 mm i.d.) was used as stationary phase. The samples were injected by auto sampler system. pH of the solutions was adjusted by a pH meter (Mettler Toledo MA 235).2.2. Materials and chemicals
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30.0 v/v, was prepared.
30.0 v/v, was prepared.2.3. Solutions
Stock standard solutions of 1.0 mg mL−1 of the five studied compounds were separately prepared by transferring 10.00 mg of each substance into 10 mL volumetric flasks. Dissolved and completed to the mark using mobile phase.2.4. Procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30.0 v/v). The drugs were separated using hypersil ODS column (5 mm, 250–4.6 mm i.d.) with a flow rate 1.0 mL min−1. The prepared buffer was filtered through 0.45 μm membrane filter. The injection volume was 10.0 μL and the chromatograms were scanned from 200.0–400.0 nm by the aid of DAD and it was found that at 260.0 nm optimum resolution was achieved.58
30.0 v/v). The drugs were separated using hypersil ODS column (5 mm, 250–4.6 mm i.d.) with a flow rate 1.0 mL min−1. The prepared buffer was filtered through 0.45 μm membrane filter. The injection volume was 10.0 μL and the chromatograms were scanned from 200.0–400.0 nm by the aid of DAD and it was found that at 260.0 nm optimum resolution was achieved.582.4.2.1. Calibration curves. Calibration curves of the two drugs and their impurities were constructed by transferring different aliquots from their stock standard solutions equivalent to 50.0–450.0 μg for NPZ and NPZ impurity B, 100.0–1100.0 μg for PHN, 100.0–700.0 μg for PHN impurity A, and 100.0–1200.0 μg for PHN impurity B into five sets of 10 mL volumetric flasks. The volumes were then completed to the mark using mobile phase. The detailed conditions previously described were applied. Linearity of the studied drugs was tested by plotting the integrated peak areas obtained against the used concentrations, then regression equations were computed.
2.4.2.2. Assay of laboratory prepared mixtures. Solutions containing different volumes of the two drugs along with their three impurities were prepared by serial dilutions from their corresponding stock standard ones into 10 mL volumetric flasks. The volume of each flask was then completed to the mark with mobile phase. They were analyzed using the previous chromatographic conditions.
2.4.2.3. Application to pharmaceutical dosage form. From Naphcon-A® eye drop, 1.0 mL aliquot was accurately transferred into a 25 mL volumetric flask. 15.0 mL of mobile phase was added and the flask was sonicated for 15 min. The volume was then completed to mark using the same solvent. The final concentration obtained was 10 μg mL−1 for NPZ and 120 μg mL−1 for PHN. This procedure was repeated four times. The previously described chromatographic conditions were followed for NPZ and PHN determination.
2.4.3.1. Calibration curves. Calibration curves were constructed in the range of 5.0–45.0 μg mL−1 for NPZ and NPZ impurity B and 10.0–110.0, 10.0–70.0 and 10.0–120.0 μg mL−1 for PHN, PHN impurity A and B, respectively, by transferring 1.0 mL aliquots from their standard solutions to separated test tubes, each containing 1.0 mL aqueous humor and 100.0 μg IS. The samples were vortexed for 1 min. 3.0 mL methanol was then added for protein precipitation. The supernatants were finally separated, evaporated to dryness and reconstituted using 1.0 mL aliquot of mobile phase. The detailed conditions previously described were applied. Linearity of the studied drugs was tested by plotting the ratio of the integrated peak area to the peak area of IS obtained against the used concentrations, and then regression equations were computed.
2.4.3.2. Assay of spiked rabbit aqueous humor samples. Solutions containing different concentrations the two drugs and their impurities were prepared by transferring accurately different aliquots from their respective stock solutions into a set of 10 mL volumetric flasks. The volumes were completed to the mark using mobile phase. From the previously prepared solutions, aliquots of 1.0 mL were transferred to test tubes separately. 100.0 μg IS and 1.0 mL aqueous humor were then added to each test tube. The samples were vortexed for 1 min and 3.0 mL methanol was then added for protein precipitation. Finally, the obtained solutions were vortexed vigorously for 5 min followed by centrifugation at 4500 rpm for 15 min. The supernatants were separated and evaporated to dryness using nitrogen steam. The residues were reconstituted using 1 mL aliquot of mobile phase and the solutions were passed through 0.45 μm membrane filter.59 The final concentration range obtained was 10.00–45.00, 20.00–90.00, 15.00–45.00, 25.00–70.00 and 20.00–120.00 μg mL−1 for NPZ, PHN, NPZ impurity B, PHN impurity A and B, respectively. The prepared solutions were analyzed adopting the previously mentioned chromatographic conditions.
3 Results and discussion
3.1. Method optimization
In order to achieve optimum separation, several trials have been performed, as using phosphate and/or acetate buffers with different pH values (3.0, 5.0 and 6.0). It was found that mobile phase with isocratic elution containing phosphate buffer pH 6.0 and acetonitrile with ratio (70.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
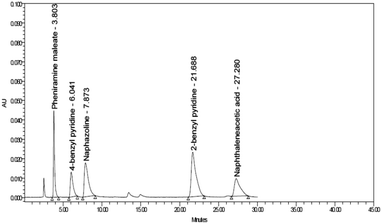
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30.0 v/v) gave the best resolution between the analyzed components. It worth noting that pH 6.0 was the one that enhance system suitability parameters regarding number of theoretical plates and tailing factor. TEA modifier was added for improving resolution and enhancing the symmetry of the separated peaks.60 After testing different types of columns including CN, C8 and C18 ones, the most separation efficiency and the best system suitability parameters were obtained upon using hypersil ODS column (5 μm, 250–4.6 mm i.d.) with run time less than 30 min using 1.0 mL min−1 flow rate. DAD was adjusted at different wave lengths from 200.0 nm to 400.0 nm and 260.0 nm was chosen as an optimum wavelength for detection of the studied compounds. Chromatograms obtained during method optimization are shown in (Fig. S1–S5, ESI†).61 The tR values were 3.82 ± 0.1, 6.04 ± 0.1, 7.87 ± 0.1, 21.68 ± 0.1 and 27.28 ± 0.1 min for PHN, PHN impurity B, NPZ, PHN impurity A and NPZ impurity B respectively (Fig. 2). To assess the system suitability, various parameters were studied as; resolution, tailing factor, retention time, selectivity, column efficiency and height equivalent to theoretical plate. The obtained results were tabulated in Table 1.
30.0 v/v) gave the best resolution between the analyzed components. It worth noting that pH 6.0 was the one that enhance system suitability parameters regarding number of theoretical plates and tailing factor. TEA modifier was added for improving resolution and enhancing the symmetry of the separated peaks.60 After testing different types of columns including CN, C8 and C18 ones, the most separation efficiency and the best system suitability parameters were obtained upon using hypersil ODS column (5 μm, 250–4.6 mm i.d.) with run time less than 30 min using 1.0 mL min−1 flow rate. DAD was adjusted at different wave lengths from 200.0 nm to 400.0 nm and 260.0 nm was chosen as an optimum wavelength for detection of the studied compounds. Chromatograms obtained during method optimization are shown in (Fig. S1–S5, ESI†).61 The tR values were 3.82 ± 0.1, 6.04 ± 0.1, 7.87 ± 0.1, 21.68 ± 0.1 and 27.28 ± 0.1 min for PHN, PHN impurity B, NPZ, PHN impurity A and NPZ impurity B respectively (Fig. 2). To assess the system suitability, various parameters were studied as; resolution, tailing factor, retention time, selectivity, column efficiency and height equivalent to theoretical plate. The obtained results were tabulated in Table 1.
| Parameter | PHN | PHN imp-B | NPZ | PHN imp-A | NPZ imp-B | Reference values |
|---|---|---|---|---|---|---|
| a Retention factor (K) = (tR− tm)/tm.b Calculation of α = K2/K1.c Column efficiency (N) = 16 (tR/w)2, where w is the peak width and tR is the retention time. | ||||||
| tR (min) | 3.80 | 6.04 | 7.87 | 21.68 | 27.28 | NA |
| Resolution (Rs) | NA | 4.73 | 2.29 | 11.99 | 4.02 | Rs > 1.5 |
| Tailing factor (T) | 1.26 | 1.55 | 1.68 | 1.52 | 1.72 | NA |
| Retention factor (k′)a | 1.11 | 2.36 | 3.37 | 11.04 | 14.16 | 1 < k′ < 10 |
| Selectivity factor (α)b | NA | 2.13 | 1.43 | 3.28 | 1.28 | α > 1 |
| Column efficiency (N)c | 3192.03 | 2533.94 | 2260.58 | 6867.68 | 7601.12 | NA |
| Height equivalent to theoretical plate (mm) | 0.08 | 0.10 | 0.11 | 0.04 | 0.03 | NA |
3.2. Method validation for dosage form analysis
For analysis of eye drops, validation of the proposed chromatographic method was conducted according to ICH guideline.62| Parameter | PHN | PHN imp-B | NPZ | PHN imp-A | NPZ imp-B |
|---|---|---|---|---|---|
| a Average of determinations of seven laboratory prepared mixtures. | |||||
| Range | 10–110 μg mL−1 | 10–120 μg mL−1 | 5–45 μg mL−1 | 10–70 μg mL−1 | 5–45 μg mL−1 |
| Slope | 3564.18 | 2988.87 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 867.08 867.08 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 527.48 527.48 |
9845.23 |
| Intercept | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 167.40 167.40 |
3498.28 | −45321.53 | −12384.81 | −16524.53 |
| SE of the slope | 34.86 | 34.43 | 181.62 | 174.23 | 117.56 |
| SE of the intercept | 2341.06 | 2509.26 | 5110.20 | 7627.92 | 3307.67 |
| Specificity (mean ± SD)a | 99.27 ± 1.52 | 100.31 ± 1.50 | 99.75 ± 1.23 | 100.06 ± 1.41 | 99.17 ± 1.74 |
| Accuracy (recovery%) | 99.62 | 99.02 | 100.35 | 100.37 | 99.94 |
| Precision (RSD%) | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Repeatability | 0.26 | 1.31 | 0.51 | 0.21 | 1.55 |
| Intermediate precision | 0.80 | 0.96 | 0.97 | 0.74 | 0.50 |
| Robustness | 1.20 | 1.49 | 0.98 | 0.77 | 1.15 |
| LOD (μg mL−1) | 3.10 | 3.00 | 1.29 | 1.98 | 1.43 |
| LOQ (μg mL−1) | 9.40 | 9.11 | 3.90 | 5.99 | 4.33 |
| Correlation coefficient (r) | 0.9992 | 0.9990 | 0.9991 | 0.9990 | 0.9990 |
3.2.3.1. Repeatability. From linearity range, three different concentrations were chosen. 10.0, 25.0, 40.0 μg mL−1 for NPZ and NPZ impurity B, 20.0, 45.0, 70.0 μg mL−1 for PHN, PHN impurity A and B. Those concentrations were analyzed three times intra-daily using the proposed HPLC-DAD method. The result expressed by relative standard deviations (RSD%) in Table 2, showing high repeatability and low deviations.
3.2.3.2. Intermediate precision. The three previously chosen concentrations were analyzed three times using the previously mentioned method inter-daily on three different days. Acceptable values of RSD% are shown in Table 2.
3.3. Application to pharmaceutical dosage form
NPZ and PHN were analyzed by the proposed HPLC-DAD method without any interference from their eye drops additives. Standard addition technique was applied with appropriate recoveries; the results are shown in Table 3.| Naphcon-A® eye drop | % found Meana ± SD | Standard addition techniquea | ||
|---|---|---|---|---|
| Taken | Added | Recovery% | ||
| a Average determinations of four eye drop dosage form solution. | ||||
| NPZ | 100.29 ± 1.47 | 20 μg mL−1 | 10 μg mL−1 | 99.20 |
| 20 μg mL−1 | 101.15 | |||
| 40 μg mL−1 | 98.90 | |||
| Mean ± SD | 99.75 ± 1.22 | |||
| PHN | 99.52 ± 1.24 | 50 μg mL−1 | 25 μg mL−1 | 99.80 |
| 50 μg mL−1 | 99.82 | |||
| 100 μg mL−1 | 101.34 | |||
| Mean ± SD | 100.32 ± 0.88 | |||
3.4. Statistical analysis
The results obtained from NPZ and PHN analysis in presence of their official pharmacopeial impurities (NPZ impurity B, PHN impurity A and B) were statistically compared with their respective BP methods of analysis.10The calculated t and F are shown in Table 4.| Parameter | HPLC | Official BP method [10] | ||
|---|---|---|---|---|
| NPZ | PHN | NPZ | PHN | |
| a Those values represent the corresponding tabulated values of t and F at p = 0.05. | ||||
| Mean of recoveries | 100.35 | 99.62 | 99.63 | 99.71 |
| SD | 0.72 | 1.15 | 0.98 | 1.15 |
| Variance | 0.51 | 1.32 | 0.96 | 1.32 |
| N | 5 | 5 | 5 | 5 |
| Student's t-test | 1.329 (2.306)a | 0.113 (2.306)a | NA | NA |
| F-test | 1.863 (6.388)a | 1.011 (6.388)a | NA | NA |
3.5. Method validation for analysis in rabbit aqueous humor
The validation of the proposed method in rabbit aqueous humor was performed according to U.S. FDA bioanalytical validation guidelines.63| Parameters | PHN | PHN impurity B | NPZ | PHN impurity A | NPZ impurity B |
|---|---|---|---|---|---|
| a CV: coefficient of variation. | |||||
| LLOQ (μg mL−1) | 10.00 | 10.00 | 5.00 | 10.00 | 5.00 |
| Recovery% (QC samples) | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| QCL | 83.73 | 88.89 | 87.14 | 82.24 | 84.12 |
| QCM | 81.58 | 88.59 | 87.34 | 83.55 | 85.70 |
| QCH | 83.98 | 87.34 | 88.19 | 83.76 | 84.90 |
| Accuracy (recovery%) | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Inter-run | 100.40 | 99.63 | 98.94 | 99.26 | 98.72 |
| Intra-run | 99.17 | 99.91 | 99.03 | 100.01 | 98.66 |
| Precision | |||||
| Inter-run (CV%)a | 8.98 | 10.43 | 9.45 | 11.43 | 8.54 |
| Intra-run (CV%) | 9.23 | 13.71 | 9.83 | 10.25 | 12.31 |
| IS – normalized MF (CV%) | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| QCL | 14.59 | 11.98 | 8.89 | 10.01 | 13.11 |
| QCH | 12.11 | 11.34 | 9.78 | 9.19 | 12.20 |
| Stability study (recovery%) | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Short – term | |||||
| QCL | 99.98 | 99.17 | 99.38 | 100.10 | 99.26 |
| QCH | 99.64 | 99.72 | 100.15 | 99.81 | 99.94 |
| Long-term | |||||
| QCL | 100.67 | 99.79 | 99.45 | 100.15 | 99.43 |
| QCH | 99.85 | 99.67 | 99.35 | 99.00 | 98.38 |
| Freeze-thaw | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| QCL | 99.79 | 99.57 | 99.24 | 98.98 | 99.38 |
| QCH | 99.90 | 99.89 | 99.44 | 99.64 | 100.16 |
| Dilution integrity (recovery%) | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| For 25% level | 99.98 | 100.42 | 100.77 | 98.90 | 99.30 |
| For 50% level | 100.34 | 99.75 | 99.14 | 100.61 | 99.69 |
3.6. Analysis of spiked rabbit aqueous humor samples
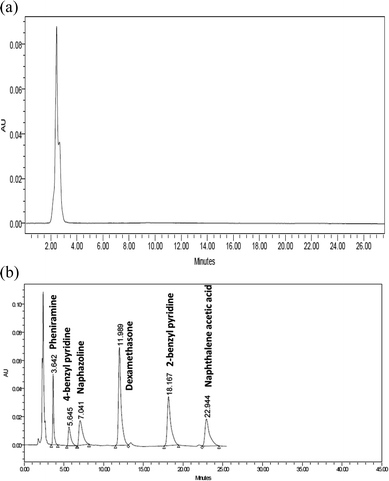
The sensitivity of this method allowed the determination of the drugs in biological fluid as rabbit aqueous humor. In spite of shifting in retention time occurred, good resolution between peaks was obtained. IS was added to ensure extraction efficiency (Fig. 3b). The values of the obtained recovery percentage are shown in Table 6.| Mixa | PHN | PHN impurity B | NPZ | PHN impurity A | NPZ impurity B | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Added conc. | Found conc. | Recovery% | Added conc. | Found conc. | Recovery% | Added conc. | Found conc. | Recovery% | Added conc. | Found conc. | Recovery% | Added conc. | Found conc. | Recovery% | |
| a Average determinations of four laboratory-prepared mixtures. | |||||||||||||||
| Mix 1 | 20.00 | 19.97 | 99.84 | 20.00 | 19.84 | 99.19 | 10.00 | 10.09 | 100.94 | 25.00 | 24.71 | 98.84 | 15.00 | 15.02 | 100.10 |
| Mix 2 | 30.00 | 29.89 | 99.62 | 40.00 | 39.60 | 98.99 | 20.00 | 20.15 | 100.76 | 35.00 | 34.73 | 99.22 | 20.00 | 20.02 | 100.09 |
| Mix 3 | 60.00 | 59.82 | 99.70 | 60.00 | 60.23 | 100.38 | 25.00 | 24.79 | 99.15 | 45.00 | 44.92 | 99.83 | 30.00 | 30.58 | 101.92 |
| Mix 4 | 70.00 | 70.11 | 100.15 | 90.00 | 90.10 | 100.11 | 35.00 | 34.99 | 99.97 | 50.00 | 50.27 | 100.54 | 40.00 | 39.95 | 99.88 |
| Mix 5 | 90.00 | 90.35 | 100.39 | 120.00 | 119.80 | 99.83 | 45.00 | 44.51 | 98.91 | 70.00 | 68.65 | 98.07 | 45.00 | 44.94 | 99.86 |
| Mean ± SD | 99.94 ± 0.32 | 99.70 ± 0.59 | 99.95 ± 0.92 | 99.30 ± 0.94 | 100.37 ± 0.87 | ||||||||||
4 Conclusion
A chromatographic HPLC-DAD method was developed for the simultaneous determination of naphazoline HCl, pheniramine maleate along with three of their pharmacopeial impurities (naphazoline impurity B, pheniramine impurity A and B). The method was also applied in analysis of the five cited components in biological fluid after spiking rabbit aqueous humor. The proposed method successfully detected trace amounts of official impurities and it could be a good candidate for impurity profiling of the two cited drugs. The method was validated according to ICH guidelines and U.S. FDA bioanalytical validation guidelines for determination of the five cited components in eye drops and spiked aqueous humor, respectively. As a result of the simplicity and successfulness of the method in determining the studied drugs in spiked aqueous humor, it is applicable for quality control laboratories, bioequivalence and bioavailability centers.Author contributions
Khadiga M. Kelani: conceptualization, methodology, software, validation, visualization, supervision, project administration, funding acquisition, writing – original draft. Maha A. Hegazy: conceptualization, methodology, software, formal analysis, data curation, visualization, supervision, project administration, funding acquisition, writing – review & editing. Amal M. Hassan: methodology, software, validation, formal analysis, investigation, funding acquisition, project administration, writing – original draft, writing – review & editing. Mahmoud A. Tantawy: methodology, software, validation, formal analysis, investigation, funding acquisition, project administration, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors express their thankful to Eva pharma pharmaceutical company for providing us the pure NPZ, PHN and IS sample.References
- N. Rahman, S. N. H. Azmi and H. Wu, Accredit. Qual. Assur., 2006, 11, 69–74 CrossRef CAS.
- ICH Harmonised Tripartite, Impurities in new drug productsQ3B (R2), current step 4, 2006 Search PubMed.
- F. Simon, For the World Allergy Organization, World Allergy Organization survey on global availability essentials for the assessment and management of anaphylaxis by allergy\immunology specialists in health care settings, 2010, vol. 104, pp. 405–412 Search PubMed.
- N. Rosario and L. Bielory, Curr. Opin. Allergy Clin. Immunol., 2011, 11, 471–476 CrossRef.
- E. R. Weeke, Monogr Allergy, 1987, 21, 1–20 CAS.
- L. L. Brunton, B. A. Chabner and B. C. Knollmann, The Pharmacological Basis of Therapeutics, McGraw-Hill, New York, 12th edn, 2011 Search PubMed.
- D. Johnson and J. Hricik, Pharmacotherapy, 1993, 13, 110S–115S CAS.
- M. Melouna, T. Syrovy and A. Vrana, Talanta, 2004, 62, 511–512 CrossRef.
- US Pharmacopeia 30, volume II, The United States Pharmacopeial Convention, Rockville, USA, 2007 Search PubMed.
- BP, British pharmacopoeia, The Stationary Office, London, UK, 2018 Search PubMed.
- S. Effat, A. Massoud, F. Hassan and A. Alma, Chem. Pharm. Bull., 2006, 54, 119–122 CrossRef.
- M. A. Korany, M. Mona and A. G. Bedairand, Drug Dev. Ind. Pharm., 1990, 16, 1555–1564 CrossRef CAS.
- N. Sayed, M. Hegazy, M. Abdelkawy and R. Abdelfatah, Bulletin of Faculty of Pharmacy, Cairo University, 2013, vol. 51, pp. 57–68 Search PubMed.
- H. C. Goicoechea, M. S. Collado, M. L. Satuf and A. C. Olivier, Anal. Bioanal. Chem., 2002, 374, 460–465 CrossRef CAS.
- B. Hemmateenejad, R. Ghavami, R. Miri and M. Shamsipur, Talanta, 2006, 68, 1222–1229 CrossRef CAS.
- P. Chocholou, D. Satınsky and P. Solich, Talanta, 2006, 70, 408–413 CrossRef.
- S. Casado-Terrones, J. F. Fernandez-Sanchez, B. Canabate Dıaz, A. Segura Carretero and A. Fernandez-Gutierrez, J. Pharm. Biomed. Anal., 2005, 38, 785–789 CrossRef CAS.
- H. Xia, H. L. Wu, H. W. Gu, X. L. Yin, H. Fang and R. Q. Yu, Chin. Chem. Lett., 2015, 26, 1446–1449 CrossRef CAS.
- A. I. Nabiyyi and M. H. Sorouraddin, Luminescence, 2014, 29, 994–1002 CrossRef.
- S. Khalil, Mikrochim. Acta, 1999, 130, 181–184 CrossRef CAS.
- T. Saito, S. Morita, I. Kishiyama, S. Miyazaki, A. Nakamoto, M. Nishida, A. Namera, M. Nagao and S. Inokuchi, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2008, 872, 186–190 CrossRef CAS.
- S. I. Sasa, I. F. Al-momani and I. M. Jalal, Anal. Lett., 1990, 23, 953–971 CrossRef CAS.
- J. Bauer and S. Krogh, Journal of Pharmaceutical Sciences, 1983, 72, 422–425 CrossRef.
- S. C. Ruckmick, D. F. Marsh and S. Duong, J. Pharm. Sci., 1995, 84, 502–507 CrossRef CAS.
- T. Korodi, M. Dulavova, E. Urban, H. K. Frank and B. Lachmann, J. Liq. Chromatogr. Relat. Technol., 2014, 37, 1321–1333 CrossRef CAS.
- V. D. Hoang, N. T. Hue, N. H. Tho and H. M. T. Nguyen, Spectrochim. Acta, Part A, 2014, 139, 20–27 CrossRef.
- A. Yesilada, N. Gokhan, M. A. Yilman and M. Ertan, Acta Pharm. Turc., 1996, 4, 101–106 Search PubMed.
- C. Chabenat and P. Boucly, Biomed. Chromatogr., 1992, 6, 241–243 CrossRef CAS.
- A. Ali, U. Farooq, M. Ahmed, M. M. Athar, K. Nadeem and G. Murtaza, Acta Chim. Slov., 2017, 64, 332–341 CrossRef CAS.
- T. Huang, N. Chen, D. Wang, Y. Lai and Z. Cao, Chem. Cent. J., 2014, 8, 1–9 CrossRef CAS.
- T. R. Koziol, J. T. Jacob and R. G. Achari, Journal of Pharmaceutical Sciences, 1979, 68, 1135–1138 CrossRef CAS.
- A. S. Sidhu, J. M. Kennedy and S. Deeble, J. Chromatogr. A, 1987, 391, 233–242 CrossRef CAS.
- M. P. Marszal, W. D. Sroka, A. Balinowska, D. Mieszkowski, M. Koba and R. Kaliszan, J. Chromatogr. Sci., 2013, 51, 560–565 Search PubMed.
- A. F. Marchesini, M. R. Williner, V. E. Mantovani, J. C. Robles and H. C. Goicoechea, J. Pharm. Biomed. Anal., 2003, 31, 39–46 CrossRef CAS.
- G. G. Mohamed, F. A. Nour El-Dien, E. Y. Z. Frag and M. E. Mohamed, J. Pharm. Anal., 2013, 3, 367–375 CrossRef CAS.
- M. M. A. C. Ribeiro, T. C. Oliveira, A. D. Batista, R. A. A. Munoz and E. M. Richter, J. Chromatogr. A, 2016, 1472, 134–137 CrossRef CAS.
- A. Yesilada, B. Tozkoparan, N. Gokhan, L. oner and M. Ertan, J. Liq. Chromatogr. Relat. Technol., 1998, 21, 2575–2588 CrossRef CAS.
- F. A. Nour El-Dien, G. G. Mohamed, E. Y. Z. Frag and M. E. Mohamed, Int. J. Electrochem. Sci., 2012, 7, 10266–10281 Search PubMed.
- S. M. Ghoreishi, M. Behpour and M. Nabi, Sens. Actuators, B, 2006, 113, 963–969 CrossRef CAS.
- T. d. C. Oliveira, J. M. Freitas, R. A. A. Munoz and E. M. Richter, Electroanalysis, 2018, 30, 868–876 CrossRef CAS.
- G. Parente, M. Pazzaglia, C. Vincenzi and A. Tosti, Contact Dermatitis, 1999, 40, 338 CrossRef CAS.
- R. P. Rosario, S. El-Gizawya, J. H. Perrin and C. M. Riley, Drug Dev. Ind. Pharm., 1986, 12, 2443–2465 CrossRef.
- S. Abdel Fattah, K. O. Kelany, B. A. El-zeanyand and M. F. El-tarras, Anal. Lett., 1987, 20, 1667–1678 CrossRef.
- H. Caglar and E. Buyuktuncel, Int. J. Pharm. Pharm. Sci., 2014, 6, 421–428 CAS.
- M. R. Louhaichi, S. Jebali, M. H. Loueslati, N. Adhoumb and L. Monser, Talanta, 2009, 78, 991–997 CrossRef CAS.
- R. Prava, G. seru, J. R. Sama and A. S. Sidhhanadham, Indo Am. J. Pharm. Sci., 2016, 3, 1521–1533 Search PubMed.
- V. D. Gupta and A. G. Ghanekar, J. Pharm. Sci., 1977, 66, 895–897 CrossRef CAS.
- I. Ali, Z. A. Al-othman, A. Al-warthan, S. D. Alam and J. A. Farooqi, Chirality, 2014, 26, 136–143 CrossRef CAS.
- O. Pirol, M. Ssukuroglu and T. Ozden, Eur. J. Chem., 2011, 8, 1275–1279 CAS.
- D. Rudolph and L. Holkup, Concordia College Journal of Analytical Chemistry, 2010, 1, 29–33 Search PubMed.
- M. Jovanovic, B. J. Stojanovic, T. Rakic, E. Malenovic, D. Ivanovic and M. Medenica, Cent. Eur. J. Chem., 2013, 11, 1150–1162 CAS.
- H. L. Wu, C. H. Huang, S. H. Chen and S. M. Wu, J. Chromatogr. Sci., 1999, 37, 24–30 CAS.
- A. K. Pandey and D. Dwivedi, Int. Res. J. Pharm., 2017, 8, 138–141 CrossRef CAS.
- R. J. Dockhorn and T. G. Duckett, Curr. Eye Res., 1994, 13, 319–324 CrossRef CAS.
- S. J. Ahn, H. K. Hong, Y. M. Na, S. J. Park, J. Ahn, J. Oh, J. Y. Chung, K. H. Park and S. J. Woo, J. Visualized Exp., 2016, 113, e53878 Search PubMed.
- E. G. C. Clarke, Clarke's Analysis of Drugs and Poisons in pharmaceuticals, body fluids and Postmortem material, Pharmaceutical Press, 2004 Search PubMed.
- N. A. El-Ragehy, M. A. Hegazy, G. Abd ElHamid and S. A. Tawfik, Bulletin of Faculty of Pharmacy, Cairo University, 2018, vol. 56, pp. 207–212 Search PubMed.
- H. M. Ahmed, Y. S. Elshamy, W. Talaat, H. F. Labib and T. S. Belal, Microchem. J., 2019, 153, 104505 CrossRef.
- M. A. Hegazy, M. H. Abdelwahab, H. A. M. Hendawy, S. A. Weshahy and S. S. Abbas, J. Liq. Chromatogr. Relat. Technol., 2018, 41, 203–222 CrossRef CAS.
- M. A. Tantawy, S. A. Weshahy, M. Wadie and M. R. Rezk, Anal. Methods, 2020, 12, 3368–3375 RSC.
- M. A. Tantawy, S. Alweshahy, D. A. Elshabasy and N. F. Youssef, J. Planar Chromatogr.–Mod. TLC, 2020, 33, 149–160 CrossRef CAS.
- International Conference on Harmonization, Validation of Analytical Procedures: Text and Methodology Q2 (R1), Current Step 4 Version, 2005 Search PubMed.
- Food and Drug Administration, USFDA, Guidance for Industry: Bioanalytical Method Validation, 2001 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra10598h |
| This journal is © The Royal Society of Chemistry 2021 |