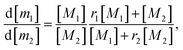Open Access Article
Open Access ArticleA highly chlorinated xylene promoter for ethylene–propylene copolymerisation over a vanadium catalyst
Ildar Salakhov *a,
Elena Tkachevaa,
Valeriy Kozlovb,
Dmitriy Galanina and
Anas Sakhabutdinova
*a,
Elena Tkachevaa,
Valeriy Kozlovb,
Dmitriy Galanina and
Anas Sakhabutdinova
aR&D Center PJSC “Nizhnekamskneftekhim”, Nizhnekamsk, 423574, Russia. E-mail: i.i.salahov@gmail.com
bInstitute of Ecology of the Volga River Basin, Russian Academy of Sciences, Togliatti, 445003, Russia
First published on 14th June 2021
Abstract
The regularities of ethylene–propylene copolymerisation over a vanadium oxytrichloride (VOCl3)–ethylaluminium sesquichloride (EASC) catalytic system in the presence of hexachloro-p-xylene (HCPX), tert-butyl chloride, trichlorotoluene, hexachlorocyclopentadiene, and trichloroacetate were studied. The main kinetic regularities of ethylene–propylene copolymerisation when using HCPX were determined and the kinetic parameters of the process were obtained. It was shown that hexachloro-p-xylene is the most efficient promoter of polymerisation that increases activity of the “vanadium” catalyst severalfold and enhances propylene reactivity in its copolymerisation with ethylene. A number of molecular characteristics and physicochemical parameters were determined for the synthesised samples of ethylene–propylene copolymers. The resulting copolymers have a higher molecular weight and a broad molecular weight distribution and are richer in propylene monomers compared to the copolymers synthesised in the catalytic system without HCPX. Modification of the “vanadium” catalyst yields the copolymer with a more homogeneous structure. Assumptions were made about the mechanism of action of the promoter under study in the VOCl3–EASC catalytic system.
Introduction
Binary ethylene–propylene copolymers (EPR) and ethylene–propylene–diene terpolymers (EPDM) are promising materials that are widely used in various industries and characterised by a steady demand and high consumption rate.1,2 These copolymers have a number of unique features, such as high resistance to chemical oxidation, thermal stability, and resistance to ozone and aggressive media. EPDMs have high filling capacity, while retaining the desired mechanical properties. This makes it possible to add significant amounts of oils and other fillers to the copolymer. Therefore, there still is keen interest in ethylene–propylene copolymerisation in the presence of various catalysts both among manufacturers of elastomers and among researchers focusing on catalytic copolymerisation of olefins.2–4In synthesis of EPDM copolymers, there has recently been a trend towards using metallocene catalytic systems as they are characterised by high activity and thermal stability, give rise to rubber with a homogeneous structure, etc.5–9 Meanwhile, the catalysts based on vanadium compounds (VOCl3, VCl4) and organoaluminium compounds such as Al(C2H5)2Cl and Al(C2H5)1.5Cl1.5 are still used in industry to produce binary ethylene–propylene copolymers and ternary copolymers of ethylene, propylene and nonconjugated diene (ethylidene-2-norbornene or dicyclopentadiene), which are amorphous highly elastic polymers characterised by random comonomer sequence distribution in the macromolecule. The “vanadium-based” catalytic systems have lower activity and thermal stability compared to metallocene catalysts. The resulting elastomer is characterised by reduced thermal stability, possibly due to the higher ash content (the residual catalytic complex) in the polymer. Nevertheless, systems based on vanadium compounds also have a number of advantages. Thus, they vary greatly in terms of the composition of the catalytic complex and synthesis conditions, as well as occurrence and stability of the technology of copolymer production, which makes them indispensable for manufacturing synthetic rubbers and elastomers.3
A feature of vanadium-based catalytic systems is that during polymerisation, aluminium alkyls reduce vanadium to the oxidation state of +2, which is inactive in polymerisation. As a result, the catalytic activity declines abruptly, up to complete termination of polymerisation.10–13 One of the ways to solve this problem is to modify vanadium catalysts with oxidising agents that promote reactivation of active sites in the polymerisation system.10,12–14 When an oxidising agent is added to the system together with the catalyst components, the monomer, or the hydrocarbon solvent, the polymerisation rate remains constant for a longer period of time, unlike during the process conducted without an oxidising agent. Many researchers10,12,13,15 have attributed these effects to regeneration of active sites of the catalyst due to oxidation of low-valent vanadium compounds, which are either inactive or poorly active during (co)polymerisation, or because the rate of reduction processes in the reaction system decreases.
Various compounds for have been tested in order to improve vanadium catalysts for olefin (co)polymerisation. Thus, A. Gumboldt et al. showed that ethylene–propylene copolymerisation can be restored by adding hexachlorocyclopentadiene (HCP) after the VOCl3–Al(C2H5)1.5Cl1.5 catalytic system had become inactive.16 V. N. Karasev and K. S. Minsker demonstrated that tetrahydrofuran reduces the rate of deactivation of the VOCl3-based catalytic complex and increases the yield of the polymer.17 D. L. J. Christman studied such compounds as HCP, hexachloroacetone, hexachloropropylene, and esters of trichloroacetic acid during polyethylene synthesis in the presence of vanadium-based pre-catalysts and alkylaluminium chlorides.18 E. Adisson et al. studied the effect of activation of halogenated hydrocarbons (tetrachloromethane, chloroform and bromoform, dichlorodibromomethane, trichloroethane, and trichlorotrifluoroethane) on high-temperature ethylene polymerisation at halogen/V ratio = 30 ÷ 300 in the presence of vanadium catalysts.12 Ethylene copolymerisation over VCl4-based systems, with chloroform and carbon tetrachloride used as promoters, was studied in ref. 11 and 19.
M. van Duin, G. van Doremaele, and N. van der Aar reported that halogenated esters are highly efficient for EPDM production.15 It was shown in ref. 13 that the use of such organic halide as dichlorophenyl(ethyl)ether increases activity of the catalyst severalfold. Earlier, G. G. Evens obtained a patent for the entire class of compounds of this type.20
Studies focused on post-metallocene vanadium-based catalytic systems have recently been published; halogenated esters were also used for catalyst reactivation in these works.21–23 Compared to the conventional VOCl3 system, metallocene complexes exhibited a much higher catalytic activity and ability to incorporate propylene.
One can see from what has been mentioned above that chlorinated esters (TCEA), cyclodienes (HCP), and aliphatic compounds (CCl4, CHCl3, etc.) exhibited the highest activity for reaction of the vanadium-based catalyst. However, these compounds are either extremely flammable or toxic (i.e., handling them is dangerous). Another drawback is their high cost. Therefore, searching for new raw-material components that would be less hazardous is very relevant for improving the industrial safety of polymer production. Hexachloro-p-xylene (HCPX), a transparent crystal powder used for manufacturing industrial rubber goods and pharmaceuticals, is the most promising product. Hexachloro-p-xylene is an inexpensive, low-toxic, non-flammable substance and exhibits a high reactivity in 1,3-butadiene polymerisation, which is comparable to that achieved by using catalysts obtained in the presence of aluminium alkyl chlorides.24 From both the fundamental and practical perspectives, it is reasonable and important to study whether the highly chlorinated xylene can have a promoting effect on vanadium-based catalyst during ethylene–propylene copolymerisation and what effect HCPX content in the system has on the characteristics of the resulting polymer. It is noteworthy that many manufacturers have a steady interest in modifying vanadium catalysts, since this technology makes it possible to develop novel improved grades of EPDM using the conventional Ziegler–Natta catalysts.3
This study focuses on ethylene–propylene copolymerisation in the presence of the vanadium oxytrichloride–EASC–HCPX catalytic system and the features of changes in molecular characteristics and structure of the resulting copolymer during this process.
Experimental
Materials
Vanadium oxytrichloride VOCl3 (manufactured by AVISMA), ethylaluminium sesquichloride (EASC) (manufactured by PJSC “Nizhnekamskneftekhim”), hexachloro-p-xylene (1,4-bis-trichloromethylbenzene, C8H4Cl6, ≥98% purity, CAS #68-36-0), 2-chloro-2-methylpropane (tert-butylchloride – TBC, C4H9Cl, ≥99% purity, CAS #507-20-0), 1,2,4-trichlorobenzene (TCB, C6H3Cl3, ≥99% purity, CAS #120-82-1), hexachlorocyclopentadiene (HCP, C5Cl6, ≥98% purity, CAS #77-47-4), and 2,2,2-trichloroethyl acetate (TCEA, C4H5CCl3O2, ≥97% purity, CAS #625-24-1) were used as components of the catalytic system.Ethylene, propylene, and hydrogen (polymerisation grade) manufactured by PJSC “Nizhnekamskneftekhim” were used in this study. They were subjected to additional purification over heterogeneous catalysts with promoters of chemical sorption, as well as by using molecular sieves 3A and 4A. Ethylidene-2-norbornene (ENB) (manufactured by INEOS) of polymerisation grade without additional purification was used as the third comonomer. n-Hexane (purified and dehydrated by distillation over metallic sodium in nitrogen flow) was used as a solvent.
Ethylene–propylene copolymerisation
Ethylene–propylene copolymerisation was carried out in a 1.0 dm3 steel reactor equipped with a mixing impeller (rotation speed, 250 rpm), a jacket for maintaining the reactor temperature, and a device for feeding gaseous or liquid monomers and the catalytic complex, as well as a polymer sampling device.The reactor was vacuum-treated before loading the components. The solvent (450 cm3) containing the specified amount of EASC solution was fed into the vacuum-treated reactor under nitrogen atmosphere at a given temperature. Next, the solvent started to be saturated with ethylene, propylene, and hydrogen at a ratio calculated to produce the copolymer of desired composition, with the mixing impeller switched on. Hydrogen concentration in the gas phase was 0.005 vol%.
Once the target pressure in the reactor (0.6 MPa) had been attained, n-hexane solutions containing the calculated amounts of VOCl3 and HCPX were added. The catalyst was fed into the reaction zone with ethylene flow (pressure, 1.0 MPa) through a dosing unit. The n-hexane solution of HCPX was fed into the reaction zone using a dosing pump. The C8H4Cl6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VOCl3 molar ratio was varied from 1 to 15. The EASC/VOCl3 molar ratio was 50 (EASC = 9.8 mmol; VOCl3 catalyst = 0.196 mmol). The weight ratio between propylene (C3) and ethylene (C2) in the mixture fed to the saturation stage was kept equal to 1.5. During the study, the polymerisation temperature was varied from 20 to 50 °C. Copolymerisation duration was 60 min. The system was deactivated with ethanol; the remaining catalytic complex was removed by washing with water. The polymer solution was stabilised with Irganox® 1520L.
VOCl3 molar ratio was varied from 1 to 15. The EASC/VOCl3 molar ratio was 50 (EASC = 9.8 mmol; VOCl3 catalyst = 0.196 mmol). The weight ratio between propylene (C3) and ethylene (C2) in the mixture fed to the saturation stage was kept equal to 1.5. During the study, the polymerisation temperature was varied from 20 to 50 °C. Copolymerisation duration was 60 min. The system was deactivated with ethanol; the remaining catalytic complex was removed by washing with water. The polymer solution was stabilised with Irganox® 1520L.
Characterisation of ethylene–propylene copolymers
The molecular weight distribution (MWD) of the copolymer samples was determined on an HT-GPC350 Viscotek high-temperature liquid chromatography system equipped with a refractive index detector and a light scattering detector. 1,2,4-Trichlorobenzene was used as an eluent; the elution rate was 1.0 cm3 min−1; the operating temperature was 150 °C. A PLgel Mixed-B LS column was employed. The instrument was calibrated using the polystyrene reference material (KDSI Instruments).The phase transitions of the ethylene–propylene copolymer samples were studied by differential scanning calorimetry (DSC) on a DSC 204F1 Phoenix instrument. The glass transition temperature was measured according to the standard test method ASTM Eh 1356.25 The sample was heated to 100 °C, cooled down to −100 °C, and heated again to 100 °C. By the end of each heating and cooling cycle, the sample was exposed to the current temperature for 5 min. The heating and cooling rates were 20 °C min−1. All the heating–cooling cycles were performed in an argon atmosphere. The flow rate of argon was 75 mL min−1. The differential DSC curve was additionally used to perform accurate graphical calculation of the enthalpy of fusion of the sample under study (ΔHm). The degree of crystallinity was calculated using the formula: χ = (ΔHm/ΔH100%)·100%, where χ is the degree of crystallinity of the sample under study, %; ΔH100% is the enthalpy of fusion of the entirely crystalline PE (χ = 100%),26 and ΔHm is the enthalpy of fusion of the sample under study.
The content of propylene monomers (±2 wt%) in the samples of ethylene–propylene copolymers was determined on a VECTOR FT-IR spectrometer according to the standard test method ASTM D 3900-2000. The polymer structure was also studied using a Spotlight 400 Series FT-IR spectrometer (Perkin Elmer): a single-beam scanning interferometer with DTGS, MCT, InGaAs detectors; 4000–600 cm−1 spectral range. Ethylene–propylene copolymer films (0.1 mm thick) for the experiments were prepared by forge rolling at 105 °C; the 20 × 20 cm samples were then cut out. Polarised radiation that allows one to identify the structurally different zones was used to determine the orientation of molecular groups.27
The NMR spectra were recorded on a Bruker Avance-600 NMR spectrometer with a magnetic field of 14.1 T; the resonance frequency of 13C nuclei was 150 MHz. The samples were rubber solutions in 1,2,4-trichlorobenzene with a concentration of about 10% w/v. The power of the exciting pulse corresponded to the “turnover” angle of the nuclear magnetisation vector ≈ 40–45°. Duration of the time interval between pulses was no less than 12 s. The spectra were recorded with suppression of the spin–spin interaction between 13C nuclei and protons in the inverse-gated decoupling mode to exclude the influence of the Overhauser effect. The measurements were carried out at a temperature of 340 K. Chemical shifts were determined relative to the signal from tetramethylsilane (δ = 0). The number of scans was at least 2000.
The kinetic parameters of ethylene–propylene copolymerisation over the VOCl3–EASC and VOCl3–EASC–HCPX catalytic systems were calculated using the Fineman–Ross28,29 and Mayo–Lewis30 methods. The copolymerisation constants were determined using the method proposed by Fineman–Ross29 according to the content of monomeric units in a copolymer. The constants k11, k12, k22, and k21 were calculated according to the equations of elementary chain propagation reactions involving two monomers, M1 and M2, and two growing active sites, ∼m1* (the chain with the terminal monomeric unit M1) and ∼m2* (the chain with the terminal monomeric unit M2):
| V11 = k11[m1*][M1]; |
| V12 = k12[m1*][M2]; |
| V21 = k21[m2*][M1]; |
| V22 = k22[m2*][M2]; |
According to the copolymerisation constants r1 and r2 and concentrations of monomeric units, the composition of the resulting copolymer is described by the Mayo–Lewis equation:
Results and discussion
The effect of the nature and structure of chlorine-containing agents
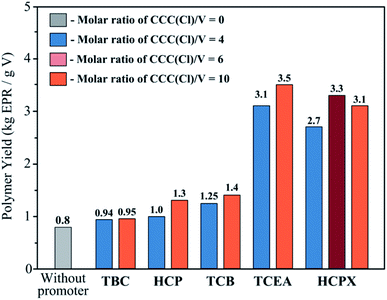
In order to determine which compound acts as the most active promoter of vanadium catalyst for ethylene–propylene copolymerisation, we tested chlorine-containing compounds (CCCs) differing in the type of hydrocarbon backbone and the number of chlorine atoms per molecule (ranging from 1 to 6). An aliphatic compound TBC, a cyclic compound HCP, aromatic compounds HCPX and TCB, as well as halogenated ester TCEA, were added to the polymerisation system. The Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V molar ratio was varied from 4 to 10. A chlorine-containing compound was added to the catalytic system at the early polymerisation stage. Fig. 1 shows the data on the effect of the studied chlorine-containing compounds on activity of vanadium catalyst.
V molar ratio was varied from 4 to 10. A chlorine-containing compound was added to the catalytic system at the early polymerisation stage. Fig. 1 shows the data on the effect of the studied chlorine-containing compounds on activity of vanadium catalyst.
Fig. 1 demonstrates that the activity of the vanadium catalyst increased for all the chlorine-containing compounds being used; however, the activity level (the yield of the ethylene–propylene copolymer) depends on the chemical nature of a CCC.
One can see that the presence of three chlorine atoms at carbon atom (–CCl3) in the promoter (TCEA and HCPX) is a favourable condition for vanadium catalyst activation.
On the other hand, HCPX carries two –CCl3 groups, while TCEA carries only one this group. Nonetheless, activity of the catalytic system in the presence of TCEA is somewhat higher compared to that in the presence of HCPX. Apparently, the presence of the ester moiety (–COO–) is a more important factor because the contribution of this group to oxidation of divalent vanadium (or catalyst activation) is greater than the contribution of the aromatic ring in HCPX. The presence of three chlorine atoms bound directly to the aromatic ring is clearly a more efficient combination compared to two trichlorocarbon moieties at the para position of HCPX.
As for HCP, it is well known that cyclopentadiene acts as catalyst poison for Ziegler–Natta catalyst during diene and olefin polymerisation; therefore, its presence in the catalytic system can slow down polymerisation. The presence of six chlorine atoms within the HCP molecule does not inhibit the copolymerisation reaction and even somewhat accelerates the process (Fig. 1), but to a much smaller extent than HCPX, which also contains six chlorine atoms. A. Gumboldt et al. demonstrated that the optimal activity of the VOCl3–EASC–hexachlorocyclopentadiene system upon ethylene–propylene copolymerisation at Tp = 30 °C was observed at the molar ratio between HCP and vanadium lying in the range of 60–100.16 This ratio (and, therefore, the amount of HCP) is an order of magnitude higher than that in the case when HCPX is used. TBC exhibits also no activating effect on ethylene–propylene copolymerisation over the VOCl3–EASC system. In general, it was demonstrated in ref. 12 that activation of copolymerisation over the VCl3–1/3AlCl3/AlEt3 catalytic system strongly increases with rising number of halogen atoms, while the number of carbon atoms remains the same: CH2Cl2 < CHCl3 < CCl4.
Our findings show that HCPX is an efficient promoter, and its activating effect is closer to that of TCEA among the studied chlorine-containing compounds. Taking into account that, unlike HCPX, TCEA has been studied appreciably well as an activator,15,19 further studies of the modified VOCl3–EASC–HCPX catalytic system for ethylene–propylene copolymerisation were continued in order to reveal its features and benefits.
The copolymerisation kinetics
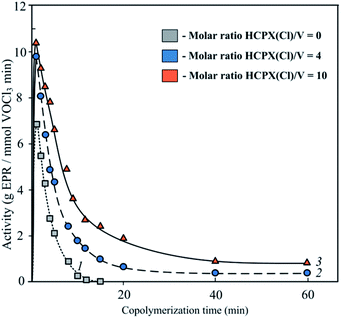
The dependences of catalyst activity on duration of ethylene–propylene copolymerisation (Fig. 2) are characteristic of these systems.17 Addition of HCPX to the system does not significantly change the shape of the curves, although catalyst activity and stability tend to increase with hexachloro-p-xylene concentration.For polymerisation durations shown in Fig. 2, there is no induction period, and the catalytic activity is maximal from the very beginning of the process.
The Fineman–Ross method28 was used to calculate the kinetic parameters of ethylene–propylene copolymerisation over the VOCl3–EASC and VOCl3–EASC–HCPX catalytic systems; the results are summarised in Table 1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VOCl3 = 4 molar ratio) calculated using the Fineman–Ross method28
VOCl3 = 4 molar ratio) calculated using the Fineman–Ross method28
| Catalytic system | kia, L mol−1 s−1 | (k0)b, s−1 | Propagation rate constants (L mol−1 s−1) (1 – ethylene; 2 – propylene) | |||
|---|---|---|---|---|---|---|
| k11 ± 450 | k12 ± 11 | k22 ± 1 | k21 ± 12 | |||
| a The rate constant of chain initiation involving ethylene.b Rate constant of deactivation of copolymerization sites. Copolymerization conditions: see Fig. 1. | ||||||
| VOCl3/EASC | 0.24 | 0.0243 | 2150 | 76 | 7 | 91 |
| VOCl3/EASC/HCPX | 0.54 | 0.0100 | 4550 | 231 | 26 | 410 |
As one can see in Table 1, the rate constant of chain initiation is several orders of magnitude lower than the propagation rate constants for any combination of factors (the nature of the active site – the monomer type). Even for the lowest constants, the k22/ki value is higher than one order of magnitude. This ratio between the rate constants of chain propagation and initiation suggests that there must be a region during the initial stage of copolymerisation where the polymerisation rate increases as the concentration of active sites rises. However, this increase seems to be very short and has not been detected in this experiment (Fig. 2). It is worth mentioning that the data on the kinetic constants obtained in this study are close to the kinetic data obtained earlier for a similar system.17,29
Addition of HCPX to the ethylene–propylene copolymerisation system distinctly increases ki and the propagation rate constants, while reducing the rate constant of deactivation of polymerisation sites. The decline in the rate constant of deactivation of polymerisation sites is expected to be accompanied by an increase in the number of active sites of copolymerisation, thus causing activation of the system. The changes in propagation rate constants demonstrate that the increased activity of the system was not caused solely by changes in the number of active sites. It indicates that the active sites have different chemical nature in the presence and in the absence of a promoter (HCPX) for the VOCl3–EASC system. The difference between the rate constants of chain initiation and propagation reactions virtually remains unhanged. Changes in these constants observed after HCPX had been added agree well with the data shown in Fig. 1, which clearly demonstrate that activity of the system increases, while the overall shape of the curve remains the same regardless of whether the system contains HCPX or not. It is noteworthy that addition of HCPX to the system increases the (k22 + k12)/(k11 + k21) value from 0.037 to 0.052; i.e., the probability that propylene monomer is inserted into the growing polymer chain increases almost 1.5-fold, so it is expected that the copolymer will become rich in the second monomer.
Variation of the HCPX/VOCl3 molar ratio
Table 2 demonstrates that the polymer yield rises as the C8H4Cl6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VOCl3 molar ratio is increased from 0 to 6, all other conditions being identical. In the presence of HCPX, the polymer yield is 0.8 kg EPR g−1 V−1, while the maximum yield (3.3 kg EPR g−1 V−1) is observed at the C8H4Cl6
VOCl3 molar ratio is increased from 0 to 6, all other conditions being identical. In the presence of HCPX, the polymer yield is 0.8 kg EPR g−1 V−1, while the maximum yield (3.3 kg EPR g−1 V−1) is observed at the C8H4Cl6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VOCl3 molar ratio = 6 (i.e., the yield increases more than fourfold).
VOCl3 molar ratio = 6 (i.e., the yield increases more than fourfold).
| No | C6H4(CCl3)2/VOCl3 molar ratio | Polymer yield, kg EPR g−1 V−1 | Mw, kg mol−1 | Mn, kg mol−1 | Mw/Mn | Propylene content in copolymer, wt% | Degree of crystallinity, % | Glass transition temperature, °C | Colour of the polymer |
|---|---|---|---|---|---|---|---|---|---|
| a Copolymerisation conditions: Tp = 30 °C, P = 0.6 MPa, τ = 60 min; the Al/V molar ratio = 50; * the third comonomer, ENB, was added (content, 5%). | |||||||||
| 1 | 0 | 0.8 | 172 | 96 | 1.8 | 29 | 13.2 | −48 | White |
| 2 | 0.5 | 0.9 | — | — | — | 29 | — | — | — |
| 3 | 1 | 1.0 | 198 | 71 | 2.8 | 29 | — | — | — |
| 4 | 4 | 2.7 | 217 | 72 | 3.0 | 33 | 11.8 | −51 | — |
| 5* | 4 | 1.9 | — | — | 30 | — | — | — | |
| 6 | 5 | 3.0 | 250 | 83 | 3.0 | 34 | — | — | Violetish-white |
| 7 | 6 | 3.3 | 264 | 91 | 2.9 | 36 | — | — | Purplish-white |
| 8 | 10 | 3.1 | — | — | — | 40 | 9.2 | −57 | Yellow with a purplish tint |
| 9 | 15 | 2.9 | — | — | — | — | — | — | |
The polymer yield is not significantly changed as the C8H4Cl6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VOCl3 molar ratio is further increased to 10 and 15 (Table 2). Addition of the third comonomer, ENB, reduces activity of the polymerisation system. The polymer yield declines from 2.7 to 1.9 kg g−1 V−1, which correlates with the findings obtained in ref. 20 showing that addition of ENB during terpolymerisation is also accompanied by activity reduction. However, the yield of the terpolymer is more than twofold compared to that in exp. 1 (Table 2).
VOCl3 molar ratio is further increased to 10 and 15 (Table 2). Addition of the third comonomer, ENB, reduces activity of the polymerisation system. The polymer yield declines from 2.7 to 1.9 kg g−1 V−1, which correlates with the findings obtained in ref. 20 showing that addition of ENB during terpolymerisation is also accompanied by activity reduction. However, the yield of the terpolymer is more than twofold compared to that in exp. 1 (Table 2).
Therefore, there are good reasons for regarding that HCPX is an efficient promoter for the VOCl3–EASC catalytic system for ethylene–propylene copolymerisation, as well as for ternary copolymerisation of ethylene, propylene, and ENB.
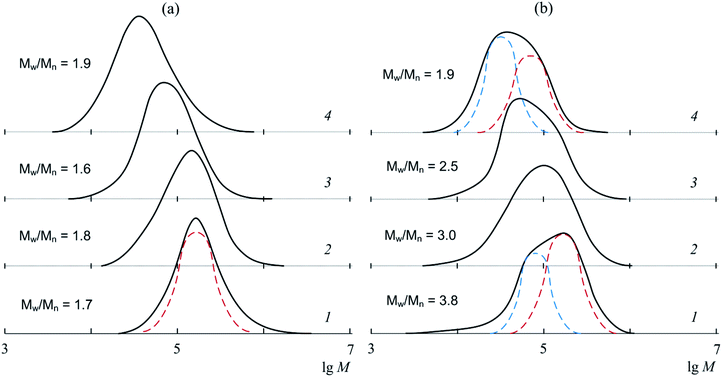
It was found by analysing the molecular characteristics of ethylene–propylene copolymer that the presence of HCPX in the system (regardless of its content for the analysed concentration range) broadened the molecular weight distribution (MWD) of the copolymer from ∼2 to ∼3 (Table 2). This can probably be attributed to the changes in the ratio between the rates of chain initiation and active site deactivation (Table 1). The weight-average molecular weight (Mw) somewhat rises with HCPX concentration (Table 2).
However, the examination of changes in the number-average molecular weight (Mn) shows that addition of HCPX reduces Mn, but at the C8H4Cl6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VOCl3 molar ratio of ∼6, Mn tends to increase and approach the value that is typical of the systems not containing HCPX.
VOCl3 molar ratio of ∼6, Mn tends to increase and approach the value that is typical of the systems not containing HCPX.
The data listed in Table 2 demonstrate that the content of propylene monomers in the copolymer increases with HCPX content in the VOCl3–EASC–HCPX catalytic system during ethylene–propylene copolymerisation. According to the propagation rate constants listed in Table 1, it is a quite reasonable result, since addition of HCPX increases the likelihood of propylene insertion into the polymer chain. The changes in the propagation rate constants demonstrate that active sites differ in their chemical nature in the presence and in the absence of a promoter (HCPX) when the VOCl3–EASC system is used. The revealed effect allows one to optimise the initial monomer ratio, since one of the features of synthesising EPR and EPDM is that the propylene content in the initial monomer blend needs to be increased to ensure the target content of propylene monomers in the copolymer. The degree of crystallinity and the glass transition temperature of the copolymers predictably decrease as the content of propylene monomers in the copolymer rises (Table 2) due to the fact that shorter crystallisable ethylene monomer sequences are formed in the macromolecule during random copolymerisation (see the r1 and r2 values in Table 4).
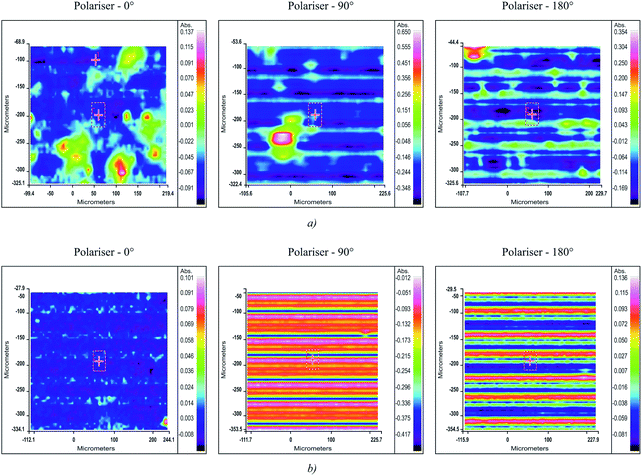
The effect of HPCX on the structure of the resulting copolymer was studied using polarised infrared radiation, which made it possible to obtain information about the chemical composition of the samples, detect homogeneous regions and heterogeneities in the composition and structure of the tested materials.27
The structural differences can be clearly seen from the images recorded using a microscope coupled with an IR spectrometer (Fig. 3). One can see from the images shown in Fig. 3 a and b that modifying the VOCl3–EASC catalytic system with hexachloro-p-xylene yields a copolymer with homogeneous composition (Fig. 3b).
13C NMR spectroscopy was used to describe the microstructure of the samples under study. The weak-field spectral region (150–100 ppm) was not taken into account as it contains no signals from 13C nuclei of ethylene or propylene monomers. The high-field region (Fig. 4; signal assignment is shown in Table 3) contains such signals, as well as low-intensity signals from 13C nuclei of ENB monomers (see the chemical shifts at 50, 46–45, 41.4, 36.1, 33.5, and 13.7 ppm).
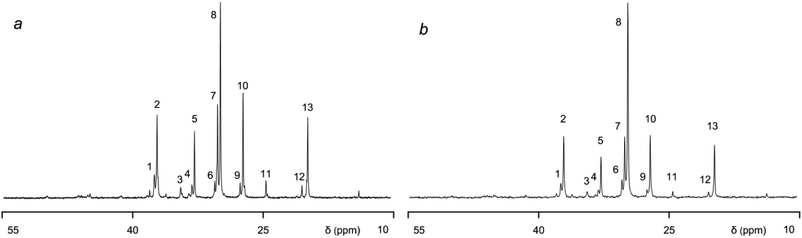 | ||
| Fig. 4 The 13C NMR spectra of EPDM rubber: the pristine (a) and modified (b) samples. The modified sample synthesised using the VOCl3/EASC/HCPX catalytic system. | ||
| No | Chemical shift, ppm | Signal assignment |
|---|---|---|
| 1 | 37.5 | Methylene groups in PEP triad sequences |
| 2 | 37.2 | Methylene groups in PEEP tetrad sequences |
| 3 | 34.4 | Methylene groups in PEP triad sequences |
| 4 | 33.1 | Methylene groups in EEP triad sequences |
| 5 | 32.8 | Methine groups in EPE triad sequences |
| 6 | 30.5 | Methine groups in EPPE tetrad sequences |
| 7 | 30.1 | Methylene groups in EEPE tetrad sequences |
| 8 | 29.8 | Methylene groups in EEE triad sequences |
| 9 | 27.5 | Methine groups in EPPE tetrad sequences |
| 10 | 27.2 | Methylene groups in EPE triad sequences |
| 11 | 24.5 | Methylene groups in EPE triad sequences |
| 12 | 20.3 | Methyl groups in EPPE tetrad sequences |
| 13 | 19.7 | Methyl groups in EPE triad sequences |
The spectra of the pristine (Fig. 4a) and modified (Fig. 4b) samples contain almost identical signals, but the ratios between signal intensity are different. Thus, signal intensities of 13C nuclei of EEE and EPE triads are noticeably higher in the spectrum of the modified sample, being indicative of quantitative changes in distribution (randomization) of ethylene and propylene monomers in macromolecules.
It is worth mentioning that the disturbance of regularity of the EPDM chain is ensured by different variants of the propylene monomer sequence. Thus, the meso and racemic combinations can be obtained at the level of dyads, while syndio-, iso-, and atactic combinations are possible for triads. Disturbance of chain regularity as reverse (head-to-head and tail-to-tail) combinations can also occur starting from the dyadic level. No signals from triadic or larger sequences of propylene monomers in the spectra of the analyzed samples have been revealed (Fig. 4). Therefore, by following the approach proposed in the recent study [3], we used the relative content of individual propylene monomers and dyadic combinations as a criterion for randomization and regularity of the EPDM chain. This value can be estimated quantitatively from the ratio between the intensities of signals 13 and 12. For the pristine and modified samples, this ratio has changed from 83/17 to 90/10, respectively.
It should be mentioned that at the C8H4Cl6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VOCl3 molar ratio higher than 5 (Table 1), the colour of the resulting copolymer changes from white to light purple. The changes in the copolymer colour are most likely to be related to vanadium oxidation by HCPX. It is known that the V+4 ion is dark blue, while the V+5 ion is reddish yellow. It is quite possible that the mixture of these two ions has purple colour. The copolymer was transparent at a C8H4Cl6
VOCl3 molar ratio higher than 5 (Table 1), the colour of the resulting copolymer changes from white to light purple. The changes in the copolymer colour are most likely to be related to vanadium oxidation by HCPX. It is known that the V+4 ion is dark blue, while the V+5 ion is reddish yellow. It is quite possible that the mixture of these two ions has purple colour. The copolymer was transparent at a C8H4Cl6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VOCl3 molar ratio = 4, so further experiments were conducted at this molar ratio, which is optimal in terms of catalyst activity and colour of the copolymer. It was also found in ref. 18 that the polymer can change its colour in the presence of chlorinated hydrocarbons: polyethylene colour was changed during polymerisation in the presence of HCP, while this phenomenon was not observed during polymerisation in the presence of chlorinated esters of trichloroacetic acid.
VOCl3 molar ratio = 4, so further experiments were conducted at this molar ratio, which is optimal in terms of catalyst activity and colour of the copolymer. It was also found in ref. 18 that the polymer can change its colour in the presence of chlorinated hydrocarbons: polyethylene colour was changed during polymerisation in the presence of HCP, while this phenomenon was not observed during polymerisation in the presence of chlorinated esters of trichloroacetic acid.
Variation of the copolymerisation temperature
Polymerisation temperature plays a crucial role in copolymerisation, since it affects monomer solubility, stability of the active site, and the reaction rate constant. Varying the temperature of ethylene–propylene copolymerisation (Tp) from 20 to 50 °C revealed (Table 4) that the polymer yield decreases with rising Tp. However, activity of the polymerisation system modified with HCPX remains higher than that of the non-modified VOCl3–EASC system within the entire studied temperature range. The temperature of 30 °C is optimal for the system used in this study. Side processes causing catalyst deactivation and reducing the copolymer yield occur at higher temperatures.| HCPX/VOCl3 molar ratio | Tp, °C | Polymer yield, kg EPR g−1 V−1 | Mw, kg mol−1 | Mn, kg mol−1 | Mw/Mn | Content of propylene monomers in the copolymer, wt% |
|---|---|---|---|---|---|---|
| a Copolymerisation conditions: P = 0.6 MPa; τ = 60 min; the Al/V molar ratio = 50. | ||||||
| 0 | 20 | 1.3 | 230 | 135 | 1.7 | 30 |
| 30 | 0.8 | 172 | 96 | 1.8 | 29 | |
| 40 | 0.4 | 86 | 54 | 1.6 | 29 | |
| 50 | 0.2 | 68 | 36 | 1.9 | 28 | |
| 4 | 20 | 3.6 | 260 | 68 | 3.8 | 35 |
| 30 | 2.7 | 217 | 72 | 3.0 | 33 | |
| 40 | 1.2 | 114 | 46 | 2.5 | 31 | |
| 50 | 0.7 | 63 | 33 | 1.9 | 29 | |
The average molecular weights (both Mw and Mn) of the copolymer decline with increasing temperature of copolymerisation over the VOCl3–EASC catalytic system. The MWD of the copolymer remains almost unchanged and is equal to ∼2 (Table 4). The MWD curves consistently shift towards lower molecular weights with increasing copolymerisation temperature (Fig. 5). The shape of the MWD curves is unimodal and is not significantly altered. Similar results on changes in molecular characteristics were obtained in ref. 31 during polymerisation over the catalytic systems containing VCl4 and VOCl3 in the presence of EASC.32
The decline the average molecular weights with increasing Tp is also typical of the copolymer produced over the VOCl3–EASC–HCPX catalytic system. The copolymers synthesised at 50 °C both in the presence of HCPX promoter and without it eventually have almost identical molecular characteristics. Nonetheless, activity of the catalytic system containing HCPX is 3.5-fold higher than that for the system without a promoter even at 50 °C. It was demonstrated above that ethylene–propylene copolymerisation over VOCl3–EASC in the presence of HCPX broadens the MWD of the copolymer (Table 2). Broadening of the molecular weight distribution resulted from the asymmetric shape of the MWD curves of the copolymer (Fig. 5b).
The copolymer synthesised at low polymerisation temperature is characterised by certain bimodality. Addition of hexachloro-p-xylene to the system does not change the course of copolymerisation that is typical of this process (Fig. 2). Therefore, MWD broadening is very likely to indicate that the number of active sites changes during copolymerisation in the presence of HCPX.
As it has been shown in a number of studies,10,12,13 hexachloro-p-xylene can oxidise inactive vanadium in the oxidation state +2 reduced by alkyls to higher oxidation states. Hence, the system may become more unstable with respect to concentration of active sites during copolymerisation than it is in the absence of a promoter, when it is less likely that vanadium reduced to the oxidation state +2 undergoes oxidation.
Ethylene–propylene copolymerisation over the VOCl3–EASC catalytic system can be represented using the following simplified Scheme 1 (adopted from ref. 10).
In the absence of HCPX, the process occurs via the following reactions: (1) formation of active sites (+EASC); (2) copolymerisation (Monomers); and (3) the side reaction of vanadium reduction (+EASC). In the presence of HCPX, there is an additional process (4): the oxidation of V+2 and reactivation of some vanadium so that it could form new active sites (+HCPX). This very process probably leads to broadening of the MWD of the copolymer. In addition, a new component, HCPX, should be added to reaction 1, since the rate of the process increases due to changes both in the number of active sites and the propagation rate constants (Table 1). This fact indicates that it is quite likely that HCPX is involved in the formation of copolymerisation sites (Scheme 2).
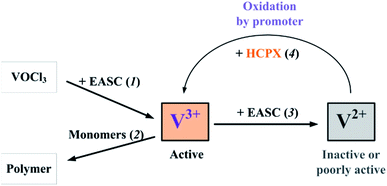 | ||
| Scheme 2 Simplified reaction pathways involved in ethylene and propylene copolymerisation performed with vanadium catalyst system and hexachloro-p-xylene. | ||
The average molecular weights of the copolymer produced over the VOCl3–EASC–HCPX catalytic system also decrease with rising Tp. Eventually, the copolymers synthesised at 50 °C both in the presence of HCPX promoter and without it the have almost identical molecular characteristics. Nonetheless, activity of the catalytic system with HCPX is 3.5-fold higher than that of the system in the absence of the promoter even at 50 °C. It is noteworthy that the content of propylene monomers in the resulting copolymer remains virtually unchanged when the catalytic system contains no HCPX. As mentioned above, addition of HCPX (Table 2) increases the content of propylene monomers in the macromolecule, but propylene content in the copolymer decreases with temperature. At Tp = 50 °C, the content of propylene monomers in the copolymers produced both in the presence and in the absence of HCPX in the catalytic system is almost the same. Hence, activation of the VOCl3–EASC system by the promoter rather than modification of the resulting copolymer occurs at Tp = 50 °C.
Having taken a close look at changes in the copolymerisation rate constants with increasing copolymerisation temperature (Table 5), one can also see that the r1 and r2 values for the catalytic systems under study are rather similar at copolymerisation temperature of 50 °C. Therefore, it is quite predictable that there were almost no differences in the copolymer composition at this temperature.
| Catalytic system | Copolymerisation temperature, °C | r1 ± 1.25 | r2 ± 0.002 | r1r2 |
|---|---|---|---|---|
| a Where r1 = k11/k12; r2 = k22/k21; r1 and r2 are the constants of relative comonomer activity or copolymerisation constants. | ||||
| VOCl3/EASC | 20 | 13.6 | 0.099 | 1.35 |
| 30 | 16.5 | 0.082 | 1.35 | |
| 40 | 21.3 | 0.063 | 1.34 | |
| 50 | 21.0 | 0.064 | 1.34 | |
| VOCl3/EASC/HCPX | 20 | 8.4 | 0.049 | 0.41 |
| 30 | 12.5 | 0.047 | 0.59 | |
| 40 | 17.5 | 0.056 | 0.98 | |
| 50 | 22.5 | 0.060 | 1.35 | |
The copolymerisation rate constants were quantitatively altered as temperature increased, but no fundamental changes in mutual reactivity of the comonomers were observed (the r1 >1 and r2 <1 values were retained).
A quite important change that has already been mentioned above is that the likelihood of propylene incorporation during copolymerisation in the presence of hexachloro-p-xylene added to the VOCl3–EASC catalytic system at 20 °C increased significantly (see the r1 and r2 values in Table 5).
Conclusions
Studying ethylene–propylene copolymerisation, as well as terpolymerisation of ethylene, propylene, and ENB, over the VOCl3–EASC catalytic system in the presence of a novel promoter hexachloro-p-xylene showed that addition of the highly chlorinated xylene increases the catalytic activity severalfold and allows one to increase the content of propylene monomers in the copolymer and produce rubber with a more homogeneous structure. The rise in the overall activity of the polymerisation system and reactivity of the vanadium catalyst with respect to propylene in the presence of the promoter are caused by the features of modification of active sites of polymerisation by HCPX (including changes in their kinetic parameters).The observed positive changes in the structure of the ethylene–propylene copolymer seem to be due to the fact that a more perfect and ordered polymer is synthesised over the modified catalyst. The reason is that the presence of the highly chlorinated xylene, active sites work steadily without deactivation and the copolymer characterized by high composition homogeneity at a high yield, while the contents of ash and residues of catalytic components in the polymer are significantly reduced.
The reported findings and conclusions suggest reasonably that enhancement of the efficiency of Ziegler–Natta vanadium catalysts and modification of the final properties of the copolymers by using hexachloro-p-xylene is of significant interest for industrial-scale production of new grades of EPDM and other rubbers, as well as polyolefins.
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Salinas, IISRP: World Rubber Statistics, 2018, vol. 8 Search PubMed.
- M. van Duin, G. van Doremaele and N. van der Aar, KGK, Kautsch. Gummi Kunstst., 2017, 11–12, 14 Search PubMed.
- K. Watanabe and S. Nakano, Sumitomo Chemical R&D Reports, 2018, vol. 1 Search PubMed.
- S. Zhang, Z. Zhang and Y. Wu, Chin. Sci. Bull., 2018, 63, 3530 CrossRef.
- W. Kaminsky, Macromol. Symp., 2001, 174, 269 CrossRef CAS.
- K. Nomura and S. Zhang, Chem. Rev., 2011, 111, 2342 CrossRef CAS PubMed.
- Y. Huang, Z. Fu, X. Gu, L. Feng and Z. Fan, J. Polym. Mater., 2013, 30, 145 CAS.
- Z. Q. Zhang, J. T. Qu, S. Z. Zhang, Q. P. Miao and Y. X. Wu, Polym. Chem., 2018, 9, 48 RSC.
- G. van Doremaele, M. van Duin, M. Valla and A. Berthoud, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2877 CrossRef CAS.
- H. X. Zhang, Y. M. Hu, C. Y. Zhang, D. H. Lee, K. B. Yoon and X. Q. Zhang, Catal. Commun., 2016, 83, 39 CrossRef CAS.
- F. J. Karol, S. C. Kao and K. J. Cann, J. Polym. Sci., Part A: Polym. Chem., 1993, 31, 2541 CrossRef CAS.
- E. Adisson, A. Deffieux, M. Fontanille and K. Bujadoux, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 1033 CrossRef CAS.
- L. D'Agnillo, J. B. P. Soares and G. H. J. van Doremaele, Macromol. Mater. Eng., 2005, 290, 256 CrossRef.
- M. K. Reinking, P. D. Bauer and J. W. Seyler, Appl. Catal., A, 1999, 189, 23 CrossRef CAS.
- M. van Duin, G. van Doremaele and N. van der Aar, Rubber World, 2018, 258, 20–29 Search PubMed.
- A. Gumboldt, J. Helberg and G. Schleitzer, Makromol. Chem., 1967, 101, 229 CrossRef CAS.
- V. N. Karasev and K. S. Minsker, Polym. Sci. U.S.S.R., 1971, 13, 1652 CrossRef.
- D. L. Christman, J. Polym. Sci., Part A-1: Polym. Chem., 1972, 10, 471 CrossRef CAS.
- G. H. Zohuri, M. Vakili, R. Jamjah, S. Ahmadjo and M. Nekomanesh, Rubber Chem. Technol., 2005, 78, 682–693 CrossRef CAS.
- G. G. Evens, E. M. J. Pijpers and R. H. M. Seevens, EP 0044119B1, 1985.
- V. A. Tuskaev, N. A. Kolosov, D. A. Kurmaev, S. C. Gagieva, V. N. Khrustalev, N. S. Ikonnikov, N. N. Efimov, E. A. Ugolkov, V. V. Minin and B. M. Bulychev, J. Organomet. Chem., 2015, 798, 393 CrossRef CAS.
- N. A. Kolosov, V. A. Tuskaev, S. C. Gagieva, I. V. Fedyanin, V. N. Khrustalev, O. V. Polyakova and B. M. Bulychev, Eur. Polym. J., 2017, 87, 266–276 CrossRef CAS.
- X. Hao, C. Zhang, L. Li, H. Zhang, Y. Hu, D. Hao and X. Zhang, Polymers, 2017, 9, 325 CrossRef PubMed.
- I. I. Salakhov, I. G. Akhmetov and V. G. Kozlov, Polym. Sci., Ser. B, 2011, 53, 385 CrossRef CAS.
- ASTM E1356-08, Standard Test Method for Assignment of the Glass Transition Temperatures by Differential Scanning Calorimetry, 2014 Search PubMed.
- J. Brandrup, E. H. Immergut and E. A. Grulke. Polymer Handbook, John Wiley & Sons, Inc., 1989, vol. 12, p. 2366 Search PubMed.
- R. Bhargava, S. Q. Wang and J. Koenig Advances in Polymer Science, Springer, Berlin, Heidelberg, 1970, vol. 163, p. 137 Search PubMed.
- M. Fineman and S. D. Ross, J. Polym. Sci., 1950, 5, 259 CrossRef CAS.
- Y. B. Podol’nyi, G. V. Solov’yeva and Y. G. Kamenev, Polym. Sci. U.S.S.R., 1974, 16, 3224 CrossRef.
- F. R. Mayo and F. M. Lewis, J. Am. Chem. Soc., 1944, 66, 1594 CrossRef CAS.
- V. N. Pronyayev, I. D. Afanas’ev and G. A. Kovaleva, Polym. Sci. U.S.S.R., 1985, 27, 1448 CrossRef.
- C. Cozewith and G. Ver Strate, Macromolecules, 1971, 4, 482 CrossRef CAS.
- I. Tritto, Z.-Q. Fan, P. Locatelli, M. C. Sacchi, I. Camuratti and M. Galimberti, Macromolecules, 1995, 28, 3342 CrossRef CAS.
- A. C. Kolbert and J. C. Didier, J. Appl. Polym. Sci., 1999, 71, 523 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |