Open Access Article
Open Access ArticleMn(II)-Catalysed ortho-alkenylation of aromatic amines and its application in reproductive diseases†
Jinfei Yang‡
 *,
Xiaolong Wu‡,
Banghua Yang,
Yirong Liu,
Rui Cheng,
Zijun Gong and
Fei Sun*
*,
Xiaolong Wu‡,
Banghua Yang,
Yirong Liu,
Rui Cheng,
Zijun Gong and
Fei Sun*
Medical School, Institute of Reproductive Medicine, Nantong University, Nantong 226019, China. E-mail: jfyang@ntu.edu.cn; sunfei@ntu.edu.cn
First published on 22nd December 2020
Abstract
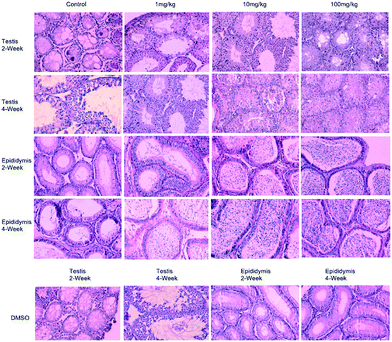
A Mn(II)-catalysed ortho-alkenylation of aromatic amines and its application in reproductive diseases were developed. The use of MnCl2 was critical for the ortho-alkenylation of aromatic amines. The general applicability of this procedure was highlighted by the synthesis of 27 vinylanilines, with good regioselectivities. The value of our approach in practical applications was investigated by studying the effects of one of the compounds 3m on 8 week-old adult male rats with azoospermia as a mammalian model. The results show that a small amount of sperm will gradually be produced in the epididymis and testes by treatment of 8 week-old adult male rats with azoospermia with 1 mg kg−1 3m after two weeks, while treatment with 10 mg kg−1 3m led to obvious sperm production. Notably, if we increase the dose to 100 mg kg−1, there will be a lot of sperm production in the epididymis and testes after two weeks of treatment. The results of this study will be of great significance in research on drugs for treating azoospermia and oligospermia diseases.
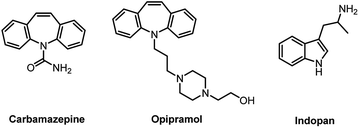
Alkenyl arylamines are very important intermediates for the synthesis of drug molecules, such as carbamazepine,1 opipramol,2 and indopan3 (Scheme 1). O-Alkenyl arylamines have attracted much attention because of their widespread presence in a variety of heterocycles including indoles, quinolines and cinnolines, which are key structural units for many biologically important compounds.4 Moreover, they have also been used as synthetic intermediates in several total synthetic methods.5 Therefore, it is of great significance to carry out the ortho-alkenylation reaction of aromatic amines. But it is not an easily accessible process under normal Friedel–Crafts conditions due to the coordination of the Lewis acid with the nitrogen atom of amino group, which leads to the deactivation of the aromatic ring.6 To overcome this challenge, chemists began to try other methods to achieve ortho-alkenylation of aromatic amines. Subsequently, several related ortho-alkenylation of aromatic amines with phenylacetylene was reported in the literature.4a,7 However, the ortho-alkenylation of aromatic amines catalyzed by base metals has not yet been developed, especially manganese. Therefore, it is still very important to develop a Mn-catalyzed ortho-alkenylation reaction of aromatic amines. Notably, there have been more C–H bond functionalization reactions catalyzed by monovalent manganese in recent years, but few of them were catalyzed by divalent manganese.8 Ackermann's research group reported several Mn(II)-catalyzed ortho-functionalization reactions of arylamides.9 In addition, a Mn(II)-catalysed dehydrogenative annulation of N-aryl anilines with alkenes or alkynes was reported by our group last year.10
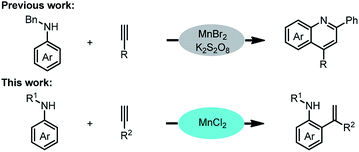
Herein, we report an example of Mn(II)-catalysed ortho-alkenylation of aromatic amines and its application in reproductive diseases (Scheme 2). In our previous work, divalent manganese is easily oxidized to tetravalent manganese by K2S2O8.10 This work, we hypothesis the ortho-alkenylation product of aromatic amine will be generated after the oxidant was removed in the system. Based on this hypothesis, we tried a series of manganese catalysts.
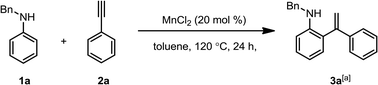
We began by treating N-benzylaniline and phenylacetylene with toluene as a solvent. Initially, we attempted using various Mn(II) catalysts to catalyse the ortho-alkenylation of aromatic amines at 120 °C, i.e., MnO, MnSO4, Mn(OAc)2, and Mn(acac)2, as detected by GC analysis, while 73% and 61% yields were observed when MnBr2 and MnI2 were used as catalysts (Table 1, entries 1–7). Fortunately, a 80% yield of N-benzyl-2-(1-phenylvinyl)aniline was obtained when using MnCl2. To improve the reaction efficiency, different solvents, including N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), acetonitrile (CH3CN), 1,4-dioxane, tetrahydrofuran (THF), 1,2-dichloroethane (DCE), p-xylene, mesitylene and n-hexane were tested (Table 1, entries 8–16). The optimal reaction solvent was found to be toluene. To increase conversion to the N-benzyl-2-(1-phenylvinyl) aniline, we examined a wide range of reaction temperatures (Table 1, entries 17–22). The results show that 120 °C was the optimum temperature, and the corresponding N-benzyl-2-(1-phenylvinyl)aniline was obtained the best yield. It is worth noting that more cyclization products were formed when the temperature is raised to 130 °C. Therefore, temperature is another key factor that drives the reaction forward. Notably, the addition of oxidant will terminate this reaction (see the ESI Table 1† for details). These results therefore support our initial hypothesis that the ortho-alkenylation product of aromatic amine will be generated after the oxidant was removed.
| Entry | Catalyst | Solvent | T (°C) | Yield 3ab (%) |
|---|---|---|---|---|
| a The reactions were carried out in sealed tubes.b Yields were determined by GC analysis.c Without MnCl2. | ||||
| 1 | Mn(OAc)2 | Toluene | 120 | 20 |
| 2 | MnO | Toluene | 120 | 0 |
| 3 | MnSO4 | Toluene | 120 | 0 |
| 4 | Mn(acac)2 | Toluene | 120 | 50 |
| 5 | MnBr2 | Toluene | 120 | 73 |
| 6 | MnI2 | Toluene | 120 | 61 |
| 7 | MnCl2 | Toluene | 120 | 80 |
| 8 | MnCl2 | DMF | 120 | 0 |
| 9 | MnCl2 | DMSO | 120 | 0 |
| 10 | MnCl2 | CH3CN | 120 | 3 |
| 11 | MnCl2 | Dioxane | 120 | 28 |
| 12 | MnCl2 | THF | 120 | 14 |
| 13 | MnCl2 | DCE | 120 | 26 |
| 14 | MnCl2 | p-Xylene | 120 | 50 |
| 15 | MnCl2 | Mesitylene | 120 | 52 |
| 16 | MnCl2 | n-Hexane | 120 | 10 |
| 17 | MnCl2 | Toluene | 80 | 12 |
| 18 | MnCl2 | Toluene | 90 | 29 |
| 19 | MnCl2 | Toluene | 100 | 51 |
| 20 | MnCl2 | Toluene | 110 | 63 |
| 21 | MnCl2 | Toluene | 130 | 70 |
| 22 | MnCl2 | Toluene | 140 | 63 |
| 23c | None | Toluene | 120 | 0 |
With the optimum reaction conditions in hand, a series of aromatic amine were investigated for extending the substrate scope (Scheme 3). This Mn(II)-catalysed ortho-alkenylation of aromatic amines shows good functional group tolerance. Aromatic amine with electron-neutral or electron-donating groups such as alkyl, phenyl, and methoxy on the aryl rings all gave the corresponding ortho-olefination products with high selectivities and in good yields. Aryls containing an electron-withdrawing group such as chloro, and bromo were also tolerated and afforded the corresponding ortho-olefination products 3v–3ab in moderate yields with highly ortho-selectivities. Moreover, the reaction of aromatic amine containing naphthyl of the aromatic rings also gave the corresponding ortho-olefination products 3k and 3l in good yields. Unfortunately, it does not give good yields for meta-substituted aromatic amines.
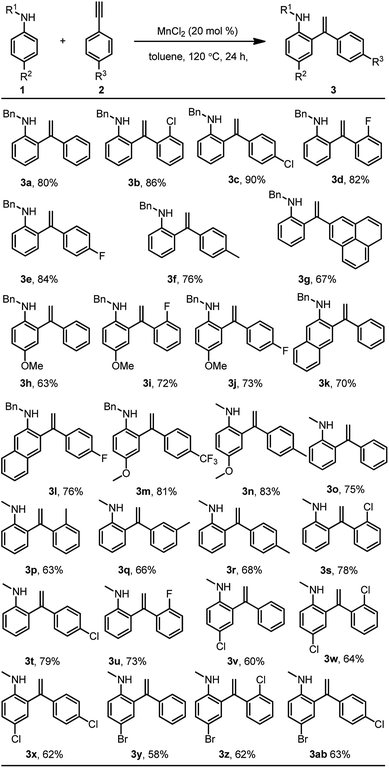 | ||
| Scheme 3 Reaction conditions: substrate 1 (0.2 mmol), aryl acetylene (0.4 mmol), MnCl2 (0.04 mmol), toluene (2.0 mL), at 120 °C for 24 h, and isolated yields for products. | ||
In addition, aryl acetylene with electron-neutral or electron donating groups such as alkyl and anthryl on the aryl rings all gave the corresponding ortho-olefination products with high selectivities and in good yields. Aryls containing an electron-withdrawing group such as fluoro, chloro, bromo and trifluoromethyl were also tolerated and afforded the corresponding ortho olefination products 3b–3e, 3i, 3j, 3l, 3m, 3s–3u, 3w and 3z in moderate to good yields. More importantly, retention of the fluorine, chlorine, and bromine atoms in the products makes the products of considerable use in organic transformations.
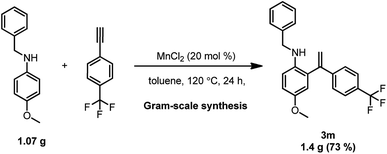
The synthetic utility of the current method was tested by performing a gram-scale ortho-alkenylation of aromatic amines under the optimum conditions. The target N-benzyl-4-methoxy-2-(1-(4-(trifluoromethyl)phenyl)vinyl)aniline 3m was obtained in 73% yield (Scheme 4).
Azoospermia is the medical condition of a man whose semen contains no sperm.11 Pre- and post-testicular azoospermia are frequently correctible, while testicular azoospermia is usually permanent.12 In humans, azoospermia affects about 1% of the male population and may be seen in up to 20% of male infertility situations in Canada.13 However, there is no specific drugs for azoospermia currently on the market. The empirical drug commonly used in clinical practice is clomiphene.14 Clomiphene is a non-steroidal drug with a chemical structure similar to diethylstilbestrol. Its mechanism of action may be that the molecule competitively occupies the ER, thereby blocking the negative feedback effect of circulating endogenous estradiol, leading to increased secretion of GnRH released by the hypothalamus, stimulating the secretion of FSH and LH, and promoting spermatogenesis. Although it has been widely used by clinicians, clinical studies show that long-term use of clomiphene may increase the risk of cancer. Therefore, it is necessary to develop a safer drug that can replace clomiphene. Given that the structure of the 3m is similar to clomiphene, we envision that 3m may also have the effect of promoting spermatogenesis.
To verify the potential of 3m in promoting spermatogenesis, we study the effects of 3m, with 8 week-old adult male rats with azoospermia as a mammalian model (Scheme 5). The test results show that a small amount of sperm will gradually be produced in the epididymis and testis by treatment of 8 week-old adult male rats with azoospermia with 1 mg kg−1 3m after two weeks later, and treatment with 10 mg kg−1 3m led to obvious sperm production. To our delight, a lot of sperm will be produced in the epididymis and testis after four weeks. Notably, if we increase its dose to 100 mg kg−1, there will be a lot of sperm production in the epididymis and testis after two weeks of treatment. In addition, to exclude the influence of DMSO, we made a group of control test, and the test results showed that the sperm number has no change in epididymis and testis. So DMSO has no effect on spermatogenesis. Accordingly, we have discovered a drug molecule that can effectively promote spermatogenesis. The results of this study will be of great significance in research on drugs for treating azoospermia and oligospermia diseases.
Additional experiments were performed to gain a better understanding of the roles of MnCl2 in the ortho-alkenylation of aromatic amines. Control experiments showed that the absence of MnCl2 shut down the reaction (Table 1, entry 23). These results imply that MnCl2 is essential to this reaction. We propose the catalytic cycle shown in the ESI.† Phenylacetylene and aromatic amine first undergoes ligand coordination with the metal center, and subsequent electrophilic addition with aromatic amine provides an intermediate A. and then the target product is obtained through proton migration, with regeneration of the catalytic Mn(II) (see the ESI Fig. 1† for details).
Conclusions
In summary, we have developed a highly efficient Mn(II)-catalysed ortho-alkenylation of aromatic amines and its application in reproductive diseases. This method is safer, more convenient, and more economical than traditional strategies. It is compatible with a range of functional groups and is suitable for gram-scale reactions. The value of our approach in practical applications was shown by studying the effects of treatment with 3m, with 8 week-old adult male rats with azoospermia as a mammalian model. The test results show that 3m significantly affect spermatogenesis. The results of this study will be of great significance in research on drugs for treating azoospermia and oligospermia diseases. Further investigations of the drug molecular mechanism of action, and other reaction types are underway in our laboratory.Ethical statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Nantong University and Experiments were approved by the Animal Ethics Committee (approval no. 20171220-005).Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Key Research and Development Program of China (2018YFC1003500 to F. S.), the High Level Talent Foundation of “Six Talent Peaks” in Jiangsu Province (131219631004 to J. Y.) for financial support.Notes and references
- (a) S. J. Nevitt, A. G. Marson, J. Weston and C. Smith Tudur, Cochrane Database Syst. Rev., 2018, 8, CD001769 Search PubMed; (b) N. Kaniwa and Y. Saito, J. Hum. Genet., 2013, 58, 317–326 CrossRef CAS; (c) L. Liu, T. Zheng, M. J. Morris, C. Wallengren, A. L. Clarke, C. A. Reid, S. Petrou and T. J. O'Brien, J. Pharmacol. Exp. Ther., 2006, 319, 790–798 CrossRef CAS; (d) A. Tateno, K. Sawada, I. Takahashi and Y. Hujiwara, Pediatr. Neurol., 2006, 35, 131–134 CrossRef.
- (a) H. J. Möller, H. P. Volz, I. W. Reimann and K. D. Stoll, J. Clin. Psychopharmacol., 2001, 21, 59–65 CrossRef; (b) M. Hanner, F. F. Moebius, A. Flandorfer, H. G. Knaus, J. Striessnig, E. Kempner and H. Glossmann, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 8072–8077 CrossRef CAS.
- (a) J. Wilcox, J. Psychoact. Drugs, 2012, 44, 274–276 CrossRef; (b) F. Nagai, R. Nonaka and K. H. K. Satoh, Eur. J. Pharmacol., 2007, 559, 32–137 CrossRef; (c) X. M. Huang, M. P. Johnson and D. E. Nichols, Eur. J. Pharmacol., 1991, 200, 187–190 CrossRef CAS.
- (a) A. Chatupheeraphat, M. Rueping and M. Magre, Org. Lett., 2019, 21, 9153–9157 CrossRef CAS; (b) L. G. Qiang and N. H. Baine, J. Org. Chem., 1988, 53, 4218–4222 CrossRef CAS; (c) C. Hausch and G. Helmkamp, J. Am. Chem. Soc., 1951, 73, 3080–3082 CrossRef; (d) C. Hausch, D. G. Crosby, M. Sadoski, A. Leo and D. Percival, J. Am. Chem. Soc., 1951, 73, 704–706 CrossRef.
- (a) J. B. Jiang, D. P. Hesson, B. A. Dusak, D. L. Dexter, G. J. Kang and E. Hamel, J. Med. Chem., 1990, 33, 1721–1728 CrossRef CAS; (b) R. Smith and T. livinghouse, J. Org. Chem., 1983, 48, 1554–1555 CrossRef CAS; (c) P. D. Magnus and N. L. Sear, Tetrahedron, 1984, 40, 2795–2797 CrossRef CAS.
- T. Sugasawa, J. Synth. Org. Chem., Jpn., 1978, 36, 480 CrossRef CAS.
- A. Arienti, F. Bigi, R. Maggi, E. Marzi, P. Moggi, M. Rastelli, G. Sartori and F. Tarantola, Tetrahedron, 1997, 53, 3795–3804 CrossRef CAS.
- (a) S. Waiba and B. Maji, ChemCatChem, 2020, 12, 1891–1902 CrossRef CAS; (b) Y. Hu and C. Wang, ChemCatChem, 2019, 11, 1167–1174 CrossRef CAS; (c) X. Yang and C. Wang, Chem.–Asian J., 2018, 13, 2307–2315 CrossRef CAS; (d) Y. Hu, B. Zhou and C. Wang, Acc. Chem. Res., 2018, 51, 816–827 CrossRef CAS; (e) Q. Lu, S. Cembellín Santos, S. Greßies, S. Singha, C. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2018, 57, 1399–1403 CrossRef CAS; (f) C. Zhu, J. L. Schwarz, S. Cembellín, S. Greßies and F. Glorius, Angew. Chem., Int. Ed., 2018, 57, 437–441 CrossRef CAS; (g) Q. Lu, S. Greßies, S. Cembellín, F. J. R. Klauck, C. G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2017, 56, 12778–12782 CrossRef CAS; (h) Q. Lu, S. Greßies, F. J. R. Klauck and F. Glorius, Angew. Chem., Int. Ed., 2017, 56, 6660–6664 CrossRef CAS.
- (a) Z. Shen, H. Huang, C. Zhu, S. Warratz and L. Ackermann, Org. Lett., 2019, 21, 571–574 CrossRef CAS; (b) C. Zhu, J. C. A. Oliveira, Z. Shen, H. Huang and L. Ackermann, ACS Catal., 2018, 8, 4402–4407 CrossRef CAS; (c) W. Liu, G. Cera, J. C. A. Oliveira, Z. Shen and L. Ackermann, Chem.–Eur. J., 2017, 23, 11524–11528 CrossRef CAS.
- C. Wang, J. Yang, X. Meng, Y. Sun, X. Man, J. Li and F. Sun, Dalton Trans., 2019, 48, 4474–4478 RSC.
- (a) H. Wang, R. Zhao, C. Guo, S. Jiang, J. Yang, Y. Xu, Y. Liu, L. Fan, W. Xiong, J. Ma, S. Peng, Z. Zeng, Y. Zhou, X. Li, Z. Li, X. Li, D. C. Schmitt, M. Tan, G. Li and M. Zhou, Sci. Rep., 2016, 6, 21776 CrossRef CAS; (b) N. Sermondade, C. Faure, L. Fezeu, A. G. Shayeb, J. P. Bonde, T. K. Jensen, M. Van Wely, J. Cao, A. C. Martini and M. Eskandar, et al., Hum. Reprod. Update, 2013, 19, 221–231 CrossRef CAS; (c) B. M. Berookhim and P. N. Schlegel, Urol. Clin. North Am., 2014, 41, 97–113 CrossRef.
- Male Infertility Best Practice Policy Committee of the American Urological Association; Practice Committee of the American Society for Reproductive Medicine. Infertility: report on evaluation of the azoospermic male. American Urological Association, American Society for Reproductive Medicine, 2001 Search PubMed.
- K. Jarvi, K. Lo, A. Fischer, J. Grantmyre, A. Zini, V. Chow and V. Mak, Can. Urol. Assoc. J., 2010, 4, 163–167 CrossRef.
- S. Yilmaz, N. Y. Sezer, İ. M. Gönenç, S. E. İlhan and E. Yilmaz, Cytotechnology, 2018, 70, 489–495 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra10172a |
| ‡ J.-F. Y. and X.-L. W. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |