Open Access Article
Open Access ArticleDesign, synthesis and application in biological imaging of a novel red fluorescent dye based on a rhodanine derivative†
Zijing Lia,
Bin Huang *b,
Yuan Wangc,
Wenbo Yuana,
Yijing Wua,
Ruitao Yua,
Guichuan Xing
*b,
Yuan Wangc,
Wenbo Yuana,
Yijing Wua,
Ruitao Yua,
Guichuan Xing *d,
Taotao Zou
*d,
Taotao Zou *c and
Youtian Tao
*c and
Youtian Tao *a
*a
aKey Laboratory of Flexible Electronics, Institute of Advanced Materials, Nanjing Tech University, Nanjing, P. R. China. E-mail: iamyttao@njtech.edu.cn
bCollege of Life Sciences and Chemistry, Jiangsu Key Laboratory of Biofunctional Molecule, Jiangsu Second Normal University, Nanjing, P. R. China. E-mail: huangbinhb31@sina.com
cGuangdong Key Laboratory of Chiral Molecule and Drug Discovery, School of Pharmaceutical Sciences, Sun Yat-Sen University, Guangzhou, P. R. China. E-mail: zoutt3@mail.sysu.edu.cn
dInstitute of Applied Physics and Materials Engineering, University of Macau, Macao SAR 999078, China. E-mail: gcxing@um.edu.mo
First published on 23rd December 2020
Abstract
A novel acceptor–donor–acceptor type molecule, namely 2-triphenylamine-1,3-dia[2-(3-ethyl-4-oxo-thiazolidin-2-ylidene)-malononitrile] (2RDNTPA), is designed and synthesized. 2RDNTPA exhibits a large Stokes shift of 244 nm and red fluorescence emission of 629 nm with a decent photoluminescence quantum yield of 13%. Furthermore, as a potential red fluorescent dye, 2RDNTPA can be applied in fluorescence imaging of living cancer cells (HepG2) with negligible cytotoxicity and a half maximal inhibitory concentration much more than 100 μM.
In recent years, imaging technology has achieved remarkable development, which can convert chemical and biological information into monitorable signals, making it possible to visually monitor different biological components and various physiological processes in living organisms.1 Among various imaging technologies, fluorescence imaging has been considered as a promising technology for elucidating biological functions.1–7 The usually utilized fluorescent dyes for fluorescence imaging are molecules with emission wavelength beyond 600 nm that allow for slight photo-damage on samples and low levels of auto fluorescence from biomolecules.2,3 It is widely reported that heavy-metal complexes8–10 and organic dyes are candidates for achieving red emission of fluorescence imaging. However, most heavy-metal complexes possess some drawbacks such as high cost, serious pollution and strong toxicity, which limit their applications.8–10 Therefore, the development of fluorescent dyes with metal-free small organic dyes for efficient fluorescence imaging attracted more and more attention.
In general, the core properties of an efficient dye for fluorescence imaging include the following: (1) a large Stokes shift between excitation and emission;3 (2) high photo-luminescence quantum yield (PLQY); (3) red or infrared emission to eliminate the backgrounds from biological environments; (4) good biocompatibility and low toxicity; (5) easy to synthesize. To date, some red or infrared light-emitting fluorophores such as Cy7-1,2 AHGa,4 DCF-MPYM,5 et al. showed great potentials in fluorescence imaging. However, compared with conventional fluorescence dyes, fluorophores for efficient fluorescence imaging are still limited in number. It remains a challenge to develop brand-new fluorescent dyes for fluorescence imaging.
Rhodanine,23–25 a five-membered S,N-heterocycle, can serve as a building block for organic dyes in biomedical fields such as biomolecule fluorescent labeling,11 proteomics,12 photo-dynamic therapy,13–15 live cell imaging,16–19 antitumor drugs,20 DNA detection,8 pH probes,21 fluorescent sensors,22 etc. Owning to their strong electron-withdrawing properties, rhodanine derivatives have been broadly utilized as acceptors for the design of donor–acceptor (D–A) type photovoltaic materials in organic solar cells (OSCs).39 However, up to now, there is no report on D–A type dyes based on rhodanine derivatives with efficient red emission for fluorescence imaging.
Herein, we designed and synthesized a new acceptor–donor–acceptor (A–D–A) type molecule 2-triphenylamine-1,3-dia[2-(3-ethyl-4-oxo-thiazolidin-2-ylidene)-malononitrile] (2RDNTPA, Scheme 1), in which triphenylamine (TPA) and rhodanine derivative of 2-(3-ethyl-4-oxo-thiazolidin-2-ylidene)-malononitrile (RDN) act as the electron donor and acceptor group, respectively. TPA unit is widely used as electron donor in emitters for its suitable steric hindrance and strong electron-donating ability,26,27 while RDN unit with two cyan groups possesses strong electron-accepting ability and high molecular rigidity. 2RDNTPA exhibits a large Stokes shift of 244 nm and red fluorescence emission of 629 nm with a decent PLQY of 13%. More importantly, it is demonstrated that 2RDNTPA can be applied in fluorescence imaging of the living cancer cells (HepG2) with negligible cytotoxicity.
Synthetic routes of 2RDNTPA were shown in Scheme 1 and the detailed procedure can be found in the ESI.† First, the key intermediate 2-triphenylaminebenzene-1,3-dialdehyde (2AIDTPA) was prepared by Suzuki coupling reaction between 2-bromobenzene-1,3-dialdehyde and N,N-diphenyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline. Then 2AIDTPA was converted to 2RDNTPA via Knoevenagel condensation30 with 2-(3-ethyl-4-oxo-thiazolidin-2-ylidene)-malononitrile. The intermediate and target compound were fully identified by 1H NMR (Fig. S1 and S2, ESI†), MS (Fig. S3, ESI†) and elemental analysis. Due to the substituent effects on the double bonds between RDN groups and the central benzene cycle, 2RDNTPA may have three kinds of cis–trans isomers (e.g.: EE, ZZ and EZ). In general, E-isomer is more stable than the corresponding Z-isomer. In addition, the 1H NMR of two RDN groups are the same (Fig. S2†), indicating that 2RDNTPA possesses a symmetrical structure. As a result, we can conclude that the reported structure of 2RDNTPA belongs to an EE isomer (Scheme 1).
Thermal gravimetric analysis showed the decomposition temperature was about 369 °C with 5% loss of weight (Fig. S4, ESI†), while the glass transition temperature was not found during the second heating of differential scanning calorimetry measurement. The thermal analysis results clearly demonstrated the favorable thermal stability of 2RDNTPA for biological imaging.
To predict the electronic distribution of 2RDNTPA, density functional theory (DFT) calculations were performed. As shown in Fig. 1a, 2RDNTPA displayed a highly twisted molecular structure with large torsion angles of 60° and 30° between RDN units and the central phenyl unit. As expected, the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of 2RDNTPA were located on the donor and acceptor unit, respectively.33 The HOMO and LUMO energy levels of 2RDNTPA were estimated to be −5.27 and −2.95 eV, respectively (Fig. 1b). Accordingly, the optical bandgap (Eg) of 2RDNTPA was calculated to be 2.32 eV.
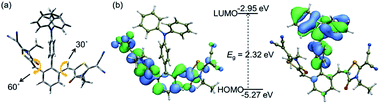 | ||
| Fig. 1 (a) Molecular structure, (b) HOMO (left) and LUMO (right) distributions, and theoretically calculated HOMO, LUMO of 2RDNTPA. | ||
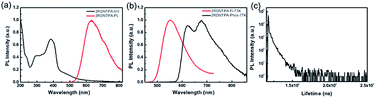
The photophysical properties of 2RDNTPA in powder were investigated. From the UV-vis absorption spectra (Fig. 2a), the absorption peak at 298 nm can be attributed to the intense absorption from TPA centered n–π* transition, while the broad absorption band in the range of 350–500 nm with peak at 385 nm originate from intramolecular charge transfer transition (ICT).33–38 From the onset of the absorption spectra, Eg of 2RDNTPA was calculated to be 2.25 eV, which agrees well with the theoretical result. As shown in Fig. 2a, 2RDNTPA exhibited an obviously red emission peak at 629 nm with PLQY of 13% (Fig. S5†). The Stokes shift of 2RDNTPA between excitation (385 nm) and emission (629 nm) was calculated to be 244 nm, which may facilitate the elimination of background interference effectively. From the fluorescence and phosphorescence spectra of 2RDNTPA at 77 K (Fig. 2b), the singlet energy level and triplet energy level of 2RDNTPA were estimated to be 2.25 and 1.99 eV, respectively. The transient PL decay curve of 2RDNTPA was also depicted in Fig. 2c, which exhibited two components decay with an average lifetime of 9.26 ns.
We also measured UV-vis and fluorescence spectra of 2RDNTPA in different solvents. As shown in Fig. S6 and Table S1,† polarity of the solvents has negligible influence on the UV-vis spectra of 2RDNTPA. In contrast, the emission spectra of 2RDNTPA is gradually red-shifted with increasing solvent polarity, indicating typical ICT feature (Fig. S7 and Table S1†). To explore the aggregated luminescence properties, the PL intensity of 2RDNTPA in the mixture of chloroform and methanol with various methanol ratio fractions was measured. As shown in Fig. S8,† the PL intensity showed an overall slow decline when methanol fractions (fw) increased from 0% to 90%, showing obvious aggregation-caused quenching properties of 2RDNTPA in the chloroform/methanol system. To evaluate the light stability of 2RDNTPA, the absorption intensity in dimethyl sulfoxide solution with various irradiation times of 365 nm ultraviolet lamp was characterized. The absorption intensity showed slow decline when irradiation time increased from 0 to 24 h (Fig. S9†). It was evident that 2RDNTPA proved to be highly photo-stable.
The electrochemical properties of 2RDNTPA were investigated by cyclic voltammetry (CV) measurements. As depicted in Fig. S10,† 2RDNTPA displayed quasi-reversible oxidation process assigned to the triphenylamine units.36,37
Based on the onset of oxidation from CV curve, the HOMO energy level was estimated to be −5.29 eV. From the equation ELUMO = EHOMO + Eg, here the Eg value was estimated from the edge of UV-vis absorption, the LUMO energy level was estimated to be −3.04 eV, which are in accordance with the calculated results.
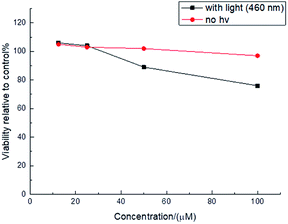
The unique photophysical properties of 2RDNTPA led us to investigate its application in biological imaging. We treated living HepG2 liver cancer cells with 2RDNTPA solution. The detailed procedure can be found in the ESI.† Then confocal laser scanning microscopy (CLSM) imaging was carried out by utilizing the red emission of 2RDNTPA to investigate the cellular uptake and sub-organelle localization in cancer cells.31,32 As shown in Fig. 3, CLSM imaging can clearly characterize the situation inside the cell with strong red fluorescence close to 620 nm, which agrees well with the excellent red light-emitting performance of 2RDNTPA. It is worth noting that the cells did not show obvious morphology change, indicating good biocompatibility of 2RDNTPA in living HepG2 cell. As we all know, the low cytotoxicity of organic dyes is a key criterion for live cell imaging.1 So we conducted 2RDNTPA in vitro cytotoxicity experiments.28,29 As shown in Fig. 4, when the concentration of 2RDNTPA reach to 100 μM, more than 95% cells still survive at no hν environment. According to U.S. National Cancer Institute recommends,40 IC50 (inhibitory concentrations) greater than 50 μM can be considered as invalid during cell toxicity assay. The results suggest that 2RDNTPA shows negligible toxicity in living HepG2 liver cancer cells whether under illuminated conditions or not. The good biocompatibility and low cytotoxicity of 2RDNTPA indicate that it can be utilized as an efficient dye for live cell imaging to replace traditional heavy-metal complexes with high cytotoxicity.41
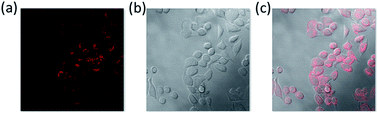 | ||
| Fig. 3 (a) CLSM (excitation of 407 nm and emission of 620 nm) images of 2RDNTPA in living HepG2 cell (10 μM incubation overnight). (b) Bright field. (c) Merged image. | ||
In summary, we have developed an A–D–A structured red fluorescent dye 2RDNTPA, in which TPA and rhodanine derivative RDN act as the electron donor and acceptor group, respectively. Based on theoretical calculations, photophysical and electrochemical tests, the photophysical properties of 2RDNTPA have been systematically investigated. 2RDNTPA exhibits a large Stokes shift of 244 nm and red fluorescence emission of 629 nm with a decent photoluminescence quantum yield of 13%. By using 2RDNTPA as a red dye in biological imaging of the living HepG2 cancer cells, we have obtained commendable imaging results with IC50 of much more than 100 μM. The present work provides a new strategy for the design and application of organic fluorescent dyes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank National Natural Science Foundation of China (No. 51103023, 91833304 and 61761136013), NSF of Jiangsu Province (No. XYDXX-026) and the Natural Science Foundation of the Jiangsu Higher Education Institutions (No. 19KJB150006). G. C. X. acknowledges the Science and Technology Development Fund, Macao SAR (File no. FDCT-0044/2020/A1, FDCT-091/2017/A2, FDCT-014/2017/AMJ), UM's research fund (File no. MYRG2018-00148-IAPME), the Natural Science Foundation of China (91733302, 61935017), Natural Science Foundation of Guangdong Province, China (2019A1515012186).Notes and references
- Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2013, 113, 192–270 CrossRef CAS.
- Y. Li, Y. Sun, J. Li, Q. Su, W. Yuan, Y. Dai, C. Han, Q. Wang, W. Feng and F. Li, J. Am. Chem. Soc., 2015, 137, 6407–6416 CrossRef CAS.
- S. Zhang, J. Fan, Z. Li, N. Hao, J. Cao, T. Wu, J. Wang and X. Peng, J. Mater. Chem. B, 2014, 2, 2688–2693 RSC.
- B. Lozano-Torres, I. Galiana, M. Rovira, E. Garrido, S. Chaib, A. Bernardos, D. Muñoz-Espín, M. Serrano, R. Martínez-Máñez and F. Sancenón, J. Am. Chem. Soc., 2017, 139, 8808–8811 CrossRef CAS.
- X. Xiong, F. Song, J. Wang, Y. Zhang, Y. Xue, L. Sun, N. Jiang, P. Gao, L. Tian and X. Peng, J. Am. Chem. Soc., 2014, 136, 9590–9597 CrossRef CAS.
- T. Li, D. Yang, L. Zhai, S. Wang, B. Zhao, N. Fu, L. Wang, Y. Tao and W. Huang, Adv. Sci., 2017, 4, 1600166 CrossRef.
- F. Ni, Z. Zhu, X. Tong, M. Xie, Q. Zhao, C. Zhong, Y. Zou and C. Yang, Chem. Sci., 2018, 9, 6150–6155 RSC.
- M. R. Gill and J. A. Thomas, Chem. Soc. Rev., 2012, 41, 3179–3192 RSC.
- K. Hanaoka, K. Kikuchi, S. Kobayashi and T. Nagano, J. Am. Chem. Soc., 2007, 129, 13502–13509 CrossRef CAS.
- A. Gorman, J. Killoran, C. O'Shea, T. Kenna, W. Gallagher and D. O'Shea, J. Am. Chem. Soc., 2004, 126, 10619–10631 CrossRef CAS.
- A. Toutchkine, V. Kraynov and K. Hahn, J. Am. Chem. Soc., 2003, 125, 4132–4145 CrossRef CAS.
- K. Janina, J. Katarzyna and O. Łukasz, J. Mol. Struct., 2015, 1084, 114–121 CrossRef.
- P. Nowaksliwinska, A. Karocki, M. Elas, A. Pawlak, G. Stochel and K. Urbanska, Biochem. Biophys. Res. Commun., 2006, 349, 549–555 CrossRef CAS.
- D. L. Traul and F. Sieber, J. Photochem. Photobiol., B, 2015, 153, 153–163 CrossRef CAS.
- G. S. Anderson, K. Miyagi, R. W. Sampson and F. Sieber, J. Photochem. Photobiol., B, 2002, 68, 101–108 CrossRef CAS.
- X. Zhang, Z. Gu, L. Liu, S. Wang and G. Xing, Chem. Commun., 2015, 51, 8606–8609 RSC.
- A. Nahain, J. E. Lee, J. H. Jeong and S. Y. Park, Biomacromolecules, 2013, 14, 4082–4090 CrossRef CAS.
- H. H. Pham, C. Szentgyorgyi, W. L. Brotherton, B. F. Schmidt, K. J. Zanotti, A. S. Waggoner and B. A. Armitage, Org. Biomol. Chem., 2015, 13, 3699–3710 RSC.
- E. E. Rastede, M. Tanha, D. Yaron, S. C. Watkins, A. S. Waggoner and B. A. Armitage, Photochem. Photobiol., 2015, 14, 1703–1712 RSC.
- D. Kaminskyy, B. Bednarczykcwynar, O. Vasylenko, O. B. Kazakova, B. Zimenkovsky, L. Zaprutko and R. Lesyk, Med. Chem. Res., 2012, 21, 3568–3580 CrossRef CAS.
- L. He, W. Lin, Q. Xu and H. Wei, ACS Appl. Mater. Interfaces, 2014, 6, 22326–22333 CrossRef CAS.
- K. Krumova and G. Cosa, J. Am. Chem. Soc., 2010, 132, 17560–17569 CrossRef CAS.
- H. Li, J. Yang, S. Ma and C. Qiao, Bioorg. Med. Chem., 2012, 20, 4194–4200 CrossRef CAS.
- W. Li, C. Zheng, L. Sun, M. Song, Y. Wu, Y. Li, Y. Liu and H. Piao, Arch. Pharmacal Res., 2014, 37, 852–861 CrossRef CAS.
- Y. Zhang, S. Wang, S. Wu, S. Zhu, G. Dong, Z. Miao, J. Yao, W. Zhang, C. Sheng and W. Wang, ACS Comb. Sci., 2013, 15, 298–308 CrossRef CAS.
- A. Mishra, M. Fischer and P. Bäuerle, Angew. Chem., Int. Ed., 2009, 48, 2474–2499 CrossRef CAS.
- Y. Shirota and H. Kageyama, Chem. Rev., 2007, 107, 953–1010 CrossRef CAS.
- K. Takasu, H. Inoue, H. Kim, M. Suzuki, T. Shishido, Y. Wataya and M. Ihara, J. Med. Chem., 2002, 45, 995–998 CrossRef CAS.
- K. Takasu, K. Pudhom, M. Kaiser, R. Brun and M. Ihara, J. Med. Chem., 2006, 49, 4795–4798 CrossRef CAS.
- G. Angajala, V. Aruna and R. Subashini, J. Mol. Struct., 2021, 1224, 129286 CrossRef.
- J. Xiang, Y. Liu, D. Sun, S. Zhang, Y. Fu, X. Zhang and L. Wang, Dyes Pigm., 2012, 93, 1481–1487 CrossRef CAS.
- D. Gao, A. Li, L. Guan, X. Zhang and L. Wang, Dyes Pigm., 2016, 129, 163–173 CrossRef CAS.
- Y. Tao, K. Yuan, T. Chen, P. Xu, H. Li, R. Chen, C. Zheng, L. Zhang and W. Huang, Adv. Mater., 2014, 26, 7931–7958 CrossRef CAS.
- W. Zeng, T. Zhou, W. Ning, C. Zhong, J. He, S. Gong, G. Xie and C. Yang, Adv. Mater., 2019, 31, 1901404 CrossRef.
- J. Chen, K. Wang, C. Zheng, M. Zhang, Y. Shi, S. Tao, H. Lin, W. Liu, W. Tao, X. Ou and X. Zhang, Sci. Adv., 2018, 5, 1800436 CrossRef.
- W. Zeng, H. Lai, W. Lee, M. Jiao, Y. Shiu, C. Zhong, S. Gong, T. Zhou, G. Xie, M. Sarma, K. Wong, C. Wu and C. Yang, Adv. Mater., 2017, 30, 1704961 CrossRef.
- F. Xie, H. Li, G. Dai, Y. Li, T. Cheng, M. Xie, J. Tang and X. Zhao, ACS Appl. Mater. Interfaces, 2019, 11, 26144–26151 CrossRef CAS.
- Y. Luo, S. Li, Y. Zhao, C. Li, Z. Pang, Y. Huang, M. Yang, L. Zhou, X. Zheng, X. Pu and Z. Lu, Adv. Mater., 2020, 32, 2001248 CrossRef CAS.
- Q. Wei, W. Liu, M. Leclerc, J. Yuan, H. Chen and Y. Zou, Sci. China: Chem., 2020, 63, 1352–1366 CrossRef CAS.
- J. L. Sebaugh, Pharm. Stat., 2011, 10, 128–134 CrossRef CAS.
- L. Xian, F. Xu, J. Liu, N. Xu, H. Li, H. Ge, K. Shao, J. Fan, G. Xiao and X. Peng, J. Am. Chem. Soc., 2019, 141, 20490–20497 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08998b |
| This journal is © The Royal Society of Chemistry 2021 |