Open Access Article
Open Access ArticleThe selective synthesis of N-arylbenzene-1,2-diamines or 1-arylbenzimidazoles by irradiating 4-methoxy-4′-substituted-azobenzenes in different solvents†
Po-Yi Chenb,
Chi-Wei Hsu*ac,
Tong-Ing Ho‡
a and
Jinn-Hsuan Ho *b
*b
aDepartment of Chemistry, National Taiwan University, Taiwan. E-mail: Vincenthsu631230@gmail.com
bDepartment of Chemical Engineering, National Taiwan University of Science and Technology, Taiwan. E-mail: jhho@mail.ntust.edu.tw
cEdBrother Biotechnology Ltd, Taiwan
First published on 10th February 2021
Abstract
The solvent-controllable photoreaction of 4-methoxyazobenzenes to afford 1-aryl-1H-benzimidazoles or N-arylbenzene-1,2-diamines has been studied. The irradiation of 4-methoxyazobenzenes in DMF containing 0.5 M hydrochloric acid provided N2-aryl-4-methoxybenzene-1,2-diamines as the major product, while irradiation in acetal containing 0.16 M hydrochloric acid led to 1-aryl-6-methoxy-2-methyl-1H-benzimidazoles as the major product. A possible reaction mechanism explaining the selectivity was also discussed.
Compounds with 1-phenyl-1H-benzimidazole skeleton1 have gained attention for a long time and been studied for the applications of platelet-derived growth factor receptor (PDGFR),2 cyclooxygenase (COX),3 and phosphodiesterase 10A4 inhibitors, as well as for many optoelectronic materials.5 N-Aryl-1,2-phenylenediamines are also important for synthesizing various benzimidazole derivatives, and 1,3,5-tris(N-phenyl-benzimidazol-2-yl)benzene (TBPI) is an important electronic material for OLED applications that is still widely used nowadays.6
The synthesis of 1-aryl-1H-benzimidazoles can be achieved via many synthetic strategies, including single and multiple molecular reactions. Single-molecule synthetic methods include the intramolecular cyclization of arylamino and oxime groups,7 the coupling of amidine and halogen (or hydrogen) groups,8 and the condensation of amide and amino groups.9 Methods involving bimolecular reactions include intermolecular coupling reactions of benzimidazoles,10 the cyclocondensation of o-diaminoarenes and aldehydes obtained from the oxidation of alcohols,11 and the catalytic cyclization of aryl halides/amidines12 or anilines/oxime acetates.13 Methods involving trimolecular reactions include one-pot reactions of arenediazonium salts, anilines, and nitriles,14 and the catalytic cascade cyclization of aryl halides, anilines, and amides.15 These synthetic methods provide solutions for the preparation of 1-phenyl-1H-benzimidazoles but they still present some difficulties, such as the use of transition metal catalysts and unsymmetrical starting materials.
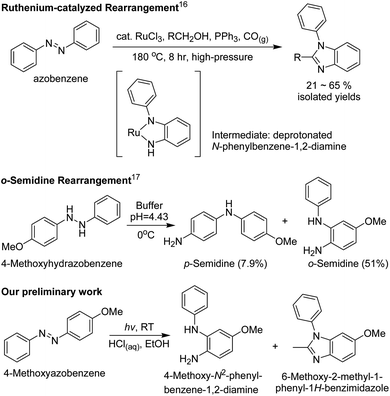
Using azobenzenes as reactants to afford 1-phenyl-1H-benzimidazoles16 and N-phenylbenzene-1,2-diamines17 was reported three decades ago. Compared to the above-mentioned synthetic methods, synthesis from azobenzenes showed the benefits of a simple preparation procedure and the good stability of symmetrical azobenzenes as starting materials (Scheme 1). The synthesis of a 1-phenyl-1H-benzimidazole16 from an azobenzene started from α-metallation with ruthenium trichloride, triggering the o-semidine rearrangement to produce the intermediate; this was then further transformed to a 1-phenyl-1H-benzimidazole upon reacting with an aldehyde that was produced via oxidizing an alcohol with ruthenium trichloride. However, the reaction needs high temperature, high pressure, transition-metal catalysts, and dangerous carbon monoxide, making it risky and environmentally unfriendly. Moreover, N-phenylbenzene-1,2-diamines17 could be obtained via the o-semidine rearrangement of hydrazobenzenes, which are the reductive products of azobenzenes. However, tributyltin hydride, which is used for the reduction of azobenzenes, is toxic, and the hydrazobenzenes were unstable in the presence of air because they were easily oxidized back to azobenzenes. Considering the previously discovered reactions involving azobenzenes and hydrazobenzenes, it would be worth developing a modified synthetic method that could be used to synthesize N-phenylbenzene-1,2-diamines and 1-phenyl-1H-benzimidazoles from azobenzenes more safely and easily.
 | ||
| Scheme 1 The synthesis of 1-phenyl-1H-benzimidazoles and N-phenylbenzene-1,2-diamines from azobenzenes and hydrazo-benzenes. | ||
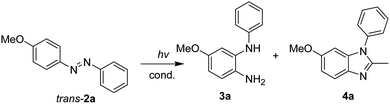
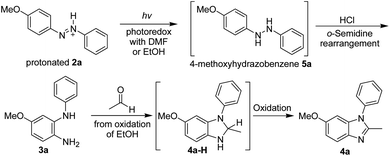
Inspired by research reporting the photoreduction of imine-containing compounds to amines in the presence of alcohols,18 a possible idea was suggested: the photoreduction of azobenzenes to hydrazobenzenes with ethanol followed by the instant transformation of hydrazobenzenes to N-phenylbenzene-1,2-diamines in acidic media. To investigate this synthetic idea, the irradiation of 4-methoxyazobenzene in ethanol containing 0.5 M hydrochloric acid with UV light at 365 nm was performed (Scheme 1). Surprisingly, this irradiation afforded both 4-methoxy-N2-phenylbenzene-1,2-diamine and 6-methoxy-2-methyl-1-phenyl-1H-benzimidazole as the two major products. This synthetic method seemed to have many advantages, including the use of stable and symmetrical azobenzenes as starting materials, the transition-metal-free synthesis, and the obtaining of two products in a one-pot synthesis process. Therefore, we report the study of the irradiation of 4-methoxy-4′-substitutedazobenzenes (2a–2g) to afford 4-methoxy-N2-(substitutedphenyl)benzene-1,2-diamines (3a–3g) and 6-methoxy-2-methyl-1-(4-substitutedphenyl)-1H-benzimidazoles (4a–4g).
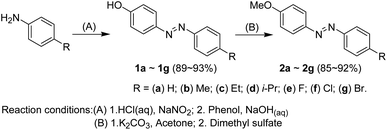
A series of 4-methoxy-4′-substituted azobenzenes (2a–2g) was synthesized via a two-step synthesis process (Scheme 2). The first step19 was an azo coupling reaction between phenol and the corresponding aryl diazonizum salt, which was prepared via a diazotization reaction between 4′-substituted aniline and sodium nitrite, to afford the 4-hydroxy-4′-substituted azobenzene (1a–1g) with a yield of 89–93%. The second step20 was the methylation of the hydroxyl group of 1a–1g using potassium carbonate and dimethyl sulfate, with yields of 94–99%. The total yields from these two steps are ca. 85–92%.
In order to investigate suitable reaction conditions for the formation of N-arylbenzene-1,2-diamines and 1-aryl-1H-benzimidazoles, a series of test reactions involving 4-methoxy-azobenzene (2a) under different reaction conditions was performed (Scheme 3). The detailed experimental procedure for entry 10 in Table 1 is described as follows. A solution containing 20 mg of compound 2a, 0.45 mL of concentrated HCl aqueous solution (12 M) and 10.55 mL of DMF was sealed in a quartz tube with a septum. The prepared solution was bubbled with nitrogen gas for 15 min and irradiated with a 365 nm UV lamp. After 2.5 h, the reaction solution was neutralized and extracted using water and ethyl acetate several times. The organic layers were collected, and the crude sample was obtained after removing the organic solvent under vacuum. The 1H-NMR spectrum of the crude sample was obtained and the trans/cis-2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a
3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a ratio was calculated based on the integration of the feature peaks of the compound (Table 1).
4a ratio was calculated based on the integration of the feature peaks of the compound (Table 1).
| En. | WL (nm) | Solvent | Acid (conc.) | t (h) | Ratio: t/c-2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a 4a |
|---|---|---|---|---|---|
| a Aniline is observed in an amount of 20–30%.b Aniline is present in an amount of ca. 10%.c Aniline is present within an amount of 2%. | |||||
| 1 | 365 | CH3CN | HCO2H (1.26 M) | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 2 | 365 | CH3CN | AcOH (1.59 M) | 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 3 | 365 | DMF | None | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 4 | 365 | DMF | HCl (0.05 M) | 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 5 | 365 | DMF | HCl (0.1 M) | 1 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 6 | 365 | DMF | HCl (0.3 M) | 1 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 7 | 365 | DMF | HCl (0.5 M) | 1 | 0.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 8 | 365 | DMF | HCl (0.7 M) | 1 | 0.38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 9 | 365 | DMF | HCl (0.9 M) | 1 | 0.47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 10 | 365 | DMF | HCl (0.5 M) | 2.5 | 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 11 | 365 | CH3CN | HCl (0.5 M) | 3 | 0.49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02 0.02 |
| 12 | 365 | EtOH | HCl (0.5 M) | 4 | 0.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.23a 1.23a |
| 13 | 419 | EtOH | HCl (0.47 M) | 0.33 | 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35a 0.35a |
| 14 | 419 | EtOH | HCl (0.77 M) | 0.33 | 0.76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.71a 2.71a |
| 15 | 419 | EtOH | HCl (0.91 M) | 0.33 | 0.74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.96a 1.96a |
| 16 | 419 | Acetal | HCl (0.77 M) | 2 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a 1a |
| 17 | 419 | Acetal | HCl (0.48 M) | 2 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0:1b 0:1b |
| 18 | 419 | Acetal | HCl (0.16 M) | 2 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1b 1b |
| 19 | 419 | Acetal | HCl (0.16 M) | 0.66 | 0.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c 1c |
| 20 | 419 | Acetal | HCl (0.16 M) | 0.33 | 0.98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c 1c |
Based on the experimental results (Table 1), this photoreaction could not progress effectively without strong acid (entries 1–3) or at a low concentration of hydrochloric acid (entries 4–6). When the reaction solution was DMF containing hydrochloric acid with a strength of at least 0.5 M, the photoreaction effectively afforded 4-methoxy-N2-phenyl-benzene-1,2-diamine (3a) as the major product (entries 7–10), and the best conditions led to a trans/cis-2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a ratio of 0.25
3a ratio of 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as shown in entry 10, Table 1. Subsequently, using acetonitrile and ethanol as the reaction solution could produce a mixed product containing 3a, 4a, and anilines (decomposed side products) (entries 11–15), and conditions for obtaining a higher amount of 6-methoxy-2-methyl-1-phenyl-1H-benzimidazole (4a) involved the use of ethanol as the solvent with 0.77 M hydrochloric acid under 419 nm irradiation (entry 14). Finally, using acetal as the solvent could produce 4a as the major product with some aniline as an impurity (entries 16–20). It could be observed that the amount of impurities (anilines) increased as the concentration of hydrochloric acid increased; therefore, the best conditions for the formation of 4a in acetal solution involved 0.16 M HCl under 419 nm irradiation for 40 min.
1, as shown in entry 10, Table 1. Subsequently, using acetonitrile and ethanol as the reaction solution could produce a mixed product containing 3a, 4a, and anilines (decomposed side products) (entries 11–15), and conditions for obtaining a higher amount of 6-methoxy-2-methyl-1-phenyl-1H-benzimidazole (4a) involved the use of ethanol as the solvent with 0.77 M hydrochloric acid under 419 nm irradiation (entry 14). Finally, using acetal as the solvent could produce 4a as the major product with some aniline as an impurity (entries 16–20). It could be observed that the amount of impurities (anilines) increased as the concentration of hydrochloric acid increased; therefore, the best conditions for the formation of 4a in acetal solution involved 0.16 M HCl under 419 nm irradiation for 40 min.
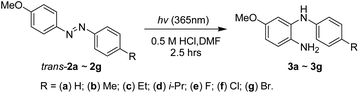
The selective synthesis of the 4-methoxy-N2-arylbenzene-1,2-diamines 3a–3g could be achieved through the 365 nm irradiation of the 4-methoxyazobenzenes 2a–2g in DMF containing 0.5 M hydrochloric acid under ambient nitrogen conditions in a quartz tube for 2.5 h (Scheme 4 and Table 2). To avoid the dramatic loss of amine products due to silica gel column chromatography, conversions and yields were determined based on the weights of crude samples and the molar ratios of compounds (trans-2:cis-2:3) from 1H-NMR spectrum integration. The reaction conversions of irradiated 2a–2g were 61–82%, and the yields of 3a–3g were 58–79%. No substitution effects were observed during this photoreaction. Considering the overall synthesis from the starting materials of phenol and substituted anilines, the total yields of 3a–3g via this 3-step synthesis were 51–72%.
| En. | Starting | Conv. (%) | Yield (%) based on conversion | Yield of 3 (%) | 3-Step yield (%) |
|---|---|---|---|---|---|
| 1 | 2a: H | 82 | 97 | 79.54 | 72.47 |
| 2 | 2b: Me | 61 | 95 | 57.95 | 51.15 |
| 3 | 2c: Et | 65 | 98 | 63.7 | 54.99 |
| 4 | 2d: i-Pr | 74 | 98 | 72.52 | 62.72 |
| 5 | 2e: F | 72 | 98 | 70.56 | 60.29 |
| 6 | 2f: Cl | 76 | 97 | 73.72 | 65.74 |
| 7 | 2g: Br | 77 | 96 | 73.92 | 68.06 |
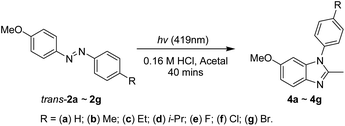
The selective synthesis of the 1-aryl-2-methyl-6-methoxy-1H-benzimidazoles 4a–4g could be achieved via the 419 nm irradiation of the 4-methoxyazobenzenes 2a–2g in acetal containing 0.16 M hydrochloric acid under ambient nitrogen conditions in a quartz tube for 40 min (Scheme 5). The reaction results are listed in Table 3, and the conversions and yields are determined based on the weights of crude samples and the molar ratios of compounds (trans-2:cis-2:4) from 1H-NMR spectrum integration. The irradiation of 2a–2g to afford 4a–4g had conversions of 66–93% and yields of 60–89%. No substitution effects were observed in this photoreaction either. The 3-step reaction yields for the synthesis of 4a–4g (including the preparation of compounds 1 and 2) were 45–81%.
| En. | Starting | Conv. (%) | Yield (%) based on conversion | Yield of 4 (%) | 3-Step yield (%) |
|---|---|---|---|---|---|
| 1 | 2a: H | 93 | 96 | 89.28 | 81.37 |
| 2 | 2b: Me | 76 | 86 | 65.36 | 57.69 |
| 3 | 2c: Et | 75 | 86 | 64.50 | 55.68 |
| 4 | 2d: i-Pr | 72 | 73 | 52.56 | 45.45 |
| 5 | 2e: F | 67 | 91 | 60.97 | 52.09 |
| 6 | 2f: Cl | 66 | 91 | 60.06 | 53.56 |
| 7 | 2g: Br | 77 | 90 | 69.30 | 63.80 |
The proposed mechanism includes three main steps: (1) a photoredox reaction between a protonated azobenzene and the solvent to produce the hydrazobenzene; (2) an o-semidine rearrangement of the hydrazobenzene to afford the N2-arylbenzene-1,2-diamine; and (3) a condensation reaction between the N2-arylbenzene-1,2-diamine and acetaldehyde to produce the 1-arylbenzimidazole via oxidative aromatization (Scheme 6), and these are described below. 4-Methoxyazobenzene (2a) is protonated by hydrochloric acid in acidic media, and then protonated 2a is irradiated and undergoes a photoredox reaction with DMF or EtOH to produce 4-methoxy-hydrazobenzene (5a) as the photoreduction product. Afterwards, an o-semidine rearrangement17,21 immediately takes place to transform 5a into 4-methoxy-N2-phenylbenzene-1,2-diamine (3a), which is the major product when using DMF as the reaction solvent. However, when EtOH is the reaction solvent, acetaldehyde may be produced during the photoredox reaction; this can consequently react with the di-amino groups of 3a to afford the intermediate 4a-H, and then 6-methoxy-2-methyl-1-phenyl-1H-benzimidazole (4a) can be quickly formed via aromatization with an appropriate oxidative reagent.22 In addition, when irradiation occurs in acidic acetal, the hydrolysis of acetal23 results in the production of a large amount of acetaldehyde in the reaction system, which leads to the formation of 4a in high yields.
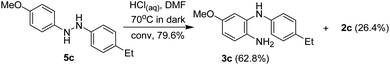
In order to confirm the possibility of the existence of 4-methoxyhydrazobenzene, the derivative 2c was reduced to hydrazobenzene (5c)24 with tributyltin hydride. When 5c reacted with hydrochloric acid in DMF without irradiation, the product 3c could be obtained as the major product (62.8%) with the oxidative product 2c (26.4%) as the side product (Scheme 7). The formation of 3c is consistent with the results from the irradiation of 2c in DMF containing hydrochloric acid, which may explain the existence of hydrazobenzenes.
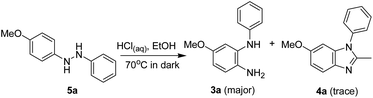
To understand the possible source of acetaldehyde, the o-semidine rearrangement (without irradiation) of 4-methoxyhydrazobenzene (5a) was performed in acidic EtOH, and compound 3a was formed as the major product with a trace amount of 4a (Scheme 8). However, when 2a was irradiated in acidic EtOH, both 3a and 4a could be obtained as major products (entries 13–15, Table 1). The difference between the reactions with and without irradiation could be used to deduce the fact that acetaldehyde, which reacted with 3a to afford 4a, may come from the photoredox reaction between 4-methoxyazobenzene (2a) and ethanol. This is also the reason why benzimidazoles are barely obtained when irradiation occurred with DMF and acetonitrile as the reaction solvent (entries 7–11, Table 1); it is because DMF and acetonitrile were barely oxidized to aldehyde.
In summary, we have successfully developed a solvent-controllable photoreaction involving 4-methoxyazobenzenes that could be used to synthesize 1-aryl-1H-benzimidazoles or N-arylbenzene-1,2-diamines in moderate to good yields.
Conflicts of interest
We have no conflicts of interest to declare.Acknowledgements
We thank the Ministry of Science and Technology (MOST) of Taiwan (R.O.C.) for financial support. We also thank Ms S.-L. Huang and Dr Iren Wang of MOST (National Taiwan University) for assistance with NMR experiments.Notes and references
- D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS.
- (a) A. R. Katritzky, D. A. Dobchev, D. C. Fara and M. Karelson, Bioorg. Med. Chem., 2005, 13, 6598 CrossRef CAS; (b) C. Zhong, J. He, C. Xue and Y. Li, Bioorg. Med. Chem., 2004, 12, 4009 CrossRef CAS.
- C. Z. Gómez-Castro, M. López-Martínez, J. Hernández-Pineda, J. G. Trujillo-Ferrara and I. I. Padilla-Martínez, J. Mol. Recognit., 2019, 32, e2801 CrossRef.
- W. Hamaguchi, N. Masuda, M. Isomura, S. Miyamoto, S. Kikuchi, Y. Amano, K. Honbou, T. Mihara and T. Watanabe, Bioorg. Med. Chem., 2013, 21, 7612 CrossRef CAS.
- (a) E. R. Sauve, J. Paeng, S. Yamaguchi and Z. M. Hudson, J. Org. Chem., 2020, 85, 108 CrossRef CAS; (b) B. Jiao, J. Wang, J. Huang, M. Cao, C. Liu, G. Yin, Y. Zhu, B. Zhang and C. Dua, Org. Electron., 2019, 64, 158 CrossRef CAS.
- (a) D. Yin, Z.-Y. Chen, N.-R. Jiang, Y.-F. Liu, Y.-G. Bi, X.-L. Zhang, W. Han, J. Feng and H.-B. Sun, Org. Electron., 2020, 76, 105494 CrossRef CAS; (b) Z. Chen, C.-L. Ho, L. Wang and W.-Y. Wong, Adv. Mater., 2020, 32, 1903269 CrossRef CAS.
- (a) Z.-H. Li, X.-M. Sun, J.-J. Qin, Z.-Y. Tan, W.-B. Wang and Y. Ma, Tetrahedron, 2020, 76, 130945 CrossRef CAS; (b) H. Li, J. Qin, Z. Yang, X. Guan, L. Zhang, P. Liao and X. Li, Chem. Commun., 2015, 51, 8637 RSC.
- (a) Y. Shi, Q. Zhou, F. Du, Y. Fu, Y. Du, T. Fang and G. Chen, Tetrahedron Lett., 2019, 60, 151082 CrossRef CAS; (b) H. Baars, A. Beyer, S. V. Kohlhepp and C. Bolm, Org. Lett., 2014, 16, 536 CrossRef CAS.
- Y. Chen, F. Xu and Z. Sun, RSC Adv., 2017, 7, 44421 RSC.
- (a) D. Koseki, E. Aoto, T. Shoji, K. Watanabe, Y. In, Y. Kita and T. Dohi, Tetrahedron Lett., 2019, 60, 1281 CrossRef CAS; (b) D. T. Ziegler, J. Choi, J. M. Munoz-Molina, A. C. Bissember, J. C. Peters and G. C. Fu, J. Am. Chem. Soc., 2013, 135, 13107 CrossRef CAS.
- (a) K. Selvam and M. Swaminathan, Tetrahedron Lett., 2011, 52, 3386 CrossRef CAS; (b) G. M. Raghavendra, A. B. Ramesha, C. N. Revanna, K. N. Nandeesh, K. Mantelingu and K. S. Rangappa, Tetrahedron Lett., 2011, 52, 5571 CrossRef CAS.
- (a) D. Zhao, J. Hu, N. Wu, X. Huang, X. Qin, J. Lan and J. You, Org. Lett., 2011, 13, 6516 CrossRef CAS; (b) D. Yang, H. Fu, L. Hu, Y. Jiang and Y. n Zhao, J. Org. Chem., 2008, 73, 7841 CrossRef CAS.
- Z. Zhu, J. Cen, X. Tang, J. Li, W. Wu and H. Jianga, Adv. Synth. Catal., 2018, 360, 2020 CrossRef CAS.
- S. W. Youn and E. M. Lee, Org. Lett., 2016, 18, 5728–5731 CrossRef CAS.
- N. T. Jui and S. L. Buchwald, Angew. Chem., Int. Ed., 2013, 52, 11624 CrossRef CAS.
- (a) A. Spencer, J. Organomet. Chem., 1985, 295, 79–89 CrossRef CAS; (b) M. F. Mackay, G. J. Trantino and J. F. K. Wilshire, Aust. J. Chem., 1993, 46, 417 CrossRef CAS.
- (a) H. J. Shine, H. Zmuda, H. Kwart, A. G. Horgan and M. Brechbiel, J. Am. Chem. Soc., 1982, 104, 5181 CrossRef CAS; (b) H. J. Shine, C. M. Baldwin and J. H. Harris, Tetrahedron Lett., 1968, 977 CrossRef CAS.
- M. Ortega, M. A. Rodriguez and P. J. Campos, Tetrahedron, 2005, 61, 11686 CrossRef CAS.
- K. Haghbeen and E. W. Tan, J. Org. Chem., 1998, 63, 4503 CrossRef CAS.
- D. E. Zembower and H. Zhang, J. Org. Chem., 1998, 63, 9300 CrossRef CAS.
- (a) G. Liu, S. Hou and J. Xu, Org. Biomol. Chem., 2019, 17, 10088 RSC; (b) M. E. Bouillon and H. H. Meyer, Tetrahedron, 2016, 72, 3151 CrossRef CAS.
- Y.-C. Lin, N.-C. Li and Y.-J. Cherng, J. Heterocycl. Chem., 2014, 51, 808 CrossRef CAS.
- (a) T. H. Fife and R. Natarajan, J. Am. Chem. Soc., 1986, 108, 8050 CrossRef CAS; (b) A. J. Kresge and D. P. Weeks, J. Am. Chem. Soc., 1984, 106, 7140 CrossRef CAS.
- A. Alberti, N. Bedogni, M. Benaglia, R. Leardini, D. Nanni, G. F. Pedulli, A. Tundo and G. Zanard, J. Org. Chem., 1992, 57, 607 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra10068d |
| ‡ This paper is dedicated to the memory of the late Prof. Tong-Ing Ho. |
| This journal is © The Royal Society of Chemistry 2021 |