Open Access Article
Open Access ArticleHighly sensitive and rapid responding humidity sensors based on silver catalyzed Ag2S–TiO2 quantum dots prepared by SILAR
Zu-Yin Deng a,
Ping-Chang Chianga,
Kuen-Lin Chenab,
Jau-Han Chenc and
Chiu-Hsien Wu*ab
a,
Ping-Chang Chianga,
Kuen-Lin Chenab,
Jau-Han Chenc and
Chiu-Hsien Wu*ab
aDepartment of Physics, National Chung Hsing University, Taichung 402, Taiwan. E-mail: chwu@phys.nchu.edu.tw; Fax: +886-4-2286253; Tel: +886-4-22840427
bInstitute of Nano-Science, National Chung Hsing University, Taichung 402, Taiwan
cDepartment of Materials Science and Engineering, Da-Yeh University, Changhua 55, Taiwan
First published on 10th March 2021
Abstract
We developed a resistive humidity sensor based on a heterojunction of silver sulfide (Ag2S) quantum dots (QDs) and TiO2 because of its specificity to water vapor adsorption and its insensitivity to environmental gases. The QDs were grown on a mesoporous TiO2 layer using the successive ionic layer adsorption and reaction (SILAR) method. The boundary condition between TiO2 and Ag2S provides a tunable energy gap by adjusting the number of SILAR cycles. Besides, the large surface-to-volume ratio of QDs provides a strong water vapor adsorption ability and electron transfer. Nano-silver precipitated during the SILAR process provides free electrons and lowers the Fermi level to between n-type TiO2 and p-type Ag2S. The resistance response increased significantly to 4600 and the reaction equilibrium time decreased greatly to 7 seconds due to the presence of nano-silver. Finally, the Ag2S QDs possess a best sensing range of 13–90%. To sum up, Ag2S QDs are high sensitivity and selectivity humidity sensors.
1. Introduction
Humidity sensors have been widely employed in various fields, including ecological, mineral processing, and food processing.1–3 The moisture in the environment can cause oxidation reactions, which change the properties of materials. It may also cause problems such as bacterial growth and decomposition. Therefore, a fast-responding and high-sensitivity humidity sensor is useful for monitoring and regulating applications.Humidity sensors can be classified into five different types: hygrometric, resistive, capacitive, gravimetric, and optical sensors. An example of a capacitive humidity sensor based on nano-gold is reported in ref. 4. A resistive sensor based on CA-NH4BF4-PEG600 thin-films responds to relative humidity (RH) due to proton transfer, known as the Grotthuss mechanism.1,5 Gravimetric humidity sensors are based on piezoelectric PVDF polymer materials.6 Most commercial humidity sensors are based on the capacitive method because of low power consumption and high output signals. However, resistive sensors also have numerous advantages such as low-cost, fast response, ease of mass production, easy of fabrication, and adjustable properties. Thus, they have significant potential for application as humidity sensors. Many previous reports used metal oxide resistive humidity sensors, such as TiO2, WO3, ZnO, and Fe2O3.6,7 This type of material provides a stable detection range of about RH 20–80%
Heterogeneous junctions formed by composite different type materials can beneficial to the electron transfer. Ag2S is a p-type semiconductor with a low energy gap (∼1.1 eV).8,9 Ag2S nanowire have been reported based on photoswitches and room temperature oxygens sensors as ultralong single-crystalline.10 Further, TiO2@Ag2S has excellent chemical stability and electron transfer characteristics.11 It was used in photovoltaic devices and solar cells due to its high mobility.12 In recent years, numerous studies on QDs have been reported due to many advantages. Owing to their tunable energy gap, low-cost and simplicity of preparation, QDs are usually decorated with different materials, including thin-films, nanowires, and nanorods. Herein, method the particle size by adjusting the number of the successive ionic layer adsorption and reaction (SILAR) cycles. And consequently, the energy gap of the sample was adopted to control due to the quantum-size effect.13,14 Compared to other synthesis methods, the SILAR method has several merits: (i) it provides a low-cost and convenient means for large area deposition on the substrate, (ii) vacuum and high-quality substrates are not required.15,16
In this study, we develop a semiconductor humidity sensor based on the Ag2S–TiO2 heterostructure. We develop SILAR methods to synthesize Ag2S QDs on a mesoporous TiO2 layer to enhance the sensitivity and response rate. The sensing characteristics for different numbers of SILAR cycles was studied. At the same time, we study the repeatability and selectivity of the Ag2S QDs humidity sensors.
2. Experimental
2.1 Material preparation
A gel of 30 nm TiO2 (Dyesol TiO2 Paste DSL 30NR-D) is dissolved in ethanol (anhydrous 99.5 UP) solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
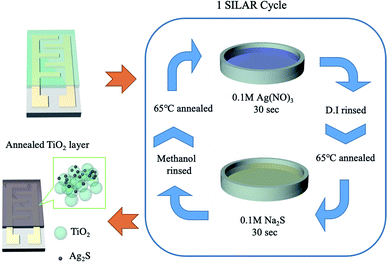
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 w/w), and was spin-coated on the surface of the finger electrode shown in Fig. 1. It was subsequently annealed at 500 °C for 15 min. Then, the substrate with TiO2 was immersed in 0.1 M Ag(NO)3 (Honeywell Fluka) solution for 30 seconds, rinsed with deionized water, and annealed at 65 °C. Next, the sample was immersed in 0.1 M Na2S (Acros Organics) solution for 30 seconds, to allow a replacement reaction with sulfur to occur. It was rinsed with methanol (Macron Fine Chemicals) and then annealed at 65 °C. These immersion steps were repeated for 4–12 cycles. Finally, the samples were annealed at 400 °C for 10 min in a nitrogen environment.
1.5 w/w), and was spin-coated on the surface of the finger electrode shown in Fig. 1. It was subsequently annealed at 500 °C for 15 min. Then, the substrate with TiO2 was immersed in 0.1 M Ag(NO)3 (Honeywell Fluka) solution for 30 seconds, rinsed with deionized water, and annealed at 65 °C. Next, the sample was immersed in 0.1 M Na2S (Acros Organics) solution for 30 seconds, to allow a replacement reaction with sulfur to occur. It was rinsed with methanol (Macron Fine Chemicals) and then annealed at 65 °C. These immersion steps were repeated for 4–12 cycles. Finally, the samples were annealed at 400 °C for 10 min in a nitrogen environment.
2.2 Humidity sensing device setup
Humidity was measured in a chamber. Water vapor was provided by a vapor-generator and was exhausted by using a pump. The reference gases including oxygen, carbon-monooxide and nitrous oxide was from gas cylinders. The entire experiment was carried out at room temperature (25 ± 1.5 °C). The resistance of the sensing device was measured by a high resistance meter (HP4349A) and the reference humidity was monitored by a commercial capacitive humidity sensor (DHT22). The material properties of Ag2S were measured by a UV-visible spectrometer (Jasco-V-550), X-ray diffractometer (XRD, Bruker d8SSS, Cu Kα1), and transmission electron microscopy (TEM, Jeol JEM-2010).3. Results and discussion
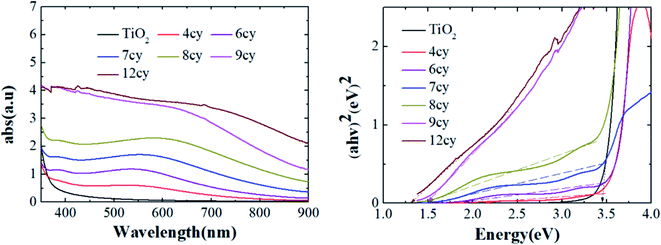
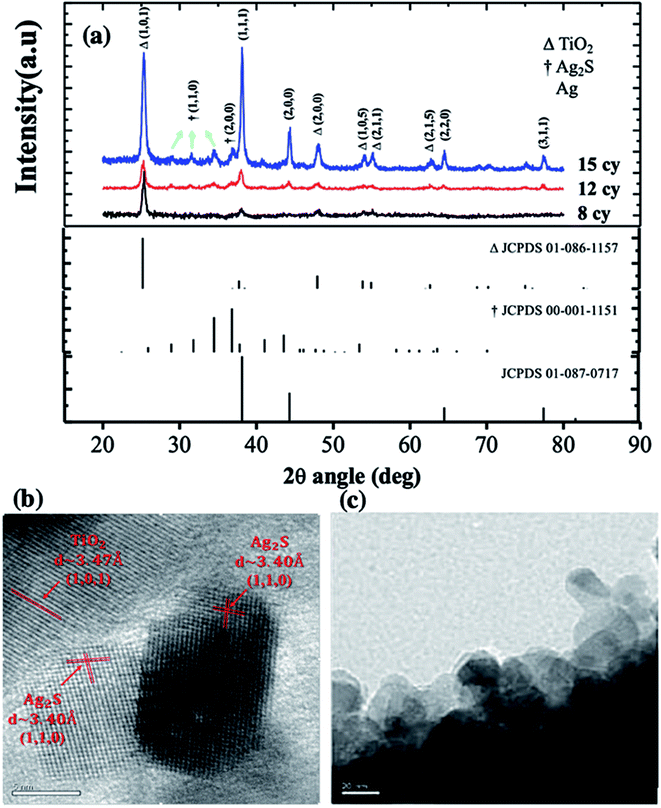
The UV-Vis spectrum of TiO2 and six Ag2S/TiO2 samples with different numbers of successive ionic layer adsorption and reaction (SILAR) cycles are shown in Fig. 2(a). The absorption intensity was increases depends on the cycles increasing. The cycle number; thus, it with. The absorption rate is depicted in the Tauc plot. The calculated energy gaps (Eg) for 4, 6, 7, 8, 9, and 12 cycle samples are 2.1, 1.9, 1.6, 1.5, 1.4, and 1.3 eV, respectively (Fig. 2(b)). The energy gap increased due to the quantum size effect, and the particle size gradually increases because of the reduction reaction and deposition in SILAR step.14XRD was used to determine the phases of crystals and crystallite size. The XRD spectra of TiO2 layers and Ag2S quantum dots (QDs) are shown in Fig. 3(a). The XRD spectra of Ag2S, Ag and TiO2 are in perfect accordance with JCPDS 00-001-1151, 01-087-0717 and 01-086-1157, respectively. Crystallite sizes were calculated by the semi-empirical Debye–Scherrer formula.
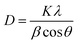 | (1) |
The Ag2S QDs have a high response to H2O molecules in the atmosphere. The ·OH was dissociated from H2O and absorbed on positively charged Ag atoms following eqn (2). This provides more free electrons to TiO2 shrinking the depletion and decreasing the overall resistance of the material.
| Ag2S(h+) + H2O → Ag2S + H+ + ·OH | (2) |
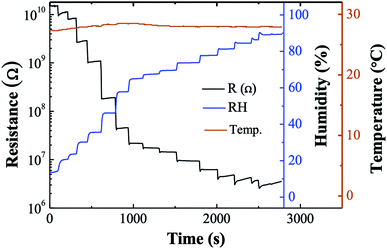
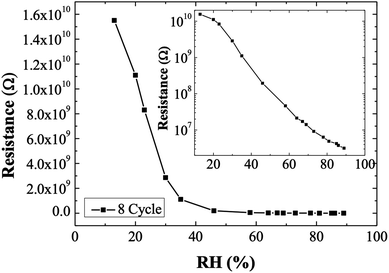
Fig. 4 exhibits the resistance response to relative humidity (RH) of 8-cycle Ag2S, which was considered to have the broadest sensing range of 13–90% RH. The lowest measured humidity of 13% corresponds to 1.6 × 1010 Ω which decreased to 6.8 × 106 Ω at 90% RH. The resistance response, as defined by eqn (3), was approximately 4600.
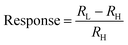 | (3) |
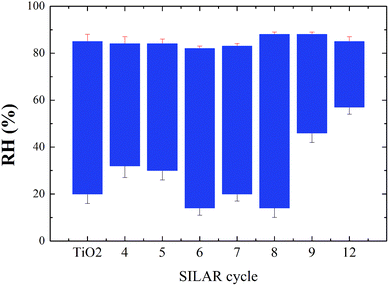
The TiO2 film coated on substrate has response between RH 20–85%, the response was 423, with the response/recovery time ∼50/100 s. Of all SILAR depositions performed in this experiment, the 5-cycle sample had the highest resistance. The measurement range was RH 30–85% (Fig. 5), and the resistance varied over the range 3 × 1010 to 1 × 107 Ω. However, this sample has poor stability at high humidity. The resistance value gradually increases when the RH exceeds 88%. Despite adsorbed steam still causing the resistance to change, the increasing baseline caused a loss of correspondence between humidity and resistance. As the numbers of SILAR cycles increases, the problem of instability was gradually ameliorated. The sensing range of the 4-cycle sample was smaller than that of the 5-cycle sample under low RH. This might be regarded as being due to a smaller concentration of Ag2S. On the other hand, smaller QDs have a larger energy gap, hence the difficulty of electron migration is increased. For this reason, the low-cycle samples provide higher response, but poor stability.
The measurement range decreased gradually for more than 8-cycles. The range of the 9-cycle sample is merely RH 46–88%. The Fermi-level is reduced as the particle size increases. This improves electron transport and reduces resistance. Since there are more free electrons, the low humidity cannot be identified by resistance change. For the same reason, the 12-cycle sample has an even worse response (RH 55–82%).
Fig. 6 shows the correlation between humidity (RH) and resistance (R) of the 8-cycle sample. For the range RH 13–90%, the response trend is clearly divided into two regions. The resistance increases linearly and significantly relative to the humidity decreases over the range 13–30% RH. The linear equation was R = −7.49 × 108RH + 2.55 × 1010. The resistance change up to 14 gigaohms and provided an accurate RH measurement. Further, this sample has good humidity dependence over the range 30–90% RH. According to eqn (2), the water vapor adsorption and dissociation reduces the number of Ag2S holes and decreases the resistance. The conductivity is proportional to the free electrons obtained by Ag2S from H2O.20 The RH range between 20–90% Ag2S QDs and H2O have a high response (∼1000). The high reaction rate is due to the large surface area of the QDs. The QDs provide a large number of carriers to react with H2O.
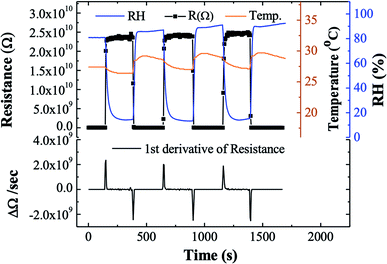
The 8-cycle sample resistance was measured for three consecutive humidity cycles of the same RH values as shown in Fig. 7. The sample exhibited a stable resistance value over the range 13–90% RH. According to the first derivative of the resistance, the Ag2S sample presents an instantaneous response and recovery times of 7 and 19 seconds respectively (Fig. 7). In comparison to the recently reported humidity sensors of different types listed in Table 1, the proposed Ag2S QDs possess a high response and fast response/recovery time. Thus, Ag2S QDs are well-suited to humidity sensors.
| Sensor classification | Sensing material | Range (% RH) | Sensitivity (1% RH) | Response | Response/recovery time(s) | Ref. |
|---|---|---|---|---|---|---|
| Resistive | Ag2S | 13–90 | 195.3MΩ | 4600 | 7.23/19.03 | This work |
| Capacitive | Au-PVA | 11.3–93 | 0.05 nF | 0.53 | 113–188/53–94 | 4 |
| Resistive | Cellulose acetate-CuO | 0–90 | 3.8 MΩ | 1093 | 13/17 | 17 |
| Gravimetric | CNCs | 11.3–97.3 | 55.3/275Hz | 1.28 | 60/15 | 18 |
| Piezoresistive | ZnO NRs | 30–80 | 3.35–15 Hz | — | 46/167 | 19 |
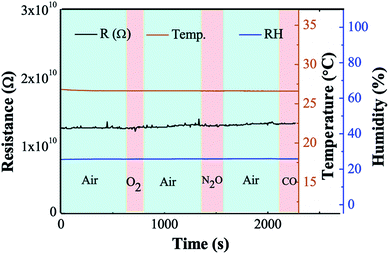
In order to observe the effect of ambient gases, several arid gases from cylinder were pumped into the chamber, including oxygen, carbon monoxide (reducing gas) and nitrous oxide gas (oxidizing gas) (Fig. 8). The humidity was fixed at 26% RH. The resistance has no response to gases with different electron affinity which ensured this sample was stable and unaffected by the ambient gas.
The ·OH from the water vapor was oxidized on the surface of Ag2S, hence providing free electrons. According to the catalytic literature of TiO2@Ag2S, the coexistence of Ag and Ag2S can further enhance the transfer of electrons (Fig. 9a).21–23 TiO2 is an n-type material with a wide energy gap. On the other hand, Ag2S is a p-type material with wide energy gap which provides reaction sites. Compared with NHE, the conduction band of TiO2 was 0.8 eV higher than the valence band of Ag2S. This energy gap can be adjusted by adjusting the number of SILAR cycles. The smaller the energy gap is, the more efficient electron transfer is. The presence of silver particles lowers the Fermi-level, which increase electron transfer (Fig. 9b). Therefore, in our experiment, the 8-cycle SILAR sample exhibits the best humidity sensing range of 13–90% RH. Furthermore, the response time of the absorption process due to the catalysis of silver particles is barely seven seconds. To verify the contributions of silver particles to the RH measurement, the dipping time of sodium sulfide solution was increased from 30 s to 90 s and thus more Ag2S were synthesized from Ag particles. There was a significant decrease of absorption peak of Ag particles in UV-Vis spectrum. The resistance response were reduced to 39. Besides, the response time also increased significantly. This result confirms that the silver particles have a catalytic effect which contribute to the humidity sensing.
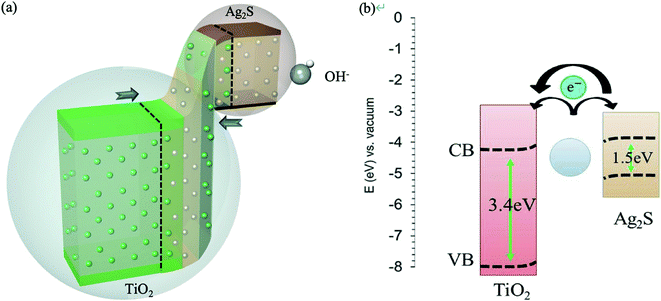 | ||
| Fig. 9 Diagram of electron-transfer mechanism. (a) Electron transfer of OH− adsorption (b) catalysis of silver particles. | ||
4. Conclusion
In this experiment, chemical methods were used to synthesize TiO2@Ag2S quantum dots (QDs), and the 8-cycle sample achieved the highest sensing range of 13% to 90%. The energy gap of was tuned to 1.5 eV based on the quantum-size effect of Ag2S. Owing to the catalytic effect of metallic silver, it provides a high resistance-based response of 4600, an accuracy of 1% RH, and high reaction speed. The Ag2S QDs has the features of uninfluenced by environmental pollutants and reproducibility. Therefore, Ag2S QDs have high practical value as a humidity sensor owing to its high efficiency and simple-operation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the financial supports of the Ministry of Science and Technology of Taiwan (MOST 108-2112-M-005-001, MOST 109-2112-M-005-007).References
- H. Farahani, R. Wagiran and M. N. Hamidon, Sensors, 2014, 14(5), 7881–7939 CrossRef PubMed.
- A. Tripathy, S. Pramanik, J. Cho, J. Santhosh and N. A. A. Osman, Sensors, 2014, 14(9), 16343–16422 CrossRef PubMed.
- Z. Chen and C. Lu, Sens. Lett., 2005, 3(4), 274–295 CrossRef CAS.
- W. Yao, X. Chen and J. Zhang, Sens. Actuators, B, 2010, 145(1), 327–333 CrossRef CAS.
- N. I. Harun, R. M. Ali, A. M. M. Ali and M. Z. A. Yahy, Phys. Procedia, 2012, 25, 221–226 CrossRef CAS.
- A. Glück, W. Halder, G. Lindner, H. Müller and P. Weindler, Sens. Actuators, B, 1994, 19(1–3), 554–557 CrossRef.
- P. V. Shinde, S. Gagare, C. S. Rout and D. J. Late, RSC Adv., 2020, 10(49), 29378–29384 RSC.
- F. Jiang, Q. Tian, M. Tang, Z. Chen, J. Yang and J. Hu, CrystEngComm, 2011, 13(24), 7189–7193 RSC.
- H. Dlala, M. Amlouk, S. Belgacem, P. Girard and D. Barjon, Eur. Phys. J.: Appl. Phys., 1998, 2(1), 13–16 CrossRef CAS.
- D. Wang, C. Hao, W. Zheng, Q. Peng, T. Wang, Z. Liao, D. Yu and Y. Li, Adv. Mater., 2008, 20(13), 2628–2632 CrossRef CAS.
- W. P. Lim, Z. Zhang, H. Y. Low and W. S. Chin, Angew. Chem., Int. Ed., 2004, 43(42), 5685–5689 CrossRef CAS PubMed.
- Y. Xie, S. H. Yoo, C. Chen and S. O. Cho, Mater. Sci. Eng., B, 2012, 177(1), 106–111 CrossRef CAS.
- D. Yan, Y. He, Y. Ge and G. Song, Sens. Actuators, B, 2017, 240, 863–869 CrossRef CAS.
- A. I. Ekimov, Al. L. Efros and A. A. Onushchenko, Solid State Commun., 1985, 56(11), 921–924 CrossRef CAS.
- H. M. Pathan and C. D. Lokhande, Bull. Mater. Sci., 2004, 27(2), 85–111 CrossRef CAS.
- S. I. Sadovnikov and E. Y. Gerasimov, Nanoscale Adv., 2019, 1(4), 1581–1588 RSC.
- M. T. S. Chani, K. S. Karimov, S. B. Khan and A. M. Asiri, Sens. Actuators, A, 2016, 246, 58–65 CrossRef CAS.
- Y. Yao, X. H. Huang, B. Y. Zhang, Z. Zhang, D. Hou and Z. K. Zhou, Sens. Actuators, B, 2020, 302, 127192 CrossRef CAS.
- J. Xu, M. Bertke, X. Li, H. Mu, H. Zhou, F. Yu, G. Hamdana, A. Schmidt, H. Bremers and E. Peiner, Sens. Actuators, B, 2018, 273, 276–287 CrossRef CAS.
- A. K. Kralj, J. Ind. Eng. Chem., 2007, 13(4), 631–636 CAS.
- H. Yu, W. Liu, X. Wang and F. Wang, Appl. Catal., B, 2018, 225, 415–423 CrossRef CAS.
- A. Badawi, Phys. E, 2019, 109, 107–113 CrossRef CAS.
- W. L. Ong, Y. F. Lim, J. L. T. Ong and G. W. Ho, J. Mater. Chem. A, 2015, 3(12), 6509–6516 RSC.
| This journal is © The Royal Society of Chemistry 2021 |