Open Access Article
Open Access ArticleSuperior anti-infective potential of eugenol–casein nanoparticles combined with polyethylene glycol against Colletotrichum musae infections
Xueyan Hao†
,
Shuya Han†,
Dingkui Qin,
Yahui Zhang,
Peng Jin * and
Qizhen Du*
* and
Qizhen Du*
The Key Laboratory for Quality Improvement of Agricultural Products of Zhejiang Province, The College of Agricultural and Food Sciences, Zhejiang A & F University, Linan, 311300, China. E-mail: jinpeng@zafu.edu.cn; qizhendu@163.com
First published on 25th January 2021
Abstract
The aim of this study was to improve the stability of eugenol–casein nanoparticles (EL–CS-NPs) through polyethylene glycol (PEG) modification. The results show that modifying the EL–CS-NPs with PEG after loading with eugenol (EL) gives PEG–EL–CS-NPs, with increased stability. The NPs modified with higher-molecular-weight PEG showed better stability. A CS/PEG ratio of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) yielded the NPs with the best stability. A PEG20 K–EL–CS-NP dispersion remained stable in cold storage for over one year, and also exhibited stronger inhibitory effects against Colletotrichum musae inoculated on bananas than an EL–CS-NP dispersion, since it showed more prolonged sustained release of EL than the EL–CS-NP dispersion. Lyophilized PEG20 K–EL–CS-NP powder showed better effectiveness against mold on bread than lyophilized EL–CS-NPs powder. Using PEG to modify CS-NPs shows potential for improving the stability of CS-NPs loaded with hydrophobic substances for delivery in the fields of food and agriculture.
1 (w/w) yielded the NPs with the best stability. A PEG20 K–EL–CS-NP dispersion remained stable in cold storage for over one year, and also exhibited stronger inhibitory effects against Colletotrichum musae inoculated on bananas than an EL–CS-NP dispersion, since it showed more prolonged sustained release of EL than the EL–CS-NP dispersion. Lyophilized PEG20 K–EL–CS-NP powder showed better effectiveness against mold on bread than lyophilized EL–CS-NPs powder. Using PEG to modify CS-NPs shows potential for improving the stability of CS-NPs loaded with hydrophobic substances for delivery in the fields of food and agriculture.
1. Introduction
The transportation of hydrophobic compounds through hydrophilic food matrices remains a challenge, particularly in the case of bioactive molecules, such as antioxidants, vitamins, fatty acids, and probiotics, which are susceptible to changes in pH and ionic strength, and to oxidation. Nanostructured carriers are considered promising vehicles for delivering hydrophobic substances, allowing for the transportation of hydrophobic compounds while remaining stable in the food matrix to ensure safe, functional, and targeted delivery.1–3 The encapsulation of sensitive ingredients into nanostructures increases their stability and maintains their bioavailability, protecting them against external degradation factors and ensuring controlled release.4,5Protein-based food materials have been shown to be safe and they are considered appropriate for delivering bioactive compounds and maintaining their functionality in the body. Caseins have been proven to be one of the most reliable nanovehicles in terms of cost, nutrition, and safety.6–9 Curcumin has been encapsulated in casein micelles to form nanoparticles with an average size distribution of <200 nm, with the potential to provide an oral dose of curcumin.10 Docosahexaenoic acid (DHA) entrapped in casein micelles has been found to be protected against oxidation, and the micelles have good colloidal stability when stored at 4 °C.11 The encapsulation of vitamin D3 in casein micelles has been found to stabilize vitamin D3 in aqueous systems, improve the accuracy and uniformity of distribution, protect it during thermal treatment (80 °C, 1 min) and 28 days of cold storage,12 shield it from harmful UV radiation damage,13 prevent its degradation in the stomach,14 and improve its bioavailability.15,16 Casein nanoparticles formed from re-assembled casein micelles have been used as nanovehicles for β-carotene, enabling water-insoluble β-carotene to be applied during different processing technologies in the food industry; this is because encapsulating β-carotene reduces its exposure to peroxyl radical oxidation and improves its bioaccessibility.17,18 Thymol encapsulated in sodium caseinate is more effective than unencapsulated thymol in inhibiting foodborne pathogens in milk owing to its enhanced distribution and solubility.19 Spray-dried zein/casein nanoparticles in which thymol and eugenol are co-encapsulated can be easily rehydrated to produce a stable dispersion. The encapsulated essential oils show controlled release over 24 h, exhibiting enhanced bactericidal and bacteriostatic effects in milk whey against E. coli O157:H7 and L. monocytogenes, respectively.20 However, based on existing studies, obtaining stable target substance-loaded nanoparticles is critical for applying casein nanoparticles as nanocarriers for functional substances.21 More fundamental research is therefore required to fully understand and control the assembly and disassembly mechanisms of structures and the stability of nanoparticles during processing and storage.22
Eugenol (4-allyl-2-methoxyphenol), a major constituent of clove essential oil, has been found to inhibit various pathogens. Eugenol (EL) has been considered as an ideal antibacterial agent in the food industry since it is classified as “generally regarded as safe”.23,24 However, the efficiency of EL is substantially limited due to its high volatility, low water solubility, and high susceptibility to environmental stress.25 Embedding and encapsulation technologies have being used for improving the physicochemical stabilities of volatile active agents.26 Recently, several studies have also revealed that the encapsulation of EL into nanoparticles can enhance its stability, permeability, and antimicrobial activity.27–29
Polyethylene glycol is a chain molecule that can be synthesized with different chain lengths depending on requirements. The long chain of polyethylene glycol may bind to nanoparticles with polar surfaces, increasing their stability. In the present study, we exhibit the effects of polyethylene glycol on the stability of eugenol–casein nanoparticles, which exhibit an improvement in their effectiveness at inhibiting pathogenic microorganisms in bananas and bread.
2. Materials and methods
2.1. Chemicals and reagents
Casein sodium (C8654-1KG) and polyethylene glycol (PEG, HO(CH2CH2O)nH) with average molecular weights of 2000 (2 K), 4000 (4 K), 8000 (8 K), 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (20 K), and 35
000 (20 K), and 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (35 K) Da were purchased from Sigma-Aldrich (Shanghai, China). Eugenol (C10H12O2), high-performance liquid chromatography (HPLC)-grade acetonitrile and methanol, analytical-grade ethyl acetate, formic acid, NaCl, and MgCl2 were purchased from Shanghai Yuanye Biotechnology Co., Ltd (Shanghai, China).
000 (35 K) Da were purchased from Sigma-Aldrich (Shanghai, China). Eugenol (C10H12O2), high-performance liquid chromatography (HPLC)-grade acetonitrile and methanol, analytical-grade ethyl acetate, formic acid, NaCl, and MgCl2 were purchased from Shanghai Yuanye Biotechnology Co., Ltd (Shanghai, China).
The banana pathogen Colletotrichum musae used in this study has been reported in previous work,29 and it was used as the indicator microorganism for antifungal assays. Stock cultures were then maintained on potato dextrose agar (PDA) slants at 25 ± 2 °C for further study. The fungi were transferred to fresh PDA and incubated at room temperature before any experiments were carried out.
2.2. Preparation of PEG–EL–CS-NP dispersions
PEG–EL–CS-NP dispersions were prepared using casein sodium at a concentration of 20 mg mL−1. EL was pre-dissolved in absolute ethanol at a concentration of 100 mg mL−1. A known amount of the resulting solution was then added dropwise to the protein micellar dispersion under stirring at 25 °C. Following the addition of the EL solution, the dispersion was ultrasonicated for 3 min in an ice bath, then a given amount of aqueous PEG solution at a concentration of 20 mg mL−1 was added to the dispersion, and ultrasonication in the ice bath was continued for a further 3 min. The dispersion was filtered through a 0.46 μm film to yield the PEG–EL–CS-NP dispersion.2.3. Effects of CS-NP PEG modification on the release of loaded EL
The % entrapment efficiency (EE) of EL in PEG–EL–CS-NPs exposed to heat (37 °C) was determined to evaluate the effectiveness of PEG in controlling EL release from casein NPs, since EL remained in the EL–CS-NPs at 37 °C for approximately 1 day.2.4. Nanoparticle physicochemical characterization
The mean particle size, size distribution, and zeta potential of the nanoparticles were measured using a Zetasizer Nano ZSE instrument (Malvern Instruments, Malvern, U.K.), employing a nominal 5 mW He–Ne laser operated at a wavelength of 633 nm and a scattering angle of 173°, as described in a previous report.30 For characterization, 1 mL of the NP dispersion was diluted with 10 mL of water at room temperature. All data are expressed as mean values of three independent batches of samples. Transmission electron microscopy (TEM) was used for the morphological characterization of PEG–EL–CS-NPs using a process described previously. Samples were prepared via dilution, and they were then placed on a film-coated 200-mesh copper specimen grid for 10 min, stained with 3% phosphotungstic acid and dried prior to TEM observations using a Hitachi H-9500E electron microscope.2.5. Entrapment efficiency (EE) of EL in NPs
The amount of EL remaining in the NPs was determined using a previously reported procedure.31 Briefly, a NP dispersion was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g to separate the free EL. Then, the clear aqueous phase (50 μL) was added to 450 μL of methanol to denature the protein. After removing the protein through filtering, the methanol solution was then subjected to high-performance liquid chromatography (HPLC) analysis for EL. HPLC analysis was performed using a Shimadzu HPLC system (Shimadzu, Kyoto, Japan) with two LC-10A pumps, an SIL-10Avp autosampler, an SPD-M10Avp UV detector, and a Symmetry C18 (5 m, 4.6 mm × 250 mm) column. The column was eluted using a mobile phase composed of water–methanol (30
000g to separate the free EL. Then, the clear aqueous phase (50 μL) was added to 450 μL of methanol to denature the protein. After removing the protein through filtering, the methanol solution was then subjected to high-performance liquid chromatography (HPLC) analysis for EL. HPLC analysis was performed using a Shimadzu HPLC system (Shimadzu, Kyoto, Japan) with two LC-10A pumps, an SIL-10Avp autosampler, an SPD-M10Avp UV detector, and a Symmetry C18 (5 m, 4.6 mm × 250 mm) column. The column was eluted using a mobile phase composed of water–methanol (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) at a flow rate of 1 mL min−1 at 30 °C. The chromatograms were recorded via UV absorbance at 280 nm. The EE (%) of EL in the NPs was calculated from the results of HPLC analysis using the equation:
70) at a flow rate of 1 mL min−1 at 30 °C. The chromatograms were recorded via UV absorbance at 280 nm. The EE (%) of EL in the NPs was calculated from the results of HPLC analysis using the equation:| EE (%) = (EL concentration of the clear aqueous phase/EL concentration of the dispersion) × 100 |
2.6. Evaluation of the storage stability of the NP dispersion
To confirm the stability of PEG20 K–EL–CS-NP during storage, NP dispersions were kept in sealed amber-colored glass vials in a cold environment (4–10 °C). The particle size, polydispersity index (PDI), zeta potential (ZP), and EE of the dispersions were then measured at predetermined time intervals and compared with the values obtained from fresh dispersions.2.7. The lyophilization of NP dispersions and calculations of the remaining EL
The PEG20 K–EL–CS-NP and EL–CS-NP dispersions were pre-frozen to −80 °C and then lyophilized using a lyophilizer with a cold trap at −80 °C (Beijing Biocool Experimental Instrument Co., Ltd, China). Some of the powder was subjected to EL extraction with methanol. The methanol solution was injected into the HPLC system for EL determination. The amount of EL remaining in the lyophilized powder was calculated based on the EL concentration of the methanol solution.2.8. Determination of EL release from lyophilized NP powder
EL release from NP powder samples at room temperature (20–25 °C) was determined via the following procedure. Mini bags (0.5 cm × 1 cm) containing lyophilized PEG20 K–EL–CS-NP or EL–CS-NP powder (5 mg) were placed in empty bread packages without being completely sealed. After a given number of days of treatment, the remaining EL in the lyophilized powder was extracted using methanol for HPLC analysis. The release of EL was calculated based on the remaining EL in the lyophilized powder.2.9. Determination of the inhibitory effects of NP dispersions on C. musae on banana
The mycelium strain Colletotrichum musae was pre-cultured on Luria–Bertani (LB, g L−1; 10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl) agar media at 28 °C for 7 d as a seed culture. A spore suspension was prepared via washing 20 day-old seed culture with sterile water containing 0.01% (v/v) Tween-80 and then diluting this to 1 × 105 spores per mL with the aid of a hemocytometer. The minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) values of the prepared nanoparticles were investigated via turbidimetric and micro-dilution methods. Seed cultures were inoculated with 5% (v/v) inoculum in fresh LB media with final eugenol amounts of 0.05–2.50 mg mL−1 from eugenol, EL–CS-NPs, and PEG20 K–EL–CS-NPs, respectively. Additionally, dilution separation methods were used to examine the presence of bacteria in the original MIC tubes via transferring 50 μL of solution from the non-turbid tubes to the appropriate broth medium and permitting growth after overnight incubation.32 The minimum concentration in a test tube with no C. musae cell growth gave the MFC value.The inhibitory activity of PEG–EL–CS-NP dispersions toward C. musae on bananas was determined using a previously reported procedure.29 Briefly, 10 μL of C. musae spore suspension was injected into a site under the peel of one half of a banana, and pure water was injected into a site under the peel on the other half as a control. The treated bananas were coated with the NP dispersion at an EL concentration of 0.1 g L−1 via brushing. After treatment, all bananas were stored at 25 °C in a breathable plastic basket. The lesions were observed and recorded after peeling at intervals of 2 days. Each treatment had three replicates, and the entire experiment was repeated three times.
2.10. Determination of the effectiveness of the lyophilized powder against mold growth on bread
The preparation of bread samples (without preservative) was carried out according to the procedure described by Gao et al.33 Briefly, bread dough was prepared via mixing ingredients in a dough mixer for 1 min at 45 rpm and 5 min at 100 rpm. After resting for 15 min, the bread dough was divided into 25 g pieces, which were rounded and proofed at 40 °C and 85% RH for 70 min. After proofing, the dough pieces were baked at 200 °C for 10 min, and then the bread was cooled down at ambient temperature for 1 h. Mini bags (0.5 cm × 1 cm) containing lyophilized PEG20 K–EL–CS-NPs, EL–CS-NPs, or CS-NPs (control) powder (1 mg) were placed under the bread, which was packaged in a hemispherical plastic package with an incomplete seal. A free-EL sample was prepared via dissolving EL in acetone, dropping this solution onto a piece of filter paper, and then removing the acetone via spontaneous evaporation. Bread was placed in packages without fungal inoculation in order to determine the effects of the lyophilized powder against naturally occurring mold in bread. The packed bread samples were stored at 25 ± 2 °C and 75% RH until mold was observed on the last group. The experiments were done in triplicate.2.11. Statistical analysis
All samples were prepared and analyzed in triplicate. The data are presented as mean ± standard-deviation values from three determinations. Statistical differences between two groups were evaluated using Student's t-test. Multiple comparisons of means were done via LSD (least significant difference) testing. A probability value of <0.05 was considered significant. All computations were carried out using the SAS system for Windows V8.3. Results and discussion
3.1. Effects of PEG molecular weight on PEG–EL–CS-NPs stability
The EL–CS-NPs are only stable for one day at 37 °C, which limits the use of these NPs for EL delivery in food-related processes. To address this limitation, we modified EL–CS-NPs with different types of PEG. According to pre-experiments, an EL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) casein–sodium (20 mg mL−1) ratio of 1
casein–sodium (20 mg mL−1) ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
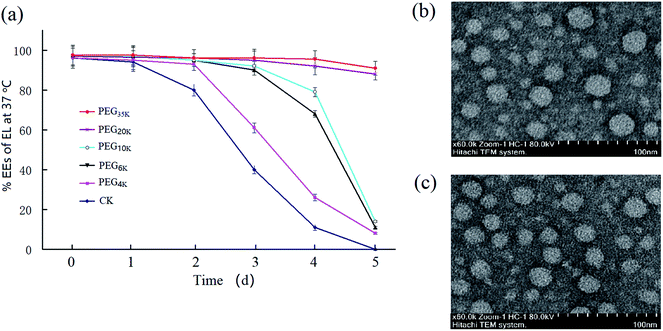
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 yielded EL–CS-NPs with an ideal EE (>90%). Thus, we used PEG samples with different molecular weights (MWs) to modify the NPs to improve their stability. The results showed that when PEG was used, the obtained PEG–EL-CS-NPs was more stable at 37 °C than EL–CS-NPs (Fig. 1a). The clear increase in stability upon increasing the PEG MW from 4 K to 20 K Da indicates that the molecular chain length of PEG plays a key role in stabilizing the NPs, which implies that PEG can fill spaces on the surfaces of the NPs. The PEG35 K–EL–CS-NPs exhibit a slight increase in stability over PEG20 K–EL–CS-NPs. Interestingly, when the loading amount of EL was increased, the diameter of PEG35 K–EL–CS-NPs decreased (Table 1). Therefore, we suggest that EL displaced water from inside the NPs to yield a more compact structure, and PEG filled the spaces on the surfaces of the NPs as a result of interactions between PEG and the polar residues on the surfaces of the NPs (Fig. 1b and c). PEG encapsulation effectively blocked the leakage of eugenol from NP holes,12 thus effectively improving the slow-release efficiency of eugenol nanoparticles and enhancing their antibacterial efficiency.29
10 yielded EL–CS-NPs with an ideal EE (>90%). Thus, we used PEG samples with different molecular weights (MWs) to modify the NPs to improve their stability. The results showed that when PEG was used, the obtained PEG–EL-CS-NPs was more stable at 37 °C than EL–CS-NPs (Fig. 1a). The clear increase in stability upon increasing the PEG MW from 4 K to 20 K Da indicates that the molecular chain length of PEG plays a key role in stabilizing the NPs, which implies that PEG can fill spaces on the surfaces of the NPs. The PEG35 K–EL–CS-NPs exhibit a slight increase in stability over PEG20 K–EL–CS-NPs. Interestingly, when the loading amount of EL was increased, the diameter of PEG35 K–EL–CS-NPs decreased (Table 1). Therefore, we suggest that EL displaced water from inside the NPs to yield a more compact structure, and PEG filled the spaces on the surfaces of the NPs as a result of interactions between PEG and the polar residues on the surfaces of the NPs (Fig. 1b and c). PEG encapsulation effectively blocked the leakage of eugenol from NP holes,12 thus effectively improving the slow-release efficiency of eugenol nanoparticles and enhancing their antibacterial efficiency.29
| Composition of NPs | Diameter (nm) at different EL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CS ratios CS ratios |
||
|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
|
| a Values in a column followed by different letters are significantly different (p < 0.05). | |||
| EL–CS-NPs | 180.4 ± 2.7a | 142.9 ± 1.6a | 117.4 ± 1.8a |
| PEG35 K–EL–EL-NPs | 162.8 ± 2.1b | 125.8 ± 1.4b | 96.4 ± 1.1b |
To improve the stabilities of casein-nanoparticle-loaded substances and the entrapment efficiencies, dextran, pectin, and chitosan have been used for co-assembly with caseins. A casein-graft-dextran copolymer was used for β-carotene encapsulation, resulting in stable β-carotene nanoparticles, although the encapsulation efficiency was relatively low.19 Casein was conjugated with maltodextrin and co-assembled with hydrophobic nutraceuticals to create nanovehicles for the enrichment of clear beverages.14 Moreover, nanocomplexes were prepared with sodium caseinate/pectin via acidification, using glucono-δ-lactone and thermal treatment to yield stable compact spherical nanocomplexes, in which pectin delayed the hydrolysis of casein by pepsin under gastric conditions for the controlled release of rutin.34 Therefore, we think that PEG20 K is an excellent material for enhancing the stability of casein-nanoparticles since PEG20 K possesses the properties of being food-safe and inexpensive.
3.2. Effects of the amount of PEG used and the EL-loading level on the stability of PEG–EL–CS-NPs
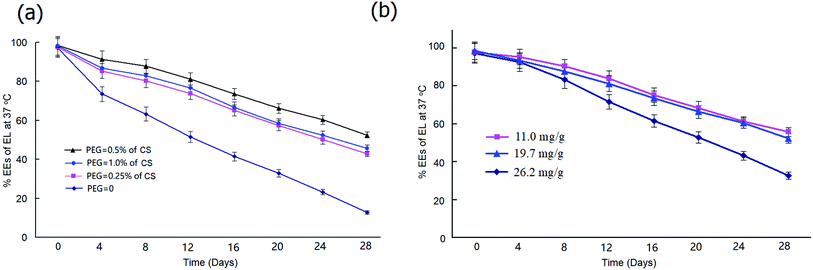
To determine the most effective stabilization approach, we prepared PEG20 K–EL–CS-NPs with different amounts of PEG20 K. Stability tests showed that particles containing PEG20 K at a level that was 0.5% of the amount of CS exhibited better NP stability than those containing PEG20 K at levels that were 0.25% and 1.0% of the amount of CS (Fig. 2a), although particles containing 1.0% PEG20 K were more stable than those containing 0.25%. Under heating at 37 °C, PEG20 K–EL–CS-NPs yielded long-term sustained release.We also determined the stabilities of NPs with different EL-loading amounts. The results showed that high EL loading led to a significant reduction in the stability of the NPs. As shown in Fig. 2b, the stability of PEG20 K–EL–CS-NPs with a loading amount of 26.2 mg g−1 sharply decreased compared to samples with loading amounts of 14.9 mg g−1 and 19.7 mg g−1.
3.3. Stability of PEG–EL–CS-NPs dispersions during cold storage (4–10 °C)
The long-term stability of PEG20 K–EL–CS-NPs dispersions in cold storage (4–10 °C), which is critical for their eventual application, was determined. The results demonstrate that PEG20 K–EL–CS-NPs was stable for more than 12 months based on changes in the particle size, zeta potential, and PDI values of the NPs (Fig. 3). Therefore, the PEG–EL–CS-NPs dispersion could be used to deliver EL to prevent the fungal decay of fresh fruits and vegetables.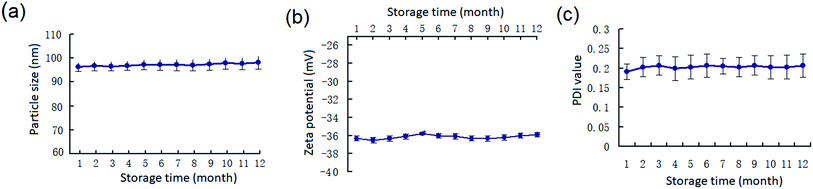 | ||
| Fig. 3 (a) Changes in the particle size, (b) zeta potential, and (c) PDI values of the PEG20 K–EL–CS-NPs during cold storage (4–10 °C). | ||
3.4. Remaining EL in and EL-release from lyophilized NP powder
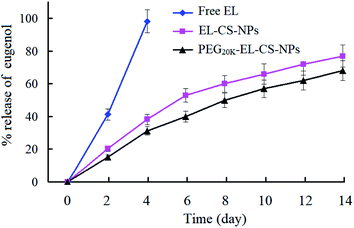
The amounts of EL remaining in EL–CS-NPs lyophilized powder and PEG20 K–EL–CS-NPs lyophilized powder were 63.1 ± 4.2% and 86.7 ± 6.3%, respectively, compared with the initial usage of EL for the preparation of the NP dispersions. It is clear that PEG20 K significantly prevented the volatilization of EL during the preparation and lyophilization processes. The release levels of EL from PEG20 K–EL–CS-NPs lyophilized powder and EL–CS-NPs lyophilized powder were determined and compared with the release of free EL from filter paper. The results showed that more than 98% of the free EL volatilized after 4 days while the EP lyophilized powder samples exhibited the sustained release of EL (Fig. 4).3.5. Enhanced inhibitory effects of a PEG20 K–EL–CS-NP dispersion against C. musae on bananas
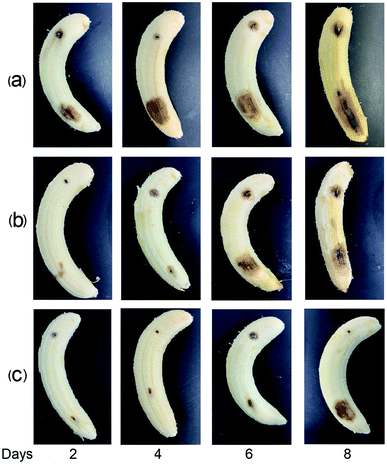
The inhibitory activities of the EL–CS-NPs and PEG20 K–EL–CS-NPs against C. musae were tested. Antibacterial analysis showed that the MIC and MFC values of PEG20 K–EL–CS-NPs against C. musae were distinctly lower than those of eugenol casein nanoparticles without PEG assistance (Table 2), indicating that stronger antibacterial activity arose from the PEG coating of nanoparticles. C. musae on bananas causes decay. Two days after the bananas were inoculated with C. musae, the control group showed decay at the site of inoculation. However, the decay of the groups treated with the EL–CS-NP and PEG20 K–EL–CS-NP dispersions was delayed until the sixth and eighth days, respectively (Fig. 5). The results demonstrate that the PEG20 K–EL–CS-NP dispersion yielded enhanced inhibitory activity against C. musae compared with the EL–CS-NP formulation. In the above experiments, we demonstrated that PEG20 K–EL–CS-NPs exhibited the better sustained release of EL than the EL–CS-NPs since the PEG20 K–EL–CS-NP are more stable than the EL–CS-NPs. However, the release of EL from the dispersion was very slow (Fig. 2a), and this was not synchronous with the anti-fungal effects on the banana. This result indicates that the stability of the NPs decreased due to the evaporation of water from the dispersions brushed onto the banana, which accelerated the release of EL. Nevertheless, the PEG20 K–EL–CS-NPs exhibited more effective release than the EL–CS-NPs. Pathogenic microorganisms play a significant role in affecting the quality of food products; in particular, they can lead to food poisoning and the production of other mycotoxins, with associated health risks. The eco-friendly encapsulation system showed obvious controlled release behavior, thereby extending the duration and extent of the microbial inhibition effect. Thus, the PEG20 K–EL–CS-NPs may have promising potential for applications relating to the control of anthracnose in food storage.| MIC (mg mL−1) | MFC (mg mL−1) | |
|---|---|---|
| a Values in a column followed by different letters are significantly different (p < 0.05). | ||
| Eugenol (EL) | 1.35 ± 0.11a | 1.85 ± 0.15a |
| EL–CS-NPs | 1.05 ± 0.09b | 1.30 ± 0.12b |
| PEG20 K–EL–CS-NPs | 0.85 ± 0.07c | 0.95 ± 0.09c |
3.6. The enhanced inhibitory effects of PEG20 K–EL–CS-NPs lyophilized powder against mold on bread
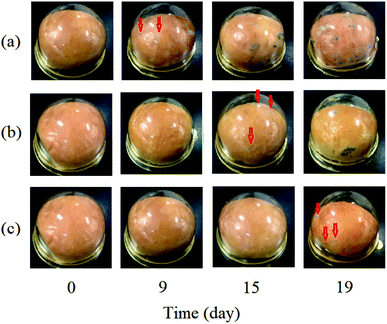
The anti-mold effectiveness of the PEG20 K–EL–CS-NP lyophilized powder was evaluated and compared with free EL (control) and EL–CS-NP lyophilized powder through being used for the packaging of bread. Visually observed shelf-lives and mold-counts on bread during storage are shown in Fig. 6. Mold growth on bread packed with EL on filter paper, EL–CS-NP lyophilized powder, and PEG20 K–EL–CS-NP lyophilized powder was visually observed after 9, 15, and 19 days of storage, respectively. The results indicated that the PEG20 K–EL–CS-NPs effectively prolonged the shelf-life of bread, compared with free EL and the EL–CS-NPs. Previous studies have demonstrated that lyophilized AgCl nanoparticles remain as intact spheres with an outer covering of poly(vinyl alcohol), which preserves their spatial integrity.35 Therefore, that the lyophilized PEG20 K–EL–CS-NP powder in this study maintained its antibacterial activity can probably be attributed to the PEG coating on the nanoparticles.4. Conclusions
In conclusion, this study was aimed at improving the stability and antimicrobial efficiency of eugenol–casein nanoparticles via PEG modification. The formulated eugenol-entrapping casein nanoparticles modified with PEG of various molecular weights showed that the PEG molecular weight was positively correlated with the stability of PEG–EL–CS-NPs. The PEG20 K–EL–CS-NP dispersion obtained using PEG with a MW of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da exhibited remarkable stability for over one year at low temperature, and it possesses a satisfactory sustained-release gradient, enhancing the inhibitory effects against C. musae on bananas and mold on bread. This work shows potential for improving the stability of casein NPs loaded with hydrophobic substances for delivery in the food and agriculture fields; however, further studies are needed, such as clarifying the interaction mechanism between PEG and casein NPs, and testing any improvements in the stabilities of casein NPs loaded with other hydrophobic substances.
000 Da exhibited remarkable stability for over one year at low temperature, and it possesses a satisfactory sustained-release gradient, enhancing the inhibitory effects against C. musae on bananas and mold on bread. This work shows potential for improving the stability of casein NPs loaded with hydrophobic substances for delivery in the food and agriculture fields; however, further studies are needed, such as clarifying the interaction mechanism between PEG and casein NPs, and testing any improvements in the stabilities of casein NPs loaded with other hydrophobic substances.
Author contributions
Conceptualization: Q. D., X. H. and P. J.; methodology: X. H., S. H., D. Q. and Q. D.; software: X. H., S. H. and D. Q; validation: Q. D., X. H. and P. J.; formal analysis: X. H., S. H., D. Q. and Y. Z.; investigation: X. H. and S. H.; resources: Q. D.; data curation: Q. D., X. H. and P. J.; writing – original draft preparation: Q. D. and P. J.; writing – review and editing: Q. D. and P. J.; visualization: Q. D. and P. J.; supervision: Q. D.; project administration: Q. D.; funding acquisition: D. Q. and Q. D. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
Authors declare no conflicts of interest.Acknowledgements
This research was funded by the Key R&D Program Project of Zhejiang Province, China (Grant 2019C02072) and the Natural Science Foundation of Zhejiang Province, China (Grant Q19C200024). We thank Sarah Dodds, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the english text of a draft of this manuscript.References
- F. Rehan, N. Ahemad, R. A. Islam, M. Gupta, S. H. Gan and E. H. Chowdhury, Optimization and formulation of nanostructured and self-assembled caseinate micelles for enhanced cytotoxic effects of paclitaxel on breast cancer cells, Pharmaceutics, 2020, 12, 984 CrossRef CAS.
- J. Zhang, Y. Liu, X. Liu, Y. Li, X. Yin, M. Subirade, P. Zhou and L. Liang, The folic acid/β-casein complex: characteristics and physicochemical implications, Food Res. Int., 2014, 57, 162–167 CrossRef CAS.
- S. M. Jafari, M. Fathi and I. Mandala, Emerging product formation, in Food Waste Recovery: Processing Technologies and Industrial Techniques, Elsevier Inc., 2015, pp. 293–317 Search PubMed.
- E. Assadpour, S. M. Jafari and Y. Maghsoudlou, Evaluation of folic acid release from spray dried powder particles of pectin-whey protein nano-capsules, Int. J. Biol. Macromol., 2017, 95, 238–247 CrossRef CAS.
- E. Assadpour, Y. Maghsoudlou, S. M. Jafari, M. Ghorbani and M. Aalami, Evaluation of folic acid nano-encapsulation by double emulsions, Food Bioprocess Technol., 2016, 9, 2024–2032 CrossRef CAS.
- G. M. Tavares, T. Croguennec, A. F. De Carvalho and S. Bouhallab, Milk proteins as encapsulation devices and delivery vehicles: applications and trends, Trends Food Sci. Technol., 2014, 37, 5–20 CrossRef CAS.
- F. Kimpel and J. J. Schmitt, Review: milk proteins as nanocarrier systems for hydrophobic nutraceuticals, J. Food Sci., 2015, 80, 2361–2366 CrossRef.
- Y. D. Livney, Milk proteins as vehicles for bioactives, Curr. Opin. Colloid Interface Sci., 2010, 15, 73–83 CrossRef CAS.
- M. H. Tunick and D. L. Van Hekken, Dairy products and health: recent insights, J. Agric. Food Chem., 2015, 63, 9381–9388 CrossRef CAS.
- A. Sahu, N. Kasoju and U. Bora, Fluorescence study of the curcumin-casein micelle complexation and its application as a drug nanocarrier to cancer cells, Biomacromolecules, 2008, 9, 2905–2912 CrossRef CAS.
- P. Zimet, D. Rosenberg and Y. D. Livney, Re-assembled casein micelles and casein nanoparticles as nano-vehicles for ω-3 polyunsaturated fatty acids, Food Hydrocolloids, 2011, 25, 1270–1276 CrossRef CAS.
- M. Haham, S. Ishshalom, M. Nodelman, I. Duek, E. Segal, M. Kustanovich and Y. D. Livney, Stability and bioavailability of vitamin D nanoencapsulated in casein micelles, Food Funct., 2012, 3, 737–744 RSC.
- E. Semo, E. Kesselman, D. Danino and Y. D. Livney, Casein micelle as a natural nano-capsular vehicle for nutraceuticals, Food Hydrocolloids, 2007, 21, 936–942 CrossRef CAS.
- G. Markman and Y. D. Livney, Maillard-conjugate based core–shell co-assemblies for nanoencapsulation of hydrophobic nutraceuticals in clear beverages, Food Funct., 2012, 3, 262–270 RSC.
- Y. Levinson, S. Ishshalom, E. Segal and Y. D. Livney, Bioavailability, rheology and sensory evaluation of fat-free yogurt enriched with VD3 encapsulated in re-assembled casein micelles, Food Funct., 2016, 7, 1477–1482 RSC.
- Y. Cohen, S. Ishshalom, E. Segal, O. Nudelman, A. Shpigelman and Y. D. Livney, The bioavailability of vitamin D3, a model hydrophobic nutraceutical, in casein micelles, as model protein nanoparticles: human clinical trial results, J. Funct. Foods, 2017, 30, 321–325 CrossRef CAS.
- J. Yi, T. I. Lam, W. Yokoyama, L. W. Cheng and F. Zhong, Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells, Food Hydrocolloids, 2015, 43, 31–40 CrossRef CAS.
- Y. Zhang, S. He, Y. Ma, W. Xu and H. Tang, Characterization and bioaccessibility of β-carotene in re-assembled casein, RSC Adv., 2015, 5, 77595–77600 RSC.
- X. Pan, P. Yao and M. Jiang, Simultaneous nanoparticle formation and encapsulation driven by hydrophobic interaction of casein-graft-dextran and β-carotene, J. Colloid Interface Sci., 2007, 315, 456–463 CrossRef CAS.
- H. Chen, Y. Zhang and Q. Zhong, Physical and antimicrobial properties of spray-dried zein–casein nanocapsules with co-encapsulated eugenol and thymol, J. Food Eng., 2015, 144, 93–102 CrossRef CAS.
- S. Haratifar and A. Guri, 5-Nanocapsule formation by caseins A2-Jafari, Seid Mahdi, in Nanoencapsulation Technologies for the Food and Nutraceutical Industries, Academic Press, 2017, pp. 140–164 Search PubMed.
- D. S. Horne, Casein micelle structure and stability, in Milk proteins: From expression to food, ed. M. B. A. Thompson and H. Singh, Academic Press/Elsevier, Paris-Amsterdam-Boston, 2020, pp. 133–162 Search PubMed.
- S. Ribes, M. Ruiz-Rico, É. Pérez-Esteve, A. Fuentes, P. Talens, R. Martínez-Máñez and J. M. Barat, Eugenol and thymol immobilised on mesoporous silica-based material as an innovative antifungal system: application in strawberry jam, Food Control, 2017, 81, 181–188 CrossRef CAS.
- P. Suwanamornlert, S. Sangchote, W. Chinsirikul, A. Sane and V. Chonhenchob, Antifungal activity of plant-derived compounds and their synergism against major postharvest pathogens of longan fruit in vitro, Int. J. Food Microbiol., 2018, 271, 8–14 CrossRef CAS.
- L. Gong, T. Li, F. Chen, X. Duan, Y. Yuan, D. Zhang and Y. Jiang, An inclusion complex of eugenol into β-cyclodextrin: preparation, and physicochemical and antifungal characterization, Food Chem., 2016, 196, 324–330 CrossRef CAS.
- Y. Bai, Y. Cui, G. C. Paoli, C. Shi, D. Wang and X. Shi, Nanoparticles affect PCR primarily via surface interactions with PCR components: using amino-modified silica-coated magnetic nanoparticles as a main model, ACS Appl. Mater. Interfaces, 2015, 7, 13142–13153 CrossRef CAS.
- K. A. Abd-Elsalam and A. R. Khokhlov, Eugenol oil nanoemulsion: antifungal activity against Fusarium oxysporum f. sp. vasinfectum and phytotoxicity on cottonseeds, Appl. Nanosci., 2015, 5, 255–265 CrossRef CAS.
- S. Woranuch and R. Yoksan, Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation, Carbohydr. Polym., 2013, 96, 578–585 CrossRef CAS.
- P. Jin, R. Yao, D. Qin, Q. Chen and Q. Du, Enhancement in antibacterial activities of eugenol-entrapped ethosome nanoparticles via strengthening its permeability and sustained release, J. Agric. Food Chem., 2019, 67, 1371–1380 CrossRef CAS.
- Z. Hong, Y. Q. Xu, J. F. Yin, J. Jin and Q. Du, Improving the effectiveness of (−)-epigallocatechin gallate (EGCG) against rabbit atherosclerosis by EGCG-loaded nanoparticles prepared from chitosan and polyaspartic acid, J. Agric. Food Chem., 2014, 62, 12603–12609 CrossRef CAS.
- L. Xv, X. Qian, Y. Wang, C. Yu, D. Qin, Y. Zhang, P. Jin and Q. Du, Structural modification of nanomicelles through phosphatidylcholine: the enhanced drug-loading capacity and anticancer activity of celecoxib-casein nanoparticles for the intravenous delivery of celecoxib, Nanomaterials, 2020, 10, 451 CrossRef CAS.
- W. B. Suleiman, In vitro estimation of superfluid critical extracts of some plants for their antimicrobial potential, phytochemistry, and GC-MS analyses, Ann. Clin. Microbiol. Antimicrob., 2020, 19, 29 CrossRef CAS.
- J. Gao, J. X. Wong, J. C. Lim, J. Henry and W. Zhou, Influence of bread structure on human oral processing, J. Food Eng., 2015, 167, 147–155 CrossRef.
- Y. Luo, K. Pan and Q. Zhong, Casein/pectin nanocomplexes as potential oral delivery vehicles, Int. J. Pharm., 2015, 486, 59–68 CrossRef CAS.
- N. D. Trinh, T. T. B. Nguyen and T. H. Nguyen, Preparation and characterization of silver chloride nanoparticles as an antibacterial agent, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2015, 6, 045011 Search PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2021 |