Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Corrosion and biocompatibility behaviours of microarc oxidation/phytic acid coated magnesium alloy clips for use in cholecystectomy in a rabbit model
Qiuxia Zheng†
 ,
Zongbin Sun†,
Zhanhui Wang
,
Zongbin Sun†,
Zhanhui Wang *,
Tinghe Duan,
Kai Xu,
Mengmeng Cai and
Bi Wang
*,
Tinghe Duan,
Kai Xu,
Mengmeng Cai and
Bi Wang
Department of Surgery, Luoyang Central Hospital Affiliated to Zhengzhou University, 288 Zhongzhou Road, Luoyang, 471000, China. E-mail: zhanhuiwang868@163.com; Fax: +86 379 6389 2095; Tel: +86 379 6389 2095
First published on 10th June 2021
Abstract
With the popularisation of laparoscopic cholecystectomy, ligation clips have been commonly used for ligating the cystic duct and cystic artery. However, non-degradable clips remain in the body long-term, which significantly increases the risk of the clip becoming detached. Thus, magnesium alloys have attracted tremendous attention owing to their biodegradability and good biocompatibility. However, the poor corrosion resistance hinders the clinical application of magnesium alloys with microarc oxidation/phytic acid (MAO/PA) composite coatings as protective coatings. Here, these alloys were used to hinder the rapid material degradation in aqueous solution. Electrochemical tests were conducted to evaluate the in vivo degradation behaviour in simulated body fluid (SBF) for Mg–Zn–Y–Nd alloys, and scanning electron microscopy (SEM) was used to observe the micromorphology of in vivo clip degradation. Cell toxicity, cell adhesion, and flow cytometry were performed in vitro to detect cytocompatibility. Biochemical detection of serum magnesium, serum creatinine (CREA), blood urea nitrogen (BUN), alanine transaminase (ALT), and alanine aminotransferase (AST), and haematoxylin–eosin (HE) staining of the heart, liver, and kidney tissues in vivo was conducted to determine the biocompatibility properties after surgery. Electrochemical measurements and SEM images revealed that the MAO/PA-coated magnesium alloy delayed corrosion in SBF. The apoptosis rate increased slightly with increased extract concentration. Nevertheless, MAO/PA-coated magnesium alloys still exhibited good cytocompatibility. No obvious abnormality was observed in the blood biochemical test or HE staining. Thus, MAO/PA-coated magnesium alloys exhibit better corrosion than bare magnesium. In addition, Mg–Zn–Y–Nd and MAO/PA-coated magnesium alloys exhibited no cytotoxicity, good adhesion, and biosafety.
Introduction
Laparoscopic cholecystectomy (LC) is currently the first choice for treating symptomatic cholelithiasis.1 Hem-o-lok and titanium clips, which are non-degradable materials, are extensively used for ligation during the procedure.2,3 However, permanent metal implants inevitably cause side effects, including thrombosis, permanent physical stimulation, and local response to chronic inflammation.4,5 In addition, titanium clips can cause a local inflammatory response in the body,6 which can form metallic artefacts in imaging examination.7 Consequently, magnesium alloys have been extensively considered as promising alternatives owing to their good biocompatibility and biodegradability.8 Recently, magnesium alloys have been extensively investigated as implants for orthopaedic and cardiovascular applications, and in oral and maxillofacial surgery.7,9,10 One reason is that magnesium alloys and human bone tissue exhibit a similar elastic modulus, and magnesium ions can induce the activation of osteoblasts.11,12 Another reason is that magnesium ions play an effective role in preventing late thrombosis.13 In addition, Mg–Zn–Y–Nd alloys have been investigated as biomaterials for intestinal, vascular, and oesophageal stents, which indicates their potential feasibility for clinical applications.14–16However, the major limitation of magnesium alloys is their poor corrosion resistance.17 Thus, the mechanical strength of the magnesium alloy biomaterials decreases after implantation; further, the local implant position is in an alkaline environment. If the large amount of hydrogen released is not absorbed in time, emphysema will occur in the loose tissue.18 Accordingly, the low corrosion resistance of magnesium alloy materials limits their clinical applications. Hence, numerous approaches, such as surface modification of the coatings and adequate alloying elements, have been developed to address these limitations, such as surface modification of the coatings and selection of adequate alloying elements.17 Magnesium alloys significantly reduce the degradation rate by surface protective coatings.18 In our previous study, we used a microarc oxidation (MAO) treatment as an economic surface treatment method to improve the corrosion resistance,19 because it relates to the high-pressure plasma-assisted anodic oxidation process and promotes the formation of highly adhesive ceramic oxide coatings. Therefore, the mechanical strength and excellent corrosion resistance of magnesium alloys have been improved.20 Nevertheless, since the porous surface of the MAO causes heterogeneous degradation, it can only improve the early corrosion resistance; in the later stage after implantation, the degradation rate was accelerated.21 Phytic acid (PA) is a natural, non-toxic organic acid that exists in the cytoplasm and nucleus of eukaryotic cells and has a strong chelating capacity with metal ions such as magnesium and zinc ions.22,23 In addition, as a surface conversion film, PA has a good protective function on the surface of magnesium alloys.24 Assuming that the pores of MAO form the first layer on the surface of the magnesium alloy, PA covered the MAO to form the outermost layer, which is the fabrication process for MAO/PA composite coatings, which can achieve a better corrosion resistance than single coatings.
Thus, MAO/PA composite coatings were synthesised on the Mg–Zn–Y–Nd alloy. The corrosion resistance was investigated by electrochemical tests and scanning electron microscopy (SEM) images, and the effects of biocompatibility were detected by in vitro cell experiments and tests. Through the analysis of corrosion and biocompatibility performances, this study aims to investigate the feasibility of dual-coated magnesium alloy clips for use in cholecystectomy in a rabbit model. It provides theoretical support for the clinical application of this type of biodegradable magnesium alloy clip.
Materials and methods
Processing of the materials
The extruded Mg–2.0% Zn–0.5% Y–0.5% Nd alloy was prepared at Zhengzhou University and contained master alloys of induction heating high-purity Mg, high-purity Zn, Mg–25Y (wt%) (99.99% purity), and Mg–25Nd (wt%) (99.97% purity), which were mixed thoroughly and melted at 740 °C under a CO2/SF6 atmosphere (volume fraction, 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in an electronic resistance furnace.25 Then, the Mg–Zn–Y–Nd alloy was extruded into rods and cut into round disks (Φ 8 × H3 mm).
1) in an electronic resistance furnace.25 Then, the Mg–Zn–Y–Nd alloy was extruded into rods and cut into round disks (Φ 8 × H3 mm).
Thereafter, the samples were mechanically ground to 2000# with grit SiC paper and cleaned sequentially in acetone, ethanol, and deionised (DI) water. After drying, some samples underwent electrochemical tests, and others were sterilised with ultraviolet rays for 1 h and prepared for in vitro cell experiments. A number of clips (cross-section diameter: 0.9 mm, side length: 10 mm) were fabricated (Fig. 1) using a drawing machine, and V-shaped clips were formed by weaving. Eventually, all clips were treated by annealing to obtain better mechanical properties in the animal experiments.
Coating preparation
The prepared specimens were anodised using an MAO machine (YS9000D-300-40), after DI water was added to trisodium phosphate, sodium hydroxide, and glycerol and the MAO electrolyte was prepared. The magnesium alloy samples underwent MAO treatment (20 min). A stainless steel sheet was used as the negative electrode with a frequency of 800 Hz, a voltage of 260 V, and a duty cycle of 15%. The specimens were subsequently washed with DI water and air-dried. The phytic acid solution was adjusted to pH 5 with sodium hydroxide to a concentration of 7.5 g L−1, and the magnesium alloy coating with MAO samples was placed in the PA solution in a constant-temperature water bath at 40 °C, rinsed with DI water again, and dried in cold air. The Mg–Zn–Y–Nd alloy coated with MAO/PA was set as the experimental group, and the uncoated samples were used as the control group.Detection of corrosion properties
Cytocompatibility in vitro
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cm2. The specimens were then placed into the cell incubator and cultured for 24 h at 37 °C in a 5% CO2 incubator. The extracts were collected, centrifuged, and subsequently diluted to concentrations of 25%, 75%, and 100% with DMEM. The DMEM medium was used as the negative control and 0.64% phenol DMEM medium was used as the positive control.
1 cm2. The specimens were then placed into the cell incubator and cultured for 24 h at 37 °C in a 5% CO2 incubator. The extracts were collected, centrifuged, and subsequently diluted to concentrations of 25%, 75%, and 100% with DMEM. The DMEM medium was used as the negative control and 0.64% phenol DMEM medium was used as the positive control.| Relative cell survival rate = (average absorbance value of experimental group − average absorbance value of positive control group)/(average absorbance value of negative control group − average absorbance value of positive control group). |
In vivo test
Results
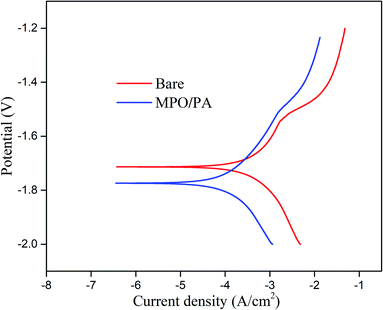
Corrosion resistance results
| Sample | Ecorr (V) | icorr (A cm−2) |
|---|---|---|
| Bare sample | −1.71 | 1.2 × 10−4 |
| MAO/PA-coated Mg alloy | −1.77 | 3.5 × 10−5 |
In vitro biocompatibility properties
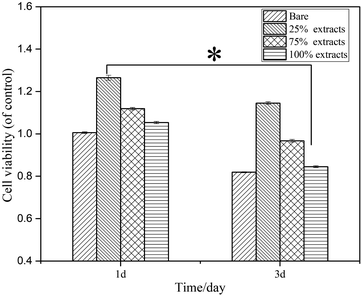 | ||
| Fig. 4 Relative survival rate of human fibroblasts cultured in the bare and MAO/PA-coated magnesium alloy extracts for 1 and 3 d (*P < 0.05). | ||
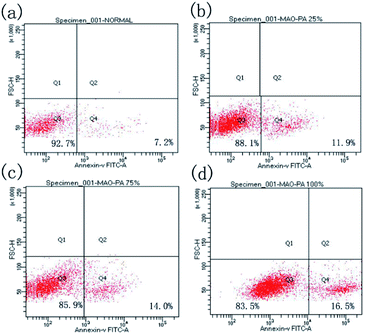
| Groups | Q3 | Q4 |
|---|---|---|
| Negative control | 92.7% | 7.2% |
| 25% | 88.1% | 11.9% |
| 75% | 85.9% | 14.0% |
| 100% | 83.5% | 16.5% |
In vivo biocompatibility properties
Discussion
Degradation behaviour
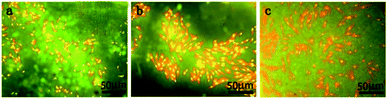
Electrochemistry is extensively used to determine the instantaneous corrosion performance.27 When magnesium alloys react with the SBF solution, the current density represents the kinetics of the corrosion process in the electrolyte, which is an important parameter affecting the corrosion resistance of the magnesium alloy. This is directly related to the corrosion rate; that is, the lower the current density, the better the corrosion resistance.28 Thus, the strength of the ongoing corrosion process in a particular electrolyte is revealed.29 Although the corrosion potential can also impact the corrosion performance, taking into account the standard deviation of the measured data and the apparent negative value of the established corrosion potential, the general thermodynamic corrosion properties can be negligible.30 In this study, the corrosion current of the MAO/PA-coated magnesium alloy decreased by one order of magnitude compared with that of the bare samples (Table 1). One reason is that the MAO technology can form an oxide ceramic coating on the surface of the magnesium alloy.31 Another reason is that PA exhibits a strong chelating ability, which can be combined with magnesium ions, zinc ions, and other cations through chemical bonds, thereby reducing the reaction between magnesium ions and corrosion solvents.32 Therefore, the MAO/PA-coated magnesium alloy is more corrosion resistant than the bare specimens in SBF. In addition, SEM observations confirmed that the MAO/PA-coated magnesium alloy clips were implanted into rabbits (Fig. 3). However, the corrosion performance must be observed for a long time in clinical applications; at least, it should be degraded stably during the service period to ensure that the clips cannot become detached.In vitro biocompatibility
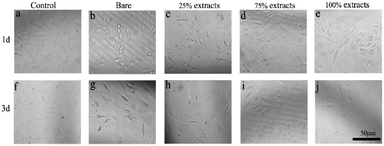
According to the ISO 10993-5 standard, a relative cell survival rate above 75% indicates no evident toxicity.26 The CCK-8 cytotoxicity test revealed that the MAO/PA-coated magnesium alloy was suitable for biomaterials. Although the morphology of the cells was relatively normal (Fig. 5), the relative survival rate of the cells gradually decreased with increased extract concentration (Fig. 4). Thus, our results demonstrated that high MAO/PA extract concentrations promoted early cell apoptosis in the flow cytometry apoptosis test (Fig. 6 and Table 2). This may be because, as the extract concentration increased, the Mg2+ concentration and pH also increased; further, the activation of Mg2+ in cells is an early event in cell apoptosis, which is earlier than DNA fragmentation.33 Tsao et al.34 postulated that higher pH values may be related to apoptosis. Ito et al.35 confirmed that zinc ions released from zinc oxide could cause cytotoxicity when the zinc content in the ceramics exceeds 1.20 wt%, the zinc ions released from zinc oxide could cause cytotoxicity. In addition, it has been found that the element Nd in magnesium alloys can also increase apoptosis.36 However, further experimental investigations are required to determine the underlying mechanism. In brief, although the apoptosis rate gradually increased with the extract concentration, the relative survival rate of the cells remained relatively high in a short time, indicating that the MAO/PA-coated magnesium alloy mildly impacted the cells. Therefore, MAO/PA-coated magnesium alloys are promising for use as biomaterials.The cells are extremely sensitive to changes in the surrounding environment.37 However, acridine orange fluorescence staining revealed that the cytoplasm and nucleus of the cells were clear in the early cell adhesion test of the uncoated, PA-coated, and MAO/PA-coated magnesium alloy groups, and the cell morphologies were not significantly abnormal. The results indicated that magnesium alloy supports cell adhesion in the early stages, and the cell compatibility was excellent.
In vivo compatibility
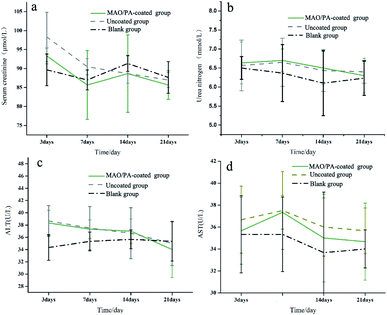
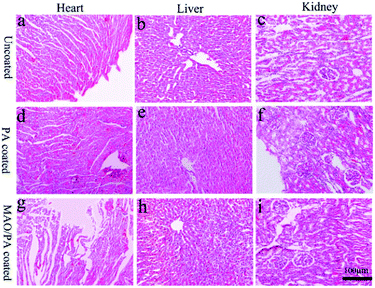
ALT and AST levels are important parameters that can be used to evaluate liver function,38 while CREA and BUN are the most widely used parameters to assess renal function; increases in these blood biochemical levels suggest liver and kidney injury.39 Furthermore, the main metabolic pathway of magnesium ions is the kidney,40 and the liver is the organ that first filters toxins, nutrients, and bacterial metabolites.41 Thus, it can be inferred that these two organs are easily damaged when clips are implanted into the body. However, our results suggest that liver and kidney functions were not affected (Fig. 8). Moreover, the results of HE staining confirmed that the clips did not impair the cells and tissues of the heart, liver, and kidney, as shown in Fig. 9. Therefore, the MAO/PA-coated and uncoated Mg–Zn–Y–Nd alloys exhibited good tissue compatibility.In this study, the MAO/PA-coated magnesium alloy clip exhibits potential feasibility in cholecystectomy due to its good biocompatibility and corrosion resistance. However, there are several limitations: the short experimental period (three weeks) and mechanical properties, which are the major factors used in clinical applications, require further investigation. Therefore, it is necessary to extend the experimental period and further investigate the mechanical properties of the clips in future experiments.
Conclusions
In this study, in vitro and in vivo tests were used to assess the corrosion resistance and biocompatibility properties of MAO/PA-coated Mg–Zn–Y–Nd alloys. In the in vitro experiments, the MAO/PA coatings significantly improved the corrosion resistance of the magnesium alloy and reduced the corrosion current by approximately one order of magnitude, indicating that the MAO/PA-coated groups had better corrosion properties than the uncoated samples in the rabbits at three weeks. However, the long-term degradation performance requires further investigation. The apoptosis rate of the MAO/PA-coated group increased with increased extract concentration. Further, the cell survival rate satisfied the requirements of biomaterials and exhibited excellent cell adhesion. Moreover, the MAO/PA-coated alloy clips were implanted into rabbits without significant tissue damage or signs of severe foreign body inflammation. Overall, the MAO/PA-coated Mg–Zn–Y–Nd alloy is a safe and biocompatible surgical clip, and there are unlimited possibilities for future clinical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 1404825) and the Luoyang Science and Technology Development Plan Project (No. 1503006A-3). The authors are also grateful to the laboratory at Zhengzhou University for providing material support.References
- J. Bueno Lledó, P. Granero Castro, I. G. I. Gomez, J. L. Ibañez Cirión, R. López Andújar and E. García Granero, Cir. Esp., 2016, 94, 429–441 CrossRef PubMed.
- M. V. Meng, J. Endourol., 2007, 20, 1054–1057 CrossRef PubMed.
- N. Simforoosh, R. Sarhangnejad, A. Basiri, S. A. Ziaee, F. Sharifiaghdas, A. Tabibi, A. Nouralizadeh, A. H. Kashi and N. Moosanejad, J. Endourol., 2012, 26, 1009–1012 CrossRef PubMed.
- P. Erne, M. Schier and T. J. Resink, Cardiovasc. Intervent. Radiol., 2006, 29, 11–16 CrossRef PubMed.
- M. Moravej and D. Mantovani, Int. J. Mol. Sci., 2011, 12, 4250–4270 CrossRef CAS PubMed.
- D. Gonganau-Nitu, R. R. Scurtu, C. G. Precup, G. Dindelegan, A. Biro, C. Crisan, S. Cocu, C. Popa and C. Ciuce, Chirurgia, 2010, 105, 501–508 CAS.
- T. Yoshida, T. Fukumoto, T. Urade, M. Kido, H. Toyama, S. Asari, T. Ajiki, N. Ikeo, T. Mukai and Y. Ku, Surgery, 2017, 16, 1553–1560 CrossRef PubMed.
- Y. Qu, M. Kang, R. Dong, J. Liu, J. Liu and J. Zhao, J. Mater. Sci.: Mater. Med., 2015, 26, 5342 CrossRef PubMed.
- O. Charyeva, O. Dakischew, U. Sommer, C. Heiss, R. Schnettler and K. S. Lips, J. Orthop. Traumatol., 2016, 17, 63–73 CrossRef PubMed.
- F. Witte, Acta Biomater., 2010, 6, 1680–1692 CrossRef PubMed.
- F. Witte, V. Kaese, H. Haferkamp, E. Switzer and H. Windhagen, Biomaterials, 2005, 26, 3557–3563 CrossRef CAS PubMed.
- D. Liu, C. Guo, L. Chai, V. R. Sherman, X. Qin, Y. Ding and M. A. Meyers, Mater. Sci. Eng., B, 2015, 195, 50–58 CrossRef CAS.
- M. Echeverry-Rendon, J. P. Allain, S. M. Robledo, F. Echeverria and M. C. Harmsen, Mater. Sci. Eng., C, 2019, 102, 150–163 CrossRef CAS PubMed.
- S. Wang, X. Zhang, J. Li, C. Liu and S. Guan, Bioact. Mater., 2020, 5, 1–8 CrossRef PubMed.
- Z. Wang, Q. Zheng, S. Guan, Z. Sun, S. Liu, B. Zhang, T. Duan and K. Xu, Mater. Sci. Eng., C, 2019, 105, 110087 CrossRef CAS PubMed.
- P. Wang, J. Liu, X. Luo, P. Xiong, S. Gao, J. Yan, Y. Li, Y. Cheng and T. Xi, J. Mater. Chem. B, 2019, 7, 7314–7325 RSC.
- M. Rahman, N. K. Dutta and N. Roy Choudhury, Front. Bioeng. Biotechnol., 2020, 8, 564 CrossRef PubMed.
- P. Tian, X. Liu and C. Ding, Colloids Surf., B, 2015, 128, 44–54 CrossRef PubMed.
- Z. Wang, Q. Zheng, S. Guan, Z. Sun, S. Liu, B. Zhang, T. Duan and K. Xu, Mater. Sci. Eng., C, 2019, 105, 110087 CrossRef CAS PubMed.
- R. C. Zeng, L. Y. Cui, K. Jiang, R. Liu, B. D. Zhao and Y. F. Zheng, ACS Appl. Mater. Interfaces, 2016, 8, 10014–10028 CrossRef CAS PubMed.
- S. F. Fischerauer, T. Kraus, X. Wu, S. Tangl, E. Sorantin, A. C. Hänzi, J. F. Löffler, P. J. Uggowitzer and A. M. Weinberg, Acta Biomater., 2013, 9, 5411–5420 CrossRef CAS PubMed.
- F. Crea, C. D. Stefano, D. Milea and S. Sammartano, Coord. Chem. Rev., 2008, 252, 1108–1120 CrossRef CAS.
- J. Torres, S. Domínguez, M. F. Cerdá, G. Obal, A. Mederos, R. F. Irvine, A. Díaz and C. Kremer, J. Inorg. Biochem., 2005, 99, 828–840 CrossRef CAS PubMed.
- X. Cui, L. Ying, Q. Li, J. Guo, M. Ding and F. Wang, Mater. Chem. Phys., 2008, 111, 503–507 CrossRef CAS.
- J. Wang, L. Wang, S. Guan, S. Zhu, C. Ren and S. Hou, J. Mater. Sci.: Mater. Med., 2010, 21, 2001–2008 CrossRef CAS PubMed.
- ISO 10993-5, International Organization of Standards, 2009 Search PubMed.
- Y. Liu, J. Xue, D. Luo, H. Wang and L. Ren, J. Colloid Interface Sci., 2017, 491, 313–320 CrossRef CAS PubMed.
- Y. Ren, E. Babaie, B. Lin and S. B. Bhaduri, Biomed. Mater., 2017, 12, 045026 CrossRef PubMed.
- B. Hadzima, M. Mhaede and F. Pastorek, J. Mater. Sci.: Mater. Med., 2014, 25, 1227–1237 CrossRef CAS PubMed.
- S. Bagherifard, D. J. Hickey, S. Fintova, F. Pastorek, I. Fernandez-Pariente, M. Bandini, T. J. Webster and M. Guagliano, Acta Biomater., 2018, 66, 93–108 CrossRef CAS PubMed.
- L. U. Yutong, L. I. Tao, X. Shang, Y. Wang and X. Cai, Shengwu Yixue Gongchengxue Zazhi, 2016, 33, 1016–1019 Search PubMed.
- X. Cui, Q. Li, L. Ying, F. Wang, J. Guo and M. Ding, Appl. Surf. Sci., 2008, 255, 2098–2103 CrossRef CAS.
- M. M. Chien, K. E. Zahradka, M. K. Newell and J. H. Freed, J. Biol. Chem., 1999, 274, 7059–7066 CrossRef CAS PubMed.
- N. Tsao and H. Y. Lei, J. Immunol., 1996, 157, 1107–1116 CAS.
- A. Ito, K. Ojima, H. Naito, N. Ichinose and T. Tateishi, J. Biomed. Mater. Res., 2000, 50, 178–183 CrossRef CAS PubMed.
- F. Feyerabend, J. Fischer, J. Holtz, F. Witte, R. Willumeit, H. Drucker, C. Vogt and N. Hort, Acta Biomater., 2010, 6, 1834–1842 CrossRef CAS PubMed.
- Z. Wei, P. Tian, X. Liu and B. Zhou, J. Biomed. Mater. Res., Part B, 2015, 103, 342–354 CrossRef PubMed.
- A. Zubrzycki, A. Wrońska, A. Kotulak-Chrząszcz, P. M. Wierzbicki and Z. Kmieć, Arch. Gerontol. Geriatr., 2020, 91, 104244 CrossRef CAS PubMed.
- X.-X. Li, F.-Y. Du, H.-X. Liu, J.-B. Ji and J. Xing, J. Ethnopharmacol., 2015, 162, 238–243 CrossRef CAS PubMed.
- S. Zhang, X. Zhang, C. Zhao, J. Li, Y. Song, C. Xie, H. Tao, Y. Zhang, Y. He, Y. Jiang and Y. Bian, Acta Biomater., 2010, 6, 626–640 CrossRef CAS PubMed.
- T. I. S. Nguepi and H. Song, Int. J. Med. Mushrooms, 2020, 22, 509–519 CrossRef PubMed.
Footnote |
| † These two authors should be considered equal first authors. |
| This journal is © The Royal Society of Chemistry 2021 |