Open Access Article
Open Access ArticleStudy on contact angles and surface energy of MXene films†
Hang Zhoua,
Fuqiang Wanga,
Yuwei Wang b,
Changping Lib,
Changrui Shia,
Yu Liua and
Zheng Ling
b,
Changping Lib,
Changrui Shia,
Yu Liua and
Zheng Ling *a
*a
aKey Laboratory of Ocean Energy Utilization and Energy Conservation of Ministry of Education, School of Energy & Power Engineering, Dalian University of Technology, Dalian 116024, China. E-mail: zling@dlut.edu.cn
bResearch Center for Eco-Environmental Engineering, Dongguan University of Technology, Dongguan 523808, PR China
First published on 1st February 2021
Abstract
MXene is a growing two-dimensional material family of transition metal carbides and nitrides and showing great promise in various applications, such as energy storage, water treatment, composites, electromagnetic interference shielding, etc. In all these applications, at least one of the MXene flake sides is in contact with a medium of interest. So the wetting behaviors of MXene are critical for the performance of MXene. Although the hydrophilicity of MXene is unquestionable, the reported contact angles of MXene have covered an extensive range. Additionally, the surface energy of MXene flakes is surprisingly poorly known and rarely studied. In this work, the static contact angles of MXene films were studied using water, glycerol, and diiodomethane. The surface energy of MXene films has a range of 49.92 ± 2.01 to 62.44 ± 0.25 mJ m−2, calculated based on the measured contact angles. The loading, drying and storage condition of the MXene films have various impacts on their contact angles and surface energy. The root cause for the wide-range of contact angles is related to the surface chemistry of the MXene films. Organic contamination and surface oxidation are responsible for the scattering water contact angle. The contact angles are mass loading-independent for MXene films with loadings from 0.3 to 2 mg cm−2. The surface energy and its acid–base component are sensitive to the delamination methods and MXene compositions, while the dispersion component of the surface energy is stable. These findings will provide valuable insight and guidance for measuring contact angles of MXene films and the rational design and synthesis of MXene-based films, composites, coatings, and energy storage devices.
1. Introduction
Two dimensional (2D) materials, such as graphene, have an atomic-level thickness and outstanding physicochemical properties, providing various promising applications, such as in coatings, composites, energy storage, water treatment, etc.1,2 The wetting behavior of 2D materials is critical for their applications since the solid–liquid interaction is indispensable for their synthesis, device fabrication, and proper working. The contact angle (CA) is one of the most important parameters measured experimentally for characterizing the wetting features of various solids,3 including 2D materials. The CA values describe a junction among three phases and are determined by the used solvents as well as the composition and structure of the solid surface involved.3 Generally, a solid surface with a water contact angle (WCA) less than 90° is considered hydrophilic, while a surface is considered hydrophobic with a WCA larger than 90°.Although some 2D materials are thought to be inherently hydrophilic, their wetting behaviors are often perturbed by their substrates, contaminants, and intercalated ions,4–6 which can shift the WCA in a wide range. The unintentional contamination at the surfaces causes the scattering values of WCAs for 2D materials, challenging the long-held belief on the wettability of some materials, such as graphene and graphite.7 However, modulation of wetting behaviors of 2D materials also provides unexpected and unique properties, endowing promising applications in various fields, such as water treatment.6 The potential applications of different 2D materials require understanding and manipulating their wettability. However, the wetting behavior of 2D materials except for graphene has been seldom studied.8 Even for the most studied graphene, there is a longstanding and ongoing debate on its inherent hydrophilicity or hydrophobicity,8 indicating there are still knowledge gaps for understanding the wettability of graphene, the most studied 2D materials. The knowledge gap in wettability of other 2D materials is much larger than that of graphene due to less attention to these newly emerging 2D materials, such as MXene.
MXene is a large and growing family of 2D transition metal carbides, carbonitrides, and nitrides,9 which was first invented in 2011 and has rapidly expanded since then.10,11 The versatile chemistry of the MXene family allows the choosing and tuning of properties for desired applications, such as water treatment, energy storage, composites, and catalysis.12,13 Solid–liquid interaction plays critical roles in these applications; however, there are few reports focusing on the wetting behaviors of MXene. Although many works reported the WCAs of MXenes, the scattering results range from 18.6° to 91°.14,15 Additionally, there is a lack of explanation for these scattering WCAs, making it difficult to draw any precise conclusion on which parameters tunes the WCAs of MXene. More research is needed to understand the wetting behavior of the MXene family fully.
The surface energy (SE) indicates the interaction between a solid surface and the contacted liquid. The 2D material–liquid interactions would perturb liquid structures, resulting in distinctive behaviors and performance for sensors, energy storage, and other applications.8 Although numerous studies on the assembly and application of MXene films or papers have been reported,16 to date, only two published articles have studied the surface free energy of MXene,17,18 while the focuses of the two articles are not the SE.
In this work, MXene films assembled from delaminated MXene nanoflakes were used for CA testing. Three test liquids, including water, diiodomethane, and glycerol, were used. The Lifshitz–van der Waals and Lewis acid–base (LW–AB) model19,20 was utilized for determining the SE from the obtained CA data. MXene films with a large range of mass loadings were tested. The impacts of MXene loading, drying conditions, storage time, delamination process and MXene composition on the CAs and SEs were investigated. The reasons for the scattering CAs of MXene films were analyzed, their condition-dependent SEs were reported for the first time.
2. Experimental
2.1 Materials
Ti3AlC2 powder and Ti2AlC (500 mesh and with purity over 99%) were purchased from Laizhou Kai Kai Ceramic Materials Co., Ltd. Lithium fluoride (LiF) was purchased from Alfa Aesar (China) Chemicals Co., Ltd. Hydrochloric acid (HCl), diiodomethane (CH2I2), and glycerol (C3H5(OH)3) were bought from Sinopharm Chemical Reagent Co., Ltd. All the chemical agents were used as received. Deionized (DI) water (18.2 MΩ cm) obtained from AquaPro laboratory ultrapure water apparatus was used in all experiments.2.2 Selectively etching and delamination of MXene
The Ti3C2Tx MXene used in this study was obtained using the same procedure reported in our previous work.21 In a typical run, LiF (1.32 g) was added to 20 mL of 6 mol L−1 HCl solution and stirred for 10 min. Then, 1 g of Ti3AlC2 powder was slowly added into the etching solution, and the mixture was magnetically stirred at 45 °C for 72 h. After etching, the product was washed using DI water and centrifuged at 3500 rpm 6 times to allow the pH of the supernatant to be neutral. After vacuum dried for 48 h, the sediment was delaminated in DI water using tip ultra-sonication for 1 h under flowing N2. The colloidal suspension of exfoliated MXene was acquired after centrifugation at 3500 rpm for 30 min. The obtained colloidal suspension had a concentration of 4.0–5.0 mg mL−1.For the preparation of Ti2AlC based MXene, 3 g of LiF was dissolved in 20 mL of 9 mol L−1 HCl solution. Then, 2 g of Ti2AlC powder was slowly added into the etching solution under magnetic stirring. The etching was conducted at 35 °C for 18 h. After etching, the mixture was washed with DI water and centrifuged at 3500 rpm several times, until the pH value of supernatant reached 6.5–7.0. Half of sediment was dispersed in 50 mL DI water using hand-shaken violently for 10 min. The suspension of delaminated Ti2CTx (where T stands for functional group) was obtained after centrifugation at 3500 rpm for 30 min. The concentration of Ti2CTx suspension had a concentration of 8.0–8.5 mg mL−1.
2.3 Film fabrication and drying procedure
The MXene films were made via vacuum-assisted filtration of the obtained MXene colloidal suspension through a polypropylene separator membrane (Celgard) with a diameter of 4 cm. The loading of MXene films was regulated by the amount of the colloidal suspension of delaminated MXene with known concentration. The Ti3C2Tx MXene films with loading of 0.3, 0.4, 0.5, 1.0, 1.5 and 2.0 mg cm−2 were assembled using the colloidal suspension with a concentration of 4.66 mg mL−1. The Ti2CTx films with loadings of 0.4 and 1.0 mg cm−2 were made using the same procedure with a concentration of 8.0 mg mL−1.Three different drying processes, including vacuum drying, natural drying, oven-drying, were used for drying the as-prepared MXene membranes. The MXene film with definite loading, respectively, was put in a vacuum desiccator with maintaining stable pressure at −0.8 kg cm−2 at room temperature (MXene-V), or immersed in the laboratory environment for natural drying (MXene-N), or put in an oven at 60 °C (MXene-O). After a certain period of storage, the dried MXene films were used for contact angle measurement.
2.4 Characterization
Raman spectra were obtained in the region from 100 cm−1 to 800 cm−1 using LabRAM HR Evolution, with a 532 nm laser. The MXene films were characterized via X-ray diffraction to analyze the crystal structure using a Bruker D8 Advanced with filtered Cu Kα radiation (λ = 0.154 nm, operated at 40 kV and 40 mA). The micro-morphology was analyzed using scanning electron microscopy (SU8200, 5.0 kV). The chemical composition of MXene films was characterized by FTIR Spectroscopy (Nicolet 6700, US) in the region from 4000 cm−1 to 400 cm−1 and X-ray photoelectron spectroscopy (Thermo, ESCALAB XI+, UK) in the region from 1350 eV to 0 eV. The surface roughness of the sample was obtained by contourgraph (Bruker, DektakXT).2.5 Contact angle measurement
Static contact angles were measured at room temperature using an industrial camera (UI-1220LE-M-GL, IDS-Germany) and Surface Meter™ software (Ningbo NB Scientific Instruments Co., Ltd). DI water, diiodomethane, and glycerol were used as the testing liquids.24 The total surface energy and their components of three testing liquids are list in Table 1. Liquid droplets of 10 μL were slowly placed on the dried film and then photographed using the industrial camera after 10 s. An average value of contact angles was obtained from four or five droplets to minimize measurement error.| γLW (mJ m−2) | γ+ (mJ m−2) | γ− (mJ m−2) | γL (mJ m−2) | |
|---|---|---|---|---|
| Water | 21.8 | 25.5 | 25.5 | 72.8 |
| Diiodomethane | 50.8 | 0 | 0 | 50.8 |
| Glycerol | 34 | 3.92 | 57.4 | 64 |
2.6 Lifshitz–van der Waals and (Lewis) acid–base (LW–AB) model
In the LW–AB model, the total SE is composed of a dispersion component γLW due to Lifshitz–van der Waals interactions (SE–LW) and an acid–base component γAB due to Lewis interactions (SE–AB).19,20Therefore, the SE is expressed as:
| γ = γLW + γAB | (1) |
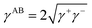 | (2) |
Furthermore, the liquid–solid interface energy is calculated as:
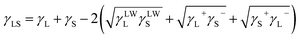 | (3) |
Combining the Young equation, it is acquired that:
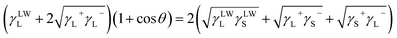 | (4) |
3. Results and discussion
It is well-known MXene has an attractive combination of excellent electrical conductivity and hydrophilia, making the MXene family attractive and promising for coating, composite fillers, and functional materials in an aqueous environment.9 The WCA has been used as the indicator of MXene's hydrophilicity in most reported papers (see Fig. 1). However, the scattering results of the WCAs, as shown in Fig. 1, suggest that the reported hydrophilic features of MXene based materials would be different from each other, although most of the WCAs are smaller than 90° with only one exception.15 There are few researches or explanations for these scattering WCAs of MXene.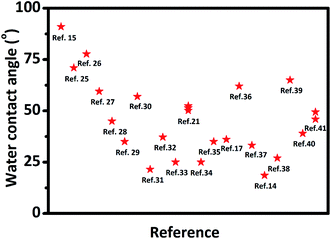 | ||
| Fig. 1 Water contact angles of reported MXene films from literature. Wang N, et al.,15 Yang W, et al.,25 Liu J, et al.,26 Liu J, et al.,27 Ding L, et al.,28 Ling Z, et al.,29 Fan Z, et al.,30 Ghidiu M, et al.,31 Jin X, et al.,32 Zhang T, et al.,33 Zhou H, et al.,21 Du F, et al.,34 Zhao M, et al.,35 Lorencova L, et al.,17 Kang K. M, et al.,36 Luo J, et al.,37 Shen J, et al.,14 Chen S, et al.,38 Wei S, et al.,39 Bian R, et al.,40 Liu G, et al.41 | ||
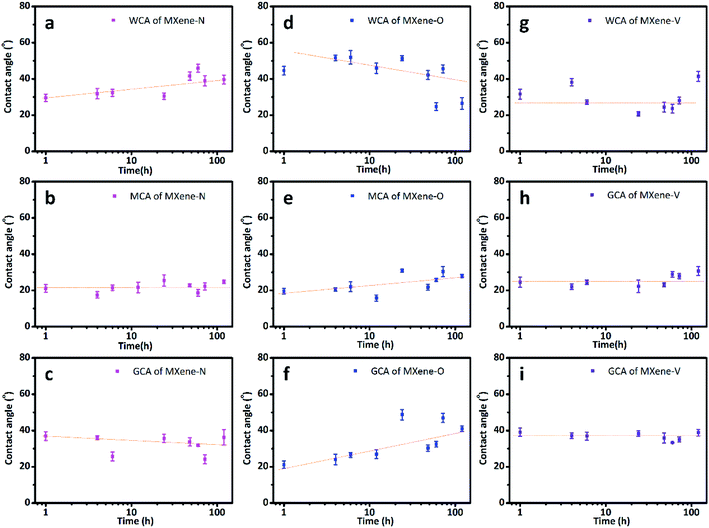
The CAs of MXene films stored under three different conditions as stated in experiment section, were tested over a storage period of 168 h. All the tested MXene films are wettable to the used solvents as the CAs are all smaller than 90°. While their CAs show different time-dependent changes due to the impact of the stored conditions. The MXene-N films show WCAs ranging from 29.55° ± 2.06° to 41.55° ± 2.26°, with an increasing trend over stored time (Fig. 2a). While their diiodomethane contact angles (MCAs) and glycerol contact angles (GCAs) almost keep constant during storage (Fig. 2b and c). The WCAs for the MXene-O films decrease with the exposure time in air at 60 °C, with a maximum of 51.94° ± 3.79° and a minimum of 24.70° ± 2.11°. Both the MACs and the GCAs of the MXene-O films show increasing trends during storage at 60 °C in air. It is also worth noting that the CA data for the MXene-O films show a more obvious scattering feature. Most of the WCAs of MXene-V films concentrate at around 27.12° while there is no clear changing-trend over the storage. In other words, the WCAs of the MXene-V films relatively keep constant compared with MXene-N and MXene-O films. The constant features are more notable for the MCAs and GCAs of the MXene-V films.
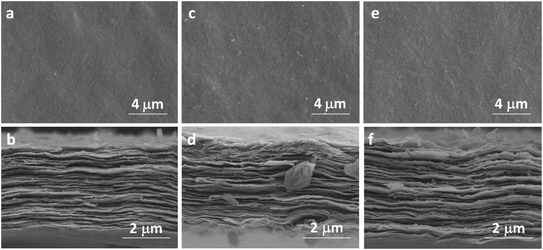
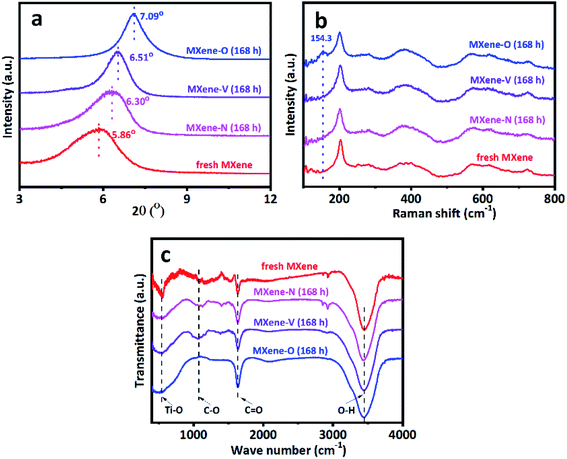
The characterization sheds light on the drying procedure-dependent CAs for the MXene-N, MXene-O, and MXene-V films. The SEM images show that all the tested MXene films share a similar front surface and cross-section morphology as the fresh MXene film, independent of their drying procedures (Fig. 3 and S1†). Similarly, the surface roughness of MXene film was obtained by contourgraph; compare to the fresh MXene films, arithmetic mean roughness of MXene films after drying shows a tiny difference, also unrelated of their drying procedures (Fig. S2†). The XRD patterns indicate the crystal structures of MXene-N, MXene-O, and MXene-V stored for 168 h remain the same as the fresh one (Fig. 4a). The peaks locating at 5.86° for the fresh MXene film have moved to 6.30°, 6.51°, and 7.09° for MXene-N, MXene-V, and MXene-O, respectively. The diffraction peak shifted to higher angles corresponds to a decreased interlayer distance due to the removal of intercalated water molecules during drying. Raman spectroscopy was used to analyze the surface changes of the MXene films due to its surface-sensitivity. As shown in Fig. 4b, MXene-N and MXene-V films have the same Raman peaks like those of the fresh MXene film, indicating natural and vacuum storages over 168 h have no visible impact on MXene films, while the MXene-O film shows an extra peak at 154.3 cm−1 besides the peaks corresponding to MXene. The new peak at 154.3 cm−1 corresponds to anatase TiO2,42 indicating the inevitable oxidation of MXene when stored in air at 60 °C. The MXene films were analyzed with X-ray photoelectron spectra (XPS) to further study the change in surface chemistry. It shows an obvious increase in the oxygen groups on the surface of the MXene films. Compared with fresh MXene film, O/C and O/Ti atomic ratios of the MXene-O films increase by 15.8% and 119.7%, the MXene-N film increased by only 8.7% and 47.5%, and the MXene-V film increased merely by 5.3% and 29.5%, respectively (Fig. S3†). The oxidation, which often heterogeneously occurs on MXene flakes and films, results in the changed CAs with a large scatter range.
The FTIR spectra show the composition change due to the storage in different conditions (Fig. 4c). All the tested samples share similar characteristic peaks at 3345 cm−1, 1640 cm−1, 1060 cm−1 and 550 cm−1, which correspond to the stretching vibration of O–H,14,43 the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O,44 the stretching vibrations of C–O bond45 and the stretching vibrations of Ti–O bond,46 respectively. While MXene-O shows a much wider peak around 550 cm−1 and the peak at 1640 cm−1 is stronger than the counterpart of other samples. These changes have resulted from the surface oxidation, which introduces more Ti–O and C
O,44 the stretching vibrations of C–O bond45 and the stretching vibrations of Ti–O bond,46 respectively. While MXene-O shows a much wider peak around 550 cm−1 and the peak at 1640 cm−1 is stronger than the counterpart of other samples. These changes have resulted from the surface oxidation, which introduces more Ti–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The MXene-N film shows two extra peaks locating at 2850 cm−1 and 2921 cm−1, which correspond to the symmetric vibration and the asymmetric oscillation of –CH2–.47 The new peaks do not belong to the intrinsic composition of MXene, suggesting the organic contamination happened during natural storage in the lab for MXene-N. The contamination is quite universal for 2D materials,8 since they have large surface areas and tend to adsorb other molecules onto their surfaces. The organic contamination would disrupt the intrinsic wetting behaviors of 2D materials. It has been reported that isolated MoS2 demonstrates hydrophilic behavior with a WCA of 69.0 ± 3.8°. However, its behavior turns to hydrophobic showing a WCA of 89.0 ± 3.8° under ambient conditions due to organic contamination.48 The organic contamination results in the drying procedure- and time-dependent WCAs of MXene-N, which could be one of the reasons for the scattering values for the reported WCAs of the MXene materials. It should take care when the preparation of samples for WCA testing. Since the unintentional contamination would keep the intrinsic wettability of novel 2D materials from the unambiguous measurement. We showed that this problem could be solved via storing the 2D materials under vacuum before the test. The measured WCAs of the MXene-V over long term storage have shown relatively stable values without visible changing-trends (see Fig. 2g).
O. The MXene-N film shows two extra peaks locating at 2850 cm−1 and 2921 cm−1, which correspond to the symmetric vibration and the asymmetric oscillation of –CH2–.47 The new peaks do not belong to the intrinsic composition of MXene, suggesting the organic contamination happened during natural storage in the lab for MXene-N. The contamination is quite universal for 2D materials,8 since they have large surface areas and tend to adsorb other molecules onto their surfaces. The organic contamination would disrupt the intrinsic wetting behaviors of 2D materials. It has been reported that isolated MoS2 demonstrates hydrophilic behavior with a WCA of 69.0 ± 3.8°. However, its behavior turns to hydrophobic showing a WCA of 89.0 ± 3.8° under ambient conditions due to organic contamination.48 The organic contamination results in the drying procedure- and time-dependent WCAs of MXene-N, which could be one of the reasons for the scattering values for the reported WCAs of the MXene materials. It should take care when the preparation of samples for WCA testing. Since the unintentional contamination would keep the intrinsic wettability of novel 2D materials from the unambiguous measurement. We showed that this problem could be solved via storing the 2D materials under vacuum before the test. The measured WCAs of the MXene-V over long term storage have shown relatively stable values without visible changing-trends (see Fig. 2g).
LW–AB model was used to calculate the SE of MXene-N, MXene-O, and MXene-V using the obtained CA data. This model allows us to dissociate the total SE into nonpolar and acid–base components, giving further insight into the surface properties of the measured MXene films. The nonpolar component comes from the dispersive forces or van der Waals forces,8 while the acid–base component has resulted from electrostatic interactions and structural forces, such as hydrogen bonding.8 These components control the adhesive forces between 2D materials and the contacted solvents. The cohesive force maintains the solvent in the form of drops with minimal surface areas. The balance between adhesive and cohesive forces determine the wetting behavior of the solvent on the 2D material surface. It is worth to note that the calculated SE depends on the used models. The validity of the proposed models is a long-going debate for decades.5 LW–AB model is one of the most commonly used models to calculate the SE of 2D materials, such as graphene.
Fig. 5 shows the total SE and its nonpolar (dispersive) and acid–base components for MXene-N, MXene-O, and MXene-V. It can be found that MXene-O has the largest total SE of 61.56 ± 0.97 mJ m−2, while MXene-V has the smallest one (54.37 ± 1.12 mJ m−2) at the first 12 h. The SE values of MXene based films are comparable to those of graphene (with a SE range of 65–120 mJ m−2) and other 2D materials, such as BN, MoS2, WS2, and MoSe2 with a SE range of 65–75 mg m−2.49 The acid–base component contributes to the increased SE of MXene-O, as the dispersive components of the three samples share a similar value of 47 mJ m−2 and keep almost consistent over the storage period of 168 h. MXene-V shows the most stable total SE and acid–base component, because it was kept under vacuum, having little chance to contact with contaminants and oxidants. While MXene-N and MXene-O show scattering SE values, which could be the results of heterogeneous contamination and oxidation. The FTIR, XPS and Raman spectra confirm the speculation. The acid–base component of MXene-O increased by 96.94%, compared with the counterpart of MXene-V stored for 1 h. As the storage time prolongs, SE of MXene-O decreases to 50.65 ± 1.85 mJ m−2, approaching that of TiO2.50,51 The decreased total SE of MXene-O is caused by the minished acid–base component. The oxidation and covering with oxygen-containing groups could passivate the surface and weak the non-dispersive interaction. The unintentional modification suggests it is possible to tune the SE via delicate surface engineering of MXene. All the results indicate that great care should be taken to obtain a faithful and repeatable WCAs and SE.
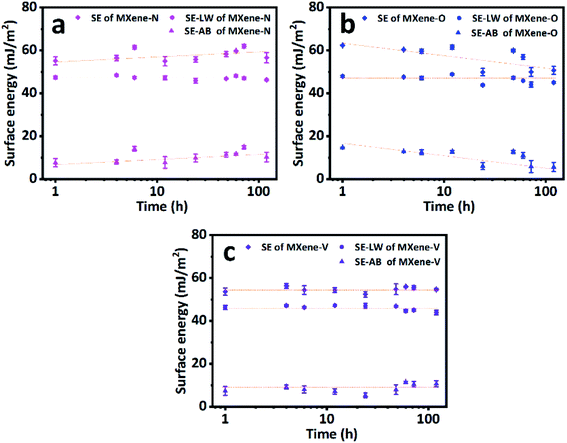 | ||
| Fig. 5 The surface energies (SE) and their nonpolar (SE–LW) and acid–base (SE–AB) components of (a) MXene-N, (b) MXene-O and (c) MXene-V films. Dash lines are a visual guide. | ||
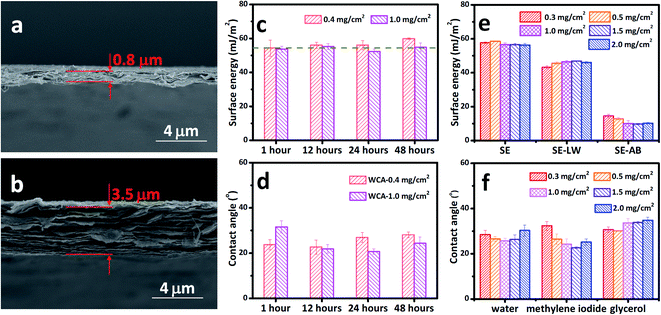
The MXene films with loadings of 0.4 mg cm−2 and 1.0 mg cm−2 were taken as the representative samples since they have typical thicknesses ranging from 0.8 μm to 3.5 μm as shown in Fig. 6a and b. The thickness range is quite common for various applications, such as treatment, electrodes for energy storage, composites, electromagnetic shielding, etc.29,52,53 It can be seen that the total SE of MXene films with 0.4 mg cm−2 is as stable as the one with a loading of 1.0 mg cm−2 (Fig. 6c). Both films have a SE of about 55.27 ± 2.20 mJ m−2, with the thinner film having a slightly larger SE, which results from its acid–base component. The SE keeps almost constant over 48 h storage under vacuum no matter the loading or thickness of the films. The measured WCAs (Fig. 6d) also indicates that the problem of scattering CAs can be eliminated to a large extent. The MCA and GCA also show thickness- and storing time-independent performance, as shown in Fig. S4.† The WCA, MCA, and GCA were also measured for a large loading range from 0.3 mg cm−2 to 2.0 mg cm−2, corresponding to a larger thickness range (Fig. 6f). The measured CAs also confirm the thickness-independent wetting behavior of MXene films stored under vacuum. The calculated total SEs of the MXene films with loadings of 0.3–2.0 mg cm−2 are shown in Fig. 6e, with their dispersive and acid–base components. The total SE is around 57.13 ± 0.93 mJ m−2 for all the films, showing thickness-independent performance.
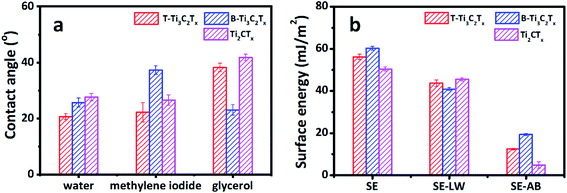
Sonication is the most common way to produce MXene flakes, although MXene can be delaminated without sonication.16 The sonication process can exfoliate and cut 2D materials,54 inevitably introducing defects into the as-produced 2D materials. Therefore, the detailed sonication process could have critical impacts on the surface chemistry of the as-prepared 2D materials and their wetting behavior, being one of the underlying causes for the scattering CAs of MXene based materials. We used both tip and bath sonication to produce Ti3C2Tx MXene flakes and assembled them into free-standing films (labeled as T-T3C2Tx and B-Ti3C2Tx, respectively) with the same loading of 1.0 mg cm−2. As shown in Fig. 7a, B-Ti3C2Tx has a larger WCA and MCA compared with T-Ti3C2Tx. While the GCA for B-Ti3C2Tx is smaller than that of T-Ti3C2Tx. The calculated total SE and the acid–base component of B-Ti3C2Tx are larger than those of T-Ti3C2Tx, while B-Ti3C2Tx has a smaller dispersive component than that of T-Ti3C2Tx (Fig. 7b). The different CAs and SEs indicate that the sonication types have direct impacts on the wetting behavior and the interaction between MXene surface and the tested solvents. The XRD and Raman spectra show no visible difference between B-Ti3C2Tx and T-Ti3C2Tx, as shown in Fig. S5.† The underlying reason could be due to the various working pattern of sonication. We tried to keep the sonication power and time consistent, but the power can only be turned in a stepsize mode rather than the continuous one. The bath sonication has a larger power (270 W) compared with that of tip-sonication (260 W). Additionally, tip-sonication worked on a plus mode. The power distribution and local overheating could contribute to the heterogeneity of MXene flakes, causing the difference in the CA and SE. It should be noted that quantifying the heterogeneity of 2D material surface is very challenge,55 so does the specific contributions of the acid–base components. More detailed and systematic work is needed.
As mentioned above, the MXene family has a large number of members with tunable composition.9,11 We etched and delaminated Ti2AlC based MXene (labeled as Ti2CTx) and measured its CAs. The composition and crystal structure of Ti2CTx were characterized via X-ray diffraction and Raman spectroscopy (see Fig. S6†). The SE was calculated using the measured CAs. Since Ti2CTx is peculiarly susceptible to oxidative damage in water (see Fig. S7†), it was delaminated by hand-shaking without sonication. To our best knowledge, it is the first time to report the CA and SE of Ti2CTx based MXene films (see Fig. 7). The WCA of Ti2CTx film is 27.68 ± 1.33°, with an MCA of 26.60 ± 1.82° and a GCA of 41.80 ± 1.17°. The calculated SE is 50.40 ± 0.96 mJ m−2, 90.15% of it contributed by the dispersive component. The acid–base component accounts for only 9.85% of the SE. Similarly, the CAs and SE of Ti2CTx film with the loading of 0.4 mg cm−2 are accordant with those of 1.0 mg cm−2, showing the uniform thickness-independent performance (see Fig. 7 and S8†). The CA and SE of Ti2CTx are different from those of Ti3C2Tx. Specifically, there is a clear difference between the B-Ti3C2Tx and Ti2CTx on MCA and GCA, yet the difference between the T-Ti3C2Tx and Ti2CTx is slight. The SE of Ti2CTx is 16.31% and 10.27% smaller than the B-Ti3C2Tx and T-Ti3CTx, respectively. Commercial Ti4AlC3 is unavailable at present, so we cannot measure CA and SE of Ti4C3Tx MXene. However, MXene, with a growing material pool, provides a great opportunity to systematically study the wetting behavior and SE of this promising material. The standard procedure is highly needed to measure and report the faithful and reproducible CA and SE.
4. Conclusions
Using water, glycerol, and diiodomethane, we have measured the CAs of MXene films and calculated their SE and the corresponding dispersion and acid–base components. The storage condition- and time-dependent variation of CAs and SEs of MXene films are found to be caused by hydrocarbon contamination and surface oxidation. The contamination and oxidation could be the reasons for the scattering CAs reported in the literature. The problems of contamination and oxidation can be eliminated by storing MXene films under vacuum, producing reproducible and faithful CAs and SEs. Moreover, we have studied how the CAs and SEs of MXene films are affected by different mass loading (corresponding thickness), delamination methods, and MXene composition. Our measurements and findings give a new insight into the wetting behavior of MXenes, these exciting materials with growing family members.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. 51606027, 51436003, 51890911), the Fundamental Research Funds for the Central Universities of China (DUT17RC(4)23), the National Key Research and Development Program of China (2017YFC0307300, 2016YFC0304001), the project funded by China Postdoctoral Science Foundation (2018T110219).References
- N. Yousefi, X. Lu, M. Elimelech and N. Tufenkji, Nat. Nanotechnol., 2019, 14, 107–119 CrossRef CAS.
- B. Wang, T. Ruan, Y. Chen, F. Jin, L. Peng, Y. Zhou, D. Wang and S. Dou, Energy Storage Materials, 2020, 24, 22–51 CrossRef.
- J. W. Drelich, L. Boinovich, E. Chibowski, C. Della Volpe, L. Holysz, A. Marmur and S. Siboni, Surf. Innovations, 2020, 8, 3–27 CrossRef.
- Y. J. Shin, Y. Y. Wang, H. Huang, G. Kalon, A. T. S. Wee, Z. X. Shen, C. S. Bhatia and H. Yang, Langmuir, 2010, 26, 3798–3802 CrossRef CAS.
- A. Kozbial, Z. T. Li, C. Conaway, R. McGinley, S. Dhingra, V. Vahdat, F. Zhou, B. D'Urso, H. T. Liu and L. Li, Langmuir, 2014, 30, 8598–8606 CrossRef CAS.
- K. Huang, P. Rowe, C. Chi, V. Sreepal, T. Bohn, K. G. Zhou, Y. Su, E. Prestat, P. B. Pillai, C. T. Cherian, A. Michaelides and R. R. Nair, Nat. Commun., 2020, 11, 10 CrossRef.
- L. A. Belyaeva and G. F. Schneider, Surf. Sci. Rep., 2020, 75, 100482 CrossRef CAS.
- P. Snapp, J. M. Kim, C. Cho, J. Leem, M. F. Haque and S. Nam, NPG Asia Mater., 2020, 12, 22 CrossRef.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS.
- Y. Gogotsi and B. Anasori, ACS Nano, 2019, 13, 8491–8494 CrossRef CAS.
- J. Pang, R. G. Mendes, A. Bachmatiuk, L. Zhao, H. Q. Ta, T. Gemming, H. Liu, Z. Liu and M. H. Rummeli, Chem. Soc. Rev., 2019, 48, 72–133 RSC.
- K. Rasool, R. P. Pandey, P. A. Rasheed, S. Buczek, Y. Gogotsi and K. A. Mahmoud, Mater. Today, 2019, 30, 80–102 CrossRef CAS.
- J. Shen, G. Liu, Y. Ji, Q. Liu, L. Cheng, K. Guan, M. Zhang, G. Liu, J. Xiong, J. Yang and W. Jin, Adv. Funct. Mater., 2018, 28, 1801511 CrossRef.
- N.-N. Wang, H. Wang, Y.-Y. Wang, Y.-H. Wei, J.-Y. Si, A. C. Y. Yuen, J.-S. Xie, B. Yu, S.-E. Zhu, H.-D. Lu, W. Yang, Q. N. Chan and G.-H. Yeoh, ACS Appl. Mater. Interfaces, 2019, 11, 40512–40523 CrossRef CAS.
- M. Alhabeb, K. Maleski, B. Anasori, P. Lelyukh, L. Clark, S. Sin and Y. Gogotsi, Chem. Mater., 2017, 29, 7633–7644 CrossRef CAS.
- L. Lorencova, T. Bertok, E. Dosekova, A. Holazova, D. Paprckova, A. Vikartovska, V. Sasinkova, J. Filip, P. Kasak, M. Jerigova, D. Velic, K. A. Mahmoud and J. Tkac, Electrochim. Acta, 2017, 235, 471–479 CrossRef CAS.
- N. Taloub, A. Henniche, L. Liu, J. Li, N. Rahoui, M. Hegazy and Y. D. Huang, Composites, Part B, 2019, 163, 260–271 CrossRef CAS.
- C. J. Vanoss, Colloids Surf., A, 1993, 78, 1–49 CrossRef CAS.
- C. J. Vanoss, L. Ju, M. K. Chaudhury and R. J. Good, J. Colloid Interface Sci., 1989, 128, 313–319 CrossRef CAS.
- H. Zhou, Y. Wang, F. Wang, H. Deng, Y. Song, C. Li and Z. Ling, Chin. Chem. Lett., 2020, 31, 1665–1669 CrossRef CAS.
- B. Janczuk, T. Bialopiotrowicz and A. Zdziennicka, J. Colloid Interface Sci., 1999, 211, 96–103 CrossRef.
- L. H. Lee, Langmuir, 1996, 12, 1681–1687 CrossRef CAS.
- C. J. Van Oss, J. Adhes. Sci. Technol., 2002, 16, 669–677 CrossRef CAS.
- W. Yang, J.-J. Liu, L.-L. Wang, W. Wang, A. C. Y. Yuen, S. Peng, B. Yu, H.-D. Lu, G. H. Yeoh and C.-H. Wang, Composites, Part B, 2020, 188, 107875 CrossRef CAS.
- J. Liu, Z. Liu, H.-B. Zhang, W. Chen, Z. Zhao, Q.-W. Wang and Z.-Z. Yu, Adv. Electron. Mater., 2020, 6, 1901094 CrossRef CAS.
- J. Liu, H.-B. Zhang, R. Sun, Y. Liu, Z. Liu, A. Zhou and Z.-Z. Yu, Adv. Mater., 2017, 29, 1702367 CrossRef.
- L. Ding, Y. Wei, Y. Wang, H. Chen, J. Caro and H. Wang, Angew. Chem., Int. Ed., 2017, 56, 1825–1829 CrossRef CAS.
- Z. Ling, C. E. Ren, M.-Q. Zhao, J. Yang, J. M. Giammarco, J. Qiu, M. W. Barsoum and Y. Gogotsi, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 16676–16681 CrossRef CAS.
- Z. Fan, Y. Wang, Z. Xie, X. Xu, Y. Yuan, Z. Cheng and Y. Liu, Nanoscale, 2018, 10, 9642–9652 RSC.
- M. Ghidiu, M. R. Lukatskaya, M.-Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, U78–U171 CrossRef.
- X. Jin, S.-J. Shin, N. Kim, B. Kang, H. Piao, J.-H. Choy, H. Kim and S.-J. Hwang, Nano Energy, 2018, 53, 841–848 CrossRef CAS.
- T. Zhang, L. Pan, H. Tang, F. Du, Y. Guo, T. Qiu and J. Yang, J. Alloys Compd., 2017, 695, 818–826 CrossRef CAS.
- F. Du, H. Tang, L. Pan, T. Zhang, H. Lu, J. Xiong, J. Yang and C. Zhang, Electrochim. Acta, 2017, 235, 690–699 CrossRef CAS.
- M.-Q. Zhao, X. Xie, C. E. Ren, T. Makaryan, B. Anasori, G. Wang and Y. Gogotsi, Adv. Mater., 2017, 29, 1702410 CrossRef.
- K. M. Kang, D. W. Kim, C. E. Ren, K. M. Cho, S. J. Kim, J. H. Choi, Y. T. Nam, Y. Gogotsi and H.-T. Jung, ACS Appl. Mater. Interfaces, 2017, 9, 44687–44694 CrossRef CAS.
- J. Luo, C. Fang, C. Jin, H. Yuan, O. Sheng, R. Fang, W. Zhang, H. Huang, Y. Gan, Y. Xia, C. Liang, J. Zhang, W. Li and X. Tao, J. Mater. Chem. A, 2018, 6, 7794–7806 RSC.
- S. Chen, Y. Xiang, C. Peng, J. Jiang, W. Xu and R. Wu, J. Power Sources, 2019, 414, 192–200 CrossRef CAS.
- S. Wei, Y. Xie, Y. Xing, L. Wang, H. Ye, X. Xiong, S. Wang and K. Han, J. Membr. Sci., 2019, 582, 414–422 CrossRef CAS.
- R. Bian, S. Xiang and D. Cai, ChemNanoMat, 2020, 6, 64–67 CrossRef CAS.
- G. Z. Liu, J. Shen, Q. Liu, G. P. Liu, J. Xiong, J. Yang and W. Q. Jin, J. Membr. Sci., 2018, 548, 548–558 CrossRef CAS.
- B. Ahmed, D. H. Anjum, M. N. Hedhili, Y. Gogotsi and H. N. Alshareef, Nanoscale, 2016, 8, 7580–7587 RSC.
- L. Wang, L. X. Chen, P. Song, C. B. Liang, Y. J. Lu, H. Qiu, Y. L. Zhang, J. Kong and J. W. Gu, Composites, Part B, 2019, 171, 111–118 CrossRef CAS.
- G. Z. Liu, S. Liu, K. Ma, H. Y. Wang, X. Y. Wang, G. P. Liu and W. Q. Jin, Ind. Eng. Chem. Res., 2020, 59, 4732–4741 CrossRef CAS.
- Q. Xue, Z. Pei, Y. Huang, M. Zhu, Z. Tang, H. Li, Y. Huang, N. Li, H. Zhang and C. Zhi, J. Mater. Chem. A, 2017, 5, 20818–20823 RSC.
- W. Q. Zhi, S. L. Xiang, R. J. Bian, R. Z. Lin, K. H. Wu, T. W. Wang and D. Y. Cai, Compos. Sci. Technol., 2018, 168, 404–411 CrossRef CAS.
- Z. T. Li, Y. J. Wang, A. Kozbial, G. Shenoy, F. Zhou, R. McGinley, P. Ireland, B. Morganstein, A. Kunkel, S. P. Surwade, L. Li and H. T. Liu, Nat. Mater., 2013, 12, 925–931 CrossRef CAS.
- A. Kozbial, X. Gong, H. Liu and L. Li, Langmuir, 2015, 31, 8429–8435 CrossRef CAS.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS.
- B. Feng, J. Y. Chen, S. K. Qi, L. He, J. Z. Zhao and X. D. Zhang, J. Mater. Sci.: Mater. Med., 2002, 13, 457–464 CrossRef CAS.
- E. Matykina, I. Garcia, J. J. de Damborenea and M. A. Arenas, Int. J. Adhes. Adhes., 2011, 31, 832–839 CrossRef CAS.
- C. E. Ren, K. B. Hatzell, M. Alhabeb, Z. Ling, K. A. Mahmoud and Y. Gogotsi, J. Phys. Chem. Lett., 2015, 6, 4026–4031 CrossRef CAS.
- F. Shahzad, M. Alhabeb, C. B. Hatter, B. Anasori, S. M. Hong, C. M. Koo and Y. Gogotsi, Science, 2016, 353, 1137–1140 CrossRef CAS.
- C. Backes, T. M. Higgins, A. Kelly, C. Boland, A. Harvey, D. Hanlon and J. N. Coleman, Chem. Mater., 2017, 29, 243–255 CrossRef CAS.
- G. Cunningham, M. Lotya, C. S. Cucinotta, S. Sanvito, S. D. Bergin, R. Menzel, M. S. P. Shaffer and J. N. Coleman, ACS Nano, 2012, 6, 3468–3480 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra09125a |
| This journal is © The Royal Society of Chemistry 2021 |