Open Access Article
Open Access ArticleObtaining an immunoaffinity monolithic material: poly(GMA-co-EDMA) functionalized with an HPV-derived peptide using a thiol–maleimide reaction†
Diego Sebastián Insuasty-Cepeda a,
Mauricio Maldonadoa,
Javier Eduardo García-Castañeda
a,
Mauricio Maldonadoa,
Javier Eduardo García-Castañeda b and
Zuly Jenny Rivera-Monroy
b and
Zuly Jenny Rivera-Monroy *a
*a
aDepartamento de Química, Universidad Nacional de Colombia, Carrera 45 No 26-85, Building 451, Office 409, Bogotá, Bogotá 11321, Colombia. E-mail: zjriveram@unal.edu.co
bDepartamento de Farmacia, Universidad Nacional de Colombia, Carrera 45 No 26-85, Building 471, Bogotá, Bogotá 11321, Colombia
First published on 21st January 2021
Abstract
Metabolites have great potential for the design of biomarkers, since their presence or absence provides valuable information about a biological system. In this context, polyclonal antibodies are important metabolites for diagnostic procedures, but in some pathologies, it has been found that these metabolites are present at low concentrations, so it could be difficult to detect them. In this investigation, an organic monolithic material of poly(GMA-co-EDMA) was functionalized with a peptide via Michael addition (thiol–maleimide) click chemistry. The peptide, covalently bound to the monolith, contains the SPINNTKPHEAR sequence derived from the human papilloma virus L1 protein. It was determined that the obtained monolithic support allows selectively isolating polyclonal antibodies against the SPINNTKPHEAR sequence, since they are retained on the chemical surface of the material by an immunoaffinity interaction. The monolithic material functionalization protocol reported here could be applied to incorporate any peptide with a terminal cysteine in order to recover a specific analyte. A new method was developed for isolating and pre-concentrating antibodies using monolithic materials, which could contribute to the improvement of disease detection strategies based on immunoaffinity interactions.
Introduction
Metabolites can have several different applications, such as antitumor, anticancer, antibacterial, antifungal, and antioxidant agents.1–5 Another important application of metabolites is their use as biomarkers,6 making them candidates for the development of methods for the diagnosis of diseases. Some metabolites are found in very low concentrations within body fluids, which makes it difficult to detect and obtain them. For this reason, investigations have been undertaken regarding the development of methods and materials for the pre-concentration of metabolites in order to facilitate their detection.7,8 Among the materials used for the enrichment of metabolites, organic monolithic supports have gained great interest, because due to their macroporosity, it is possible for large molecules to easily flow through their internal structure, while their mesoporosity facilitates the interaction between the analyte and the functionalized support.9 Modified monolithic supports are used to pre-concentrate several large organic molecules with high selectivity, mainly using extraction and microextraction methods.10,11Cervical cancer is a public health problem worldwide that is mainly associated with HPV infection. It is the fourth most common cancer in women around the world, with an estimated 528![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 new cases and 266
000 new cases and 266![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths each year. Currently, the most important diagnostic method for cervical cancer is the Papanicolaou (Pap) test, also called vaginal cytology. Thanks to this test, it has been possible to reduce the mortality caused by cervical cancer by up to 50%; however, the test only has a sensitivity between 50% and 60%.12 A significant risk factor is a low socioeconomic and educational level, with women from developing countries being more vulnerable, where cytology coverage is limited due to technological, medical, and cultural aspects.13,14
000 deaths each year. Currently, the most important diagnostic method for cervical cancer is the Papanicolaou (Pap) test, also called vaginal cytology. Thanks to this test, it has been possible to reduce the mortality caused by cervical cancer by up to 50%; however, the test only has a sensitivity between 50% and 60%.12 A significant risk factor is a low socioeconomic and educational level, with women from developing countries being more vulnerable, where cytology coverage is limited due to technological, medical, and cultural aspects.13,14
Within this context, there is a need to design a diagnostic method complementary to cytology, as far as possible with better sensitivity, less invasive, and easier to apply, in order to increase coverage, taking into account that in certain asymptomatic and symptomatic episodes of the disease, the level of antibodies varies, generally being very low. Since the human papilloma virus L1 protein (HPV-L1) is present in 90% of the HPV capsid and is directly involved in the process of the virus' infection of host cells, the aim of the present investigation was to obtain a monolithic material that would allow enriching polyclonal antibodies against a peptide derived from HPV-L1, specifically the SPINNTKPHEAR sequence. This sequence is recognized by means of antibodies from patients infected with HPV who present lesions in the cervix.15
In previous reports16 we showed the gold electrode functionalization with peptide C-Ahx-SPINNTKPHEAR in order to build a biosensor for electrochemistry HPV detection. However, the biosensor application is limited by the antibodies concentration level in asymptomatic infected patients. In this work, we synthetized a functionalized monolith material to pre-concentrate and separate antibodies that could be then detected by electrochemistry biosensor and/or ELISA assays.
Polyclonal antibodies against the peptide SPINNTKPHEAR were obtained by immunization of New Zealand rabbits.16 For obtaining the affinity material, the monolithic copolymer poly(GMA-co-EDMA) was synthesized, and then its surface was modified, first with 6-maleimidohexanoic acid and then with a peptide that contained (i) a cysteine residue, (ii) a spacer, and (iii) the SPINNTKPHEAR sequence. Through the specific addition reaction between thiol and the maleimide group, the peptide was incorporated into the copolymer. It was determined that the functionalized monolithic support allows selective isolation of polyclonal antibodies against the SPINNTKPHEAR sequence, since they are retained on the chemical surface of the material by an immunoaffinity interaction between the antigen and the polyclonal antibodies. This investigation contributes to the development of techniques for bioseparations, using monolithic supports functionalized with peptides, which allow the isolation of antibodies by immunoaffinity.
Results and discussion
A new methodology for functionalizing a monolithic material with an HPV-derived peptide is reported. In the first research stage, a twelve-residue sequence, SPINNTKPHEAR, was selected, which was recognized by antibodies from patients infected with HPV who were diagnosed with lesions in the cervix.15 Using the selected sequence, fifteen peptides were designed (Table 1), as is described as follows: (i) peptide 1: CGSPINNTKPHEARGC contains two cysteine residues that allow obtaining the polymeric peptide (CGSPINNTKPHEARGC)n by oxidation, which was used to immunize New Zealand rabbits in order to obtain polyclonal antibodies against the SPINNTKPHEAR sequence. (ii) Peptides 2 and 10 contain the selected sequence, a spacer (Ahx), and a cysteine residue at the N-terminal or C-terminal end, respectively. (iii) Truncated peptides were designed in order to establish whether there is a short peptide that has the highest degree of recognition by polyclonal anti-SPINNTKPHEAR antibodies. For generating truncated peptides, sequentially each amino acid of the C-terminal (peptides 3 to 9) or N-terminal (peptides 11 to 15) end of SPINNTKPHEAR sequence was removed.| Code | Sequence | tR (min) | [m/z] | ELISAa |
|---|---|---|---|---|
a Peptide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) antibody (v/v). NR: no recognition. antibody (v/v). NR: no recognition. |
||||
| 1 | CG-1SPINNTKPHEAR12-GC | 3.2 | 1682.7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| 2 | C-Ahx-1SPINNTKPHEAR12 | 3.2 | 1580.1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| 3 | C-Ahx-1SPINNTKPHEA11 | 3.5 | 1409.7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
| 4 | C-Ahx-1SPINNTKPHE10 | 3.4 | 1339.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6400 6400 |
| 5 | C-Ahx-1SPINNTKPH9 | 3.6 | 1211.7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
| 6 | C-Ahx-1SPINNTKP8 | 3.5 | 1097.8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
| 7 | C-Ahx-1SPINNTK7 | 2.8 | 877.1 | NR |
| 8 | C-Ahx-1SPINNT6 | 2.8 | 750.7 | NR |
| 9 | C-Ahx-1SPINN5 | 3.3 | 752.3 | NR |
| 10 | 1SPINNTKPHEAR12-Ahx-C | 3.2 | 1580.1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| 11 | 2PINNTKPHEAR12-Ahx-C | 3.4 | N.D. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| 12 | 3INNTKPHEAR12-Ahx-C | 3.9 | N.D. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
| 13 | 4NNTKPHEAR12-Ahx-C | 3.7 | N.D. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6400 6400 |
| 14 | 5NTKPHEAR12-Ahx-C | 3.4 | N.D. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
| 15 | 6TKPHEAR12-Ahx-C | 3.4 | N.D. | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
Currently, there are several techniques to chemically attach peptides or proteins to organic monolithic supports,33 some of the most frequently used are shown in the Fig. S1 (ESI†). These techniques involve mainly anchoring via amino groups (highly nucleophilic) that attack the tight 2,3-epoxypropyl ring present in GMA motif.17 In these methodologies, peptide or protein join to material could be no specific because these molecules can have various amino groups. It is relevant attaching the peptide to the material through a specific bond for guaranteeing that peptide antigenic motifs are free, and thus, they allow antigenic recognizing by the polyclonal antibodies.
All of the designed peptides contained the spacer (Ahx) and a cysteine residue at the N- or C-terminal end, which allowed introducing a thiol group for functionalizing the monolithic material via thiol–maleimide click reaction. This design allows a specific grafting, in which the peptide is going to be bound only by its terminal cysteine, which allows controlling the conformational arrangement of the peptide, which is of utmost importance in immunoaffinity interactions.
The designed peptides were synthesized using SPPS with the Fmoc/tBu strategy and were purified via RP-SPE and characterized via RP-HPLC and MALDI TOF MS. The recognition of the peptides by the New Zealand rabbit's serum was tested using ELISA assays. Table 1 summarizes the analytical characterization of the peptides and the ELISA result.
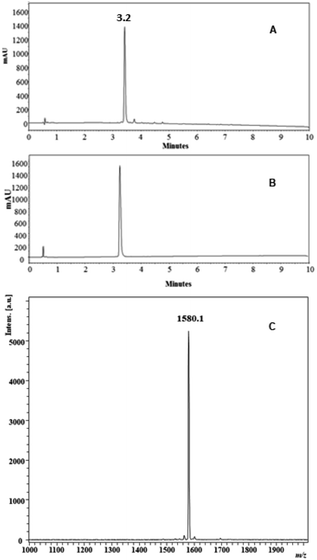
The process of synthesis of all the peptides proceeded without difficulty, and all the residues were incorporated into the resin or resin-peptide easily and quickly. In the synthesis of peptides 1 and 2, the incorporation of Arg, Lys and Ile residues required only two coupling reaction cycles for their complete incorporation into the growing chain during SPPS, while the other amino acids only needed one coupling reaction cycle. The crude peptides were obtained with nearly 80% purity and were purified via RP-SPE chromatography, obtaining the peptides with chromatographic purity greater than 95% for most of them (Table 1). As an example, the analytical characterization of peptide 2 is shown in Fig. 1. The chromatographic profile of the crude and the purified product shows a principal specie at a retention time (tR) of 3.2 min (Fig. 1A and B), and the MALDI-TOF mass spectrum has a signal at m/z = 1580.1, corresponding to the specie [M + H]+ of the desired product (Fig. 1C).
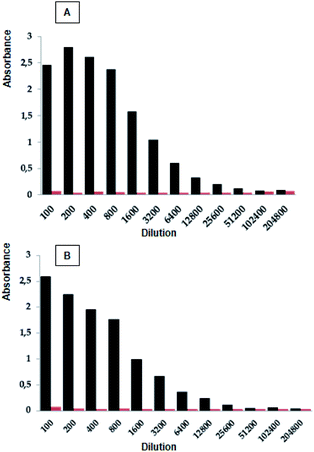
New Zealand rabbits were immunized with the polymeric peptide (CGSPINNTKPHEARGC)n, in accordance with the immunization scheme described in the Material and methods section above. The rabbits were bled, and the sera containing polyclonal antibodies were separated by centrifugation and stored at −20 °C. The ELISA assays were performed in order to establish if the polyclonal antibodies recognized the designed peptides (peptides 2 to 15). The results showed that the peptides 2, 10, and 11 exhibited the highest antibody title, i.e. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 (peptide
600 (peptide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) serum post-IV dilution v/v), being equal to that presented by the polymeric peptide used as a control (Fig. 2 and Table 1). These results suggest that the incorporation of the cysteine residue in the N- or C-terminal of the selected sequence, as well as the inclusion of the Ahx residue, does not interfere with the antibody recognition. On the other hand, when an amino acid from the N- or C-terminal was sequentially removed, the antibody recognition diminished, suggesting that all of the amino acids of the SPINNTKPHEAR sequence are important for antigen–antibody recognition. Only when 1Ser was removed (peptide 11) was the antibody title similar to that showed by peptides containing the complete SPINNTKPHEAR sequence. Our results suggest that the peptide epitope is linear instead of conformational. The antibody title indicates that the post-IV serum contains a high concentration of the polyclonal antibodies, which specifically recognize the SPINNTKPHEAR compared with the pre-immune serum. From this research stage, we selected peptide 2, C-Ahx-SPINNTKPHEAR, since: (i) it presented a high recognition title against the polyclonal anti-SPINNTKPHEAR antibodies present in the post-IV serum (1
serum post-IV dilution v/v), being equal to that presented by the polymeric peptide used as a control (Fig. 2 and Table 1). These results suggest that the incorporation of the cysteine residue in the N- or C-terminal of the selected sequence, as well as the inclusion of the Ahx residue, does not interfere with the antibody recognition. On the other hand, when an amino acid from the N- or C-terminal was sequentially removed, the antibody recognition diminished, suggesting that all of the amino acids of the SPINNTKPHEAR sequence are important for antigen–antibody recognition. Only when 1Ser was removed (peptide 11) was the antibody title similar to that showed by peptides containing the complete SPINNTKPHEAR sequence. Our results suggest that the peptide epitope is linear instead of conformational. The antibody title indicates that the post-IV serum contains a high concentration of the polyclonal antibodies, which specifically recognize the SPINNTKPHEAR compared with the pre-immune serum. From this research stage, we selected peptide 2, C-Ahx-SPINNTKPHEAR, since: (i) it presented a high recognition title against the polyclonal anti-SPINNTKPHEAR antibodies present in the post-IV serum (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 v/v), (ii) it showed good synthetic yield, and (iii) it contains the cysteine residue.
600 v/v), (ii) it showed good synthetic yield, and (iii) it contains the cysteine residue.
In this investigation, a monolithic material functionalized with an HPV-L1-derived peptide was synthesized that can be used for the concentration of antibodies through immunoaffinity. Fig. 3 shows the strategy used for obtaining the monolithic material functionalized with a peptide derived from L1-HPV: step 1, the monolithic material was synthesized by reaction between glycidyl methacrylate as the main monomer and ethylene dimethacrylate (EDMA) as the crosslinking monomer, obtaining a monolithic material of poly(GMA-co-EDMA), containing highly reactive 2,3-epoxypropyl groups exposed on its surface. Step 2, the material's surface was functionalized with the maleimide group; for this, the epoxide ring opening reaction was used, and poly(GMA-co-EDMA) was treated with 6-maleimidohexanoic acid in the presence of tetrabutylammonium chloride in DMF. Step 3, finally, in order to obtain the monolithic material functionalized with peptide 2, the copolymer containing the maleimide motif was treated with the peptide whose sequence included a cysteine residue at the N-terminal end in order to allow the selective reaction between the double bond of the maleimide group and the thiol group by Michael's addition reaction.18
The poly(GMA-co-EDMA) monolith material was obtained following the protocol reported by Okanda et al.,17 as described in the method (Fig. 3, step 1). The synthesized monolith was characterized via scanning electron microscopy (SEM), showing that the material exhibits a granular morphology, probably due to the nucleation and nuclei precipitation during the polymerization process. Also, the SEM microscopy showed that the material had mesopores (<50 nm) and macropores (>50 nm), Fig. 4A. Additionally, the material obtained has good mechanical stability, homogeneity, and ease of handling, and has an appearance similar to that obtained by other authors.19
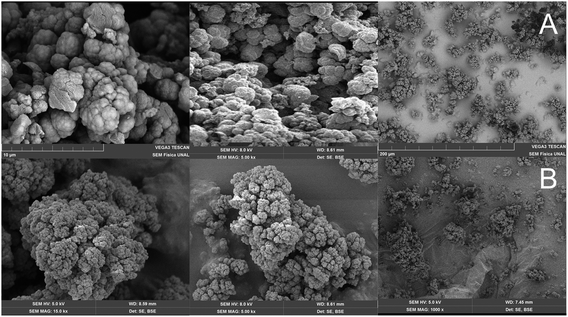 | ||
| Fig. 4 SEM images of monolithic materials: non-functionalized poly(GMA-co-EDMA) (A) and poly(GMA-co-EDMA)-Mhx (B). | ||
The granular morphology denotes a bimodal structure formed by macropores and mesopores. This can be an advantage when considering the use of this material to obtain immunoaffinity columns, since the analytes are usually large macromolecules that have difficulty in flowing through columns packed with small pore size, requiring extremely high pressures to achieve flow. The macropores of this material allow the efficient flow of large analytes, which facilitates its interaction with the active sites of the material; additionally, the mesoporosity allows a good interaction between the analyte and the functionalized material, since it has a large surface area.
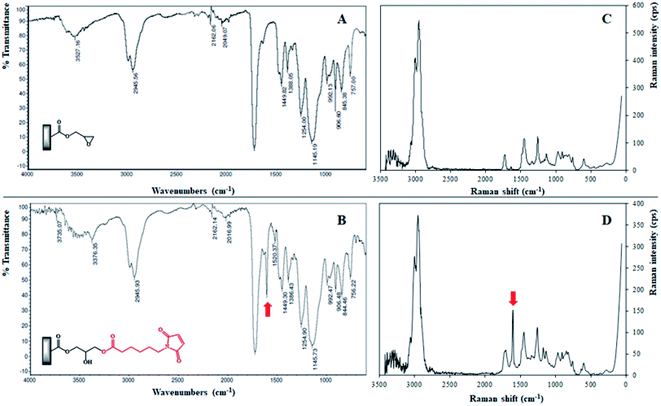
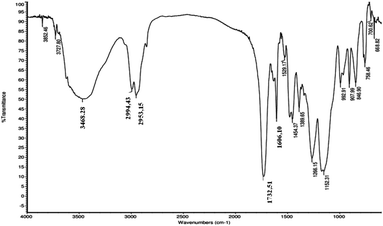
The step 2, corresponding to the functionalization of the poly(GMA-co-EDMA) monolithic support (Fig. 3), was performed through an epoxide ring opening reaction between the 2,3-epoxypropyl group and the 6-maleimidohexanoic acid (Mhx), generating the poly(GMA-co-EDMA)-Mhx material. The IR spectrum of the poly(GMA-co-EDMA)-Mhx showed signals similar to those found for poly(GMA-co-EDMA), Fig. 5: carbonyl group (1729.57 cm−1), tense C–O stretching (908.39 cm−1) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching (2958.85 cm−1). However, in the IR spectrum of poly(GMA-co-EDMA)-Mhx, a signal at 1604.48 cm−1 was observed (Fig. 5B, red arrow), which corresponds to C
C stretching (2958.85 cm−1). However, in the IR spectrum of poly(GMA-co-EDMA)-Mhx, a signal at 1604.48 cm−1 was observed (Fig. 5B, red arrow), which corresponds to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the imide group, which was reported as characteristic of the Mhx group,20 suggesting the presence of Mhx moiety in the monolithic material. The poly(GMA-co-EDMA) and poly(GMA-co-EDMA)-Mhx monolithic materials were also analyzed using Raman spectroscopy (Fig. 5C and D). In the poly(GMA-co-EDMA)-Mhx spectrum, a signal at 1606.42 cm−1 was observed, which corresponds to the C
O stretching of the imide group, which was reported as characteristic of the Mhx group,20 suggesting the presence of Mhx moiety in the monolithic material. The poly(GMA-co-EDMA) and poly(GMA-co-EDMA)-Mhx monolithic materials were also analyzed using Raman spectroscopy (Fig. 5C and D). In the poly(GMA-co-EDMA)-Mhx spectrum, a signal at 1606.42 cm−1 was observed, which corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the imide group, which is in accordance with the infrared spectrum for this material. The IR and Raman analyses confirm the presence of Mhx on the material's surface, indicating that the poly(GMA-co-EDMA)-Mhx material was successfully obtained.
O bond of the imide group, which is in accordance with the infrared spectrum for this material. The IR and Raman analyses confirm the presence of Mhx on the material's surface, indicating that the poly(GMA-co-EDMA)-Mhx material was successfully obtained.
The composition of the poly(GMA-co-EDMA) and poly(GMA-co-EDMA)-Mhx monolithic materials was determined using elemental analysis; the materials had similar carbon and hydrogen content (% w/w), Table 2. It was observed that only the poly(GMA-co-EDMA)-Mhx monolithic support contains nitrogen, due to the presence of the maleimide group. The nitrogen content percentage was 0.91% w/w, which indicates that 12.5% w/w of the poly(GMA-co-EDMA) surface was functionalized with the Mhx group. Assuming that the poly(GMA-co-EDMA)-Mhx functionalization process was 100%, the total nitrogen content should be 20%, so it can be concluded that 63% of the glycidyl groups were functionalized with the Mhx molecule. This behavior could be related to the glycidyl groups exposed over the material's surface. According to previous reports, this functionalization percentage is a favorable result.21
| % w/w | ||
|---|---|---|
| Poly(GMA-co-EDMA) | Poly(GMA-co-EDMA)-Mhx | |
| Nitrogen | 0.00 | 0.90 |
| Carbon | 61.19 | 58.79 |
| Hydrogen | 7.59 | 6.90 |
| 6-MhxA | 0.00 | 12.54 |
The poly(GMA-co-EDMA)-Mhx monolithic support was analyzed via SEM (Fig. 4B). As can be seen, the material retains the same morphological properties as poly(GMA-co-EDMA): a granular structure with the presence of nuclei, mesopores, and macropores. In summary, the infrared, Raman and elemental analyses indicate that 63% of the monolithic surface of poly(GMA-co-EDMA) was functionalized with Mhx. Additionally, the characterization via SEM shows that the poly(GMA-co-EDMA)-Mhx support exhibits morphological and structural macropores and mesopores, which facilitates the peptide's functionalization as well the flux of the solution through the material, making it useful in chromatography.
The Michael addition reaction between the thiol group (cysteine) and the maleimido group (click chemistry) is frequently used to label proteins;22 however, this reaction has been little used to functionalize porous polymeric materials.23 Therefore, it was decided to first study the reaction in solution, in order to determine the optimal experimental conditions for the reaction over the monolith's surface. The reaction between peptide 2 and 6-maleimidohexanoic acid (6-MhxA) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 eq.) was performed in methanol at RT and was monitored via RP-HPLC (Fig. 6). After 1 minute of reaction time in the chromatographic profile (Fig. 6A), signals corresponding to peptide 2 (tR = 3.2 min) and 6-MhxA reagent (tR = 4.3 min) were observed. After 24 hours of reaction, no peptide was observed, and therefore the Michael reaction was complete (Fig. 6B). Two new signals were observed at 4.0 and 4.1 min, which correspond to addition reaction products. These results indicate that the Michael addition reaction between peptide 2 and the 6-MhxA reagent: (i) is quantitative, (ii) requires 24 h reaction time to be complete, and (iii) two products were formed, possibly attributable to the tautomerism between the amino group and the thiol group of cysteine, which does not interfere with the antigen–antibody interaction, since it does not compromise the peptide epitope.24 It was also performed the reaction between the 6-MhxA and peptide 10, which contains a cysteine residue at C-terminal end. This reaction presented the same behavior that reaction described for peptide 2 (Fig. 6). Specifically, the reaction chromatographic profile showed two products between 4.0–4.1 minutes. This result indicated that Michael addition occurred with cysteine residue at N or C-terminal with no differences.
1 eq.) was performed in methanol at RT and was monitored via RP-HPLC (Fig. 6). After 1 minute of reaction time in the chromatographic profile (Fig. 6A), signals corresponding to peptide 2 (tR = 3.2 min) and 6-MhxA reagent (tR = 4.3 min) were observed. After 24 hours of reaction, no peptide was observed, and therefore the Michael reaction was complete (Fig. 6B). Two new signals were observed at 4.0 and 4.1 min, which correspond to addition reaction products. These results indicate that the Michael addition reaction between peptide 2 and the 6-MhxA reagent: (i) is quantitative, (ii) requires 24 h reaction time to be complete, and (iii) two products were formed, possibly attributable to the tautomerism between the amino group and the thiol group of cysteine, which does not interfere with the antigen–antibody interaction, since it does not compromise the peptide epitope.24 It was also performed the reaction between the 6-MhxA and peptide 10, which contains a cysteine residue at C-terminal end. This reaction presented the same behavior that reaction described for peptide 2 (Fig. 6). Specifically, the reaction chromatographic profile showed two products between 4.0–4.1 minutes. This result indicated that Michael addition occurred with cysteine residue at N or C-terminal with no differences.
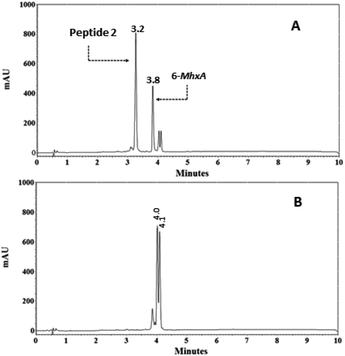 | ||
| Fig. 6 Monitoring via RP-HPLC of the reaction in solution between peptide 2 (C-Ahx-SPINNTKPHEAR) and the 6-maleimidohexanoic acid (6-MhxA). Reaction time: 1 min (A) and 24 hours (B). | ||
The Michael reaction between poly(GMA-co-EDMA)-Mhx and peptide 2 to obtain the functionalized material poly(GMA-co-EDMA)-Mhx-C-Ahx-SPINNTKPHEAR was carried out in methanol at RT for 24 h. Then the material was thoroughly washed and dried with a vacuum gun to constant weight. The monolithic material functionalized with peptide 2 was analyzed via IR spectroscopy (Fig. 7).
The IR spectrum (Fig. 7) showed a broad signal around 3500 cm−1, which can be attributed to NH stretches of the amide group corresponding to a peptide bond and/or OH stretching groups of side chains of threonine and serine residues. Furthermore, the IR spectrum showed a signal at 1606.10 cm−1, which corresponds to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the imide group, which was also observed in the poly(GMA-co-EDMA) IR spectrum. This signal is characteristic of the maleimide group.25 The IR spectra of the functionalized monolith suggest the presence of peptide 2 attached to the material. In order to corroborate this result, the interaction between poly(GMA-co-EDMA)-Mhx-C-Ahx-SPINNTKPHEAR material and polyclonal anti-SPINNTKPHEAR antibodies (1–12) was determined via ELISA assays, since this technique has high sensitivity and specificity.
O stretching of the imide group, which was also observed in the poly(GMA-co-EDMA) IR spectrum. This signal is characteristic of the maleimide group.25 The IR spectra of the functionalized monolith suggest the presence of peptide 2 attached to the material. In order to corroborate this result, the interaction between poly(GMA-co-EDMA)-Mhx-C-Ahx-SPINNTKPHEAR material and polyclonal anti-SPINNTKPHEAR antibodies (1–12) was determined via ELISA assays, since this technique has high sensitivity and specificity.
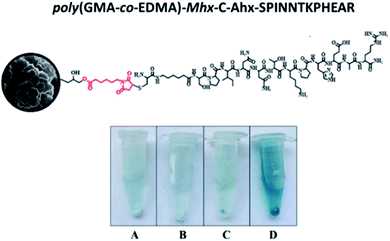
The monolithic material was placed in an Eppendorf tube, and the ELISA assay was carried out using post-IV serum in accordance with the Material and methods section (Fig. 8D). Three controls were used: (i) the empty Eppendorf tube, in order to determine if there are nonspecific interactions of the antibodies with the tube material (polypropylene) (Fig. 8A). (ii) Poly(GMA-co-EDMA) monolith, in order to establish the non-specific interaction of the polyclonal antibodies with the monolithic material without functionalizing (Fig. 8C), and (iii) poly(GMA-co-EDMA)-Mhx-C-Ahx-SPINNTKPHEAR monolith treated with pre-immune serum to determine nonspecific interactions (Fig. 8D). The procedure was similar to that used in the ELISA assays, and the Eppendorf tubes containing the solution were centrifuged in order to eliminate the washing solution and the excess of the primary and secondary antibodies. For poly(GMA-co-EDMA)-Mhx-C-Ahx-SPINNTKPHEAR incubated with the post-IV serum (D), a blue coloration in the solution and the monolithic material was observed, and the absorbance measured was 0.4, while for the controls (A, B, and C) the absorbance values were lower than 0.10. The anti-SPINNTKPHEAR polyclonal antibodies present in the post-IV serum recognized the sequence attached on the surface of the monolithic support, clearly indicating the presence of peptide 2 in the material. The antibodies were not captured by the Eppendorf material nor the non-funtionalizated monolithic support poly(GMA-co-EDMA) monolith, indicating that the antibody interaction was specific to the SPINNTKPHEAR sequence. On the other hand, poly(GMA-co-EDMA)-Mhx-C-Ahx-SPINNTKPHEAR incubated with the preimmune sera showed that it has a pale blue coloration that is very faint compared to the monolith incubated with the post-IV serum. Our results suggest that the polyclonal antibodies can be attached specifically to the monolithic support poly(GMA-co-EDMA)-Mhx-C-Ahx-SPINNTKPHEAR, allowing their separation from other proteins and/or molecules that are contained in the sera.
We envisage that the methodology presented here could be used to functionalize materials with linear, dimeric, tetrameric, cyclic or dendrimeric peptides which have at least one thiol group in their sequence, making this approach versatile and useful to get materials for immunoaffinity columns. Regarding proteins, we consider that this possibility could also be explored; however, the number of cysteines present in the protein could limit the specificity of the material functionalization.
Our results showed that it was possible to obtain a monolithic material functionalized with an HPV derived peptide. Besides, polyclonal antibodies of rabbit antisera recognized this material. We envision that this methodology could be applied to attach derived peptides from immunogenic regions from HPV-L1 and HPV-L2 proteins (16 and/or 18 serotypes) and, later on, to establish if HPV infected patients sera could recognize the functionalized material. In this research, great importance is given to the synthesis and chemical characterization, since we consider that presented design is innovative, it is a grafting type that had not been done before and it, unlike other grafting techniques, allowed attach the peptide in a specific way.33 The peptide attached was chemically join by its end facilitating the interaction antibody–antigen recognizing.
Material and methods
The resin Rink amide and the reagents Fmoc-Ser(tBu)-OH, Fmoc-Pro-OH, Fmoc-Ile-OH, Fmoc-Asn(Trt)-OH, Fmoc-Thr(tBu)-OH, Fmoc-Lys(Boc)-OH, Fmoc-His(Trt)-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Ala-OH, Fmoc-Arg(Pbf)-OH, Fmoc-Cys(Trt)-OH, Fmoc-6-aminohexanoic acid (Fmoc-Ahx-OH), dicyclohexylcarbodiimide, and 1-hydroxy-6-chlorobenzotriazole were purchased from AAPPTec (Louisville, KY, USA). The reagents acetonitrile, trifluoroacetic acid, dichloromethane, diisopropylethylamine, N,N-dimethylformamide, ethanedithiol, isopropanol, methanol, and triisopropylsilane were purchased from Merck (Darmstadt, Germany). SPE columns Supelclean™, 6-maleimidohexanoic acid (6-MhxA), tetrabutylammonium chloride, glycidyl methacrylate (GMA), ethylene dimethacrylate (EDMA), cyclohexanol, dodecanol, 1,1′-azobis(cyclohexanecarbonitrile), silica gel, Tween 20, and 3,3′,5,5′-tetramethylbenzidine (Liquid Substrate System for ELISA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Potassium diacid phosphate, sodium acid phosphate, sodium chloride, and potassium chloride were purchased from AppliChem Panreac (Barcelona, Spain).Peptide synthesis
To obtain the designed molecules, the solid-phase peptide synthesis method was used, specifically via the Fmoc/tBu strategy,26 according to the protocol established in our group (Table 1).27,28 For this, Rink amide resin (0.46 meq. g−1), which allows obtaining peptides with the amide group at the C-terminal end, was subjected to a swelling process by treatment with N,N-dimethylformamide (DMF) and dichloromethane (DCM). The Fmoc-amino acids were attached to the solid support or the growing peptide-resin sequentially until the sequence was complete. The peptide synthesis involved sequential reactions, as follows: (step 1) Fmoc group removal, (step 2) Fmoc-amino acid coupling reaction. Step 1 was carried out by treating the resin or the peptide-resin with 20% v/v 4-methylpiperidine in DMF for 10 min at RT, twice. Step 2 consisted of a peptide bond formation reaction (coupling) between the free amino group in the resin or peptide resin and the carboxylic group from the Fmoc amino acid to be incorporated. The Fmoc-amino acid was previously activated by treatment with DCC/6-Cl-HOBt in DMF for 15 min at RT, and then the reaction mixture was added to the resin or peptide-resin and the reaction mixture was gently stirred for 2 h at RT. The reactions were monitored using the Kaiser test in accordance with the method reported by Kaiser et al.29 When the sequence was complete, the removal of the amino acid side chains protecting groups and the peptide separation from the resin (cleavage) was carried out. This reaction was performed by treating the dry peptide-resin with a cocktail containing TFA/TIS/water (95/2.5/2.5% v/v) for 3 h at RT. Then the reaction mixture was filtered and the solution was treated with cool ethyl ether and stored at 4 °C for 20 min in order to allow the formation of the precipitate. All the peptides were purified via RP-SPE and characterized via RP-HPLC and MALDI-TOF MS, as will be described below. The polymerization of the precursor peptide CGSPINNTKPHEARGC was carried out by dissolving the peptide in DMSO 10% (v/v) in water (15 mg mL−1), and solid ammonium bicarbonate was added until the pH was 8.0. Then the reaction mixture was gently stirred for 24 h until the peptide oxidation was complete, as determined by Ellman's test.16Analytical methods
Synthesis of the copolymer poly(GMA-co-EDMA)
The preparation of the copolymer was performed following the protocol reported by ref. 17, 25 and 31. Briefly, first the monomer inhibitor (hydroquinone monomethyl ether, MEHQ) was removed from monomers glycidyl methacrylate (GMA) and ethylene glycol dimethacrylate (EDMA), using a silica gel column (0.5 g mL−1 of monomer). Then a mixture of 18% GMA, 12% EDMA, 58.8% cyclohexanol, and 11.2% dodecanol (% w/w) was prepared. Afterward, the initiator 1,1-azobis(cyclohexanecarbonitrile) (ABCN) was added in a proportion of 1% with respect to the GMA. The final mixture was purged with nitrogen for 5 minutes and then incubated at 57 °C for 24 h. Next, the polymer was thoroughly washed with methanol and dried in a vacuum trap. The copolymer was characterized via IR (ATR), elemental analysis, and SEM.Poly(GMA-co-EDMA) surface modification
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. Finally, the supernatant was discarded, and the precipitate was exhaustively washed with methanol and ethyl ether and stored at RT in a desiccator.
000 rpm for 5 min. Finally, the supernatant was discarded, and the precipitate was exhaustively washed with methanol and ethyl ether and stored at RT in a desiccator.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 v/v), was added, and the box was incubated at 37 °C for 2 h, washed, and dried. Subsequently, 100 μL of secondary antibody solution (anti-rabbit IgG-Sigma A0545 coupled to peroxidase, 1
100 v/v), was added, and the box was incubated at 37 °C for 2 h, washed, and dried. Subsequently, 100 μL of secondary antibody solution (anti-rabbit IgG-Sigma A0545 coupled to peroxidase, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000, v/v, in blocking solution) was added, and the incubation, washing, and drying process was repeated. Finally, 100 μL of development solution (TMB and hydrogen peroxide (H2O2) at 30%, 1
5000, v/v, in blocking solution) was added, and the incubation, washing, and drying process was repeated. Finally, 100 μL of development solution (TMB and hydrogen peroxide (H2O2) at 30%, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) was added to each well and left to stand in the dark for 5 minutes. The absorbance of each well was read at 620 nm.
1 v/v) was added to each well and left to stand in the dark for 5 minutes. The absorbance of each well was read at 620 nm.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min and the supernatant was discarded. The resulting solid was washed 5 times with 1 mL of washing solution and 5 times with 1 mL of distilled water. Subsequently, 100 μL of the primary antibody solution (serum post IV, 1
000 rpm for 5 min and the supernatant was discarded. The resulting solid was washed 5 times with 1 mL of washing solution and 5 times with 1 mL of distilled water. Subsequently, 100 μL of the primary antibody solution (serum post IV, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 v/v) was added and incubated at 37 °C for 1 h. After incubation, it was centrifuged at 15
100 v/v) was added and incubated at 37 °C for 1 h. After incubation, it was centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min, the supernatant was discarded, and the washings were performed in the same manner as described above. Next, 100 μL of secondary antibody (1
000 rpm for 5 min, the supernatant was discarded, and the washings were performed in the same manner as described above. Next, 100 μL of secondary antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 v/v) was added, and the incubation and washing processes were repeated as described. Finally, 500 μL of developing solution was added, stirred for 20 seconds, and centrifuged at 15
5000 v/v) was added, and the incubation and washing processes were repeated as described. Finally, 500 μL of developing solution was added, stirred for 20 seconds, and centrifuged at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 minutes. As a control, the following was used: poly(GMA-co-EDMA)-Mhx-C-Ahx-SPINNTKPHEAR with pre-immune serum and the Eppendorf treated with post IV serum.
000 rpm for 5 minutes. As a control, the following was used: poly(GMA-co-EDMA)-Mhx-C-Ahx-SPINNTKPHEAR with pre-immune serum and the Eppendorf treated with post IV serum.Conclusions
A copolymer of GMA and EDMA, poly(GMA-co-EDMA), was produced and characterized via scanning electron microscopy (SEM) and RAMAN and ATR-FTIR spectroscopy. The copolymer was treated with 6-maleimidohexanoic acid, and the ATR-FTIR and Raman spectroscopy results showed that the maleimide group was successfully introduced through an ester bond, generating poly(GMA-co-EDMA)-Mhx monolithic material. The elemental analysis indicated that this functionalization had a yield of 63%. Then, via Michael addition (thiol–maleimide) click chemistry over the monolithic surface of poly(GMA-co-EDMA)-Mhx, a peptide derived from L1-HPV protein was attached. It was determined that the resulting monolithic support, poly(GMA-co-EDMA)-Mhx-C-Ahx-SPINNTKPHEAR, allows selectively isolating polyclonal anti-SPINNTKPHEAR antibodies, since they were retained on the chemical surface of the material by an immunoaffinity interaction.The material obtained in this research is interesting since it could be subsequently studied in human sera from people infected with HPV, as a contribution to an alternative strategy for the early diagnosis of this disease. Additionally, this new grafting method is a model that could be extrapolated to other peptides of biological interest, incorporating an Ahx and a cysteine in the N or C terminal position. Our results suggest that the material functionalized through of this methodology could be used for the separation and concentration of antibodies from sera of people infected with HPV contributing in the development of HPV detection methods. Additionally, this grafting method could be extrapolated using other antigenic or immunogenic peptides of other pathogens.
Conflicts of interest
The authors declare no conflicting financial interest.Acknowledgements
The authors wish to thank COLCIENCIAS (project: 110165843141, contract: FP44842-037-2015) for its financial support.Notes and references
- S. K. Palanisamy, D. Trisciuoglio, C. Zwergel, D. Del Bufalo and A. Mai, J. Enzyme Inhib. Med. Chem., 2017, 32(1), 614–623 CrossRef CAS.
- S. Sunkar, V. Sibitha, C. Valli Nachiyar, P. Prakash and K. Renugadevi, Res. J. Biotechnol., 2017, 12(2), 46–56 CAS.
- J. Jacob, R. U. Rajendran, S. H. Priya, J. Purushothaman and S. A. Dileep Kumar, PLoS One, 2017, 12(4), e0175919 CrossRef.
- M. E. Aristimuño Ficoseco, C. J. Sequin, P. G. Aceñolaza, M. A. Vattuone, C. A. N. Catalán and D. A. Sampietro, Nat. Prod. Res., 2016, 6419, 1–4 Search PubMed.
- S. Damiano, M. Forino, A. De, L. A. Vitali, G. Lupidi and O. Taglialatela-Scafati, Food Chem., 2017, 230, 24–29 CrossRef CAS.
- Z. Cieslarova, F. S. Lopes, C. L. do Lago, M. C. França and A. V. Colnaghi Simionato, Talanta, 2017, 170, 63–68 CrossRef CAS.
- T. Madrakian, A. Afkhami, M. Rahimi, M. Ahmadi and M. Soleimani, Talanta, 2013, 115, 468–473 CrossRef CAS.
- L. Y. Thang, M. C. Breadmore and H. H. See, J. Chromatogr. A, 2016, 1461, 185–191 CrossRef CAS.
- F. Svec, Electrophoresis, 2008, 29(8), 1593–1603 CrossRef CAS.
- J. Cai, G. T. Zhu, X. M. He, Z. Zhang, R. Q. Wang and Y. Q. Feng, Talanta, 2017, 170, 252–259 CrossRef CAS.
- S. Meseguer-Lloret, S. Torres-Cartas, M. Catalá-Icardo, E. F. Simó-Alfonso and J. M. Herrero-Martínez, Anal. Bioanal. Chem., 2017, 409(14), 3561–3571 CrossRef CAS.
- A. G. Waxman and M. M. Zsmelye, Med. Clin. North Am., 2008, 92, 1059–1082 CrossRef.
- M. J. Khan, E. E. Partridge, S. S. Wang and M. Schiffman, Cancer, 2005, 104(1), 61–70 CrossRef.
- L. E. Markowitz, E. F. Dunne, M. Saraiya, H. W. Lawson, H. Chesson and E. R. Unger, Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP), MMWR Recomm. Rep. (Morb. Mortal. Wkly. Rep.), 2007, 56(RR-2), 1–24 Search PubMed.
- H. Santamaria, K. Manoutcharian, L. Rocha, E. Gonzalez, G. Acero, T. Govezensky, L. I. Uribe, A. Olguin, J. Paniagua and G. Gevorkian, Clin. Immunol., 2001, 101(3), 296–302 CrossRef CAS.
- J. A. Lara Carrillo, R. Fierro Medina, J. Manríquez Rocha, E. Bustos Bustos, D. S. Insuasty Cepeda, J. E. García Castañeda and Z. J. Rivera Monroy, Molecules, 2017, 22(11), 1970 CrossRef.
- F. M. Okanda and Z. El Rassi, Electrophoresis, 2006, 27(5–6), 1020–1030 CrossRef CAS.
- B. H. Northrop, S. H. Frayne and U. Choudhary, Polym. Chem., 2015, 6(18), 3415–3430 RSC.
- S. Xu, R. Mo, C. Jin, X. Cui, R. Bai and Y. Ji, J. Pharm. Biomed. Anal., 2017, 140, 190–198 CrossRef CAS.
- J. Bai, J. Ou, H. Zhang, S. Ma, Y. Shen and M. Ye, J. Chromatogr. A, 2017, 1514(8), 72–79 CrossRef CAS.
- D. Wu, B. Zhao, Z. Dai, J. Qin and B. Lin, Lab Chip, 2006, 6(7), 942–947 RSC.
- Z. Rivera-Monroy, G. K. Bonn and A. Guttman, Electrophoresis, 2009, 30(7), 1111–1118 CrossRef CAS.
- S. Belbekhouche, M. Guerrouache and B. Carbonnier, Macromol. Chem. Phys., 2016, 217(8), 997–1006 CrossRef CAS.
- Z. Chen, Q. Sun, Y. Yao, W. Zhang and J. Qian, Biosens. Bioelectron., 2017, 91, 553–559 CrossRef CAS.
- B. A. Velásquez-Silva, A. Castillo-Aguirre, Z. J. Rivera-Monroy and M. Maldonado, Polymers, 2019, 11(7), 1147–1160 CrossRef.
- P. Lloyd-Williams, F. Albericio and E. Giralt Chemical approaches to the synthesis of peptides and proteins, CRC Press, 1997 Search PubMed.
- M. A. León-Calvijo, A. L. Leal-Castro, G. A. Almanzar-Reina, J. E. Rosas-Pérez, J. E. García-Castañeda and Z. Rivera-Monroy, BioMed Res. Int., 2015, 2015, 1–8 CrossRef.
- C. F. Vergel Galeano, Z. J. Rivera Monroy, J. E. Rosas Pérez and J. E. García Castañeda, J. Mex. Chem. Soc., 2014, 58(4), 386–392 Search PubMed.
- E. Kaiser, R. L. Colescott, C. D. Bossinger and P. I. Cook, Anal. Biochem., 1970, 34(2), 595–598 CrossRef CAS.
- D. S. Insuasty-Cepeda, A. V. Rodríguez-Mayor, H. M. Pineda-Castañeda, J. E. García-Castañeda, M. Maldonado-Villamil, R. Fierro-Medina and Z. J. Rivera-Monroy, Molecules, 2019, 24(7), 1215 CrossRef.
- A. A. Castillo-Aguirre, B. A. Velásquez-Silva, C. Palacio, F. Baez, Z. J. Rivera-Monroy and M. Maldonado, J. Braz. Chem. Soc., 2018, 29(9), 1965–1972 CAS.
- M. H. Nasirtabrizi, S. Khodabandlou, L. Zargin and A. Parchehbaf Jadid, Int. J. Ind. Chem., 2014, 5(1), 1–8 CrossRef.
- E. L. Pfaunmiller, M. L. Paulemond, C. M. Dupper and D. S. Hage, Anal. Bioanal. Chem., 2014, 405(7), 2133–2145 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra09095f |
| This journal is © The Royal Society of Chemistry 2021 |