Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Second derivative synchronous fluorescence determination of avanafil in the presence of its acid-induced degradation product aided by powerful Lean Six Sigma tools augmented with D-optimal design†
Khalid A. M. Attiaa,
Ahmad A. Mohamadab,
Mohamed S. Emara *a,
Ahmed M. Abdel-Raoof
*a,
Ahmed M. Abdel-Raoof a,
Mohamed A. Hasan
a,
Mohamed A. Hasan a,
Ahmed W. Madkoura and
Ebrahim A. El-Desouky
a,
Ahmed W. Madkoura and
Ebrahim A. El-Desouky a
a
aPharmaceutical Analytical Chemistry Dept., Faculty of Pharmacy, Al-Azhar University, Nasr City, Cairo 11751, Egypt. E-mail: Mohamed.emara83@yahoo.com; Tel: +201002429533
bPharmaceutical Chemistry Department, Heliopolis University for Sustainable Development, 3 Belbis Disert Road, Alsalam City, Cairo 11777, Egypt
First published on 19th January 2021
Abstract
In this work, the quantitative determination of an erectile dysfunctional drug avanafil in the presence of its acid-induced degradation product was achieved via the application of a pre-optimized novel spectrofluorimetric method. The fluorescence emission wavelength was recorded at 370 and 407 nm, after being excited at 268 and 271 nm for avanafil and its acid-induced degradation product, respectively. Direct determination of avanafil based on its native fluorescence is restricted because the emission spectra of both components are heavily overlapped. Therefore, to overcome this constraint, a novel second derivative synchronous fluorescence method was evolved to eliminate this overlapping. The ideal determination wavelength was found to be 377 nm. Augmentation of lean six sigma (LSS) with response surface methodology (RSM) play a significant role in the development of robust specifications to ensure quality at the six sigma level with a high level of statistical confidence and targeted performance. All of the experimental conditions were optimized using D-optimal design as a RSM to select the optimal parameters. In addition, this work includes a graphical representation of the relationships between various variables that can greatly affect the results and the intensity of the synchronous fluorescence.
Introduction
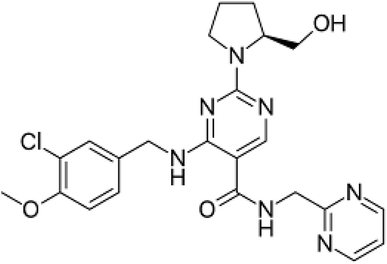
Avanafil is known chemically as (S)-4-[(3-chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide (Fig. 1). It is a white crystalline powder, with the molecular formula C23H26ClN7O3 and a molecular weight of 483.95. Additionally, it is soluble in 0.1 M hydrochloric acid, practically insoluble in water and slightly soluble in ethanol. Avanafil is used for the treatment of erectile dysfunction owing to its inhibiting effect on specific phosphodiesterase type 5 (PDE5) enzymes present in various body tissues, which are particularly found in the corpus cavernosum in the penis. The advantage of avanafil is that it has a very fast onset of action compared with other PDE5 inhibitors (sildenafil, tadalafil and vardenafil). Within fifteen minutes, the patient is able to perform in sexual activity owing to its higher absorption following oral administration. In terms of overdose, avanafil may cause vision changes, sudden vision or hearing loss, shortness of breath and palpitations. Inappropriate storage may cause degradation of the drug, which may contribute to and exaggerate these side effects. However, in spite of these side effects, its use is still preference over the other PDE5 drugs.1–5 For the determination of avanafil, the literature reveals that many methods have been reported for the quantitative estimation of the studied drug avanafil including multivariate chemometric methods6 and different chromatography techniques.7–12Fluorescence spectroscopy is associated with major advantages such as good selectivity, low detection limit and hence high sensitivity. Many drugs have been determined using this technique.13–19
Synchronous fluorescence spectroscopy (SFS) was firstly described by Lloyd,20 and has numerous advantages over traditional fluorescence, with advantages including a contracted spectral range, narrow spectral bands and simplified emission spectra.21
Since there are a lot of variable factors and values of each variable, the efforts and time consumed to optimize them will be considerable until optimal values are reached that lead to ideal results for the method. To this aim, we turned to fractional factorial designs [FrFD], a powerful tool that has the ability to theoretically check all variables and values without them being practically determined. By applying this tool significant values of variables can be selected.
The equation that represents the total number of runs is N = 2k−p, where N is the number of levels of each factor investigated, k is the number of factors investigated, and p describes the size of the fraction of the full factorial used. Using the adjusted settings of the experiment, a FrFD was used to check the significant relationships between many factors and the responses.22,23
After the successful application of FrFD we started to utilize response surface methodology (RSM) to optimize all of the experimental parameters.24,25 D-optimal designs are successfully used for decreasing the number of design runs and qualitative factors that have a number of levels above two. The D-optimal design in our experiment has the prime advantage of decreasing the number of runs into just 17 experiments.26
In the past few years, Lean Six Sigma (LSS) has affirmed its effectiveness in developing its shareholders and bottom lines through a quality enhancement approach owing to its recognition by greater numbers of corporations. LSS depends on a collaborative team effort to enhance the performance of a method. LSS not only presents a framework for an overall organizational change in culture, but also decreases process defects and waste.27,28 The process capability (Cp) and process capability index (Cpk) have been explored as short-term potential capability statistical tools measures for process capability. Different capability processes use LSS as a common mathematical framework to describe process quality in terms of sigma. In other words, in terms of different processes, it allows us to compare apples to oranges. As we have just seen, the performance concept is incorporated to achieve competitive capacity, efficacy, efficiency, productivity and performance.29,30
Many mixtures in their dosage forms and biological fluids samples have been measured and quantified via the aid of derivative SFS.31–36
The aim of the current work is to develop an accurate, specific, sensitive and capable 2nd derivative SFS method for the determination and resolution of avanafil in pure and dosage forms using a highly optimized area via an experimental design approach augmented with LSS as a collaborative process to improve method capability and performance.
Experimental
Instrumentation
The instruments and software used in this work include a Jasco spectrofluorometer FP-6200 (Japan); a hot plate (Torrey pines Scientific, USA); a UV lamp (Germany); a Jenway pH meter (USA); a Scilogex-RE 100-pro (USA) rotary evaporator; Minitab® 18.1.0 software, which was used to process capability and performance; and Design-Expert® trial version 11.0 software, which was used for designing and optimizing the proposed method.Materials and reagents
Pure samples
Avanafil (99.48%), prepared using a reported method,10 was supplied by Atco Pharma for Pharmaceutical Industries, Egypt.Pharmaceutical formulation
Atconafil® 200 mg tablets (batch no. 180667) were used in this work, supplied by Atco Pharma, Egypt.Chemicals
Analytical grade chemicals were used in this work, including sodium dodecyl sulphate (SDS) and cetyltrimethylammonium bromide (CTAB) (0.5% aqueous solutions for both, Winlab, UK); methyl cellulose, boric acid, glacial acetic acid, ethyl acetate, hydrochloric acid and sodium hydroxide (El Nasr company, Egypt); triethylamine, phosphoric acid and n-hexane (Riedel-deHäen, Germany); and Britton–Robinson (BR) buffer solutions (pH from 2 to 9).Standard solutions
Procedures
Optimization of the experimental conditions
The fitted model was obtained using Design-Expert® trial version 11.0 software utilizing a full array of responses (Table 6S†). Statistics of the chosen model were determined using specific software, then the gathered data were subjected to further processing for further optimization. To investigate each factor significance, ANOVA was used (Table 7S†).
The solution was subjected to 15 minutes of shaking, 30 minutes of sonication and was then finally filtered. Methanol was used to make the volume up to the meniscus to obtain a solution equivalent to 100 μg mL−1, which was then further diluted to obtain a 10 μg mL−1 solution. By repeating this general procedure, the tablet content of avanafil was determined from the corresponding regression equation.
Results and discussion
The main aim of this study was to develop a specific and sensitive 2nd derivative SFS method to determine avanafil in pure and dosage forms without them being previously separated.Degradation of avanafil
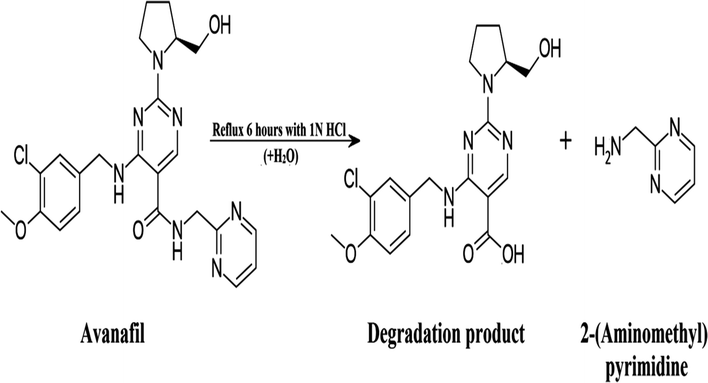
Heating avanafil under aqueous, oxidative or basic conditions does not lead to the complete degradation of the drug, whereas refluxing it for 6 h in 1 N HCl leads to its complete degradation, as shown in Scheme 1. Thin layer chromatography (TLC) was used to test the obtained solution. Separation was done using ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glacial acetic acid
glacial acetic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (7
methanol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, by volume) as a mobile phase and ultraviolet (UV) detection at 286 nm, which confirmed the different retention factor (Rf) values at which the spots appeared, with an Rf of 0.25 for avanafil and 0.63 for its acid-induced degradation product.
2.5, by volume) as a mobile phase and ultraviolet (UV) detection at 286 nm, which confirmed the different retention factor (Rf) values at which the spots appeared, with an Rf of 0.25 for avanafil and 0.63 for its acid-induced degradation product.
Confirmation of the acid-induced degradation product using infrared (IR) spectroscopy
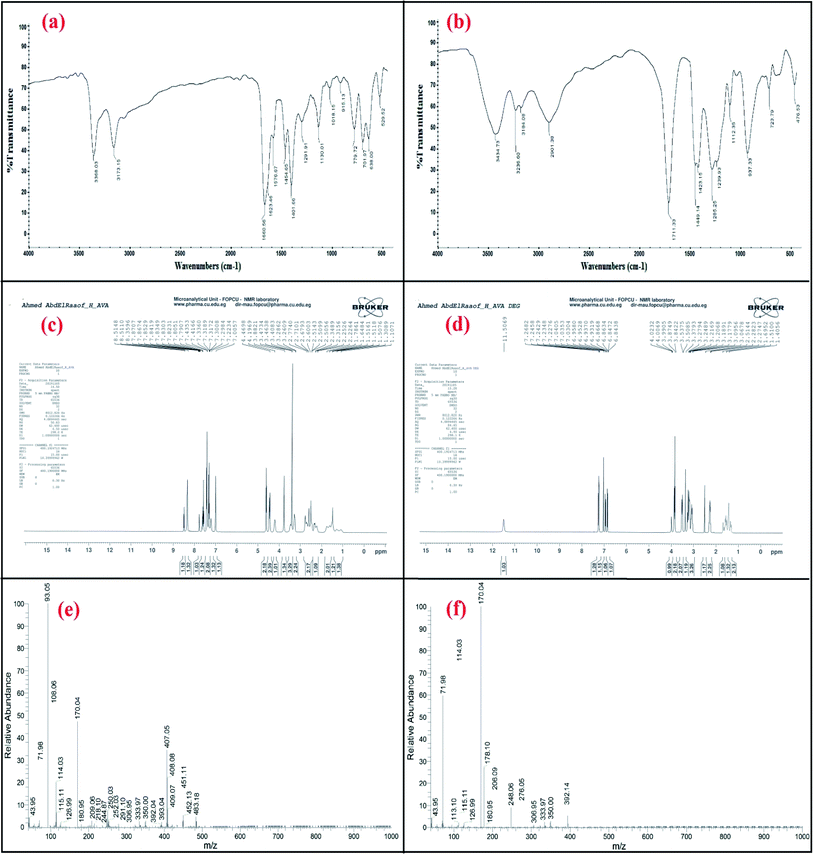
The IR spectrum of avanafil in Fig. 2a shows a peak for the carbonyl group of the amide bond (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH) at 1660.56 cm−1, while the IR spectrum of the acid-induced degradation product in Fig. 2b shows the shifting of the (C
C–NH) at 1660.56 cm−1, while the IR spectrum of the acid-induced degradation product in Fig. 2b shows the shifting of the (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretch to 1711.33 cm−1, indicating the cleavage of the amide linkage and the formation of a carboxyl group (O
O) stretch to 1711.33 cm−1, indicating the cleavage of the amide linkage and the formation of a carboxyl group (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH).
C–OH).
 | ||
| Fig. 2 Confirmation of the acid-induced degradation product using (a and b) IR spectroscopy, (c and d) 1H NMR spectroscopy and (e and f) mass spectrometry. | ||
Confirmation of the acid-induced degradation product using 1H nuclear magnetic resonance (NMR) spectroscopy
The 1H NMR spectra of the intact avanafil and its acid-induced degradation product in dimethyl sulfoxide (DMSO) reveal the disappearance of the signal of the amide group proton (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH) at 7.8707 ppm and the appearance of the signal of the carboxyl group proton (O
C–NH) at 7.8707 ppm and the appearance of the signal of the carboxyl group proton (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH) at 11.5069 ppm in the avanafil acid-induced degradation product, as shown in Fig. 2c and d.
C–OH) at 11.5069 ppm in the avanafil acid-induced degradation product, as shown in Fig. 2c and d.
Confirmation of the acid-induced degradation product using mass spectrometry
In the mass spectra, molecular ion peaks at m/z = 483.18 and m/z = 392.14 can be observed for avanafil and its acid-induced degradation product, respectively, indicating that the molecular weight of the acid-induced degradation product is 392.14, as shown in Fig. 2e and f.Spectral characteristics
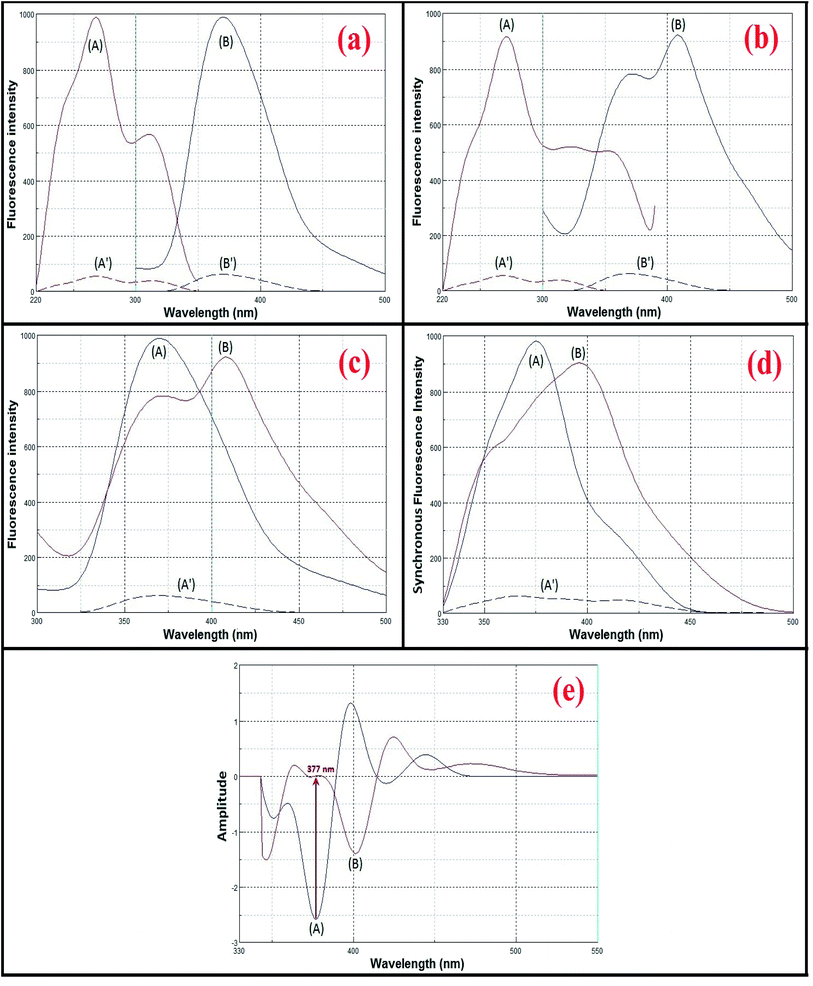
Both avanafil and its acid-induced degradation product show inherent fluorescence. The fluorescence emission spectra for avanafil and its acid-induced degradation product were observed at 370 and 407 nm, after excitation at 268 and 271 nm, respectively, as shown in Fig. 3a and b. There is heavy overlapping of the emission spectra of avanafil and its acid-induced degradation product (Fig. 3c), which prevents the accurate analysis of avanafil in the presence of its acid-induced degradation product via direct determination developed on the basis of their inherent fluorescence. Such a problem cannot be solved using synchronous method entirely, as shown in Fig. 3d. Deriving the 2nd derivative of the synchronous spectra SFS (Δλ = 110 nm) may resolve this difficulty. The 2nd derivative SFS shows a zero-crossing point with fair separation at 377 nm, which allows easy and accurate evaluation of avanafil in the presence of its acid-induced degradation product (Fig. 3e).Optimization of the experimental conditions
For the SFI, the mathematical prediction model was calculated as follows
| (SFI) = 842.73 − 15.62x1 − 9.32x2 − 15.18x3 − 110.44x12 – 110.62x22 + 10.12x1x3 |
The F value of the probability falls below 0.05, which indicates that there is no significance in the lack of fit, as shown in the ANOVA table of the quadratic model (Table 8S†). The difference between the predicted R2 and the adjusted R2 is less than 0.2, where the predicted R2 of 0.9613 is in reasonable agreement with the adjusted R2 of 0.9884. Additionally, the Adeq precision is 42.61 in adequate value as adequate precision means that the signal-to-noise ratio should be greater than 4 to be agreeable (Table 8S†). The prediction residual sum of squares exhibits a high R2 and low standard deviation values for the quadratic model.
Contour desirability, overlay plots, 3D plots and numerical optimization show that a desirability of close to 1 can be achieved utilizing a buffer with a pH of 4.2, methanol as a solvent and a constant Δλ of 107 nm, but the selected wavelength was 110 nm because it offers the best synchronous spectrum with fair separation at Δλ = 110 nm leading to an improvement in the sensitivity and resolution of the method (Fig. 3S–6S†), which is shown in the data in Table 9S.†
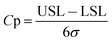 | (1) |
As the process capability improves, the variability decreases, and the data will be more tightly centered between the specification limits (Fig. 4). The current work was used to analyze and assure that the process capability index (Cpk) is > 1.33. Process capability six-pack analysis and the process capability report indicated that the reset of all of the measurements within the acceptable specification range is a 6-sigma capable process and that the data are statistically capable and more accurate (Fig. 4 and 7S†), as presented in Table 1.
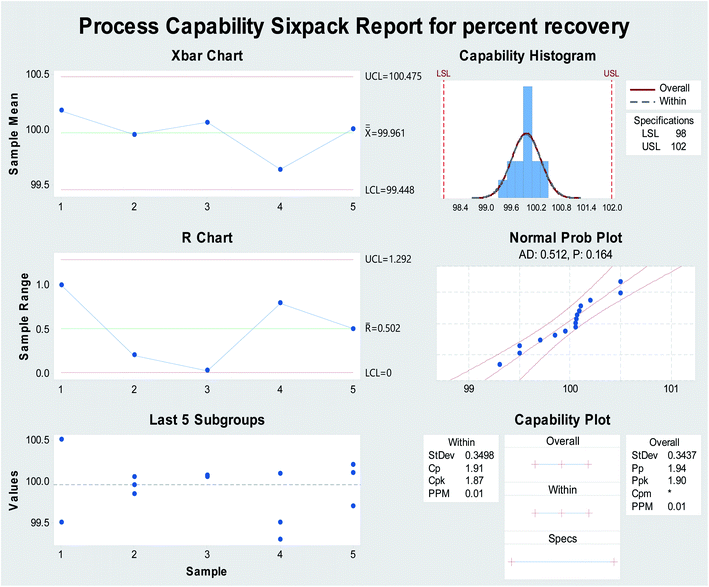 | ||
| Fig. 4 Process capability six-pack quality tools for obtaining the normally distributed assay results of percentage recovery using Minitab® 18.1.0. | ||
| Parameters | Proposed method |
|---|---|
| a LSL: lower specification limit, USL: upper specification limit, Cp: process capability, CPU: process capability within USL, CPL: process capability within LSL, Cpk: process capability centered, PP: process performance and Ppk: process performance centered. | |
| LSL | 98 |
| USL | 102 |
| Mean | 99.96 |
| SD | 0.349 |
| Sample number | 15 |
| Cp | 1.91 |
| CPU | 1.94 |
| CPL | 1.87 |
| Cpk | 1.94 |
| PP | 1.90 |
| Ppk | |
Method validation39
Linearity
The amplitude of the 2nd derivative SFS at 377 nm versus the concentrations was plotted to obtain the method calibration graph, with the data listed in Table 2.| Parameters | Proposed method |
|---|---|
| a Nine average determinations.b Nine precision determinations. | |
| Linearity range (μg mL−1) | 0.05–1.8 |
| Slope | 1.1752 |
| Intercept | 0.4776 |
| LOD (μg mL−1) | 0.01 |
| LOQ (μg mL−1) | 0.04 |
| Correlation coefficient (r) | 0.9994 |
| Accuracy (% R)a | 99.13 |
| Precision (% RSD)b | |
| Repeatability | 0.729 |
| Intermediate precision | 1.054 |
Limits of detection (LOD) and quantitation (LOQ)
LOD and LOQ values were calculated and the results were listed in Table 2.Accuracy and precision
Agreeable values of % recovery (%R) assure the accuracy of the method, as listed in Table 2. Also, elevated method precision was assured by low values % RSD listed in Table 2.Specificity
The proposed method shows high specificity for the determination of avanafil in the presence of its acid-induced degradation product, as shown in Table 3. Also, we used a standard addition technique as a specificity measure and the values listed in Table 4 indicate no interference from the matrix.| Intact (μg ml−1) | Acid-induced degradation product (μg ml−1) | Intact found (μg ml−1) | % Recovery of intact |
|---|---|---|---|
| 1.5 | 0.3 | 1.523 | 101.53 |
| 1.3 | 0.5 | 1.310 | 100.77 |
| 1.1 | 0.7 | 1.088 | 98.91 |
| 0.9 | 0.9 | 0.913 | 101.44 |
| 0.7 | 1.1 | 0.699 | 99.86 |
| 0.5 | 1.3 | 0.503 | 100.60 |
| 0.3 | 1.5 | 0.298 | 99.33 |
| 0.2 | 1.6 | 0.197 | 98.50 |
| Mean ± % RSD | 100.12 ± 1.144 | ||
| SD | 1.147 | ||
| Atconafil® tablets taken (μg mL−1) | Pure added (μg mL−1) | Pure found (μg mL−1) | % Recovery |
|---|---|---|---|
| 0.4 | 0.2 | 0.196 | 98.00 |
| 0.4 | 0.407 | 101.75 | |
| 0.6 | 0.604 | 100.67 | |
| Mean ± % RSD | 100.20 ± 1.788 | ||
| SD | 1.793 | ||
Application to the finished product
The avanafil in 200 mg Atconafil® tablets was determined using the proposed method. The obtained results show the absence of any interference from either excipients or additives. The results of the reported method10 were compared with those of the proposed method. The proposed method shows good accuracy and precision for the assay of avanafil in Atconafil® tablets, with the relevant values listed in Table 5.Conclusion
The proposed method utilizes experimental design augmented with LSS as a powerful tool for the estimation of avanafil in the presence of its acid-induced degradation product with high accuracy and precision, with fewer experiments and at low cost without any interference. The augmentation of using theoretical software alongside practical study is a promising approach by which to achieve analysis with high capability and performance. The whole procedure was checked for validity and found to be valid in various quality control laboratories.Conflicts of interest
The authors declare that they have no conflict of interest.References
- M. Limin, N. Johnson and W. J. Hellstrom, Expert Opin. Invest. Drugs, 2010, 19, 1427–1437 CrossRef.
- I. Goldstein, A. McCullough and L. Jones, J. Sex. Med., 2012, 9, 1122–1133 CrossRef CAS.
- C. Zhao, S. W. Kim, D. Y. Yang, J. J. Kim, N. C. Park, S. W. Lee, J. S. Paick, T. Y. Ahn, K. H. Moon, W. S. Chung, K. S. Min, J. K. Suh, J. S. Hyun, K. Park and J. K. Park, BJU Int., 2012, 110, 1801–1806 CrossRef CAS.
- I. Goldstein, L. A. Jones, L. H. Belkoff, G. S. Karlin, C. H. Bowden, C. A. Peterson, B. A. Trask and W. W. Day, Mayo Clin. Proc., 2012, 9, 843–852 CrossRef.
- L. H. Belkoff, A. McCullough, I. Goldstein, L. Jones, C. H. Bowden, K. DiDonato, B. Trash and W. W. Day, Int. J. Clin. Pract., 2013, 4, 333–341 CrossRef.
- M. N. Patel and C. S. Kothari, J. AOAC Int., 2016, 99, 649–663 CrossRef CAS.
- D. Jung-ah, N. Eunyoung, Y. Soon-byung, P. Hyoung-joon, C. Sooyeul, P. sung-kwan and Y. Chang-yong, Anal. Sci. Technol., 2015, 28, 278–287 CrossRef.
- J. H. Lee, J. H. Jeong, H. Park, J. Do, S. Heo, S. Cho and C. Yoon, Anal. Sci. Technol., 2016, 29, 155–161 CrossRef.
- U. A. Fahmy and B. M. Aljaeid, Int. J. Adv. Pharm., Biol. Chem., 2016, 5, 59–64 CAS.
- E. Öztürk, B. Özbek and S. Bakırdere, J. Sep. Sci., 2019, 42, 475–483 CrossRef.
- E. Oztürk, E. Akkaya, B. Zbek and S. Bakırdere, Microchem. J., 2019, 147, 269–276 CrossRef.
- E. Oztürk, E. Akkaya, B. Zbek and S. Bakırdere, Microchem. J., 2020, 152, 104302 CrossRef.
- K. A. M. Attia, A. A. Mohamad and M. S. Emara, Anal. Methods, 2016, 8, 5136 RSC.
- M. Zhu, L. Wang, H. Zhang, S. Fan, Z. Wang, Q. X. Li, Y. Wang and S. Liu, Environ. Sci. Pollut. Res., 2018, 25, 17735–17748 CrossRef CAS.
- M. Zhu, L. Wang, Y. Wang, J. Zhou, J. Ding, W. Li, Y. Xin, S. Fan, Z. Wang and Y. Wang, Int. J. Environ. Res. Public Health, 2018, 15(1), 116 CrossRef.
- Y. Wang, M. Zhu, E. Jiang, R. Hua, R. Na and Q. X. Li, Sci. Rep., 2017, 7, 4377 CrossRef.
- M. Zhu, Z. Zhao, Y. Huang, F. Fan, F. Wang, W. Li, X. Wu, R. Huaand and Y. Wang, Sci. Total Environ., 2020, 143102 Search PubMed.
- M. Zhu, Y. Xua, L. Sang, Z. Zhao, L. Wang, X. Wu, F. Fana, Y. Wang and H. Li, Environ. Pollut., 2020, 256, 113427 CrossRef CAS.
- M. Zhu, L. Wang, X. Wu, R. Na, Y. Wang, Q. X. Li and B. D. Hammock, Anal. Chim. Acta, 2019, 1058, 155–165 CrossRef CAS.
- J. F. Lloyd, Nat. Phys. Sci., 1971, 231, 64–65 CrossRef CAS.
- D. Patra and A. K. Mishra, Trends Anal. Chem., 2002, 21, 787–798 CrossRef CAS.
- C. R. T. Tarley, G. Silveira, W. N. L. dos Santos, G. D. Matos, E. G. P. da Silva, M. A. Bezerra, M. Miró and S. L. C. Ferreira, Microchem. J., 2009, 92, 58–67 CrossRef CAS.
- M. A. Bezerra, R. E. Santelli, E. P. Oliveira, L. S. Villar and L. A. Escaleira, Talanta, 2008, 76, 965–977 CrossRef CAS.
- G. E. Box, J. S. Hunter and W. G. Hunter, Statistics for experimenters: design, innovation, and discovery, Wiley-Interscience, New York, 2005 Search PubMed.
- R. E. Bruns, I. S. Scarminio and B. de Barros Neto, Statistical design-chemometrics, Elsevier, 2006 Search PubMed.
- L. Eriksson, E. Johansson, M. Müller and S. Wold, J. Chemom., 2000, 14, 599–616 CrossRef CAS.
- S. A. Albliwi, J. Antony and S. A. Limhalim, Business Process Management J., 2015, 21(3), 665–691 CrossRef.
- R. D. Snee, International Journal of Lean Six Sigma, 2010, 1(1), 9–29 CrossRef.
- Y. T. Ping, C. Jeng Feng, J. Prakash and L. Wei Ping, International Journal of Lean Six Sigma, 2012, 3(3), 206–230 CrossRef.
- N. Venkatraman and V. Ramanujam, Acad. Manag. Rev., 1986, 11(4), 801–814 CrossRef.
- N. El-Enany, F. Belal, Y. El-Shabrawy and M. Rizk, Int. J. Biomed. Sci., 2009, 5, 136–145 CAS.
- M. I. Walash, F. Belal, N. El-Enany and A. A. Abdelal, J. Fluoresc., 2008, 18, 61–74 CrossRef CAS.
- N. El-Enany, J. AOAC Int., 2008, 91, 542–550 CrossRef CAS.
- Y. F. Wei, X. H. Li and D. M. Ma, Guangpuxue Yu Guangpu Fenxi, 2005, 25, 588–590 CAS.
- Y. Xiao, H. Y. Wang and J. Han, Spectrochim. Acta, Part A, 2005, 61, 567–573 CrossRef.
- L. Garcia, S. Blazquez, M. S. Andres and S. Vera, Anal. Chim. Acta, 2001, 434, 193–199 CrossRef CAS.
- M. I. Rodrigues and A. F. Iemma, Experimental Design and Process Optimization, CRC Press, 2014 Search PubMed.
- H. Myers, M. Anderson-Cook, in Response Surface Methodology, John Wiley and Sons, New York, 3rd edn, 2009 Search PubMed.
- International Conference on Harmonization, ICH Harmonised tripartite guideline, Validation of analytical procedure: text and methodology, Q2 (R1), Geneva, International Conference on Harmonization, 2005 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08216c |
| This journal is © The Royal Society of Chemistry 2021 |