Experimental and theoretical investigation of the photothermal effect in gold nanorods
Afsaneh
Abareshi
 ,
Maghsoud
Arshadi Pirlar
and
Mahboubeh
Houshiar
,
Maghsoud
Arshadi Pirlar
and
Mahboubeh
Houshiar
 *
*
Department of Physics, Shahid Beheshti University, Evin, Tehran 1983969411, Iran. E-mail: m-houshiar@sbu.ac.ir
First published on 23rd November 2020
Abstract
In this work, gold nanorods (GNRs) were synthesized using a seed-mediated route and their photothermal properties were investigated experimentally as well as theoretically. The structure and optical properties of the samples were also characterized by their X-ray diffraction (XRD) pattern, and Ultraviolet-Visible (UV-Vis) spectroscopy, High Resolution Transmission Electron Microscopy (HRTEM) and Energy Dispersive X-ray (EDX) images. The HRTEM images confirmed that the synthesized GNRs possess a length of about 33 ± 0.3 nm and a diameter of 11.5 ± 0.2 nm; therefore the obtained GNRs have an aspect ratio of about 3 having a plasmon resonance peak at 760 nm wavelength. Since the produced photothermal heat of the synthesized GNRs by near-infrared (NIR) laser irradiation can be applied in photothermal therapy, a photothermal experiment was performed for GNR suspensions in water as well as MoS2 aqueous solution. The photothermal results confirm that the temperature of the MoS2-GNR suspension increases with GNR concentration increasing after 10 min laser irradiation. Then numerical simulation was used to compare its thermal properties with the results of the photothermal experiment for a GNR sample. Finally, the outcome indicated that GNRs and MoS2-GNR suspension can be used as two good candidates in photothermal therapy and also there is a fairly good agreement between experimental and theoretical results.
Introduction
Gold nanorods (GNRs) are one of the nanoparticle types which is extended along one direction and possesses a longitudinal and a transverse surface plasmon band. In the past few decades, GNRs have attracted much attention due to their superb intrinsic properties.1,2 First, they are able to absorb a lot of light because of their ability in absorption of intense longitudinal plasma resonance.3 Second, for GNRs, this absorption can be easily tuned by increasing the value of aspect ratio (length/width) from the visible to near-infrared (NIR) region (650–900 nm).1,4–6 These attractive intrinsic properties have made GNRs a suitable candidate to be applied in the electronics industry.7–9 On the other hand, however, in the NIR region the nanorods show low energy and high penetration in blood and tissue cells, which makes them suitable to be used in biomedical treatments.6,10–12To prepare GNRs, several synthetic methods exist which include; the hard template method,13,14 photochemistry,15 electrochemistry,14 seed-mediated growth,11 secondary growth,16 and the amorphous seed method.13,17 Among the methods mentioned above, the seed-mediated method is often applied to obtain small GNRs with high structural quality. The reducing agents often applied in this method are ascorbic acid and hydroquinone. In this synthesis cetyltrimethylammonium bromide (CTAB), gold ions and hydroquinone (reducing agent) are usually consumed.11 Attractive thermodynamic properties (strong absorption together with an increase in temperature), good biocompatibility and low toxicity make these small GNRs a qualified candidate in different applications such as photothermal therapy and drug delivery.2,5,18,19 In this field, many investigations have been performed on photothermal properties of spherical gold nanoparticles but performing the same study on GNRs can be interesting in terms of laser experimental issues. In our recent report, we have studied the photothermal properties of MoS2 nanoflakes and shown different concentrations of this colloid which indicated different hydrodynamic properties.20 In this regard, we intended to synthesize GNRs and investigate their photothermal properties through experimental and theoretical studies. As the hydrodynamic and optical properties of these nanorods strongly depend on the concentration of base fluid; the hydrodynamic properties of the GNRs at different concentrations are studied to achieve optimum concentration.
Experimental
Materials
Cetyltrimethylammonium bromide (CH3(CH2)15N(Br) (CH3)3, CTAB, ≥ 99%), hydrogen tetrachloroaurate(III) (HAuCl4·3H2O, ≥99.9%), sodium borohydride (NaBH4, ≥98%), hydroquinone (C6H4-1,4-(OH)2, 99%), silver nitrate (AgNO3), ammonium molybdate tetrahydrate (H24Mo7N6O24·4H2O, 99%), polyethylene glycol 400 (PEG, Mw 400), and thioacetamide (99%) were purchased from Sigma-Aldrich. All chemical compounds were applied without further purification. De-ionized water (18 MΩ cm) was also used in these experiments.Synthesis of MoS2 nanoflakes
MoS2 nanoflakes were prepared by a simple hydrothermal method according to the literature.20 In this method, MoS2 nanoflakes were prepared by mixing solutions of PEG 400 (2 g, 40 mL de-ionized) and thioacetamide (6 mg, 40 mL) to H24Mo7N6O24·4H2O (0.7 g, 40 mL de-ionized) solution under vigorous stirring to achieve a clear solution. This mixture was then transferred into a Teflon-lined stainless steel autoclave. After maintaining the autoclave at 210 °C for 20 h, a black solid product was obtained and centrifuged several times with de-ionized water and ethanol and finally lyophilized for 48 h.Synthesis of GNRs
A solution of GNRs was prepared according to the method reported by Picciolini et al.11 Briefly, the seed solution was prepared by mixing solutions of HAuCl4·3H2O (0.492 mg, 2.5 mL de-ionized) and NaBH4 (0.11 mg, 300 μL ice-cold) to CTAB solution (182.2 mg, 2.5 mL de-ionized) under vigorous stirring. Then, the solution's color immediately changed from yellow to light brown. The resulting brownish solution was stirred for 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min and was kept at room temperature for about 2
min and was kept at room temperature for about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h.
h.
The growth solution was prepared by the addition of 11 mg of hydroquinone to 91.1 mg of CTAB in water (2.5 mL of de-ionized). Afterwards, solutions of AgNO3 (0.0679 mg, 100 μL de-ionized) and HAuCl4·3H2O (2.5 mL, 1 mM) were added to CTAB and the hydroquinone solution prepared as above. After that, 6 μL of the seed suspension was added to this solution. The suspension color gradually changed in about 30 min. To complete the GNR growth process, the suspension was kept undisturbed overnight at 27 °C. Using Beer Lambert's method, the GNR concentration was calculated to be 0.05 nmol L−1 where in this calculation the amount of molar extinction coefficient of the GNR solution was considered to be 4.6 × 109 M−1 cm−1.10 Finally, to remove excess CTAB, the GNRs were centrifuged twice (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) rpm, 10
rpm, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min) and then they were dispersed in de-ionized water.
min) and then they were dispersed in de-ionized water.
Synthesis of MoS2–GNR nanocomposites
Finally to synthesize MoS2–GNR nanocomposites, the MoS2 (0.01 g, 20 mL de-ionized) solution was exfoliated using an ultrasonic cell disruptor (80% amplitude, 800 W, 90 min). For removing unwanted large size MoS2 flakes, centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 20 min) was performed. The synthesized GNR solution (10 mL) was then added drop by drop to the MoS2 solution (20 mL) under continuous stirring.
000 rpm, 20 min) was performed. The synthesized GNR solution (10 mL) was then added drop by drop to the MoS2 solution (20 mL) under continuous stirring.
Characterization of the samples
The crystalline structure of the sample was identified using an X-ray difffractometer (XRD) (Model STADI STOE, Germany) with CuKα irradiation in λ = 1.540 Å in 2θ = 10°–80° and then the obtained data were analyzed using X’pert software. Size and morphology characterization of the samples was carried out using a Tecnai G2 F20, 200 kV transmission electron microscope, equipped with an energy-dispersive X-ray spectrometric (EDX) element analyser. For High Resolution Transmission Electron Microscopy (HRTEM) measurements, a piece of slate was prepared by placing one drop of sample on copper grids which were covered by carbon. Optical characterization of the sample was carried out using a PerkinElmer LAMBDA 20 UV-Vis spectrometer. Photothermal experiments in solution were conducted using a Fiber Coupled Diode Laser with 808 nm wavelength and 10 W power when it was irradiated perpendicular to a quartz cell containing an aqueous solution of the samples with total volume = 2 mL.Results and discussion
Structural characterization
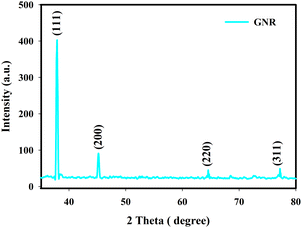
Fig. 1 illustrates a typical XRD pattern of the GNRs which have been obtained from 11 mg hydroquinone. This figure indicates the crystalline nature of these nanorods with four sharp diffraction peaks formed with an fd3m space group (JCPDS 008-0234). The mentioned peaks are located at 37.98°, 45.09°, 64.50° and 77.25° angles respectively where they are indexed to (1 1 1), (2 0 0), (2 2 0) and (3 1 1) planes which are in accordance with a face centered cubic structure.21 Two strong peaks at 37.98° and 45.09° angles are in accordance with the diffractogram of crystalline gold reported by others.21,22 The crystal size and lattice constant have been calculated from the XRD patterns which are 34.1 nm and 4.078 Å, respectively.Fig. 2(a–c) show the TEM images of the GNR sample. As seen in these figures, GNRs shape is clearly distinguishable and the distribution of transverse diameter is normally much narrower than the distribution of the longitudinal length in a way that the average aspect ratio of the synthesized GNRs is about 3.33, with a length of 30 ± 0.3 nm and diameter of 9 ± 0.2 nm.11Fig. 2(c) illustrates the inter distance of the lattice planes which are about 0.2 nm and can be indexed to the (2 0 0) surface plane of a crystal structure of the synthesized GNR sample.23
Furthermore, the EDX image was provided to confirm the elemental composition of the synthesized GNRs (Fig. 3). As shown in this figure, the EDX image of the GNR sample indicates signals of the presence of gold (Au), copper (Cu) and carbon (C) elements. The Cu peaks observed in this image are from the grid and also the detected carbon peak belongs to the grid and pollutants. As in this analysis, no other element was detected so it can be declared that the synthesized GNRs are pure in this structure.24
Optical properties
The absorption spectrum of metal nanoparticles is strongly dependent on particle shape so the presence of different degrees of polarization of electrons in all directions is due to the anisotropy of the structure in the nanorod samples.25Absorption characteristics of GNRs were investigated by UV-Vis spectroscopy as shown in Fig. 4. As seen in this figure, these nanorods illustrate independent electron vibrations along the diameter and the long axis directions with the peak ranging from 510 nm to 530 nm due to the electron vibration in the diameter axis direction (Fig. 4a), named transverse surface plasmon resonance, while the peak in range of the visible to NIR region is due to the vibration along the long axis (Fig. 4b), called longitudinal localized surface plasmon resonance.13,25 These two typical peaks have been revealed at 525 nm and 760 nm wavelengths respectively for the synthesized GNR sample.
 | ||
| Fig. 4 Schematic illustration of the localized surface plasmon resonance excitation for the GNR sample; (a) transverse plasmon bands, (b) longitudinal plasmon bands, and (c) UV-Vis spectrum of GNRs. | ||
Using the longitudinal surface plasmon peak wavelength, the aspect ratio can be calculated for the GNRs as follows:8
| Aspect ratio = 0.0078 × peak position [nm] − 3.3 | (1) |
The obtained aspect ratio from the synthesized GNRs is about 2.625 which is comparable with the gained result from the TEM images (aspect ratio = 2.81). As mentioned above, the value of aspect ratio for the nanorods changes the position of the longitudinal localized surface plasmon resonance peak, which plays an important role in the optical properties.10
Thermal properties
Due to the large value of surface area, easy surface modification, high stability, low toxicity, controllable size and morphology of GNRs, they can be applied in drug delivery and photothermal therapy.3,5,6,8,10 In our recent paper, we investigated the hydrodynamic properties of MoS2 nanoflakes and showed that these nanoflakes possess a two dimensional layered structure, strong absorbance and attractive photothermal properties in the NIR region.20 In this research, we have performed a hydrodynamic investigation for GNRs in such a way that photothermal heat was examined for de-ionized water (base sample), GNR aqueous suspension and GNR-MoS2 solution (with different concentrations) as shown in Fig. 5. In order to achieve the optimum value of concentration, different volumes of synthesized GNRs (1.2, 1.3, 1.4, and 1.5 mL) were dissolved in MoS2 aqueous solution with 200 ppm concentration. The solutions were shaken well, then 2 mL of each sample solution was irradiated under the irradiation of a continuous wave (CW) laser with 808 nm wavelength and 1 W cm−2 power density for 10 min. | ||
| Fig. 5 Temperature versus time for water, GNR, MoS2 and MoS2–GNR aqueous suspension with different concentrations under irradiation of an 808 nm CW laser with 1 W cm−2 power density after 600 s. | ||
As seen in Fig. 5, for all the samples, the temperature increases versus time after 10 min laser irradiation. It is also seen in this figure that the temperature of de-ionized water increased to only about 28.9 °C after 10 min laser irradiation but the temperature of the GNR suspension alone (with the amount of 1.5 mL of GNR dissolved in 2 mL of de-ionized water) was increased to 57.5 °C. This confirmed that NIR responsive nanorods have produced most of the photothermal heat. As a result, it can be claimed that GNRs are able to produce high photothermal heat. This amount of produced heat upon NIR laser irradiation can be used in photothermal therapy as heat higher than 45 °C generated by laser irradiation can destroy cancer cells.26–28 Then, a photothermal experiment was carried out for MoS2-GNR solution with four different concentrations of GNR suspension (1.2, 1.3, 1.4, and 1.5 mL). Fig. 5 shows the temperature curve versus time for the GNRs, all different concentrations of Mos2-GNR solution and water. It can be seen in this figure that the temperature has increased to 39.9 °C, 49.2 °C, 50.0 °C, and 51.1 °C respectively when the volume of GNRs increases in MoS2 solution after 10 min laser irradiation. According to Fig. 5, it can be seen that the MoS2-GNR sample with GNR concentrations higher than 1.3 mL can be used as an optimum value of concentration in photothermal therapy. As this temperature increase in photothermal therapy seemed to be sufficient, we did not further investigate higher values of concentrations in this research. For higher concentrations greater than 1.5 mL of GNR suspension, further searches are recommended.
To explain the effect of the suspended nanorods on temperature increase of the solution, the mathematical relation of this phenomenon can be used. Let us consider a liquid-phase medium with nanorods irradiated by a light beam with intensity I, uniformly distributed over the region. As a result of the action of a lighted area in the medium, temperature and concentration gradients arise, which then determine the processes of heat and mass transfer. These phenomena are described by a system of balanced equations for temperature and particle concentration.29–31 This can be written as a system of balanced equations of heat conduction and mass transfer of nanorods as follows:29
 | (2) |
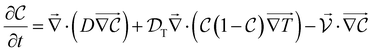 | (3) |
 is volume concentration of the medium,
is volume concentration of the medium,  is thermal conductivity of the medium,
is thermal conductivity of the medium,  is absorption coefficient of light, D is diffusion coefficient of nanorods,
is absorption coefficient of light, D is diffusion coefficient of nanorods,  is thermal diffusion coefficient,
is thermal diffusion coefficient,  is concentration convection velocity, Cp and ρ are heat capacity and density of the solution, respectively. We will not consider the pressure of the lighted area which forces on the particles. These equations are coupled equations. In the presence of a uniform liquid, convection flow velocity
is concentration convection velocity, Cp and ρ are heat capacity and density of the solution, respectively. We will not consider the pressure of the lighted area which forces on the particles. These equations are coupled equations. In the presence of a uniform liquid, convection flow velocity  along the z-axis (vertical coordinate) is perpendicular to the light beam. In this way, the thermal transport equation which describes temperature changes caused by thermal convection and conduction is given by:20
along the z-axis (vertical coordinate) is perpendicular to the light beam. In this way, the thermal transport equation which describes temperature changes caused by thermal convection and conduction is given by:20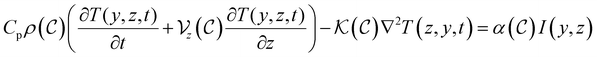 | (4) |
Using the green function for conduction and convention, the heat transfer equation in which the heat distribution changes in terms of the absorbed laser energy can be obtained as follows;32
 | (5) |
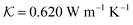 ,Cp = 4032 J kg−1 K−1 and ρ = 1032.6 kg m−3. These are the values for the case where we dissolve the volume percentage of GNRs in water (ϕ = 19.4 × 10−4). The volume percentage of ϕ is equivalent to
,Cp = 4032 J kg−1 K−1 and ρ = 1032.6 kg m−3. These are the values for the case where we dissolve the volume percentage of GNRs in water (ϕ = 19.4 × 10−4). The volume percentage of ϕ is equivalent to  which is the volume concentration of suspended nanorods in the fluid. On the other hand, one can represent the convective velocity
which is the volume concentration of suspended nanorods in the fluid. On the other hand, one can represent the convective velocity  by
by  , where η is the dynamic viscosity coefficient of the nanofluid, ρ is its density, and l is the characteristic length of the system. According to the synthesized GNR properties, we use the value of η = 8.25 × 10−7 kg mm−1 s−1 and l = 10 mm
, where η is the dynamic viscosity coefficient of the nanofluid, ρ is its density, and l is the characteristic length of the system. According to the synthesized GNR properties, we use the value of η = 8.25 × 10−7 kg mm−1 s−1 and l = 10 mm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 in our experiment.
33 in our experiment.
Fig. 6 shows the theoretical simulation of the temperature distribution on the sample with three different values of time. These figures illustrate the influence of convection velocity on heat distribution. This figure indicates that with increasing time, the heat is distributed more in the positive z direction but is symmetric in the y direction.
 | ||
| Fig. 6 The simulated temperature distribution at three different times: (a) t = 5 s, (b) t = 100 s and (c) t = 600 s. | ||
To obtain the theoretical average temperature of the sample, the temperature distribution at any value of time t, was averaged. Fig. 7 shows the calculated average temperature of the GNR solution at different times t and the values of all the hydrodynamic properties can be measured. Also, the mean temperature variation curve with the experimental results is shown in Fig. 7. As it is seen in this figure, the experimental results are in good agreement with the theoretical results.
 | ||
| Fig. 7 Temperature variations versus time for the GNR sample. The solid pink line shows the theoretical results and the blue filled circles are experimental data. | ||
It seems that by changing the hydrodynamic characteristics, the temperature distribution varies with the solution's mean temperature. On the other hand, according to eqn (4), parameters such as density ρ, convection velocity  , absorption coefficient
, absorption coefficient  and thermal conductivity
and thermal conductivity 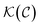 are functions of nanorod concentration. So, it is expected that by choosing different concentrations of a solution, different values from the temperature variation curve can be obtained, which is clearly seen in Fig. 5. As is seen in this figure, the results of temperature variation versus time for a GNR sample show behaviors which vary in MoS2 solution and water. Also, different concentrations of GNR-MoS2 composite show different behaviors. The hydrodynamic variables used in eqn (4), have different functionality with different nanorods concentration which was described in our previous report.20 Unlike what was observed in our last report, in the present case, the temperature distribution does not have a linear relation with the nanorod concentration. To investigate this issue, different concentrations of GNR-MoS22solution were considered and examined empirically, the results of which are seen in Fig. 5.
are functions of nanorod concentration. So, it is expected that by choosing different concentrations of a solution, different values from the temperature variation curve can be obtained, which is clearly seen in Fig. 5. As is seen in this figure, the results of temperature variation versus time for a GNR sample show behaviors which vary in MoS2 solution and water. Also, different concentrations of GNR-MoS2 composite show different behaviors. The hydrodynamic variables used in eqn (4), have different functionality with different nanorods concentration which was described in our previous report.20 Unlike what was observed in our last report, in the present case, the temperature distribution does not have a linear relation with the nanorod concentration. To investigate this issue, different concentrations of GNR-MoS22solution were considered and examined empirically, the results of which are seen in Fig. 5.
Conclusions
In the present research, the photothermal effect of gold nanorods was studied both experimentally and theoretically. GNRs were synthesized through a seed-mediated growth method using hydroquinone as a reducing agent. The XRD pattern confirmed that the synthesized nanorods possess an fd3m space group with a face centered cubic structure. The calculated crystal size and lattice constant were 34.1 nm and 4.078 Å respectively. TEM images indicated that the average aspect ratio of these nanorods is about 3.33, with a length of 30 ± 0.3 nm and diameter of 9 ± 0.2 nm. The presence of pure gold elements was confirmed by EDX image. UV-Vis spectroscopy reveals transverse and longitudinal surface plasmon peaks at 525 nm and 760 nm wavelengths, respectively, so the obtained aspect ratio was found to be about 2.625, almost similar to the value found in the TEM images.The photothermal experiment confirmed that under NIR irradiation, photothermal heat of the samples increased with increasing concentration of GNRs in the MoS2 solution. To justify and validate the obtained experimental photothermal results, the theoretical calculation was also performed using green function simulation.
Present study provided comparable photothermal effect of GNRs in experimental as well as theoretical results. Further experimentation and investigation on potential applications of GNRs in photothermal therapy are strongly recommended. In using GNRs as photothermal agents in biomedical applications, the photothermal stability test of this material is vastly suggested.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support from vice presidency for research and technology of Shahid Beheshti University.References
- X. Xu, Y. Zhao, X. Xue, S. Huo, Fei Chen, G. Zou and X. J. Liang, J. Mater. Chem. A, 2014, 2, 3528–3535 RSC.
- L. An, Y. Wang, Q. Tian and S. Yang, Materials, 2017, 10, 1372–1393 CrossRef PubMed.
- X. Yang, M. Yang, B. Pang, M. Vara and Y. Xia, Chem. Rev., 2015, 115, 10410–10488 CrossRef CAS PubMed.
- N. D. Burrows, S. Harvey, F. A. Idesis and C. J. Murphy, Langmuir, 2017, 33, 1891–1907 CrossRef CAS PubMed.
- G. Terentyuk, E. Panfilova, V. Khanadeev, D. Chumakov, E. Genina, A. Bashkatov, V. Tuchin, A. Bucharskaya, G. Maslyakova, N. Khlebtsov and B. Khlebtsov, Nano Res., 2014, 7, 325–337 CrossRef CAS.
- H. Tang, S. Shen, J. Guo, B. Chang, X. Jiang and W. Yang, J. Mater. Chem., 2012, 22, 16095–16103 RSC.
- C. Jing, F. J. Rawson, H. Zhou, X. Shi, W. H. Li, D. W. Li and Y. T. Long, Anal. Chem., 2014, 86, 5513–5518 CrossRef CAS PubMed.
- S. Thatai, S. P. Parul Khurana and D. Kumar, Microchem. J., 2014, 113, 77–82 CrossRef CAS.
- M. Runowski, S. Sobczak, J. Marciniak, I. Bukalska, S. Lis and A. Katrusiak, Nanoscale, 2019, 11, 8718–8726 RSC.
- Q. Wei, H. Ni, X. Jin and J. Yuan, RSC Adv., 2015, 5, 54971–54977 RSC.
- S. Picciolini, D. Mehn, I. Ojea-Jiménez, F. Gramatica and C. Morasso, J. Visualized Exp., 2016, 114, 1–7 Search PubMed.
- W. S. Kuo, C. N. Chang, Y. T. Chang, M. H. Yang, Y. H. Chien, S. J. Chen and C. S. Yeh, Angew. Chem., 2010, 49, 2711–2715 CrossRef CAS PubMed.
- L. Meng, J. Zhang, H. Li, W. Zhao and T. Zhao, J. Nanomater., 2019, 11, 4925702 Search PubMed.
- K. Sun, Y. Chang, B. Zhou, X. Wang and L. Liu, Int. J. Nanomed., 2017, 12, 1905–1915 CrossRef CAS PubMed.
- G. N. Abdelrasoul, R. Cingolani, A. Diaspro and A. Pignatelli, J. Photochem. Photobiol., A, 2014, 275, 7–11 CrossRef CAS.
- K. A. Kozek, K. M. Kozek, W. C. Wu, S. R. Mishra and J. B. Tracy, Chem. Mater., 2013, 25, 4537–4544 CrossRef CAS PubMed.
- P. J. Straney, C. M. Andolina and J. E. Millstone, Langmuir, 2013, 29, 4396–4403 CrossRef CAS PubMed.
- B. Jang, J. Y. Park, C. H. Tung, I. H. Kim and Y. Choi, ACS Nano, 2011, 5, 1086–1094 CrossRef CAS PubMed.
- M. A. Mackey, M. R. K. Ali, L. A. Austin, R. D. Near and M. A. El-Sayed, J. Phys. Chem. B, 2014, 118, 1319–1326 CrossRef CAS PubMed.
- A. Abareshi, M. Arshadi Pirlar and M. Houshiar, Mater. Res. Express, 2019, 6, 1 Search PubMed.
- M. Samim, C. K. Prashant, A. K. Dinda, A. N. Maitra and I. Arora, Int. J. Nanomed., 2011, 6, 1825–1831 CrossRef PubMed.
- H. W. Wang, H. Y. Chen, W. C. Shiu, B. Russo and G. Cao, Nanotechnology, 2006, 17, 2689–2694 CrossRef CAS PubMed.
- Y. Leng, X. Yin, F. Hu, Y. Zou, X. Xing, B. Li, Y. Guo, L. Ye and Z. Lu, RSC Adv., 2017, 7, 25469–25474 RSC.
- C. J. Huang, P. H. Chiu, Y. H. Wang, C. F. Yang and S. W. Feng, J. Colloid Interface Sci., 2007, 306, 56–65 CrossRef CAS PubMed.
- J. Cao, T. Sun and K. T. V. Grattan, Sens. Actuators, B, 2014, 195, 332–351 CrossRef CAS.
- S. H. Wang, C. W. Wei, S. H. Jee and P. C. Li, J. Med. Biol. Eng., 2010, 31, 387–393 CrossRef.
- D. d. Melo-Diogo, R. L. Sousa, C. Alves and I. J. Correia, Biomater. Sci., 2019, 7, 3534–3551 RSC.
- G. K. Moojoong Kim, D. Kim, J. Yoo, D. K. Kim and H. Kim, Cancers, 2019, 11, 764–783 CrossRef PubMed.
- Y. Rudyak, A. V. Minakov and M. I. Pryazhnikov, Tech. Phys. Lett., 2017, 43, 23–26 CrossRef.
- R. Karimzadeh and M. Arshadi, Laser Phys., 2013, 23, 115402 CrossRef.
- D. Razzaghi, M. Arshadi Pirlar and M. S. Ghamsari, J. Mod. Opt., 2018, 66 Search PubMed.
- R. Karimzadeh, Opt. Commun., 2013, 286, 329–333 CrossRef CAS.
- F. E. Berger Bioucas, C. Damm, W. Peukert, M. H. Rausch, T. M. Koller, C. Giraudet and A. P. Fröba, J. Phys. Chem. B, 2019, 123, 9491–9502 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2021 |