Synthesis of hydroxide-enriched cerium-doped oxy-sulfide catalyst for visible light-assisted reduction of Cr(VI)†
Misganaw Alemu
Zeleke
 ab and
Dong-Hau
Kuo
ab and
Dong-Hau
Kuo
 *b
*b
aDepartment of Materials Science and Engineering, Bahir Dar University, P.O. Box 79, Ethiopia
bDepartment of Materials Science and Engineering, National Taiwan University of Science and Technology, No. 43, Sec. 4, Keelung Road, Taipei 10607, Taiwan. E-mail: dhkuo@mail.ntust.edu.tw; Fax: +011-886-2-27303291
First published on 16th November 2020
Abstract
Semiconductor catalysts are significantly attractive materials for different cutting-edge applications, including the detoxification of toxic pollutants. Herein, a hydroxide-enriched cerium-doped nanoflower-like indium oxy-sulfide catalyst was synthesized via an efficient and simple procedural course with Ce/[Ce + In] = x precursor molar ratios, where x = 0.5, 0.6, and 0.7, and with a fixed thioacetamide molar content in the air atmosphere. All catalyst materials were characterized systematically, and their activities were studied towards the reduction of hazardous hexavalent chromium [Cr(VI)] under visible light illumination. The hydroxide-enriched nanoflower-like Ce–In2(O,S)3 catalyst, with a Ce/[Ce + In] precursor molar ratio at 0.6 designated as S-0.6, exhibited enhanced catalytic activity compared to the rest of the composition and host catalyst material. Its Cr(VI) reducing efficiency was found to be 99.3% within 6 min irradiation time with a rate constant increased by a factor of 5.42 compared to the cerium-free nanoflower catalyst. Hence, the as-synthesized nanoflower-like catalyst is very promising for the detoxification of hazardous Cr(VI) from the source environment.
1. Introduction
Semiconductor catalysts have wide-range applications and hence, researchers have exploited their skills on synthesizing these materials at different working conditions for different applications, including photocatalytic hydrogen production,1 CO2 reduction,2 water splitting,3 and the detoxification of toxic pollutants.4–7 These materials are of great interest due to the advantages of high efficiency, low cost, no secondary pollution, and energy saving properties. They accomplish photocatalytic activity by absorbing photons of enough energy for the initiation of the electrons on their filled valence band, and by adsorbing the reactant species on their surfaces.8 Their valence band and conduction band are moderately far apart to each other, and are separated by an empty space known as the bandgap. The electrons are fully distributed in the valence band, but can be promoted to the unoccupied higher-energy conduction band when the catalyst is exposed to photons of energy greater than or equal to its bandgap energy.9 In such a case, the positively charged holes remain in the valence band, which means the electron–hole pairs are formed and have migrated to the surface of the catalyst. The electrons are then taking the responsibility of reducing the electron acceptor reactants, and the holes oxidize the electron donor reactants. For this to happen, the recombination probability of the photo-generated charge carriers must be diminished, or the possible back transfer of electrons from the acceptor or holes from the donor species to the catalyst has to be evaded.10,11The treatment of toxic heavy metal cations from a variety of sources, including leather tanning, electroplating, mining, pigment, and printing industries, is an urgent issue since they are mutagenic and carcinogenic to human health.12,13 Hexavalent chromium is among the chronic heavy metal cations, and can induce severe toxicity in living organisms. Hence, its detoxification must be the prime concern. Researchers are using both reduction and adsorption methods to detoxify harmful Cr(VI). However, the latter method cannot solve the problem exhaustively; rather, it gives rise to another secondary pollution in the environment. Hence, semiconductor catalyst materials are playing significant roles for the reduction of Cr(VI) into less toxic, lower oxidation state forms under light illumination. In catalysis research, the great challenges that researchers are facing include synthesizing a photocatalyst semiconductor material with optimized bandgap energy, activated surface for the initiation of adsorption of the reactant species, tidy morphology for the proper diffusion of charge carriers over the surface, and efficient catalytic activity. Researchers have synthesized diverse types of single-phase14–16 and composite17–21 catalysts for the application of Cr(VI) reduction and others under different conditions. However, there are still limitations to reduce hazardous Cr(VI) efficiently to the lower relatively non-toxic state. Currently, researchers in different fields are devoting their attention towards the detoxification of hazardous Cr(VI). Jingwen Pan et al.22 synthesized a 3D heterostructured ZnIn2S4/SnS2 nanocomposite material, and tested it for the reduction of Cr(VI) under visible light illumination. However, it took an extended amount of time to reduce the chronic Cr(VI) into a non-toxic state. Other researchers23–25 also faced this, and other material-related challenges to detoxify Cr(VI) efficiently.
Oxy-sulfide based catalysts have been given special attention since the combination of oxygen and sulfur in the crystal system and the morphology of the semiconductor catalyst play vital roles in the efficient catalytic performance.26,27 In this research work, the hydroxide-enriched cerium-doped nanoflower-like oxy-sulfide catalyst was synthesized, and used for the reduction of chronic Cr(VI) under visible light illumination since this light source is an sufficiently available form of solar energy.28,29 The cerium dopant brought a significant change in the electrical and morphological properties of the host indium oxy-sulfide catalyst. A cotton wool ball type morphology with compact nature was obtained because of cerium incorporation. The highly positively charged cerium ion caused the excess dissemination of hydroxide ion on the surface of the catalyst material. This phenomenon facilitated adsorption, followed by a fast reduction of hazardous Cr(VI) into the less toxic Cr(III) under visible light irradiation. Generally, the as-synthesized hydroxide-enriched cerium-doped nanoflower-like oxy-sulfide catalyst is highly promising for the detoxification of Cr(VI) and well-being of human health.
2. Experimental
2.1. Materials
Indium chloride tetrahydrate (InCl3·4H2O), cerium nitrate octahydrate [Ce(NO3)3·8H2O], and thioacetamide (C2H5NS) were purchased from Aladdin and used without further purification.2.2. Synthesis of hydroxide-enriched nanoflower-like Ce–In2(O,S)3 catalysts
The synthesis of hydroxide-enriched nanoflower-like Ce–In2(O,S)3 catalysts was performed based on our previous work.30 Typically, 2 mmol indium chloride tetrahydrate, cerium nitrate octahydrate, and 4 mmol thioacetamide were dissolved in 6 mL ethylene glycol separately followed by heating at 80 °C for 30 min under uninterrupted stirring over a hot plate under an atmosphere of air. The solutions of the metal precursors were mixed with Ce/[Ce + In] = x precursor molar ratios, where x = 0.5, 0.6, and 0.7. The working temperature was then raised to 120 °C and kept for 40 min. The thioacetamide solution was then added to the above solution, and heated at a temperature of 150 °C for 2 h. To remove any unnecessary side products, the resulting yellowish precipitate was washed with DI water, followed by ethanol and dried at 80 °C overnight. The resulting samples, with the above molar ratios, were designated as S-0.5, S-0.6, and S-0.7, respectively. Pure In2(O,S)3 catalyst was synthesized with the same procedure, except for the addition of Ce(NO3)3·8H2O, and designated as S-0.2.3. Characterization of hydroxide-enriched nanoflower-like Ce–In2(O,S)3 catalysts
The phase purity, crystalline-versus-amorphous nature, and bond nature of the as-synthesized hydroxide-enriched nanoflower-like cerium-doped indium oxy-sulfide catalysts were analyzed using X-ray diffractometry (XRD, Bruker D2 phaser, Japan, Cu-Kα radiation) and Renishaw inVia micro-Raman spectrophotometer with an Ar+ laser of wavelength 532 nm. The morphological features of the S-0 and Ce–In2(O,S)3 catalysts were studied using field emission scanning electron microscopy (FE-SEM, JSM 6500F, JEOL, Tokyo, Japan) equipped with energy dispersive spectroscopy (EDS) and transmission electron microscopy (TEM, Tecnai F20 G2, Philips, Netherlands). The absorbance of all as-synthesized catalysts and Cr(VI) solutions were conducted using a UV-Vis spectrophotometer (JASCD 760). X-ray photoelectron spectroscopy (XPS, ESCALAB 250, England) characterization was performed to deal with the oxidation state of the constituent elements of the catalysts. The lifespan of the electron–hole separation in each catalyst and the conductance were accompanied by characterization using a photoluminescence (PL) spectrophotometer (JASCD-8500) at an excitation wavelength of 332 nm and electron impedance spectroscopy (EIS) (EC-Lab, SP-300, BioLogic Science Instrument), respectively.3. Results and discussion
The X-ray diffractometry analysis revealed that the monometallic nanoflower S-0 and the best efficient hydroxide-enriched nanoflower-like S-0.6 catalysts both exhibited a cubic structure, where In2S3 was found to be a major phase with JCPDS # 65-0459 [Fig. 1(a)]. No additional X-ray peaks were observed, indicating that no secondary phase was formed in the synthesizing route of the Ce–In2(O,S)3 catalysts. The principal X-ray peaks of the nanoflower S-0 catalyst that evolved at the crystal planes of (311), (400), and (440) were more intense compared to those of the peaks of the S-0.6 catalyst. The nature of the XRD peak intensity is a direct reflection of whether the catalyst material has a crystalline or amorphous dominance. The lower intensity of the diffraction peak of the material is related to its amorphous behavior compared to the more intense one.31 Herein, the peak intensities of the nanoflower-like S-0.6 catalyst at the crystal planes of (311) and (440) were determined to be decreased by a factor of 0.79 and 0.66, respectively. Hence, their atoms do not tend to form a strictly defined atomic arrangement in the lattice system of the crystal structure compared to the host material. The Amorphous materials possess short-range atomic orders with randomly oriented bonds. This atomic arrangement leads to the formation of large surface-exposed defects due to the easiest mobility of charge carriers to the neighboring active sites.32 Hence, the target toxic reactant species get reduced or degraded easily to the corresponding nontoxic products, which means that the catalytic activity of the material is enhanced.33,34 An inclusion of cerium made the catalyst material more amorphous compared to the cerium-free S-0 catalyst. This is indeed advantageous for the charge carriers to move more freely relative to those in the less amorphous catalyst due to the short-range order of interatomic distances and number of neighboring atoms in the amorphous catalyst materials that facilitate the transportation of charge carriers.35 The peak intensity of the (400) plane is almost diminished upon cerium addition, which means that the doping of cerium retarded the grain growth of the cubic S-0. As shown in the ESI† [Fig. S1(a)], the X-ray peaks of the as-synthesized S-0.5 and S-0.7 catalysts are identical with those of the S-0 peaks, confirming that only a single-phase solid solution was formed upon addition of the given molar fraction of cerium. | ||
| Fig. 1 (a) XRD patterns and (b) Raman spectra of the host nanoflower S-0, and the best efficient hydroxide-enriched nanoflower-like S-0.6 catalysts. | ||
Raman spectroscopy is an important characterization technique that is used to deal with bond vibrations, and its signals are treated as the fingerprint of a compound.36Fig. 1(b) shows the Raman spectra of commercial In2S3, and the as-synthesized nanoflower S-0 and hydroxide enriched nanoflower-like Ce–In2(O,S)3 catalysts. The peak positions of both In2S3 powder and nanoflower S-0 catalyst are invariant. They are exactly lined up with each other, indicating that the partial addition of oxygen to the lattice structure of In2S3 has no impact on the In-S bond structure. The Raman peaks of the nanoflower S-0 catalyst corresponding to wavenumbers at 129, 290, and 354 cm−1 were found to be shifted to the left as cerium was added. The shift to the lower wavenumber is related to the increase in the metal–sulfur/oxygen bond length, and this is most probably ascribed to the coulombic interaction between the cationic or anionic components of the S-0.6 catalyst and the surface hydroxide ion. The surface-enriched hydroxide ions have attractive and repulsive interactions with the cationic and anionic components of the nanoflower-like catalysts, respectively, leading to the elongation of the metal–sulfur/oxygen bond length. In general, this phenomenon is evidence for the replacement of some cationic parts of S-0 by cerium cation in the crystal system and the presence of excess hydroxide ions on the surface of the catalyst. Similar phenomena are also observed for the rest of the Ce–In2(O,S)3 catalysts [Fig. S1(b), ESI†].
As revealed from the FE-SEM analysis, the morphology of the pure S-0 catalyst [Fig. 2(a)] is purely a nanoflower. The openings of the nanoflower are observed in each bunch of flower-type morphology. On a closer checkup, the morphology of the nanoflower S-0 catalyst indicates the formation of no secondary phase. EDS analysis was performed to detect the elements present in the as-synthesized catalyst samples. Fig. 2(b) displays the characteristic EDS spectra of each element found in the nanoflower S-0 catalyst, where the inset indicates the atomic composition. According to the compositional information, the cation ratio relative to the whole atomic composition is 35.5%, whereas the anion ratio is 64.5%. In comparison to the theoretical percentage, the as-synthesized nanoflower catalyst has higher anion composition, which is more expectedly due to the adsorbed oxygen in the form of a hydroxide ion. The morphology of the hydroxide-enriched nanoflower-like S-0.6 [Fig. 2(c)] is different compared to that of a cerium-free nanoflower catalyst. It looks like a rolled cotton becoming cotton wool balls, which implies that the cerium cation brought a significant change to the morphology of the nanoflower S-0 catalyst. The formed cotton wool ball-like morphology is uniform, which means that no secondary phase was formed upon the addition of cerium cation, and supports the results obtained from XRD analysis. As displayed in the inset of the atomic composition of the S-0.6 catalyst [Fig. 2(d)], the cation composition was changed to 36.15% and that of the anion became 63.85%. This suggests that the cerium ions were diffused to the vacant sites of indium in the crystal structure. From the EDS result, the cerium metal atomic ratio is far from the recipe amount, implying that the precipitation rate of cerium in the formation route of Ce–In2(O,S)3 is lower compared to indium in the given experimental conditions. Hence, the only limited cerium contents were going to the crystal system of indium oxy-sulfide, and brought a significant change over the host catalyst. Conversely, the remaining amounts were washed out during the removal process of unwanted side products using DI water, followed by ethanol. The distribution of all the constituent elements of the hydroxide-enriched nanoflower-like S-0.6 catalyst over the selected area [Fig. S2(a), ESI†] is shown by applying FE-SEM elemental mapping [Fig. S2(b), ESI†].
 | ||
| Fig. 2 FE-SEM images of (a) the nanoflower S-0 catalyst with (b) its EDS composition, and (c) the hydroxide-enriched nanoflower-like S-0.6 catalyst with (d) its EDS composition. | ||
The nature of the morphology of the hydroxide-enriched nanoflower-like S-0.6 catalyst was further investigated using TEM characterization. As clearly seen from the TEM images at low and high magnification [Fig. 3(a) and (b)], the S-0.6 catalyst maintained a rolled cotton type morphology. The petals of the nanoflower are observed to be smaller compared to the cerium-free nanoflower oxy-sulfide catalyst, as observed from the FE-SEM analysis. Lattice fringe analysis is an important method to recognize the crystal structure of a compound.7 It is used as a fingerprint for the identification of the compound itself. The HR-TEM image was somewhat blurred because of the amorphous character of the catalyst. However, upon magnifying the selected area of the image using Gatan Digital Micrograph software, we found the measured distances of 0.21, 0.27, and 0.32 nm between the consecutive atomic layers of the as-synthesized hydroxide-enriched nanoflower-like S-0.6 catalyst, corresponding to the (511), (400), and (311) crystal planes, respectively [Fig. 3(c)]. Having a look at the SAED patterns of the best efficient S-0.6 catalysts [Fig. 3(d)], no clearly distinguished white spots were seen on the rings. Instead, they are the diffusive type, which means that the catalyst has an amorphous nature. The SAED rings of the S-0.6 catalyst towards the outer region were labeled for the (311), (400), and (440) crystal planes, and confirmed no existence of an extra phase, which supports the data obtained from the XRD, FE-SEM, and TEM analyses.
 | ||
| Fig. 3 Low (a) and high (b) magnification TEM images, (c) HR-TEM image, and (d) SAED pattern of the hydroxide-enriched nanoflower-like S-0.6 catalyst. | ||
Analysis of the optical properties of all of the as-synthesized catalyst materials was conducted using UV-Vis spectroscopy. Fig. 4(a) displays the absorption spectra of the host nanoflower S-0, and all of the hydroxide-enriched nanoflower-like Ce–In2(O,S)3 catalysts. The addition of cerium caused a blue shift of the threshold absorption wavelengths compared to the host nanoflower S-0 catalyst. The bandgap energies were calculated using the formula Eg = hc/λ = 1241.57/λ eV, where h is Plank's constant (6.63 × 10−34 J s), c is the speed of light (3 × 108 m s−1), and λ (nm) is the threshold absorption wavelength.37 Hence, the corresponding Eg values of the S-0.5, S-0.6, and S-0.7 catalysts were determined to be 2.48, 2.50, and 2.53 eV, respectively [Fig. 4(b)]. The widening between the valence band and conduction band in the hydroxide-enriched nanoflower-like catalysts is larger compared to that of the cerium-free nanoflower S-0 catalyst (Eg = 2.35 eV). This phenomenon is beneficial to reduce the recombination probability of charge carriers in the as-synthesized catalysts.
 | ||
| Fig. 4 (a) Absorption spectra of the S-0 and Ce–In2(O,S)3 catalysts, and (b) the variation of their bandgap energies with changes in the Ce/[Ce + In] precursor molar ratios. | ||
The oxidation state of each element in the most efficient as-synthesized cerium dopant-induced hydroxide-enriched nanoflower-like S-0.6 catalyst was studied using X-ray photoelectron spectroscopy (XPS) analysis. Fig. 5(a) displays the two asymmetric doublet XPS spectra of In, with a peak-to-peak separation of 7.6, in the nanoflower-like S-0.6 catalyst. The peaks appeared at 444.6 and 452.2 eV for the In 3d5/2 and In d3/2 spin orbits, respectively. As reported in the literature,38 the already mentioned binding energies for the respective spin orbits indicate that In in the catalyst existed in the 3+ oxidation state. The XPS signals suggesting the presence of cerium in the nanoflower-like S-0.6 catalyst are shown in Fig. 5(b). The peaks at binding energies of 885.3 and 903.2 eV are due to Ce 3d5/2, whereas those at 907.0 and 917.6 eV correspond to the Ce 3d3/2 spin–orbit. Hence, Ce existed as Ce4+ in the as-synthesized catalyst.39 As explained from the XRD analysis, sulfur is the major anion component, where it forms the major phase as In2S3 in the cerium-free and the rest are the nanoflower-like Ce–In2(O,S)3 catalysts. Hence, Fig. 5(c) shows the XPS signals of S 2p, where the peaks appeared at 160.6 and 161.7 eV for 2p3/2 and 2p1/2, respectively. The spin splitting was 1.1 eV, implying that sulfur existed as S2− in the nanoflower-like catalyst.40 Oxygen is the minor lattice anionic component in the as-synthesized catalyst, and its XPS signal is designated in Fig. 5(d). The lattice oxygen, as a result of interaction with indium and cerium, has the XPS peaks at a binding energy of 531.03 eV, and the one ascribed to the adsorbed hydroxide grew at 532.30 eV.41 From the XPS characterization, the surface composition of the constituent atom of the catalyst material is directly related to its XPS peak area. Herein, the XPS peaks of the lattice oxygen (Olattice) and adsorbed hydroxide (OOH−) have peak areas of 1347.848 and 1528.289, respectively, which means that the Olattice![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OOH− XPS peak area ratio is 1
OOH− XPS peak area ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.13. Therefore, they both have approximately the same peak area, implying the excess dissemination of hydroxide ion on the surface of the catalyst.42 In most research works, the XPS peak area of oxygen contributed from the adsorbed hydroxide is smaller compared to the one contributed from the lattice oxygen. Cerium, with +4 oxidation state, has more positive charge density than indium, with +3 oxidation state. Hence, it induced the excess adsorption of the hydroxide ion on the surface of the nanoflower-like catalyst. This is indeed valuable for the photocatalyst-assisted Cr(VI) reduction under visible light illumination.
1.13. Therefore, they both have approximately the same peak area, implying the excess dissemination of hydroxide ion on the surface of the catalyst.42 In most research works, the XPS peak area of oxygen contributed from the adsorbed hydroxide is smaller compared to the one contributed from the lattice oxygen. Cerium, with +4 oxidation state, has more positive charge density than indium, with +3 oxidation state. Hence, it induced the excess adsorption of the hydroxide ion on the surface of the nanoflower-like catalyst. This is indeed valuable for the photocatalyst-assisted Cr(VI) reduction under visible light illumination.
 | ||
| Fig. 5 High-resolution XPS spectra of (a) In-3d, (b) Ce-3d, (c) S-2p, and O-1s of the hydroxide-enriched nanoflower-like S-0.6 catalyst. | ||
To study the catalytic activity of all as-synthesized catalysts for the reduction of toxic Cr(VI) under visible light irradiation, 0.20 g L−1 photocatalyst was disseminated in an aluminum foil wrapped glass photo-reactor having 100 mL aqueous solution of potassium dichromate (K2Cr2O7, 20 ppm). The solution was sonicated for 30 min under the dark condition to ensure the adsorption–desorption condition, followed by vigorous stirring just after equipping the photo-reactor with a 150 W halogen lamp with wavelength and flux of 575 nm and 206.91 W m−2, respectively. Around 5 mL solution was taken out at each of 2 min irradiation time intervals, and centrifuged to get the required supernatant. The absorption spectrum of the toxic Cr(VI) solution was examined using UV-Vis absorbance spectrophotometry. For the absorbance measurement, one drop of each 1,5-diphenylcarbazide and concentrated H2SO4 was added to a transparent cuvette containing the supernatant solution.
The hazardous Cr(VI) reducing activity of nanoflower S-0 and all nanoflower-like Ce–In2(O,S)3 catalysts are displayed in Fig. 6(a). As clearly seen, the addition of cerium to the nanoflower catalyst brought significant improvement for the reduction of hazardous Cr(VI). The Cr(VI) reducing efficiencies of S-0, S-0.5, S-0.6, and S-0.7 were found to be 51.3, 96.2, 99.3, and 95.3% within 6 min irradiation time, respectively. Among all of the compositions, the hydroxide-enriched nanoflower-like S-0.6 catalyst exhibited enhanced photo-reduction activity of toxic Cr(VI) under visible light irradiation. The Cr(VI)-reducing capability of the most efficient nanoflower-like catalyst is almost double, compared to that of the nanoflower S-0. The highly positively charged cerium cation induced an excess of hydroxide ions on the surface of the catalyst, and this phenomenon resulted in the greater attraction of Cr(VI) cations towards the surface of the catalyst. As soon as the hydroxide-enriched nanoflower-like S-0.6 catalyst was exposed to the visible light source, the electrons from the valence band of the catalyst were excited to the conduction band and reduced the surface spread Cr(VI) immediately. The reduction of hazardous Cr(VI) over the most efficient catalyst under dark condition showed nothing, but only adsorption of Cr(VI) on the surface of the nanoflower-like catalyst. Therefore, the host catalyst, cerium dopant, and visible light illumination were very important for the effective reduction of Cr(VI). Fig. 6(b) shows the kinetics of the visible light-assisted Cr(VI) reduction reactions over the as-synthesized catalyst materials. From the natural logarithm versus irradiation time plot, the fitting to each reduction reaction suggests that the S-0.6 catalyst has the highest reduction reaction rate constant compared to the rest. The rate constant for the Cr(VI) reduction reaction over the most efficient catalyst is increased by a factor of approximately 5.42 compared to that of the cerium-free nanoflower S-0 catalyst. As displayed in Fig. 6(c), the nanoflower-like S-0.6 with Ce/[Ce + In] molar ratio at 0.6 exhibited the upper maxima from the plot of the rate constant versus the Ce/[Ce + In] molar ratios, signifying that it has the highest reducing activity. From the absorption spectra of the supernatant Cr(VI) solution [Fig. 6(d)], the absorption peak intensity of Cr(VI) at 542 nm became flat at 6 min irradiation time, which means that Cr(VI) was almost completely converted to Cr(III) at this short irradiation time.
The electronic properties of the as-synthesized catalysts were studied using photoluminescence (PL) and electron impedance spectroscopy (EIS) analysis. The PL spectrum is used to deal with the formation and transfer of photogenerated charge carriers. It also gives information about the electron–hole recombination nature in the catalyst material. If the excited electrons are relaxed to the valence band in a short time interval, then the corresponding PL peak intensity becomes shorter compared to the rest.43 As shown in Fig. 7(a), the nanoflower-like S-0.6 catalyst exhibited the lowest PL peak intensity compared to the others. Therefore, the accessibility of the photoexcited electrons for the detoxification of the hazardous Cr(VI) is expectedly highest, and it is the confirmation for its highest catalytic activity shown in Fig. 6(a). The EIS analysis was conducted in a three-electrode system of Ag/AgCl, platinum, and glassy carbon electrodes as the reference, counter, and working electrodes, respectively. The electrolyte solution (0.1 M KCl) was prepared in a 100 mL DI water. The required amount of catalyst sample was dispersed in a solution containing Nafion, DI water, and ethanol with a Nafion![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DI water
DI water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol volume ratio at 0.75
ethanol volume ratio at 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, followed by sonication for 10 min. The above catalyst solution was then loaded on the active tip of the working electrode, and dried in an oven at 80 °C for about 5 min. The EIS characterization was performed in the frequency range of 10 KHz–10 mHz. As clearly shown from the Nyquist plots of all catalyst samples [Fig. 7(b)], the hydroxide-enriched nanoflower-like S-0.6 exhibited a semicircle having the smallest arc compared to the others. This means that the electrons in the catalyst confronted the least resistance to transfer. Hence, this characterization is additional evidence for the best catalytic activity of the hydroxide-enriched nanoflower-like S-0.6 catalyst.
1, followed by sonication for 10 min. The above catalyst solution was then loaded on the active tip of the working electrode, and dried in an oven at 80 °C for about 5 min. The EIS characterization was performed in the frequency range of 10 KHz–10 mHz. As clearly shown from the Nyquist plots of all catalyst samples [Fig. 7(b)], the hydroxide-enriched nanoflower-like S-0.6 exhibited a semicircle having the smallest arc compared to the others. This means that the electrons in the catalyst confronted the least resistance to transfer. Hence, this characterization is additional evidence for the best catalytic activity of the hydroxide-enriched nanoflower-like S-0.6 catalyst.
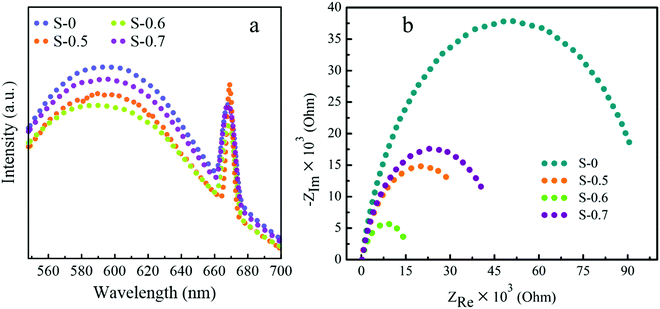 | ||
| Fig. 7 (a) Photoluminescence (PL) spectra and (b) EIS Nyquist plots of S-0, S-0.5, S-0.6, and S-0.7 catalysts. | ||
We have taken some typical previously conducted research works towards Cr(VI) reduction under different conditions, and compared these studies with our works. As summarized in Table 1, the Cr(VI) reductions have been taken at different catalyst dosages, K2Cr2O7 concentrations, and light sources. In the case of all of the mentioned previous works, Cr(VI) reduction has taken a long-reduction time even in the presence of excess catalyst dosage and highly energetic UV light source. In our case, we have used only 20 mg catalyst and completely reduced 200 mg L−1 of K2Cr2O7 under visible light irradiation, and it took only 6 min to completely reduce Cr(VI) into Cr(III), which suggests the best efficient catalytic activity of the hydroxide-enriched Ce–In2(O,S)3 catalyst material.
| Catalyst | Catalyst (mg) | K2Cr2O7 (mg L−1) | Solution (mL) | Light source | Reduction time (min) | Ref. |
|---|---|---|---|---|---|---|
| ZIS/CdS | 50 | 50 | 50 | Visible light-300 W Xe lamp | 30 | 44 |
| BiWO6 | 60 | 10 | 50 | Visible light-300 W Xe lamp | 100 | 45 |
| CC@SnS2/SnO2 | 120 | 10 | 50 | Visible light-300 W Xe lamp | 60 | 46 |
| SnO2/PANI | 300 | 50 | 300 | Visible light | 200 | 47 |
| LDH-TiO2 | 100 | 20 | 100 | Ultraviolet light-5 W Hg lamp | ∼250 | 48 |
| Ce–In2(O,S)3 | 20 | 200 | 100 | Visible light-150 W Xe lamp | 6 | This work |
The recyclability of the hydroxide-enriched nanoflower-like S-0.6 catalyst for the detoxification of the hazardous Cr(VI) was checked in three successive cycles [Fig. 8(a)]. The visible light-assisted reduction of Cr(VI) over the nanoflower-like S-0.6 catalyst in the first run was 99.3% within 6 min irradiation time. Conversely, in the second and third runs, it was found to be 94.4 and 85.6% within 12 and 20 min irradiation times, respectively. The decrease in the reduction efficiency was expectedly due to the coverage of the active sites of the catalyst by Cr(III) in the form of Cr(OH)3. Therefore, the as-synthesized hydroxide-enriched cerium-doped nanoflower-like catalyst is promising for the treatment of hazardous Cr(VI), which in turn, is used to minimize or eliminate the risk due to the exposure to this toxic heavy metal cation. The successful reduction of Cr(VI) into relatively non-toxic Cr(III) was confirmed using XPS analysis. Accordingly, Fig. 8(b) displayed the 2p3/2 and 2p1/2 asymmetric XPS peaks of Cr(III). The two peaks appeared at binding energies of 575.7 and 585.2 eV, respectively, with the peak-to-peak splitting of 9.5 eV.49 A blurred type of morphology is shown in Fig. S3(a) from the ESI,† which is due to the distribution of Cr(III) in the form of Cr(OH)3 on the surface of the hydroxide-enriched nanoflower-like S-0.6 catalyst. Fig. S3(b) (ESI†) displays the elemental mapping of the recycled nanoflower-like catalyst, which indicates the existence of all constituent elements, including Cr(III). The invariance of their oxidation state in the regenerated nanoflower-like catalyst was checked using XPS characterization. Accordingly, Fig. S4 in the ESI† verifies the presence of no noticeable changes in the characteristic XPS peaks for each element in the regenerated catalyst.
 | ||
| Fig. 8 (a) Recyclability test and (b) XPS spectra of Cr(III) in the three times used hydroxide-enriched nanoflower-like S-0.6 catalyst. | ||
The mechanistically illustrated scheme shown in Fig. 9 gives detail and precise information about how the visible light-assisted reduction of toxic Cr(VI) was accomplished over the as-synthesized nanoflower-like catalyst. The highly positively charged cerium cation induced the excess attraction of hydroxide ion towards the surface of the catalyst. The surface-adsorbed hydroxide ions caused a surplus attraction of hexavalent chromium towards the surface of the catalyst. As soon as the nanoflower-like catalyst was exposed to visible light, the electrons on the valence band gained enough energy and were excited to the conduction band. The Mulliken electronegativity theory50 of ECB = χ − Ee − 0.5Eg and EVB = ECB + Eg, where χ is the absolute electronegativity, which is the arithmetic geometric mean of the constituent elements and Ee is the energy of the free electron in the hydrogen scale (∼4.5 eV), was employed to find the valence band-edge potential (EVB) and conduction band-edge potential (ECB) of the nanoflower-like S-0.6 catalyst. Since the conduction band-edge potential of the catalyst (+0.38 eV) is less positive than the reduction potential of (Cr2O7)2− [(Cr2O7)2−/Cr(III) = +1.23 V(E°) (vs. NHE)], the photoexcited electrons easily detoxified the surface-disseminated hazardous Cr(VI).51 In contrast, the valence band-edge potential of the catalyst (+2.88 eV) is more positive compared to the standard oxidation potential of H2O [H2O/O2 = +0.82 V(E°), neutral pH (vs. NHE)], which means that the photogenerated holes easily oxidized water over there.52 Hence, the as-synthesized hydroxide-enriched cerium-doped nanoflower-like catalyst is promising for the detoxification of chronic hexavalent chromium.
 | ||
| Fig. 9 Mechanistic illustration of the visible light-assisted reduction of hazardous Cr(VI) over hydroxide-enriched nanoflower-like S-0.6 catalyst. | ||
4. Conclusion
In this research work, it was confirmed that the cerium dopant to the indium oxy-sulfide catalyst brought rolled cotton wool type morphology. It also resulted in the excess gathering of hydroxide ion on the surface of the catalyst. In the absorption spectra, a blue shift was observed because of the cerium addition to the indium oxy-sulfide catalyst. The electronic properties of the Ce–In2(O,S)3 catalysts were found to be significantly improved compared to the host material. The Cr(VI) reducing efficiency over the most efficient hydroxide-enriched nanoflower-like S-0.6 catalyst was two times greater than that of the cerium-free nanoflower S-0 catalyst. Its Cr(VI) reduction efficiency was 99.3% within 6 min visible light irradiation time. Hence, the as-synthesized hydroxide-enriched nanoflower-like catalyst is very promising for the detoxification of hazardous Cr(VI) from the source environment.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The Ministry of Science and Technology of Taiwan under Grant No. 107-2218-E-011-026 supported this work.References
- W. Zhao, J. Liu, Z. Ding, J. Zhang and X. Wang, J. Alloys Compd., 2020, 813, 152234 CrossRef CAS
.
- F. Zhang, Y.-H. Li, M.-Y. Qi, Z.-R. Tang and Y.-J. Xu, Appl. Catal., B, 2020, 268, 118380 CrossRef CAS
.
- X. Li, J. Xiong, X. Gao, J. Huang, Z. Feng, Z. Chen and Y. Zhu, J. Alloys Compd., 2019, 802, 196–209 CrossRef CAS
.
- P. Dhiman, S. Sharma, A. Kumar, M. Shekh, G. Sharma and M. Naushad, Ceram. Int., 2020, 46, 12255–12268 CrossRef CAS
.
- S. Tang, Z. Wang, D. Yuan, Y. Zhang, J. Qi, Y. Rao, G. Lu, B. Li, K. Wang and K. Yin, Int. J. Electrochem. Sci., 2020, 15, 2470–2480 CrossRef CAS
.
- S. Li, J. Chen, S. Hu, W. Jiang, Y. Liu and J. Liu, Inorg. Chem. Front., 2020, 7, 529–541 RSC
.
- M. A. Zeleke and D.-H. Kuo, Chemosphere, 2019, 235, 935–944 CrossRef CAS
.
-
U. Ashik, A. Viswan, S. Kudo and J.-I. Hayashi, Applications of Nanomaterials, Elsevier, 2018, pp. 45–82 Search PubMed
.
- D. W. Manley and J. C. Walton, Beilstein J. Org. Chem., 2015, 11, 1570–1582 CrossRef CAS
.
- X. Kang, S. Liu, Z. Dai, Y. He, X. Song and Z. Tan, Catalysts, 2019, 9, 191 CrossRef
.
- F. Wang, Q. Li and D. Xu, Adv. Energy Mater., 2017, 7, 1700529 CrossRef
.
- X. Du, X. Yi, P. Wang, J. Deng and C.-C. Wang, Chin. J. Catal., 2019, 40, 70–79 CrossRef
.
- J. Qiu, M. Li, L. Yang and J. Yao, Chem. Eng. J., 2019, 375, 121990 CrossRef CAS
.
- P. Kush, K. Deori, A. Kumar and S. Deka, J. Mater. Chem. A, 2015, 3, 8098–8106 RSC
.
- H. Li, T. Wu, B. Cai, W. Ma, Y. Sun, S. Gan, D. Han and L. Niu, Appl. Catal., B, 2015, 164, 344–351 CrossRef CAS
.
- G. Chen, J. Feng, W. Wang, Y. Yin and H. Liu, Water Res., 2017, 108, 383–390 CrossRef CAS
.
- D. Yang, Y. Sun, Z. Tong, Y. Nan and Z. Jiang, J. Hazard. Mater., 2016, 312, 45–54 CrossRef CAS
.
- L. Zhang, C.-G. Niu, C. Liang, X.-J. Wen, D.-W. Huang, H. Guo, X.-F. Zhao and G.-M. Zeng, Chem. Eng. J., 2018, 352, 863–875 CrossRef CAS
.
- Q. Zhang, M. Wang, M. Ao, Y. Luo, A. Zhang, L. Zhao, L. Yan, F. Deng and X. Luo, J. Alloys Compd., 2019, 805, 41–49 CrossRef CAS
.
- C.-R. Chen, H.-Y. Zeng, S. Xu, J.-C. Shen, G. Hu, R.-L. Zhu, J.-Z. Du and Y.-X. Sun, Appl. Clay Sci., 2018, 165, 197–204 CrossRef CAS
.
- Y. Ma, Y. Zhang, L. Wang, C. Wang, X. Diao and J. Shi, J. Alloys Compd., 2019, 784, 405–413 CrossRef CAS
.
- J. Pan, Z. Guan, J. Yang and Q. Li, Chin. J. Catal., 2020, 41, 200–208 CrossRef CAS
.
- Y. Wang, Y. Su, W. Fang, Y. Zhang, X. Li, G. Zhang and W. Sun, Powder Technol., 2020, 363, 337–348 CrossRef CAS
.
- R. Li, D. Hu, K. Hu, H. Deng, M. Zhang, A. Wang, R. Qiu and K. Yan, Sci. Total Environ., 2020, 704, 135284 CrossRef CAS
.
- Y. Wang, S. Bao, Y. Liu, W. Yang, Y. Yu, M. Feng and K. Li, Appl. Surf. Sci., 2020, 510, 145495 CrossRef CAS
.
- F. T. Bekena, H. Abdullah, D.-H. Kuo and M. A. Zeleke, J. Ind. Eng. Chem., 2019, 78, 116–124 CrossRef CAS
.
- W. L. Kebede, D.-H. Kuo, M. A. Zeleke and K. E. Ahmed, J. Alloys Compd., 2019, 797, 986–994 CrossRef CAS
.
- C. Liang, L. Zhang, H. Guo, C.-G. Niu, X.-J. Wen, N. Tang, H.-Y. Liu, Y.-Y. Yang, B.-B. Shao and G.-M. Zeng, Chem. Eng. J., 2019, 361, 373–386 CrossRef CAS
.
- Y. Ma, Y. Guo, H. Jiang, D. Qu, J. Liu, W. Kang, Y. Yi, W. Zhang, J. Shi and Z. Han, New J. Chem., 2015, 39, 5612–5620 RSC
.
- M. A. Zeleke, D.-H. Kuo, K. E. Ahmed and N. S. Gultom, J. Colloid Interface Sci., 2018, 530, 567–578 CrossRef CAS
.
- Y. Xiao, J. Lee, A. Yu and Z. Liu, J. Electrochem. Soc., 1999, 146, 3623–3629 CrossRef CAS
.
- J. S. Kim, B. Kim, H. Kim and K. Kang, Adv. Energy Mater., 2018, 8, 1702774 CrossRef
.
-
S. L. Suib, New and future developments in catalysis: activation of carbon dioxide, Newnes, 2013 Search PubMed
.
- R. Beltrán-Suito, P. W. Menezes and M. Driess, J. Mater. Chem. A, 2019, 7, 15749–15756 RSC
.
- C. Yoon and D. L. Cocke, J. Non-Cryst. Solids, 1986, 79, 217–245 CrossRef CAS
.
- A. Kudelski, Talanta, 2008, 76, 1–8 CrossRef CAS
.
- H. Chuai, D. Zhou, X. Zhu, Z. Li and W. Huang, Chin. J. Catal., 2015, 36, 2194–2202 CrossRef CAS
.
- W. Yue, F. Wei, C. He, D. Wu, N. Tang and Q. Qiao, RSC Adv., 2017, 7, 37578–37587 RSC
.
- A. Younis, D. Chu and S. Li, J. Phys. D: Appl. Phys., 2012, 45, 355101 CrossRef
.
- M. A. Zeleke and D.-H. Kuo, Environ. Res., 2019, 172, 279–288 CrossRef CAS
.
- A. Kushwaha and M. Aslam, J. Phys. D: Appl. Phys., 2013, 46, 485104 CrossRef
.
- M. S. Burke, M. G. Kast, L. Trotochaud, A. M. Smith and S.
W. Boettcher, J. Am. Chem. Soc., 2015, 137, 3638–3648 CrossRef CAS
.
- L. Zhong, W. Cai and Q. Zhong, RSC Adv., 2014, 4, 43529–43537 RSC
.
- G. Zhang, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, Appl. Catal., B, 2018, 232, 164–174 CrossRef CAS
.
- F. Xu, H. Chen, C. Xu, D. Wu, Z. Gao, Q. Zhang and K. Jiang, J. Colloid Interface Sci., 2018, 525, 97–106 CrossRef CAS
.
- G. Zhang, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, J. Colloid Interface Sci., 2018, 514, 306–315 CrossRef CAS
.
- J. Li, T. Peng, Y. Zhang, C. Zhou and A. Zhu, Sep. Purif. Technol., 2018, 201, 120–129 CrossRef CAS
.
- Y. Yang, L. Yan, J. Li, J. Li, T. Yan, M. Sun and Z. Pei, Appl. Surf. Sci., 2019, 492, 487–496 CrossRef CAS
.
- B. Qiu, C. Xu, D. Sun, Q. Wang, H. Gu, X. Zhang, B. L. Weeks, J. Hopper, T. C. Ho and Z. Guo, Appl. Surf. Sci., 2015, 334, 7–14 CrossRef CAS
.
- S. Samanta, S. Khilari, D. Pradhan and R. Srivastava, ACS Sustainable Chem. Eng., 2017, 5, 2562–2577 CrossRef CAS
.
- W. Liu, M. Wang, C. Xu and S. Chen, Chem. Eng. J., 2012, 209, 386–393 CrossRef CAS
.
- F. E. Osterloh, Chem. Mater., 2007, 20, 35–54 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nj04628k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2021 |

