Cobalt ion redox and conductive polymers boosted the photocatalytic activity of the graphite carbon nitride–Co3O4 Z-scheme heterostructure†
Tao
Li
 a,
Jiandong
Cui
b,
Yezhan
Lin
c,
Kecheng
Liu
a,
Rui
Li
a,
Bo
Wang
c,
Haiquan
Xie
a and
Kui
Li
a,
Jiandong
Cui
b,
Yezhan
Lin
c,
Kecheng
Liu
a,
Rui
Li
a,
Bo
Wang
c,
Haiquan
Xie
a and
Kui
Li
 *c
*c
aEngneering Technology Research Center of Henan Province for Solar Catalysis, School of Chemistry and Pharmaceutical Engineering, Nanyang Normal University, Nanyang 473061, Henan, China
bSchool of Fashion Media, Jiangxi Institute of Fashion Technology, Nanchang 330201, China
cSchool of Materials Science and Engineering, University of Jinan, Jinan 250022, China. E-mail: mse_lik@ujn.edu.cn
First published on 18th November 2020
Abstract
The enhanced photocatalytic hydrogen evolution performance of g-C3N4–Co3O4 2D–1D Z-scheme heterojunctions was achieved by employing the cobalt ion redox and conductive polymers (polyaniline, PANi) for the first time. Specifically, a Co3O4 1D nanobelt acting as the Z-scheme heterostructure component could promote the separation of photo-excited charge carriers and increased the redox capacity, and the conductive PANi brought about the boosted transport efficiency of the charge carriers. Notably, cobalt ions were adopted as the intermediate of the electron transfer between two components for boosting the carrier transport and utilization efficiency. Consequently, the Co3O4 nanobelt, PANi and cobalt ions exhibited a synergistic effect on facilitating the photocatalytic HER performance of the g-C3N4-based heterostructure, and the optimal ternary heterostructure (g-C3N4–PANi–Co3O4) exhibited a photocatalytic H2-evolution rate of 4.61 μmol h−1 under visible light, which further increased to 9.95 μmol h−1 by adding Co2+ solution owing to the further facilitated transport of charge carriers through the redox reaction of cobalt ions. Moreover, even in the absence of sacrificial agents, this Z-scheme system exhibited a photocatalytic hydrogen production activity of 3.35 μmol h−1 in the Co2+ aqueous solution under UV-visible light.
Introduction
Photocatalytic conversion of solar energy into clean hydrogen or carbon energy has been deemed as the most promising method to solve the global energy and environmental problems.1–6 The earth-abundant graphitic carbon nitride seems to be one of the most promising candidate photocatalysts due to its high thermal and chemical stability, environmental friendliness, low cost, and suitable bandgap for visible light absorption.7–10 The 2D layered structure of g-C3N4 could provide a large number of photocatalytic sites, which is beneficial to the surface-based photocatalytic hydrogen evolution reaction (HER).3,11–13 Moreover, the g-C3N4-based photocatalyst has been proved to produce H2O2 in the absence of sacrificial agents.14,15 However, the high carrier recombination rate, low transfer rate and utilization of the photo-generated charge carriers limited its application.3,10,13,16To overcome the shortcomings of g-C3N4, constructing heterojunctions is a feasible idea, where the semiconductors have different work functions, leading to electron transfer from the higher work function to the lower one and the opposite hole transfer process, whereas, in the normal type II heterojunction, the carrier transfer process brings about the weaker reductive and oxidizing capacity in the hydrogen and oxygen production reactions, respectively.17,18 Satisfyingly, the Z-scheme heterostructure provided a new path to inject electrons from the conductive band of one semiconductor (stronger oxidative capacity) to the valence band of the other semiconductor with better reducing capacity, and therefore the electron transfer in the “Z” shape would provide many benefits for photocatalytic reduction owing to the strong redox capacity.3,19–21 Many g-C3N4-based Z-scheme heterojunctions have been reported and selecting a suitable semiconductor to inform a Z-scheme heterojunction with g-C3N4 is a foremost question.22–25
As a classic p-type semiconductor and a versatile transition metal oxide, Co3O4 has a suitable energy band structure and excellent electronic properties, which make it a widely used cocatalyst and electrode material for supercapacitors.26 But the performance in the photocatalytic hydrogen evolution of g-C3N4–Co3O4, which has been proven to be a Z-scheme structure, was reported minimally.27,28 Further investigation of this structure is feasible.
To enhance the transport of photo-generated charge carriers in a photocatalyst, some classic conductive components have been widely reported, such as noble metals, graphene, porous carbon, conductive polymers and even highly crystallized solid solutions, which have been adopted as mediators.10,29–38 In particular, conductive polymers (CPs) have been extensively studied and are promising candidates for facilitating the transport of photo-excited charge carriers between components.34,39 In addition, the insertion of CPs between two photocatalyst components could increase the photocatalytic activity by increasing the visible light absorption, separation and transport efficiency of the photo-excited charge carriers due to their narrow bandgap.34,39,40 In particular, due to its simple synthesis, remarkable catalytic activity, good environmental stability and reversible redox characteristics, PANi is an attractive alternative to improve the carrier performance of the interface between two photocatalyst components.
Redox couples adopted for efficient Z-scheme water splitting should possess the following properties: (i) an appropriate redox potential stretching across the reduction and oxidation potentials of water, (ii) sufficient reversibility (also known as redox cyclability) under reaction conditions without deposition or precipitation, and (iii) good transparency to UV and visible light.19,41 As previously reported, Fe3+/Fe2+, IO3−/I−, [Co(bpy)3]3+/2+ redox couples were adopted as the intermediate of the electron transfer between two photocatalysts for boosting the carrier transport.42–45 However, the occurrence of backward electron transfer to the redox couple and the low redox reaction rate became the downside of this system,46 so further increasing the separation and transport efficiency of photoinduced charge carriers was of particular importance for improving the photocatalytic activity.47,48 In addition, most of the indirect Z-scheme systems are usually faced with the challenge of reaction stability.41 So searching for new redox couples for solid state direct Z-scheme heterostructures is of particular importance for the practical application of photocatalysts for solar energy conversion.49
Herein, a novel g-C3N4–PANi–Co3O4 Z-scheme heterostructure was designed and its photocatalytic performance was systematically investigated. In detail, the Z-scheme heterostructure formed between a Co3O4 nanobelt and g-C3N4 could promote the separation of photo-excited charge carriers and increase the redox capacity, the adoption of the conductive PANi brought about the boosted transport efficiency of the charge carriers, and cobalt ions as the intermediate of the electron transfer in the Z-scheme heterostructure could facilitate the carrier transport and utilization efficiency. Consequently, the photocatalytic HER performance of the optimal ternary heterostructure (g-C3N4–PANi–Co3O4) was 4.61 μmol h−1 and further increased to 9.95 μmol h−1 by adding a certain amount of Co2+ under visible light. Moreover, even in the absence of the sacrificial agent, this “Z-scheme” system exhibited a photocatalytic HER activity of 3.35 μmol h−1 with considerable H2O2 production under UV-visible light irradiation.
Experimental
Synthesis of g-C3N4
The g-C3N4 nanosheet was prepared by a traditional thermal polymerization strategy as reported previously.16,34,50 In a typical procedure, 5 g of C3N3(NH2)3 was placed in a crucible with a cover and then annealed at 650 °C for 2 h in argon. The final yellow powder was then collected.Synthesis of g-C3N4–PANi
To synthesize g-C3N4–PANi, 200 mg g-C3N4 was added to 30 mL deionized water and sonicated in an ultrasonic washer. Then, a suitable amount of aniline was introduced into the aforementioned solution with ultrasonication. 5 mL of an aqueous solution containing 50 mg ammonium persulfate was slowly added dropwise into the above-mentioned solution. After magnetic stirring for 12 hours, the resulting grey-green suspension was centrifuged and washed with water and ethanol three times, respectively. Finally, the product was dried at 60 °C overnight.Synthesis of g-C3N4–PANi–Co3O4
Typically, 200 mg g-C3N4–PANi was dispersed in a 30 mL solution containing suitable amounts of carbamide, NH4F and Co(NO3)2·6H2O. After ultrasonic dispersion, the homogeneous solution was transferred to a 50 mL Teflon-lined reactor and maintained for 10 h at 120 °C. The final grey-black products were rinsed several times with distilled water and ethanol, and dried at 60 °C overnight. The g-C3N4–Co3O4 and Co3O4 control samples were prepared with a similar experimental process using pure g-C3N4 or without the addition of g-C3N4.Results and discussion
X-ray diffraction (XRD) and Fourier Transform Infrared Spectroscopy (FTIR) were employed for confirming the formation of the g-C3N4–PANi–Co3O4 ternary heterostructure. As exhibited in Fig. 1a, the distinct characteristic peak located around 27.4° could be observed in all the g-C3N4-based heterostructures, which was indexed as the (002) crystal plane of g-C3N4.50 After the adoption of Co3O4 and PANi, there was no obvious difference with g-C3N4, except for the slightly reduced peak intensity. The diffraction peaks of the as-prepared pure Co3O4 could be matched with the standard PDF index card of cubic Co3O4 (JCPDS no. 42-1467), which could also be observed in g-C3N4–Co3O4 and g-C3N4–PANi–Co3O4, confirming the formation of the g-C3N4–Co3O4 surface heterostructure.51 The weaker peaks of Co3O4 might imply poor crystallinity, which is probably due to the hydrothermal method and lower synthesis temperature.52–57 In addition, the low content ratio and test conditions might also have caused a difference in XRD results. As displayed in Fig. 1b, four obvious peaks appearing at 810, 887, 2134, and 3000–3300 cm−1 for g-C3N4 could also be found in g-C3N4–PANi and g-C3N4–PANi–Co3O4, with the peaks at about 810 and 887 cm−1 corresponding to the out-of-plane bending vibrations of tri-s-triazine and the peak located at 2134 cm−1 belonging to the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N vibration band.58 Furthermore, the existence of PANi characteristic peaks (N–H stretching vibrations, C–N stretching of a secondary aromatic amine and the C–H benzenoid unit) in g-C3N4–PANi and g-C3N4–PANi–Co3O4 affirmed the formation of the g-C3N4–PANi heterostructure.59
N vibration band.58 Furthermore, the existence of PANi characteristic peaks (N–H stretching vibrations, C–N stretching of a secondary aromatic amine and the C–H benzenoid unit) in g-C3N4–PANi and g-C3N4–PANi–Co3O4 affirmed the formation of the g-C3N4–PANi heterostructure.59
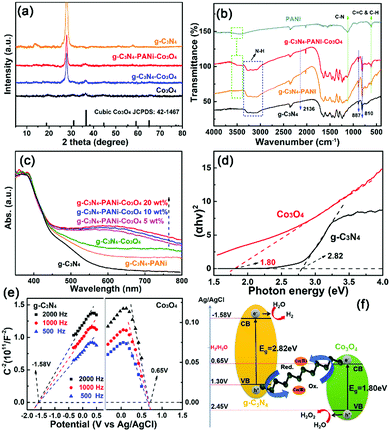 | ||
| Fig. 1 (a) PXRD patterns and (b) FTIR spectra of g-C3N4-based samples; (c) UV-vis absorption spectra and (d) (αhγ)2versus hγ curve of g-C3N4-based samples; (e) Mott–Schottky plots of g-C3N4 and Co3O4; (f) possible mechanism for the separation and transfer of charge carriers over the optimal g-C3N4-PANi-Co3O4 ternary heterostructure with cobalt ions. | ||
The optical absorption characteristics and band structures of the as-synthesized g-C3N4, g-C3N4–PANi, g-C3N4–Co3O4 and g-C3N4–PANi–Co3O4 were evaluated by UV-visible diffuse reflection spectroscopy (Fig. 1c). The loading of PANi and Co3O4 can both promote the visible light absorption, which can be further increased by the co-loading of PANi and Co3O4, whereas the bandgap of g-C3N4 was not affected by the adoption of PANi and Co3O4, indicating the successful construction of the heterostructure. The band structures of g-C3N4 and Co3O4 were also investigated through UV-visible spectroscopy, and the results in Fig. 1d show that their bandgaps were, respectively, 2.82 and 1.80 eV. Using Mott–Schottky plots, the separation and transport directions of electron–hole pairs in g-C3N4–PANi–Co3O4 were confirmed (Fig. 1e), and it could be observed that the flat band potentials of g-C3N4 and Co3O4 were calculated to be −1.52 V and 0.65 V, respectively. Based on the above results, the schematic diagram for the separation and transfer of charge carriers in the g-C3N4–PANi–Co3O4 ternary heterostructure with the participation of cobalt ions is shown in Fig. 1f. Under light irradiation, electrons were stimulated and jumped from the valence band (VB) of Co3O4 to the conduction band (CB) of g-C3N4 through the highly conductive PANi and Co2+/Co3+ pairs as the redox reaction between Co2+ and Co3+ constituted a cycle to deliver electrons. In total, the electrons and holes separately accumulated in the CB of g-C3N4 and the VB of Co3O4, possessing the reduction and oxidation capacity to provide H2 and H2O2. Notably, such a Z-scheme heterostructure promoted the separation of photo-excited charge carriers and increased the redox capacity, which was beneficial for the photocatalytic water splitting reaction.60,61
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) were used to characterize the morphologies of the as-prepared g-C3N4-based heterostructures. The nanosheet morphology of pristine g-C3N4 could be clearly observed from the TEM image (Fig. 2a), whereas Co3O4 exhibited a single layer nanobelt morphology as depicted in the TEM (Fig. 2b) and SEM images of Co3O4 (inset of Fig. 2b), which is favorable for radial carrier transmission.62 It can be observed from Fig. 2c that PANi nanoparticles were uniformly dispersed in the skeleton of g-C3N4.34 Moreover, it could be found from the TEM image of ternary g-C3N4–PANi–Co3O4 (Fig. 2d) that the Co3O4 nanobelt and PANi nanoparticles were uniformly distributed over the g-C3N4 nanosheet. High-resolution TEM (HRTEM) was employed for further investigating the morphology of Co3O4 in the ternary g-C3N4–PANi–Co3O4 heterostructure. As exhibited in Fig. 2e, the lattice fringe with a lattice constant of 0.24 nm could be indexed to the (311) plane of Co3O4. Moreover, g-C3N4–PANi–Co3O4 exhibited a similar morphology on SEM images (Fig. S4, ESI†). Furthermore, elemental mapping was adopted to investigate the distribution of different components. The results reflect that the C, N and the Co, Ni scattered according to the corresponding outline of g-C3N4–PANi and Co3O4 nanobelt.
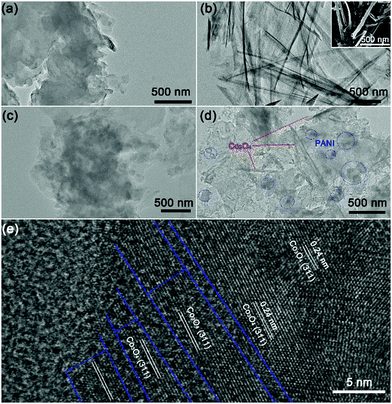 | ||
| Fig. 2 TEM images of (a) g-C3N4, (b) Co3O4 (inset showing the SEM image), (c) g-C3N4–PANi, (d) and g-C3N4–PANi–Co3O4, and (e) the HRTEM image of g-C3N4–PANi–Co3O4. | ||
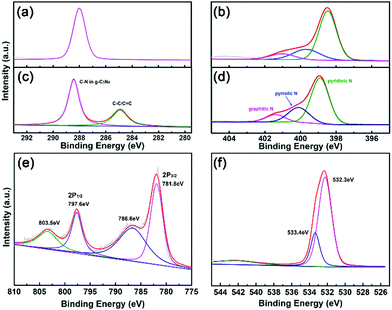
The chemical structure of g-C3N4–PANi–Co3O4 was investigated by X-ray photoelectron spectroscopy (XPS). The C 1s XPS spectra of g-C3N4–PANi–Co3O4 and pure g-C3N4 were comprehensively compared in Fig. 3a. Corresponding to the C–N bonding of g-C3N4, the main peak of g-C3N4–PANi–Co3O4 located at 288.4 eV shifted to a higher energy state than that of pure g-C3N4, which implies the stronger combination and electronic coupling between g-C3N4 and Co3O4.10 Moreover, the peak at 284.8 eV of g-C3N4–PANi–Co3O4 is ascribed to the C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C species of PANi. The N 1s spectrum in Fig. 3b reveals three peaks, including pyridinic N, pyrrolic N and graphitic N. As the majority species, pyridinic-N displays a stronger hydrogen evolution activity, which was beneficial for the hydrogen evolution reaction. Moreover, compared with pure g-C3N4, the above-mentioned peaks also showed the same trend as those in the C 1s spectrum.16,63 The Co XPS spectrum could be fitted into two peaks. As displayed in Fig. 3c, the Co 2p XPS spectra indicate two main binding energy bands at 781.8 eV (Co 2p3/2) and 797.6 eV (Co 2p1/2) with two shakeup satellite peaks at 786.6 and 803.5.64 As depicted in Fig. 3d, two mean different peaks at 532.3 and 533.4 eV correspond to lattice oxygen atoms in the Co3O4 and external water molecule, respectively. Moreover, there was no significant difference for the optimal ternary heterostructure after the photocatalytic reaction in cobalt ions as revealed in the XPS results (Fig. S1, ESI†), indicating that the cobalt ions only acted as redox mediators and are not doped into the catalyst.
C species of PANi. The N 1s spectrum in Fig. 3b reveals three peaks, including pyridinic N, pyrrolic N and graphitic N. As the majority species, pyridinic-N displays a stronger hydrogen evolution activity, which was beneficial for the hydrogen evolution reaction. Moreover, compared with pure g-C3N4, the above-mentioned peaks also showed the same trend as those in the C 1s spectrum.16,63 The Co XPS spectrum could be fitted into two peaks. As displayed in Fig. 3c, the Co 2p XPS spectra indicate two main binding energy bands at 781.8 eV (Co 2p3/2) and 797.6 eV (Co 2p1/2) with two shakeup satellite peaks at 786.6 and 803.5.64 As depicted in Fig. 3d, two mean different peaks at 532.3 and 533.4 eV correspond to lattice oxygen atoms in the Co3O4 and external water molecule, respectively. Moreover, there was no significant difference for the optimal ternary heterostructure after the photocatalytic reaction in cobalt ions as revealed in the XPS results (Fig. S1, ESI†), indicating that the cobalt ions only acted as redox mediators and are not doped into the catalyst.
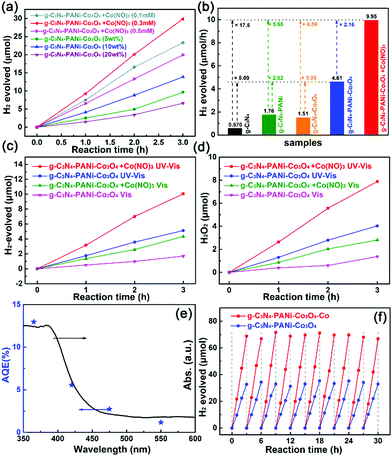
The photocatalytic HER of the g-C3N4–PANi–Co3O4 heterostructure (20 mg) with various contents of Co3O4 under visible light irradiation is compared in Fig. 4a, and the optimal contents of PANi were investigated as shown in Fig. S2 (ESI†). The sample with 10 wt% Co3O4 exhibits optimal hydrogen production activity, and further exploration of the influence of cobalt salt was based on g-C3N4–PANi–Co3O4 with the optimal ratio. The optimal concentration of cobalt salt was 0.3 mmol L−1. Notably, trace amounts of cobalt ions could dramatically improve the photocatalytic hydrogen evolution, and the optimal photocatalytic hydrogen production is 9.95 μmol h−1 under visible light and 22.89 μmol h−1 under UV-visible light (Fig. 4b and Fig. S3, ESI†), which was 2.16 and 5.65 times larger than that of g-C3N4–PANi–Co3O4 and g-C3N4–PANi, respectively. Noticeably, the optimal g-C3N4–PANi–Co3O4-Co system exhibited a photocatalytic hydrogen evolution rate that is 17.5 times larger than that of g-C3N4 and the g-C3N4–Co3O4 heterostructure, indicating the obvious synergistic effect between PANi and Co2+/Co3+ on improving the photocatalytic hydrogen evolution performance of the g-C3N4–Co3O4 heterostructure. As displayed in Table 1, the optimal g-C3N4–PANi–Co3O4 with Co2+/Co3+ exhibited competitive photocatalytic activity in comparison with those of g-C3N4-based heterostructures, g-C3N4 loaded with different types of cocatalysts, and the reported system of g-C3N4–Co3O4 based heterostructures.
| Photocatalyst | Dosage (mg) | H2-evolved (mmol h−1) | Performance improvement multiple (compared with g-C3N4) | Ref. |
|---|---|---|---|---|
| Fe2O3/g-C3N4 | 20 | 7.96 | 13 | 65 |
| Pt/g-C3N4/SrTiO | 50 | 27.6 | 1.86 | 66 |
| Co–Pi/g-C3N4 | 50 | 1.27 | 8.7 | 67 |
| PCN/PANI/BTO | 20 | 12.04 | 21 | 68 |
| Ag3PO4/Ag/g-C3N4 | 50 | 0.205 | 4.88 | 69 |
| g-C3N4/Ag/MoS2 | 100 | 10.40 | 3.51 | 70 |
| MoO3/1T-MoS2/g-C3N4 | 10 | 5.13 | 12 | 71 |
| Co3O4@g-C3N4/CNFs | 5 | 0.34 | — | 27 |
| Co3O4/g-C3N4 | 100 | 5 | 3.3 | 26 |
| g-C 3 N 4 –PANI–Co 3 O 4 with cobalt ions | 20 | 9.95 | 17.5 | This work |
More interestingly, without any sacrificial agents, the optimal “Z-scheme“ system possessed considerable HER performance of 0.62 μmol h−1 under visible light irradiation and 1.44 μmol h−1 under UV-visible light with the corresponding amount of H2O2 production (Fig. 4c and d). Even without Co2+/Co3+ ions, the g-C3N4–PANi–Co3O4 ternary heterostructure also possesses the photocatalytic hydrogen production capacity in pure water. In order to verify the electron-promoted photocatalytic hydrogen evolution reaction in the as-prepared g-C3N4–PANi–Co3O4 ternary heterostructure with the addition of cobalt ions, the apparent quantum efficiency (AQE) of the optimal sample was calculated under different specific wavelength illuminations in Table S1 (ESI†) and are compared in Fig. 4e. At the irradiation wavelengths of 365, 420, 475 and 550 (±8) nm, the corresponding apparent quantum efficiencies were 12.97, 5.56, 2.74 and 1.17%, respectively, indicating the fairly good consistency between the AQE and the UV-visible diffuse reflection spectra. Furthermore, both the ternary g-C3N4–PANi–Co3O4 heterostructure and that with an optimal Co2+ concentration exhibited excellent photocatalytic stability over 30 h (Fig. 4f), which proved the existence of the Co2+/Co3+ redox conducting carrier mechanism.
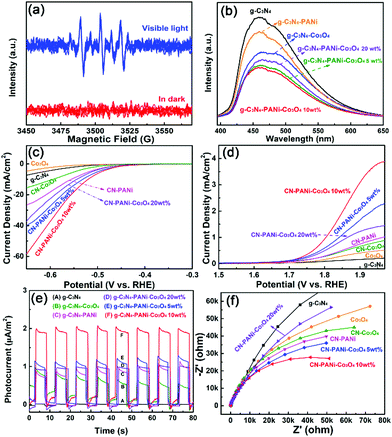
The formation of H2O2 was investigated by Electron Paramagnetic Resonance (EPR) spectroscopy in the photocatalytic reaction process, which was carried out with 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as the spin trapping agent. As depicted in Fig. 5a, under visible light irradiation, there appeared distinct characteristic signals of ˙OH, which proved the oxidation of H2O to ˙OH.16,36 Room temperature photoluminescence (PL) was adopted to prove the important role of PANi and Co3O4 loading in promoting the separation efficiency of the photo-generated charge carriers. As shown in Fig. 5b, the introduction of Co3O4 can significantly quench the strong PL intensity at about 450 nm in theoriginal g-C3N4, which may be related to the formation of g-C3N4–Co3O4 heterostructures to improve the separation efficiency of charge carriers. In particular, owing to the improvement of carrier transport between g-C3N4 and Co3O4, introducing the CPs into g-C3N4–Co3O4 further weakened the PL intensity, which indicates the inhibition of charge carrier combination. In addition, ternary g-C3N4–PANi–Co3O4 with 10 wt% Co3O4 exhibited the lowest carrier recombination rate, consistent with the photocatalytic H2-evolution results.
The electrochemical performances were further evaluated to confirm the extremely important roles of Co3O4 and PANi in the transport and utilization efficiency of photo-generated charge carriers. Electrocatalytic HER and OER measurements were employed for evaluating the behavior of photocatalytic hydrogen and the results are depicted in Fig. 5c and d. By intercalating PANi or CO3O4, the g-C3N4–PANi and g-C3N4–Co3O4 heterostructures exhibited lower HER and OER overpotential than pristine g-C3N4, indicating the improved HER and OER activity. Furthermore, g-C3N4–PANi–Co3O4 showed the lowest HER and OER overpotential. The photocurrent responses in Fig. 5e indicate that the photocurrent density of g-C3N4–PANi–Co3O4 was about 4 times as high as that of the g-C3N4–Co3O4 heterostructure. Electrochemical impedance spectroscopy (EIS) characterization was performed to evaluate the internal resistance during the charge transfer process of the g-C3N4-based samples (Fig. 5f). It could be clearly found that the addition of PANi significantly reduced the semi-circular diameter and the smallest appears in the EIS spectrum of optimal C3N4–PANi–Co3O4 with 10 wt% Co3O4 added, which indicates the important role of PANi in reducing the interfacial charge transport resistance.
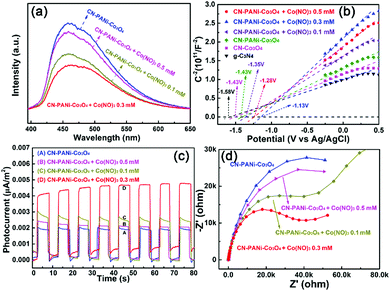
The effect of Co2+/Co3+ ions on the separation, transport and utilization efficiency of charge carriers is shown in Fig. 6. When the ternary g-C3N4–PANi–Co3O4 heterostructure is in a cobalt ionic environment, as shown in Fig. 6a, the intensity of the PL peak was further attenuated. Different degrees of attenuation can be observed, and all the PL intensities at different Co2+ concentrations were weaker than that of the optimal g-C3N4–PANi–Co3O4. Notably, C3N4–PANi–Co3O4 with 0.3 mM Co2+ presented the weakest PL intensity. As displayed in Fig. 6b, the Mott–Schottky plots of g-C3N4, g-C3N4–PANi and g-C3N4–Co3O4 indicated that Co3O4 dramatically improved the density of charge carriers, as evidenced by the increased slope of the Mott−Schottky plots. Notably, the slope of ternary g-C3N4–PANi–Co3O4 samples displayed the maximum enhancement, and the sample with 0.3 mmol L−1 Co2+ showed a larger slope than the sample without cobalt, which indicated the charge carrier density increase. As depicted in Fig. 6c, the participation of cobalt ions also increased the photocurrent density, indicating the cooperation of PANi and Co3O4 for improving the separation efficiency of photogenerated charges. In the presence of the Co2+/Co3+ redox, the ternary heterostructure displayed the smallest interfacial charge transport resistance in the EIS spectrum, which further verified the extremely important role of PANi and cobalt ions in building the “highway” of carrier transport (Fig. 6d). The above results signify that the synergy of PANi and cobalt ions can effectively inhibit the carrier recombination and enhance the transport and utilization efficiency.
Conclusions
Novel g-C3N4–PANi–Co3O4 Z-scheme heterostructures were fabricated and Co2+/Co3+ redox couples were used to achieve efficient photocatalytic hydrogen production activity with and without any sacrificial reagent. The main merits of the ternary g-C3N4–PANi–Co3O4 heterostructure could be attributed to the following aspects: (i) the Co3O4 1D nanobelt acting as the Z-scheme heterostructure component could promote the separation of photo-excited charge carriers and increase the redox capacity; (ii) the conductive PANi brought about the boosted transport efficiency of the charge carriers; (iii) cobalt ions were adopted as the intermediate of the electron transfer between two components for boosting the carrier transport and utilization efficiency. Consequently, the photocatalytic HER performance of the optimal ternary heterostructure (g-C3N4–PANi–Co3O4) was 4.61 μmol h−1 and could be further enhanced to 9.95 μmol h−1 after adding a certain amount of Co2+ under visible light. More significantly, even in the absence of the sacrificial agent, this Z-scheme system exhibited a photocatalytic HER activity of 1.44 μmol h−1 with considerable H2O2 production under visible light and 3.35 μmol h−1 under UV-visible light irradiation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC) (No. 22071081, 21902061 and 21601063), the Opening Project of Key Laboratory of Inorganic Functional Materials and Devices, Chinese Academy of Sciences (Grant No. KLIFMD202007), and the Special Research Project of Nanyang Normal University (ZX2016014).Notes and references
- M. Lu, J. Liu, Q. Li, M. Zhang, M. Liu, J.-L. Wang, D.-Q. Yuan and Y.-Q. Lan, Angew. Chem., Int. Ed., 2019, 58, 12392–12397 CrossRef CAS.
- B. Zhao, Y. Huang, D. Liu, Y. Yu and B. Zhang, Sci. China: Chem., 2020, 63, 28–34 CrossRef CAS.
- Y. Yang, J. Wu, T. Xiao, Z. Tang, J. Shen, H. Li, Y. Zhou and Z. Zou, Appl. Catal., B, 2019, 255, 117771 CrossRef CAS.
- X. Xu, L. Pan, Q. Han, C. Wang, P. Ding, J. Pan, J. Hu, H. Zeng and Y. Zhou, J. Catal., 2019, 374, 237–245 CrossRef CAS.
- X.-B. Meng, J.-L. Sheng, H.-L. Tang, X.-J. Sun, H. Dong and F.-M. Zhang, Appl. Catal., B, 2019, 244, 340–346 CrossRef CAS.
- C. Yang, Z.-D. Yang, H. Dong, N. Sun, Y. Lu, F.-M. Zhang and G. Zhang, ACS Energy Lett., 2019, 4, 2251–2258 CrossRef CAS.
- T. Su, Z. D. Hood, M. Naguib, L. Bai, S. Luo, C. M. Rouleau, I. N. Ivanov, H. Ji, Z. Qin and Z. Wu, Nanoscale, 2019, 11, 8138–8149 RSC.
- Y. Tian, L. Zhou, Q. Zhu, J. Lei, L. Wang, J. Zhang and Y. Liu, Nanoscale, 2019, 11, 20638–20647 RSC.
- K. Li, Y.-Z. Lin, Y. Zhang, M.-L. Xu, L.-W. Liu and F.-T. Liu, J. Mater. Chem. C, 2019, 7, 13211–13217 RSC.
- K. Li, Y. Zhang, Y. Z. Lin, K. Wang and F. T. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 28918–28927 CrossRef CAS.
- L. Lin, Z. Yu and X. Wang, Angew. Chem., Int. Ed., 2019, 58, 6164–6175 CrossRef CAS.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS.
- Y. Yang, Z. Tang, B. Zhou, J. Shen, H. He, A. Ali, Q. Zhong, Y. Xiong, C. Gao, A. Alsaedi, T. Hayat, X. Wang, Y. Zhou and Z. Zou, Appl. Catal., B, 2020, 264, 118470 CrossRef CAS.
- C. Zhu, M. Zhu, Y. Sun, Y. Zhou, J. Gao, H. Huang, Y. Liu and Z. Kang, ACS Appl. Energy Mater., 2019, 2, 8737–8746 CrossRef CAS.
- R. R. Wang, K. C. Pan, D. D. Han, J. J. Jiang, C. X. Xiang, Z. Q. Huang, L. Zhang and X. Xiang, ChemSusChem, 2016, 9, 2470–2479 CrossRef CAS.
- L.-Z. Qin, Y.-Z. Lin, Y.-C. Dou, Y.-J. Yang, K. Li, T. Li and F.-T. Liu, Nanoscale, 2020, 12, 13829–13837 RSC.
- F. Al Marzouqi, Y. Kim and R. Selvaraj, New J. Chem., 2019, 43, 9784–9792 RSC.
- X. Liu, Z. Ni, Y. He, N. Su, R. Guo, Q. Wang and T. Yi, New J. Chem., 2019, 43, 8711–8721 RSC.
- Y. P. Zhang, H. L. Tang, H. Dong, M. Y. Gao, C. C. Li, X. J. Sun, J. Z. Wei, Y. Qu, Z. J. Li and F. M. Zhang, J. Mater. Chem. A, 2020, 8, 4334–4340 RSC.
- M. Zhang, M. Lu, Z.-L. Lang, J. Liu, M. Liu, J.-N. Chang, L.-Y. Li, L.-J. Shang, M. Wang, S.-L. Li and Y.-Q. Lan, Angew. Chem., Int. Ed., 2020, 59, 6500–6506 CrossRef CAS.
- D. Liu, Y. Xu, M. Sun, Y. Huang, Y. Yu and B. Zhang, J. Mater. Chem. A, 2020, 8, 1077–1083 RSC.
- C. Zhao, L. Tian, Z. Zou, Z. Chen, H. Tang, Q. Liu, Z. Lin and X. Yang, Appl. Catal., B, 2020, 268, 118445 CrossRef CAS.
- X. Yang, L. Tian, X. Zhao, H. Tang, Q. Liu and G. Li, Appl. Catal., B, 2019, 244, 240–249 CrossRef CAS.
- L. Tian, X. Yang, X. Cui, Q. Liu and H. Tang, Appl. Surf. Sci., 2019, 463, 9–17 CrossRef CAS.
- W. Liu, J. Shen, X. Yang, Q. Liu and H. Tang, Appl. Surf. Sci., 2018, 456, 369–378 CrossRef CAS.
- L. Yang, J. Liu, L. Yang, M. Zhang, H. Zhu, F. Wang and J. Yin, Renewable Energy, 2020, 145, 691–698 CrossRef CAS.
- R. F. He, H. O. Liang, C. P. Li and J. Bai, Colloids Surf., A, 2020, 586, 124200 CrossRef CAS.
- X. X. Zhao, Z. Y. Lu, R. Ji, M. H. Zhang, C. W. Yi and Y. S. Yan, Catal. Commun., 2018, 112, 49–52 CrossRef CAS.
- H. Tada, T. Mitsui, T. Kiyonaga, T. Akita and K. Tanaka, Nat. Mater., 2006, 5, 782–786 CrossRef CAS.
- K. Li, R. Chen, S.-L. Li, M. Han, S.-L. Xie, J.-C. Bao, Z.-H. Dai and Y.-Q. Lan, Chem. Sci., 2015, 6, 5263–5268 RSC.
- K. Chang, Z. Mei, T. Wang, Q. Kang, S. Ouyang and J. Ye, ACS Nano, 2014, 8, 7078–7087 CrossRef CAS.
- Q. Xiang, J. Yu and M. Jaroniec, J. Am. Chem. Soc., 2012, 134, 6575–6578 CrossRef CAS.
- M. D. A. Khan, A. Akhtar and S. A. Nabi, New J. Chem., 2015, 39, 3728–3735 RSC.
- K. Li, Y.-Z. Lin, K. Wang, Y. Wang, Y. Zhang, Y. Zhang and F.-T. Liu, Appl. Catal., B, 2020, 268, 118402 CrossRef CAS.
- M. O. Ansari, M. M. Khan, S. A. Ansari and M. H. Cho, New J. Chem., 2015, 39, 8381–8388 RSC.
- Y.-Z. Lin, K. Wang, Y. Zhang, Y.-C. Dou, Y.-J. Yang, M.-L. Xu, Y. Wang, F. Liu and K. Li, J. Mater. Chem. C, 2020, 8, 10071–10077 RSC.
- S. Li, T. Zhu, L. Dong and M. Dong, New J. Chem., 2018, 42, 17644–17651 RSC.
- S. Nilforoushan, M. Ghiaci, S. M. Hosseini, S. Laurent and R. N. Muller, New J. Chem., 2019, 43, 6921–6931 RSC.
- Q. Zhou and G. Shi, J. Am. Chem. Soc., 2016, 138, 2868–2876 CrossRef CAS.
- Y.-H. Yao, J. Li, H. Zhang, H.-L. Tang, L. Fang, G.-D. Niu, X.-J. Sun and F.-M. Zhang, J. Mater. Chem. A, 2020, 8, 8949–8956 RSC.
- Y. O. Wang, H. Suzuki, J. J. Xie, O. Tomita, D. J. Martin, M. Higashi, D. Kong, R. Abe and J. W. Tang, Chem. Rev., 2018, 118, 5201–5241 CrossRef CAS.
- K. Sayama, K. Mukasa, R. Abe, Y. Abe and H. Arakawa, Chem. Commun., 2001, 2416–2417 RSC.
- R. Abe, K. Shinmei, K. Hara and B. Ohtani, Chem. Commun., 2009, 3577–3579 RSC.
- Y. Sasaki, H. Kato and A. Kudo, J. Am. Chem. Soc., 2013, 135, 5441–5449 CrossRef CAS.
- Y. Miseki, S. Fujiyoshi, T. Gunji and K. Sayama, Catal.: Sci. Technol., 2013, 3, 1750 RSC.
- O. Tomita, S. Nitta, Y. Matsuta, S. Hosokawa, M. Higashi and R. Abe, Chem. Lett., 2017, 46, 221–224 CrossRef CAS.
- H. Dong, X.-B. Meng, X. Zhang, H.-L. Tang, J.-W. Liu, J.-H. Wang, J.-Z. Wei, F.-M. Zhang, L.-L. Bai and X.-J. Sun, Chem. Eng. J., 2020, 379, 122342 CrossRef CAS.
- B. Wang, S. He, L. L. Zhang, X. Y. Huang, F. Gao, W. H. Feng and P. Liu, Appl. Catal., B, 2019, 243, 229–235 CrossRef CAS.
- H. Dong, X. Zhang, Y. Lu, Y. Yang, Y.-P. Zhang, H.-L. Tang, F.-M. Zhang, Z.-D. Yang, X. Sun and Y. Feng, Appl. Catal., B, 2020, 276, 119173 CrossRef CAS.
- G. Zhang, S. Zang and X. Wang, ACS Catal., 2015, 5, 941–947 CrossRef CAS.
- M. Kang, H. Zhou, N. Zhao and B. L. Lv, CrystEngComm, 2020, 22, 35–43 RSC.
- Q. Xu, P. Zhao, Y. K. Shi, J. S. Li, W. S. You, L. C. Zhang and X. J. Sang, New J. Chem., 2020, 44, 6261–6268 RSC.
- Y. Zhu, T. Wan, X. Wen, D. Chu and Y. Jiang, Appl. Catal., B, 2019, 244, 814–822 CrossRef CAS.
- J. Ganesamurthi, M. Keerthi, S. M. Chen and R. Shanmugam, Ecotoxicol. Environ. Saf., 2020, 189, 110035 CrossRef CAS.
- M. Y. Zhu, S. J. Yu, R. Y. Ge, L. Y. Feng, Y. X. Yu, Y. Li and W. X. Li, ACS Appl. Energy Mater., 2019, 2, 4718–4729 CrossRef CAS.
- C. Qian, X. Guo, W. Zhang, H. Yang, Y. Qian, F. Xu, S. Qian, S. Lin and T. Fan, Microporous Mesoporous Mater., 2019, 277, 45–51 CrossRef CAS.
- Q. Guo, C. W. Zhang, C. F. Zhang, S. Xin, P. C. Zhang, Q. F. Shi, D. W. Zhang and Y. You, J. Energy Chem., 2020, 41, 185–193 CrossRef.
- F. He, G. Chen, Y. Yu, S. Hao, Y. Zhou and Y. Zheng, ACS Appl. Mater. Interfaces, 2014, 6, 7171–7179 CrossRef CAS.
- L. Ge, C. Han and J. Liu, J. Mater. Chem., 2012, 22, 11843–11850 RSC.
- X. Zhao, Z. Lu, R. Ji, M. Zhang, C. Yi and Y. Yan, Catal. Commun., 2018, 112, 49–52 CrossRef CAS.
- H. Wu, C. Li, H. Che, H. Hu, W. Hu, C. Liu, J. Ai and H. Dong, Appl. Surf. Sci., 2018, 440, 308–319 CrossRef CAS.
- K. Li, M. Han, R. Chen, S.-L. Li, S.-L. Xie, C. Mao, X. Bu, X.-L. Cao, L.-Z. Dong, P. Feng and Y.-Q. Lan, Adv. Mater., 2016, 28, 8906–8911 CrossRef CAS.
- Y. Sun, J. Jiang, Y. Liu, S. Wu and J. Zou, Appl. Surf. Sci., 2018, 430, 362–370 CrossRef CAS.
- H.-L. Zhu and Y.-Q. Zheng, Electrochim. Acta, 2018, 265, 372–378 CrossRef CAS.
- Q. Xu, B. Zhu, C. Jiang, B. Cheng and J. Yu, Sol. RRL, 2018, 2, 1800006 CrossRef.
- J.-T. Lee, Y.-J. Chen, E.-C. Su and M.-Y. Wey, Int. J. Hydrogen Energy, 2019, 44, 21413–21423 CrossRef CAS.
- L. Ge, C. Han, X. Xiao and L. Guo, Appl. Catal., B, 2013, 142–143, 414–422 CrossRef CAS.
- Q. N. Li, Y. G. Xia, K. L. Wei, X. T. Ding, S. Dong, X. L. Jiao and D. R. Chen, New J. Chem., 2019, 43, 6753–6764 RSC.
- M. You, J. Pan, C. Chi, B. Wang, W. Zhao, C. Song, Y. Zheng and C. Li, J. Mater. Sci., 2017, 53, 1978–1986 CrossRef.
- D. Lu, H. Wang, X. Zhao, K. K. Kondamareddy, J. Ding, C. Li and P. Fang, ACS Sustainable Chem. Eng., 2017, 5, 1436–1445 CrossRef CAS.
- J.-W. Shi, Y. Zou, D. Ma, Z. Fan, L. Cheng, D. Sun, Z. Wang, C. Niu and L. Wang, Nanoscale, 2018, 10, 9292–9303 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nj04322b |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2021 |