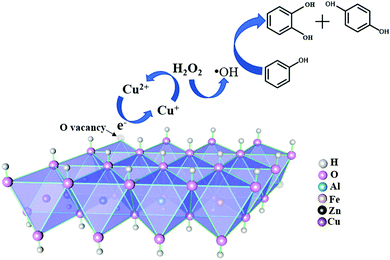
Enhanced catalytic phenol hydroxylation by CuZnFeAl layered double hydroxides: synergistic effects of Cu+ and oxygen vacancies†
Yong
Jiang‡
a,
Wenlong
Xu‡
b,
Jinhua
Liang
*b,
Jiecan
Shen
a,
Xiaomin
Fu
b,
Haimin
He
a,
Shichang
Yan
a and
Xiaoqian
Ren
 *a
*a
aCollege of Chemistry and Chemical Engineering, Nanjing Tech University, Nanjing, 211816, China. E-mail: xqren@njtech.edu.cn; Tel: +86 18915906233
bCollege of Biotechnology and Pharmaceutical Engineering, Nanjing Tech University, Nanjing, 211816, China. E-mail: jhliang@njtech.edu.cn; Tel: +86 13770598601
First published on 27th October 2020
Abstract
In this work, a series of CuZnFeAl-LDH catalysts for phenol oxidation to dihydroxybenzene have been prepared through a co-precipitation method. Versatile characterization studies are applied to reveal electron transfer from oxygen vacancies to Cu2+ on the LDH surface. The resulting Cu+ benefits the formation of hydroxyl radicals to promote the catalytic activity. Besides, through inverse gas chromatography (IGC), the acid–base hydrotalcite surface can be quantitatively determined. Both the oxygen vacancies and acid–base ratio (Ka/Kb) abide by a volcano-like tendency with the addition of copper content, which is consistent with the catalysis result. Among all these catalysts, 15/CuZnFeAl-LDH presents the optimal conversion (66.9%), selectivity (71.3%), and stable recyclability under mild conditions (60 °C, 1.0 MPa), respectively, and is environmentally-friendly and energy efficient. The high efficiency of this catalyst is mainly attributed to the synergistic effect between Cu+ and oxygen vacancies promoted by Ka/Kb.
Introduction
Phenol hydroxylation is one of the most important Fenton reactions in industrial applications.1–10 The main products are catechol (CAT) and hydroquinone (HQ) which are widely used as raw materials in photography chemicals,4 antioxidants,6 polymerization inhibitors,8 and medicine.10 The traditionally used catalysts in this field are homogenous catalysts, such as soluble iron and cobalt salt, which present great merits resulting in high catalytic activity and selectivity due to the accessibility of catalytic sites.11,12 However, the unavoidable drawbacks including the employment of harsh reaction conditions and toxic metallic reagents and the difficulty in separation of the used catalysts and recyclability restrict their commercial expansion.12–14 To solve these problems, versatile heterogeneous catalysts including zeolites,15 metal oxides,10,16 and hydrotalcites17,18 have attracted people's attention during the last few decades.19–21 Among all these catalysts, hydrotalcite exhibits significant advantages as a preferable candidate over other solid Fenton catalysts owing to its good conversion, neutral reaction conditions, and environmentally-friendly properties.17,21,22Layered double hydroxides (LDHs) can be described by the general formula [MII1−xMIIIx(OH)2] [An−]x/n·mH2O, where MII and MIII represent divalent and trivalent metal ions, respectively, while A is the interlayer anion and x is normally between 0.2 and 0.4. The layered and octahedral structure of these materials could provide a laminal platform for electron exchange.23,24 The flexibility of the composition,17 especially by adjusting the species and contents of transition metal elements in the laminate, could modulate the selective oxidation properties in the reaction. Du and colleague21 compared a series of alcohol oxidation experiments by using MMgMn-LDH (M = Zn2+, Cu2+ and Fe3+) catalysts to explore the higher catalytic effects. Zhang et al.25 recently claimed an optimum molar ratio of Zn/Al in ZnAl hydrotalcites and their calcinated products for the photocatalytic degradation of RhB, for achieving higher activity and selectivity. Therefore, the suitable arrangement of different transition elements such as Cu, Zn, Fe, and Al may bring forth a prospective catalytic effect in phenol hydroxylation.
In addition, despite hydrotalcite being a typical alkaline material, previous studies have also indicated that the acidity of the catalyst plays a critical role in the reaction process.17,26 Most of the corresponding studies on the acidity and alkalinity of hydrotalcites only focused on the pH conditions during synthesis and calcination modification.17,27,28 Besides, the traditional techniques for characterizing hydrotalcites cannot confirm the acidity resulting from their inferior stability and trace amount.27,28 Furthermore, to find out a simple way to enhance the catalytic activity by adjusting the microstructure of these basic sites is still a challenge.
In this regard, rarely reported quaternary hydrotalcites CuZnFeAl can be selected to match the request to a highly efficient and environmentally friendly catalyst in the hydroxilation of phenol. Cu2+ and Fe3+ in the hydrotalcites could provide active sites via a Fenton-like process,24 while the acidity and alkalinity can be adjusted by doping with Al and Zn elements. Besides, through investigating the equilibrium interaction of adsorption and separation between small organic molecules and the tested samples, IGC can reflect the surface property parameters of the stationary phase,29 which can further determine the acidity and basicity of materials under mild conditions efficiently and accurately. Therefore, herein, we report the catalytic performance of a series of CuZnFeAl quaternary hydrotalcites with various copper contents for the selective hydroxylation of phenol under mild conditions, which is environmentally-friendly and energy efficient. The effects of various parameters including copper content, reaction temperature, catalyst dosage and oxidant amount are investigated in detail. With the help of IGC, the accurate acidity and alkalinity details of the resulting catalysts can be determined. Moreover, the synergetic effects of Cu+ and oxygen vacancies are also explored, and a possible reaction mechanism is proposed at the end. According to the reasonable analysis and comparison with previous studies,30–32 the catalysts chosen in this work present their superiority in the field of environmental protection due to the application of green solvents and mild reaction conditions, which would further promote the revolution of green chemistry.1
Results and discussion
Structural characterization
The powder XRD patterns of the as-synthesized material with different copper contents are shown in Fig. 1. All the samples present similar patterns to those of the hydrotalcite like structure. The LDH phase is mainly observed as the crystalline phase in all the samples. The sharp and intense peaks at low diffraction angles around 11°, 23° and 34° are ascribed to the diffraction by the basal planes (003), (006) and (009), respectively. The obscure peaks at higher angles around 39°, 46°, 59° and 61° are ascribed to the (015), (018), (110) and (113) planes,24 which confirms the formation of a hydrotalcite lamellar structure (JCPDS 089-0460). The basal spacing of the (003) plane (d003) is around 0.76 nm, consistent with the value of CO32−. This result may indicate that carbonate serves as the main anion between the layers due to the existence of excess Na2CO3 in the synthesis. However, the basal spacing of the (003) peak increases from 0.753 nm for 5/CuZnFeAl-LDH to 0.760 nm for 25/CuZnFeAl-LDH. This phenomenon can be explained by an increase in the layer charge density, due to the large electronegativity of copper (1.9) compared to that of zinc (1.65).33 All the samples present sharp and symmetric reflections in low diffraction angles which may have resulted from the similarity of the octahedral ionic radii of Cu2+ (0.073 nm) and Zn2+ (0.074 nm). This indicates the good crystallinity and high purity of the synthesized CuZnFeAl-LDH samples.34 With the increasing Cu ratio, the crystallinity of CuZnFeAl-LDH declines gradually. When X reaches more than 5, an obvious reflection at 36° assigned to Cu(OH)2 (JCPDS 089-0460) can be observed due to the Jahn–Teller distortion of Cu2+.21,35 It is worth noting that the synthesized quaternary hydrotalcite shows some nonnegligible small diffraction peaks, which may have resulted from the replacement of Mg2+ and Al3+ with the addition of Cu2+, Zn2+, and Fe3+, respectively. Since the radius of Cu2+ and Fe3+ (0.055 nm) is larger than that of Mg2+ and Al3+ (0.054 nm), there are some small peaks in the XRD image, which may be due to the precipitation of one or another metal element.23The morphology of the X/CuZnFeAl-LDH surface with different copper contents is investigated by SEM. As shown in Fig. 2, the microstructures of all the samples consist of crystallites with different sizes and show a flake-like structure, which is in good agreement with the layered structure in LDH. The particle morphology of X/CuZnFeAl-LDH is found to be well-crystallized in a size of 200–400 nm, while nonuniform distribution of the particle size is noted, and upon further addition of Cu, the size of the particles would decline due to the Jahn–Teller effect.19 However, the shape of the hydrotalcite is relatively regular and the surface impurities are small, which matches well with the structure shown in the XRD patterns.
 | ||
| Fig. 2 Scanning electron microscopy images of X/CuZnFeAl-LDH: (a) 5/CuZnFeAl-LDH, (b) 10/CuZnFeAl-LDH, (c) 15/CuZnFeAl-LDH, (d) 20/CuZnFeAl-LDH, and (e) 25/CuZnFeAl-LDH. | ||
The metal composition and lattice parameters for the five samples are summarized in Table 1. As shown in ICP, the molar ratio of copper, zinc, iron and aluminum in the X/CuZnFeAl-LDH materials is close to the theoretical calculation, indicating the successful synthesis of hydrotalcite materials. The small deviation can be attributed to the preferential precipitation of one or another metal ions as hydroxide.23 The lattice parameter a is a function of the average distance of metal cations within the layers, which can be calculated by a = 2 × d110 and the lattice parameter c is the value in the crystallite size counted by using the Scherrer equation. It is clear that a almost does not change at all with the increase of copper content. This may be attributed to the similar ionic radii of Cu2+ (0.073 nm) and Zn2+ (0.074 nm). In addition, it can be found that the lattice parameter c has some concomitant relation with the charge of the layer. The higher the charge density of the layer, the smaller the lattice parameter c. An obvious increase in the lattice parameter c from 5/CuZnFeAl-LDH to 15/CuZnFeAl-LDH can be observed in Table 1. Since the electronegativity of copper is higher than that of zinc, when the copper content increases, the positive charge of the laminate falls, which leads to an increase of the interlayer distance.36 When the Cu/Zn molar ratio further improves, the lattice parameter c drops again.19 This volcano-like change in the layer charge density is consistent with the XRD analysis.
| Samples | (Cu/Zn/Fe/Al) molar ratios | Cu (%) | a (nm) | c (nm) |
|---|---|---|---|---|
| 5/CuZnFeAl-LDH | 5.15/70.90/12.21/11.74 | 5.15 | 0.309 | 2.260 |
| 10/CuZnFeAl-LDH | 10.10/65.75/12.18/11.98 | 10.10 | 0.309 | 2.264 |
| 15/CuZnFeAl-LDH | 15.05/60.40/12.37/12.18 | 15.05 | 0.310 | 2.287 |
| 20/CuZnFeAl-LDH | 20.17/55.96/12.15/11.71 | 20.17 | 0.309 | 2.275 |
| 25/CuZnFeAl-LDH | 25.63/49.76/12.50/12.12 | 25.63 | 0.309 | 2.279 |
The FTIR result of the five catalyst samples is shown in Fig. 3, which presents two broad bands around 3450 cm−1 and 1629 cm−1. They can be ascribed to the stretching vibration of the hydroxyl and the bending vibration of the interlayer water molecules,37 respectively. The position is closely related to the distribution of the charge density between the layers. A vibration peak appears near 1355 cm−1, which is attributed to the asymmetric stretching vibration of carbonate.38 This result coincides with the XRD patterns mentioned before, which could confirm that carbonate is the main anion in the interlayer. However, there are some obvious decreases in the intensity of the CO32− band vibration with the increasing copper content. This may indicate that with the reducing electron density around the hydroxyl groups, the bond strength of the hydrogen atoms binding between the OH groups in the layer and the CO32− anions would decline consequently, which is associated with the increasing number of Cu2+ cations coordinated to the OH groups in the layers.39 Moreover, a series of peaks can be observed in a range under 1000 cm−1, which is attributed to the vibrations of metal–oxygen and metal–hydroxyl groups in the lattice of LDHs.40
The thermal stability of X/CuZnFeAl-LDH is also investigated by TG-DSC experiments and is presented in Fig. 4. All the TG curves show three weight loss sections, corresponding to three exothermal peaks in the DSC curves. The first mass loss from 30 to 160 °C is mainly ascribed to dehydration. The second mass loss from 160 to 300 °C can be attributed to the dehydroxylation of the LDH lattice, which degrades its polarity gradually. Besides, the thermal decomposition of the interlayer CO32− anions can be observed from the mass loss in the range of 300–600 °C. These results are consistent with the consequent XRD and FTIR analysis, which indicates that carbonate is the counter anion between layers. According to the DSC curves, a trend can be found that the peaks corresponding to the first and second stages in the TG results shift to the lower temperature with the increase of copper content, which suggests the degraded stability of LDHs with the increase of copper content. In addition, it is intriguing to find that there are two plausible reasons dedicated to this phenomenon. First, because the Cu2+ electronegativity is greater than that of Zn2+, the electron density around the hydroxyl groups on the layer could be reduced, which would weaken the hydrogen bond strength between the interlayer anions and water molecules. The electrostatic interaction between the interlayer anions would be heavily deteriorated, facilitating further mass loss presented in the TG results.41 In addition, the occurrence of Cu(OH)2 due to the Jahn–Teller effect also plays a critical role in the decline of the hydrotalcite stability.
The textural properties of these five as prepared samples are shown in Table 2. The larger surface area provides good capacity of adsorption and more active sites accessible for the production of ˙OH. It is worth noting that the specific surface area of the most active 15/CuZnFeAl-LDH catalyst (91.8 m2 g−1) is intriguingly not the largest. This may indicate that the specific surface area of the catalyst is not the decisive factor for the activity in catalysis.
| Catalysts | S BET/m2 g−1 | V T/cm3 g−1 | Conversiona (%) | Selectivitya (%) |
|---|---|---|---|---|
| a Reaction conditions: reaction temperature 60 °C; reaction time 40 min; n(H2O2)/n(phenol) = 3; 5% catalyst dosage. | ||||
| 5/CuZnFeAl-LDH | 60.9 | 0.37 | 19.2 | 34.4 |
| 10/CuZnFeAl-LDH | 104.7 | 0.51 | 34.6 | 38.1 |
| 15/CuZnFeAl-LDH | 91.8 | 0.46 | 66.9 | 71.3 |
| 20/CuZnFeAl-LDH | 81.8 | 0.38 | 50.1 | 60.5 |
| 25/CuZnFeAl-LDH | 69.8 | 0.36 | 47.6 | 49.8 |
To further investigate the chemical states and compositions on the surface of X/CuZnFeAl-LDH, XPS measurements are performed in this work. The Cu 2p, Zn 2p, Fe 2p and O 1s spectra are shown in Fig. 5. The asymmetric Cu 2p peaks contribute to the different chemical states of Cu in CuZnFeAl-LDH. Two broad peaks ascribed to the Cu 2p spectra in the range of 930.0–937.0 eV and 950.0–957.0 eV can be observed in Fig. 5a. The convolution peaks at about 933.0 and 953.0 eV can be attributed to the binding energy of the 2p3/2 and 2p1/2 orbitals on Cu+, while the peaks located at about 934.0 and 954.0 eV can be assigned to the spin–orbit splitting of the Cu2+ 2p3/2 and 2p1/2 orbitals.32,42 It is worth noting that the presence of Cu+ could suggest electron transfer between metals in the brucite-like layer. In addition, the Cu 2p binding energy of both Cu(II) and Cu(I) shifts with the increasing copper content, indicating that the copper surface locates in a different electronic environment.43 Among all these five samples, 15/CuZnFeAl-LDH demonstrates the lowest binding energy at 933.08 eV, which indicates its surface copper receiving more electrons to form a higher amount of Cu+ species.19 However, the main 2p3/2 corresponding satellite peaks can be observed at 944.1 and 942.1 eV corresponding to the Cu(OH)2 satellite peak.37,44 The peak intensity increases with the addition of copper content, which indicates the increase of the Cu(OH)2 percentage in LDH. This result is consistent with the XRD pattern (Fig. 1). When the ratio of the Cu content is more than 5%, Cu(OH)2 will be present unavoidably.
Fig. 5b illustrates the Zn 2p XPS spectrum, and the peaks at 1045.6 and 1022.8 eV can be ascribed to the 2p1/2 and 2p3/2 of Zn2+, respectively.45 Besides, the Fe 2p spectra are deconvolved into 3 peaks: the main Fe 2p3/2 peak of Fe3+ around 712.0 eV, its satellite line around 719.0 eV, and the main Fe 2p1/2 peak of Fe3+ around 725.0 eV, as demonstrated in Fig. 5c and Table 3.46 However, no obvious Fe2+ 2p3/2 and Fe2+ 2p1/2 spectra as well as satellite peaks can be found in this work, which indicates that all the Fe species are presented as Fe3+ in all the samples.
| Samples | Binding energy (eV) | Percentage (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu 2p3/2 | Cu 2p1/2 | Fe 2p3/2 | Fe 2p1/2 | OVs | Cu+ | ||||
| Cu+ | Cu2+ | Cu+ | Cu2+ | Fe3+ | Fe3+ | Fe3+ | |||
| 5 | 933.18 | 933.98 | 952.98 | 953.88 | 711.75 | 718.60 | 725.04 | 33.1 | 48.7 |
| 10 | 933.38 | 934.23 | 953.13 | 954.53 | 711.63 | 718.46 | 725.07 | 35.3 | 44.4 |
| 15 | 933.08 | 934.88 | 953.18 | 954.73 | 711.60 | 718.46 | 724.86 | 35.9 | 53.4 |
| 20 | 933.13 | 934.98 | 952.98 | 954.83 | 711.53 | 718.08 | 724.38 | 29.6 | 51.5 |
| 25 | 933.23 | 934.98 | 953.13 | 954.83 | 711.67 | 718.20 | 724.54 | 30.3 | 38.0 |
Generally, various oxygen species play a critical role in redox reactions.47 The O species in X/CuZnFeAl-LDH are also analysed as shown in Fig. 5d. In this work, all the O 1s spectra are deconvolved into 3 divisions: the lattice oxygen and CO32− (530.9 eV), adsorbed molecular water and hydroxyls (531.7 eV), and the surface adsorbed oxygen originating from the defect site (532.4 eV).19,48 Among these three divisions, the defect oxygen ratio positively correlates to the change of surface oxygen vacancy (OV) concentration.49 Owing to their higher mobility, they are more active than lattice oxygen, which illustrates a key role in oxidation reactions.50 In terms of this, an O 1s binding energy shift from 531.73 to only 531.83 eV is observed; the maximal value 531.83 eV appearing in 15/CuZnFeAl-LDH confirms the increase of oxygen defects and electron transfer between copper and oxygen to accelerate the reaction,19,51 while the unexpected slight shift in the binding energy can be attributed to the strong synergistic interactions between Cu, Zn, Fe, and Al metal elements.52
Besides, quantitative analyses of the Cu 2p, Fe 2p and O 1s signals by integrating the peak area for the X/CuZnFeAl-LDH catalysts are shown in Table 3. The percentage of OVs and Cu+ was acquired by the calculation of Gaussian–Lorentzian (G–L) deconvolution. Compared with their pristine oxide counterparts, it indicates that all metals in LDHs present lower binding energies, which may have resulted from the lack of an M1–O–M2 binding structure in pure metal oxides. The percentage of Cu+ presents no regular change with the increase of copper content on the hydrotalcite surface. When the copper content in the hydrotalcite is more than 15%, the ratio of Cu+ rapidly drops from 53.4% to 38.0%, which can be ascribed to the Jahn–Teller effect of Cu2+ and a new Cu(OH)2 phase can be formed, as verified by the XRD spectra. Therefore, when the copper content reaches 15%, the content of oxygen vacancies reached the maximum value of 35.9%, which presents the best potential in the following evaluation of catalytic phenol hydroxylation.
The inverse gas chromatography study
The equilibrium between the acid and basic sites is a key factor to determine the activity of phenol hydroxylation.53 The IGC technique reflects the surface property parameters of the fixed phase based on the equilibrium interaction between the adsorption and separation by small organic molecules with different acid or base properties.29,54 Therefore, it can be applied to quantitatively and qualitatively detect the acidity and alkalinity of hydrotalcite. The results are shown in Table 4; among them, Ka and Kb represent the acidic and basic parameters of the hydrotalcite surface, respectively. With the increasing copper content, the value of the acidic parameters Ka presents a volcano-like variation tendency initially increasing from 0.1324 to 0.2673, followed by dropping to 0.2649. Simultaneously, the basic parameter value of Kb abides by the reverse tendency, which decreases from 1.2973 to 1.1930 followed by rising to 1.3686. This change can be attributed to the acidic reinforcement which covers the basic sites on the hydrotalcite surface.42,44 Besides, with the amount of basic metal hydroxide Cu(OH)2 increasing, the surface alkalinity begins to reinforce again. However, all the values of Ka/Kb are smaller than 1, which indicates that the alkalinity is stronger than their acidity. The hydrotalcite can serve as a kind of strong alkali but weak acidic salt in the reaction. Considering the extremely largest Ka/Kb value in 15/CuZnFeAl-LDH, it can be selected as a reasonable catalyst for the phenol catalytic hydroxylation.| Samples | K a | K b | K a + Kb | K a/Kb |
|---|---|---|---|---|
| 5/CuZnFeAl-LDH | 0.1324 | 1.2973 | 1.4297 | 0.1021 |
| 10/CuZnFeAl-LDH | 0.2144 | 1.2224 | 1.4368 | 0.1754 |
| 15/CuZnFeAl-LDH | 0.2673 | 1.1930 | 1.4603 | 0.2241 |
| 20/CuZnFeAl-LDH | 0.2670 | 1.2343 | 1.5013 | 0.2163 |
| 25/CuZnFeAl-LDH | 0.2649 | 1.3686 | 1.6335 | 0.1936 |
Catalytic performance
Phenol hydroxylation is carried out with H2O2 as an oxidant to elucidate the catalytic properties of the CuZnFeAl-LDH with different copper contents as shown in Table 2. The variation tendency of the conversion of phenol and the selectivity to the target is similar to the change of alkalinity and acidity mentioned before, and thus the 15/CuZnFeAl-LDH catalyst presents the highest conversion of 66.9% and a selectivity of 77.3%. Considering the maximum Ka/Kb value of 15/CuZnFeAl-LDH and the generation of oxygen vacancy, the synergistic influence of both the active sites may be the dominating reason to bring forth the excellent catalyst activity and selectivity together.27,55 Besides, as seen from the time-dependence plot in Fig. 6a, the conversion and selectivity increased first (conversation: from 19.2% to 66.9%; selectivity: from 34.4% to 71.3%) and then dropped with duration time. However, the selectivity ratio of Cat/HQ is always larger than 1 during the reaction, which is consistent with the previous reports in the literature.10,19,56 However, it is intriguing to find that catechol is more easily produced than hydroquinone, while this value sharply decreased from 2.06 to 1.04 only after 60 minutes. This may have resulted from the polarity of catechol, which is more difficult to desorb from the hydrotalcite surface compared to hydroquinone. The continued oxidation by surface hydroxyl radicals is promoted and frustrates the selectivity ratio.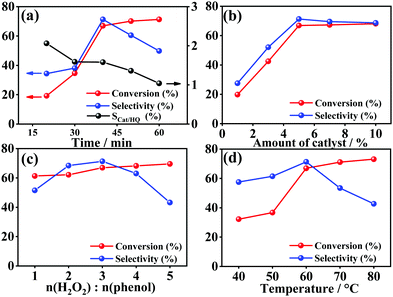 | ||
| Fig. 6 Effect of the (a) reaction time, (b) catalyst amount, (c) initial n(H2O2)/n(phenol) value, and (d) reaction temperature. | ||
The influence of the reaction conditions could decide the efficiency of phenol hydroxylation with 15/CuZnFeAl-LDH as a catalyst.57 Therefore, the catalyst dosage, the initial n(H2O2)/n(phenol) ratio, and the temperature are investigated in this work to shed light on the optimal reaction conditions (Fig. 6). As illustrated in Fig. 6b, the conversion of phenol and the selectivity to the target products rise rapidly when the catalyst dosage is from 1% to 5%, and remain almost constant when the amount of catalyst is more than 5%. However, a slight decline of the selectivity to benzenediol can also be observed, which can be attributed to the occurrence of side reactions and “sub-catalysis” of the reaction resulting from excessive catalysts.58 In addition, the continuous dosage of initial n(H2O2)/n(phenol) could benefit the conversion of phenol (see Fig. 6c). This could be due to the formation of more surface hydroxyl radicals introduced by the H2O2 dosage. However, when the initial n(H2O2)/n(phenol) ratio is more than 3, the selectivity to benzenediol would turn to the inflection point, which sharply drops from 71.3% to 51.5%. This unexpected degradation can be explained by continuous benzenediol oxidation and further increase in the by-product content in this system. The effect of the reaction temperature has also been explored as shown in Fig. 6d. The conversion of phenol could increase from 32.3% to 66.9% in the range of 40–80 °C. When the temperature increases, the decomposition rate of hydrogen peroxide would be accelerated and more hydroxyl radicals are formed. Meanwhile, the contact probability between the resulting hydroxyl radicals and reactants also improves due to the higher temperature. But, the variation tendency of the benzenediol selectivity presented a volcano-like plot in this process. The maximum selectivity of 71.3% has been observed at 60 °C. This phenomenon is related to the unavoidable decomposition of H2O2 at a higher temperature.
After the end of the reaction, the used catalyst is washed with acetone several times and centrifuged to recovery. The recyclability of the catalyst has been presented in Fig. S1 (ESI†), which presents no decline obviously after 5 runs. In the fifth run, the conversion of phenol can still be maintained at 62.5% with a high benzenediol selectivity of 66.0%. Considering the descriptions above, in summary, the 15/CuZnFeAl-LDH material shows potential as an excellent and highly stable catalyst for phenol hydroxylation under the optimal reaction conditions (5% catalyst dosage, n(H2O2)/n(phenol) ratio = 3, 60 °C), which also presents the optimal catalytic activity and reaction conditions compared with several different successful catalysts reported in the literature (see Table 5).
| No. | Catalysis | Temperature/°C | Solvent | Conversion % |
|---|---|---|---|---|
| a The catalyst used in this work. | ||||
| 1 | Co3PMo1 | 80 | Glycerol | 45.0 |
| 2 | Cu-apatite2 | 60 | Water | 52.0 |
| 3 | MCM-413 | 110 | Water | 76.0 |
| 4 | Cu-SCPNs5 | 60 | Water | 30.0 |
| 5 | Ni3Sn47 | 70 | Water | 42.0 |
| 6 | Ti-HMA9 | 27 | Acetic acid | 20.0 |
| 7 | MgO–Al2O3-HTS13 | 80 | Acetone | 38.0 |
| 8 | Al-free Mn-beta14 | 80 | Water | 35.2 |
| 9 | 15/CuZnFeAl-LDHa | 60 | Water | 66.9 |
Catalytic mechanism
It is worth noting that the best catalyst sample 15/CuZnFeAl-LDH presents the lowest binding energy (933.08 eV) which is ascribed to Cu+, and the highest binding energy (531.83 eV) is ascribed to the oxygen vacancy. This indicates a relationship between Cu species, oxygen vacancy, and the catalytic properties.19,51 Based on the analysis mentioned before, it can be confirmed that CuZnFeAl-LDH is highly active for phenol hydroxylation owing to the presence of Cu+ and oxygen vacancies through the Fenton reaction.57 However, the role of Fe3+ in the hydrotalcite is still obscure.In order to determine the role of Fe3+, a comparison experiment with CuZnAl-LDH as a catalyst in this reaction at a similar molar ratio is performed. During the procedure, the phenol conversion is only 57.5% with a selectivity of 62.6%, which are slightly lower than those of 15/CuZnFeAl-LDH (66.9% conversion and 72.3% selectivity). The decreased conversion accompanied by the opposite unchanged selectivity indicates that Fe3+ mostly also played a critical role as a Fenton active site.24
In addition, a quenching experiment has also been done under the same reaction conditions with an excess amount of tert-butanol as a radical scavenger. Different from the results that we have observed with hydroperoxide as a reactant, no obvious degrading yields can be observed, which demonstrates the degrading role of the hydroxide in the reaction.
Combining characterization and catalytic performance, the relationship between the structure and activity of CuZnFeAl-LDH can be revealed. It should be noted that different from the homogeneous counterpart, the heterogeneous Fenton reaction prefers generating surface ˙OH with a longer life time,59 where Cu+ plays the role of the key ion in accelerating this process.34,60 In addition, hydrogen peroxide is adsorbed on the active Cu+ site to generate hydroxyl radicals, which can also oxidize Cu+ into Cu2+ simultaneously due to its high oxidization properties (eqn (1)). When the copper content reaches 15%, both the surface Cu+ content (53.4%) and the catalytic activity (66.9%) reach the highest values (Table 3). Moreover, the hydroxyl groups on the sheets guarantee LDHs to be highly hydrophilic, favouring the proximity of phenol and H2O2 near the active sites, which is essential for driving the reaction.24,61 As multifunctional active sites, oxygen vacancies are energetically more favourable for the adsorption of reactants than other sites, due to their high electron density,59,62 where oxygen vacancies also accelerate the Cu2+/Cu+ cycle (eqn (2)).62 This can be the reason for explaining why hydrotalcite with the highest content of Cu+ and oxygen vacancies presents the highest activity.
| Cu+ + H2O2 → Cu2+ + ˙OH + OH− | (1) |
| OVs + Cu2+ → Cu+ | (2) |
| Fe3+ + H2O2 → Fe2+ + H+ + HO2˙ | (3) |
| Fe2+ + H2O2 → Fe3+ + OH− + ˙OH | (4) |
For further investigation on the mechanism, the relationship between OVs, Ka/Kb and catalytic properties is presented in Fig. S2 (ESI†). The benzenediol yield is proportional to the oxygen vacancy percentage, and a volcano-like plot can be observed, which is similar to the result of copper content mentioned before. This result can be explained by Hu et al.'s proposal.19 After H2O2 was adsorbed on OVs, the single O–O bond in H2O2 near OVs would be weaker than the single O–O bond in free H2O2 due to the stretching effect; it further promoted the hydroxyl radical emergence. This may indicate that the oxygen vacancies present a positive effect on the yield of benzenediol, because the oxygen vacancies and the surface defect could help in providing a significantly enlarged active surface in the reaction.19,63 However, the values of Ka/Kb continuously increase and then drop with further addition of copper content. According to the analysis results of XRD, FT-IR and TG-DSC, the hydrogen bond strength on the surface of the hydrotalcite is weakened with the addition of copper. The polarity and alkalinity of hydrotalcites can be affected by the surface hydroxyl groups. When the copper content continues to increase, the change in the percentage of oxygen vacancies matches with the Ka/Kb values. The appropriate solidification of the surface acidity percentage could be beneficial to the generation of oxygen vacancies.17,27,64
Conclusions
In summary, we have explored a X/CuZnFeAl-LDH catalyst for phenol hydroxylation under environmentally-friendly and energy efficient conditions. A series of CuZnFeAl quaternary hydrotalcites with different copper contents are synthesized by a co-precipitation method. The results of XRD, SEM, FT-IR and TG-DSC show that the catalysts present a typical hydrotalcite layered structure. With the increase of copper content, the stability of hydrotalcite gradually deteriorated accompanied by a volcano-like tendency in the activity. Among all the catalysts, 15/CuZnFeAl-LDH demonstrates the highest catalytic activity, and the conversion of phenol reached 66.9% with a selectivity of 71.3% to benzenediol. After recycling for five runs, the conversion of phenol could still be maintained at 62.5% with a selectivity of 66% to benzenediol. In the X/CuZnFeAl-LDH samples, the Cu+, Cu2+, Zn2+ and Fe3+ species are presented by XPS, and the synergism between copper and oxygen vacancies is revealed. The structure–activity relationship investigation suggests that the catalyst activity highly relies on the percentage of Cu+ and oxygen vacancies on the catalyst surface. The octahedral structure and oxygen vacancies of X/CuZnFeAl-LDH promote electron transfer on the catalyst surface, which accelerates the Cu2+/Cu+ redox properties.Experimental section
Synthesis of X/CuZnFeAl-LDH
CuZnFeAl layered double hydroxides were prepared by co-precipitation. Two solutions, namely solution A containing the desired amount of metal (Cu, Zn, Fe, and Al) nitrates and solution B with precipitating agents (NaOH and Na2CO3) were used at a molar ratio of n(M2+)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(M3+) = 3
n(M3+) = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, n(Fe3+)
1, n(Fe3+)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(Al3+) = 1
n(Al3+) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The solutions A and B were slowly dropped into a 500 mL three-necked flask and the pH value was maintained from 9 to 10. The samples were crystallized in an oil bath at 60 °C, followed by microwave crystallization for 15 minutes after 24 hours. The resulting precipitates were filtered and washed with deionized water until pH = 7. The solid was dried at 60 °C for 24 h. A series of catalysts with copper content of 5%, 10%, 15%, 20%, and 25% were obtained and recorded as X/CuZnFeAl-LDH, where X represents the mole content of Cu2+.
1. The solutions A and B were slowly dropped into a 500 mL three-necked flask and the pH value was maintained from 9 to 10. The samples were crystallized in an oil bath at 60 °C, followed by microwave crystallization for 15 minutes after 24 hours. The resulting precipitates were filtered and washed with deionized water until pH = 7. The solid was dried at 60 °C for 24 h. A series of catalysts with copper content of 5%, 10%, 15%, 20%, and 25% were obtained and recorded as X/CuZnFeAl-LDH, where X represents the mole content of Cu2+.
Catalytic hydroxylation was performed in a heating reactor equipped with a magnetic stirrer and a reflux condenser. The reactor was charged with a mixture of 0.30 g of phenol, 0.015 g of catalyst and 30 mL of deionized water. After dropwise addition of H2O2 (the molar ratio of phenol/H2O2 was 1/3), the resulting mixture was heated at 60 °C for 40 min under stirring. The reaction products were identified and quantified by high-performance liquid chromatography (HPLC Wufeng, Shanghai) equipped with a C18 column. A solution of methanol and water with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was chosen as the mobile phase; the flow rate was 1 mL min−1, and the detection wavelength was 265 nm.
1 was chosen as the mobile phase; the flow rate was 1 mL min−1, and the detection wavelength was 265 nm.
Characterization
X-ray diffraction (XRD) patterns were recorded on a Bruker D8 Advance powder diffractometer using Cu Kα radiation sources at 40 kV and 100 mA, at a scanning rate of 20° min−1, with the 2θ angle ranging from 5° to 80°. The morphologies of the catalysts were observed by SEM using Hitachi S-4800 instruments. N2 physical adsorption–desorption measurements were carried out on an ASAP 2020 surface analyzer (Micromeritics Co. USA) at 77K. Before the measurement, the catalysts were degassed at 150 °C for 4 h. The specific surface area and the pore volume were calculated by the BET method. Fourier transform infrared (FT-IR) spectra were recorded on a Nexus 870 FTIR spectrometer. The samples were ground with KBr for IR measurements in the range of 4000–400 cm−1. A STA 499 F3 type synchronous thermal analyzer manufactured by NETZSCH was used to determine the thermal stability of the sample. The measurement was carried out at an increasing temperature rate of 10 °C min−1 in the range of 30–800 °C. The process takes place in a nitrogen atmosphere. The contents of Cu, Zn, Fe and Al in the samples were determined using inductively coupled plasma atomic emission spectroscopy on a PerkinElmer ICP-OES Optima 3000 instrument using solutions prepared by dissolving the samples in dilute HNO3. X-ray photoelectron spectra (XPS) were recorded with a Kratos Axis HSi photoelectron spectrometer equipped with an Al Kα radiation source (hν = 1486.6 eV) (Shimadzu, Kyoto, Japan). A power of 250 W and a pass energy of 37.5 eV were used during the experiment. IGC was carried out to measure the acid–base interaction constants Ka and Kb. A conventional gas chromatograph (Kechuang, GC-910, China) with a thermal conductivity detector was used for the measurements. All the calculations and results of the IGC measurements are summarized in Tables S1–S16 (ESI†).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Basic Research Program of China, funded Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).Notes and references
- K. Pamin, J. Połtowicz, M. Prończuk, S. Basąg, J. Maciejewska, J. Kryściak-Czerwenka and R. Tokarz-Sobieraj, Catal. Today, 2015, 257, 80–85 CrossRef CAS.
- A. Amedlous, O. Amadine, Y. Essamlali, K. Daanoun, M. Aadil and M. Zahouily, RSC Adv., 2019, 9, 14132–14142 RSC.
- S. Ray, S. F. Mapolie and J. Darkwa, J. Mol. Catal. A: Chem., 2007, 267, 143–148 CrossRef CAS.
- Y. Y. Huang, H. Konnerth, J. Y. Yeh, M. H. G. Prechtl, C. Y. Wen and K. C. W. Wu, Green Chem., 2019, 21, 1889–1894 RSC.
- S. Thanneeru, S. S. Duay, L. Jin, Y. Fu, A. M. Angeles-Boza and J. He, ACS Macro Lett., 2017, 6, 652–656 CrossRef CAS.
- H. Li, Y. Zhai, X. Zhang, G. Lv, Y. Shen, X. Wang, T. Jiang and Y. Wu, Ind. Eng. Chem. Res., 2020, 59, 10289–10297 CrossRef CAS.
- R. Tongsri, S. Pithakratanayothin, T. Chaisuwan and S. Wongkasemjit, Catal. Sci. Technol., 2017, 7, 5413–5421 RSC.
- X. Chen, B. Li, T. Zhang, S. Jiang, G. Zhang, W. Wu and X. Ma, Appl. Surf. Sci., 2018, 439, 1047–1056 CrossRef.
- F. H. S. K. Mohapatra and P. Selvam, Catal. Commun., 2002, 4, 57–62 CrossRef.
- O. Amadine, Y. Essamlali, A. Fihri, M. Larzek and M. Zahouily, RSC Adv., 2017, 7, 12586–12597 RSC.
- B. Kumar Kundu, V. Chhabra, N. Malviya, R. Ganguly, G. S. Mishra and S. Mukhopadhyay, Microporous Mesoporous Mater., 2018, 271, 100–117 CrossRef CAS.
- D. Hermosilla, M. Cortijo and C. P. Huang, Sci. Total Environ., 2009, 407, 3473–3481 CrossRef CAS.
- K. J. Winstanley and D. K. Smith, J. Org. Chem., 2007, 72, 2803–2815 CrossRef CAS.
- Z. He, J. Wu, B. Gao and H. He, ACS Appl. Mater. Interfaces, 2015, 7, 2424–2432 CrossRef CAS.
- S. Navalon, M. Alvaro and H. Garcia, Appl. Catal., B, 2010, 99, 1–26 CrossRef CAS.
- Y. Orooji, S. Mortazavi-Derazkola, S. M. Ghoreishi, M. Amiri and M. Salavati-Niasari, J. Hazard. Mater., 2020, 400, 123140 CrossRef CAS.
- M. Xu and M. Wei, Adv. Funct. Mater., 2018, 28, 1802943 CrossRef.
- Y. Orooji, R. Mohassel, O. Amiri, A. Sobhani and M. Salavati-Niasari, J. Alloys Compd., 2020, 835, 152240 CrossRef.
- X. X. Guo, T. T. Hu, B. Meng, Y. Sun and Y.-F. Han, Appl. Catal., B, 2020, 260, 118157 CrossRef CAS.
- J. Zou, Z. Wang, W. Guo, B. Guo, Y. Yu and L. Wu, Appl. Catal., B, 2020, 260, 118185 CrossRef CAS.
- Y. Du, Q. Wang, X. Liang, P. Yang, Y. He, J. Feng and D. Li, Catal. Sci. Technol., 2017, 7, 4361–4365 RSC.
- K. Yan, Y. Liu, Y. Lu, J. Chai and L. Sun, Catal. Sci. Technol., 2017, 7, 1622–1645 RSC.
- S. Kannan, A. Dubey and H. Knozinger, J. Catal., 2005, 231, 381–392 CrossRef CAS.
- H. Wang, M. Jing, Y. Wu, W. Chen and Y. Ran, J. Hazard. Mater., 2018, 353, 53–61 CrossRef CAS.
- Z. Zhang, Z. Hua, J. Lang, Y. Song, Q. Zhang, Q. Han, H. Fan, M. Gao, X. Li and J. Yang, CrystEngComm, 2019, 21, 4607–4619 RSC.
- M. Shirotori, S. Nishimura and K. Ebitani, Chem. Lett., 2016, 45, 194–196 CrossRef CAS.
- X. Wu, C. Ci, Y. Du, X. Liu, X. Li and X. Xie, Mater. Chem. Phys., 2018, 211, 72–78 CrossRef CAS.
- Y. Du, X. Liu, X. Wu, Q. Cheng, C. Ci and W. Huang, ChemistrySelect, 2018, 3, 7996–8002 CrossRef CAS.
- X. Ren, X. Hu, F. Zhang, J. Wang, J. Liang, W. Wu, M. Jiang and J. Wang, Catal. Sci. Technol., 2015, 5, 4813–4820 RSC.
- Y. Liu, M. Zhu, Y. Yao, J. Jiang, F. Zhang, Z. Yang, Z. Lu, I. Kumakiric, X. Chen and H. Kita, Microporous Mesoporous Mater., 2018, 268, 84–87 CrossRef.
- W. Zhang, Y. Wang, Y. Shen, M. Xie and X. Guo, Microporous Mesoporous Mater., 2016, 226, 278–283 CrossRef CAS.
- M. Liu, H. Wang, P. Zhu, J. Wang, H. Tan and Z. Zheng, Catal. Commun., 2019, 129, 105752 CrossRef CAS.
- H. Daneshvar, M. S. Seyed Dorraji, A. R. Amani-Ghadim and M. H. Rasoulifard, Ultrason. Sonochem., 2019, 58, 104632 CrossRef CAS.
- C. A. Antonyraj and S. Kannan, Appl. Clay Sci., 2011, 53, 297–304 CrossRef CAS.
- G. Cui, X. Meng, X. Zhang, W. Wang, S. Xu, Y. Ye, K. Tang, W. Wang, J. Zhu, M. Wei, D. G. Evans and X. Duan, Appl. Catal., B, 2019, 248, 394–404 CrossRef CAS.
- I. M. Ahmed and M. S. Gasser, Appl. Surf. Sci., 2012, 259, 650–656 CrossRef CAS.
- F. L. L. H. Zhang, D. G. Evans and X. Duan, Ind. Eng. Chem. Res., 2010, 49, 5959–5968 CrossRef.
- F. Wang and Z. Guo, J. Alloys Compd., 2018, 767, 382–391 CrossRef CAS.
- H. Zhang, H. Chen, S. Azat, Z. A. Mansurov, X. Liu, J. Wang, X. Su and R. Wu, J. Alloys Compd., 2018, 768, 572–581 CrossRef CAS.
- X. Peng, W. Luo, M. Wang, F. Hu, F. Qiu and H. Dai, J. Mol. Liq., 2019, 290, 111201 CrossRef CAS.
- J. L. Feng Li, David G. Evans and Xue Duan, Chem. Mater., 2004, 16, 1597–1602 CrossRef.
- Z. Xu, Y. Yu, D. Fang, J. Liang and L. Zhou, Mater. Chem. Phys., 2016, 171, 386–393 CrossRef CAS.
- Q. Zhang, S. Wen, Q. Feng and S. Zhang, Sep. Purif. Technol., 2020, 242, 116760 CrossRef CAS.
- S. Ajmal, Y. Yang, K. Li, M. A. Tahir, Y. Liu, T. Wang, A.-U.-R. Bacha, Y. Feng, Y. Deng and L. Zhang, J. Phys. Chem. C, 2019, 123, 11555–11563 CrossRef CAS.
- F. Qu, T. Thomas, B. Zhang, X. Zhou, S. Zhang, S. Ruan and M. Yang, Sens. Actuators, B, 2018, 273, 1202–1210 CrossRef CAS.
- T. Yamashita and P. Hayes, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS.
- J. Hu, J. Li, J. Cui, W. An, L. Liu, Y. Liang and W. Cui, J. Hazard. Mater., 2020, 384, 121399 CrossRef CAS.
- Q. Nie, Y. Xie, J. Ma, J. Wang and G. Zhang, J. Cleaner Prod., 2020, 242, 118532 CrossRef CAS.
- T. Boningari, P. R. Ettireddy, A. Somogyvari, Y. Liu, A. Vorontsov, C. A. McDonald and P. G. Smirniotis, J. Catal., 2015, 325, 145–155 CrossRef CAS.
- L. Zhang, T. Chen, S. Zeng and H. Su, J. Environ. Chem. Eng., 2016, 4, 2785–2794 CrossRef CAS.
- D. A. Zatsepin, A. F. Zatsepin, D. W. Boukhvalov and N. V. Gavrilov, J. Alloys Compd., 2020, 829, 154459 CrossRef CAS.
- X. Han, K. Fang and Y. Sun, RSC Adv., 2015, 5, 51868–51874 RSC.
- O. D. Pavel, D. Tichit and I. C. Marcu, Appl. Clay Sci., 2012, 61, 52–58 CrossRef CAS.
- Z. Ding, W. Xu, X. Zhang, Z. Liu, J. Shen, J. Liang, M. Jiang and X. Ren, Catalysts, 2019, 9, 470–485 CrossRef CAS.
- J. Kuljiraseth, A. Wangriya, J. M. C. Malones, W. Klysubun and S. Jitkarnka, Appl. Catal., B, 2019, 243, 415–427 CrossRef CAS.
- V. R. A. Dubey and S. Kannan, J. Mol. Catal. A: Chem., 2002, 181, 151–160 CrossRef.
- Y. Chen, T. Liu, H. He and H. Liang, Appl. Organomet. Chem., 2018, 32, 4330–4340 CrossRef.
- A. M. Al-Sabagh, F. Z. Yehia, G. Eshaq and A. E. ElMetwally, ACS Sustainable Chem. Eng., 2017, 5, 4811–4819 CrossRef CAS.
- H. Li, J. Shang, Z. Yang, W. Shen, Z. Ai and L. Zhang, Environ. Sci. Technol., 2017, 51, 5685–5694 CrossRef CAS.
- M. G. Churchil, A. Antonyraj and S. Kannan, Ind. Eng. Chem. Res., 2010, 49, 6020–6026 CrossRef.
- V. Rives, J. Catal., 2003, 220, 161–171 CrossRef CAS.
- N. Zhang, E. P. Tsang, J. Chen, Z. Fang and D. Zhao, J. Colloid Interface Sci., 2020, 558, 163–172 CrossRef.
- X. Gu, H. Jing, X. Mu, H. Yang, Q. Zhou, S. Yan, S. Liu and C. Chen, J. Alloys Compd., 2020, 814, 152274 CrossRef CAS.
- W. Gao, C. Li, H. Chen, M. Wu, S. He, M. Wei, D. G. Evans and X. Duan, Green Chem., 2014, 16, 1560–1568 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nj03905e |
| ‡ These two authors contributed equally in composing it. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2021 |