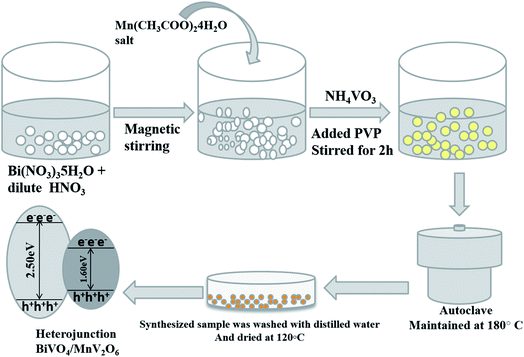
Open Access Article
Open Access ArticleSunlight driven photocatalytic degradation of organic pollutants using a MnV2O6/BiVO4 heterojunction: mechanistic perception and degradation pathways†
Karina
Bano
a,
Susheel K.
Mittal
b,
Prit Pal
Singh
 *a and
Sandeep
Kaushal
*a and
Sandeep
Kaushal
 *a
*a
aDepartment of Chemistry, Sri Guru Granth Sahib World University, Fatehgarh Sahib, Punjab, India. E-mail: dhillonps2003@gmail.com; kaushalsandeep33@gmail.com
bSchool of Chemistry & Biochemistry, Thapar Institute of Engineering and Technology, Patiala, India
First published on 1st September 2021
Abstract
In the field of photocatalysis, fabrication of a heterojunction structure with effective charge separation at the interface and charge shift to enhance the photocatalytic activity has acquired extensive consideration. In the present investigation, MnV2O6/BiVO4 heterojunction samples with excellent photocatalytic performance under sunlight irradiation were conveniently synthesized by a hydrothermal technique, and characterized by UV-Vis, FTIR, XRD, FESEM, HRTEM, PL, BET and XPS techniques. The prepared samples were investigated as photocatalysts for degrading MB and RhB dyes under sunlight. Among various samples of MnV2O6/BiVO4, the S-V hetero-junction sample exhibited maximum photocatalytic activity with 98% and 96% degradation of MB and RhB dyes, respectively, in 6 and 35 min. The high photocatalytic activity of MnV2O6/BiVO4 may be due to the successful generation and shift of charges in the presence of visible light. The average reduction of chemical oxygen demand (COD) was found to be 75% after irradiation with direct sunlight. In the degradation process of dyes, superoxide anion radicals were the main responsive species, as revealed by trapping experiments. The degradation efficiency of MnV2O6/BiVO4 heterojunction did not diminish even after four cycles. In addition, the catalytic performance of the fabricated heterojunction was also explored for reducing 4-nitrophenols (4-NP) by using NaBH4. Absolute conversion of 4-NP to 4-aminophenol (4-AP) occurred without the production of intermediate byproducts.
1. Introduction
A clean environment is highly significant for healthy living and sustaining life on earth. As a whole, all living creatures are reliant on the environment for food, air, water, and many other requirements. Consequently, it is significant that each individual must contribute to save and protect the environment from various pollutants. Very important assets for life like water and air are under continuous threat from various organic pollutants and toxic chemicals generated from the chemical, agricultural, food and textile industries that act as poisons.1,2 Carcinogenic dyes present in water restrict the path of sunrays and obstruct them from entering the aqueous system. This results in a reduced rate of photosynthesis and damage to aquatic animals.3 In addition to the impairment of body organs, dyes also affect public health by accumulation in living beings. Nitrophenol compounds are widely used in the synthesis of agrochemicals, fungicides and rubber. These are considered to be persistent pollutants and adversely affect the functioning of various body organs. An eco-friendly approach must be planned to carry out degradation reactions more precisely and efficiently for elimination of these toxic chemicals from waste water. Previously used conventional methods such as biological degradation, membrane filtration, chemical oxidation, and plasma ozonization have not been proved to be appropriate due to their complexity, low efficiency, time consuming mechanisms, disposal problems and being uneconomical.4,5Among all the natural energy resources, solar energy is considered to be the most efficient, easily available and renewable energy source on earth. In the whole solar spectrum, 43% energy is provided by visible light whereas UV region contributes only 4% of energy.6,7 In this manner, improvement of effective photocatalysts, especially noticeable light responsive systems, is fundamental for the proficient usage of sunlight-based energy in photocatalysis. The general mechanism of photocatalysis involves the generation of activated electron–hole pairs in the valence band of semiconductor, followed by electron transfer from the valence band to the conduction band in the presence of light energy.8 These charged species play a major role in photocatalysis by providing surface for adsorption of species, and generate superoxide and hydroxide radicals which further participate in oxidation–reduction degradation reactions.
In past years, removal of pollutants by the photocatalytic process has become a matter of great interest. Until now, a number of semiconductor photocatalysts have been fabricated to remove toxic chemicals from the environment. TiO2, ZnO, CuO, SnO2, ZnS, CdS, BiVO4, and g-C3N4 are considered to be the most common semiconductors utilized for light energy mediated catalytic reactions.9–12 Out of all the semiconductor photocatalyst materials, BiVO4 has gained maximum attraction because of its excellent photocatalytic activity in visible light region, low toxicity and high stability.13 BiVO4 has a band gap of 2.44 eV that corresponds to λmax value between 300 and 400 nm. Its photocatalytic behavior promotes removal of toxic dyes, pesticides, disintegration of pollutants and production of hydrogen gas by splitting of water. One major limitation concerning its efficiency was a high rate of recombination of electron–hole pairs which retards its capability. This limitation was overcome by modifying the structure of BiVO4 by doping with metals and non-metals or coupling with other semiconductors. With these efforts, the structure and morphology of BiVO4 were tuned for its utilization as a photocatalyst in the presence of visible light. To date, a number of BiVO4 based hybrid composites have been manufactured which exhibited higher photocatalytic activity towards photo-electrochemical reactions, dye degradation, mineralization of pesticides and, splitting of water as compared to that of pure BiVO4 semiconductor.14–21
In the present investigation, a sunlight activated heterojunction, MnV2O6/BiVO4, was synthesized by a one pot hydrothermal method. The p-type MnV2O6 photocatalyst comprises narrow band gap energy (∼1.6 eV) and exhibits high catalytic activity towards redox reactions involving splitting of water into hydrogen and oxygen, elimination of harmful pesticides, and degradation of toxic organic dyes under visible radiation.22–24 In addition to this, MnV2O6 also acts as an excellent anodic material in lithium-ion batteries, attributed to its continuous recycling activity.25 The prepared MnV2O6/BiVO4 heterojunction was utilized as a photocatalyst for degrading MB and RhB dyes, and reducing 4-NP in the presence of solar radiation. Photocatalytic experiments under same conditions were performed for sole BiVO4 and MnV2O6 also.
2. Experimental
2.1 Materials and methods
Bi(NO3)3·5H2O, NH4VO3, Mn(CH3COO)2·4H2O, NaBH4, polyvinylpyrrolidone (PVP), 4-nitrophenol, and MB and RhB dyes were purchased from LOBA Chemie Pvt Ltd India.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BiVO4i.e., 0.25
BiVO4i.e., 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00, 0.50
1.00, 0.50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00, 0.75
1.00, 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00, and 1.00
1.00, and 1.00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 were synthesized by varying the amounts of reagents and labeled as S-III, S-IV, S-V and S-VI, respectively (Scheme 1).
1.00 were synthesized by varying the amounts of reagents and labeled as S-III, S-IV, S-V and S-VI, respectively (Scheme 1).
3. Results and discussion
The as-synthesized MnV2O6/BiVO4 heterojunction was characterized using various sophisticated techniques like UV, FTIR, XRD, FESEM, HRTEM and XPS. UV-vis diffuse reflectance spectroscopy (DRS) of S-I to S-VI samples was performed and the results are displayed in Fig. S1 (ESI†). The band gap (Eg) values of BiVO4 and MnV2O6 were found to be 2.5 eV & 1.60 eV, respectively, which agree with previous findings.22,26 The band gap of MnV2O6/BiVO4 heterojunction samples i.e. S-III to S-VI was found to be 2.2, 2.1, 1.95 and 1.90 eV, respectively, signifying that the incorporation of MnV2O6 diminishes the band gap of BiVO4. Moreover, this diminution affirms electronic coupling between MnV2O6 and BiVO4.FTIR spectra of pure BiVO4, MnV2O6 and MnV2O6/BiVO4 heterojunctions with varied molar ratios are shown in Fig. 1. The band in the 600–800 cm−1 region was assigned to symmetric and asymmetric vibrations of the VO43− group. The peak at 750 cm−1 is attributed to the vibrations of Bi–V bonds, and stretching vibration of the V–O double bond is present at 1366 cm−1.27 The sharp peaks in 600–1000 cm−1 region for MnV2O6 are attributed to the vibrations of V–O–V bonds. Short V–O bonds gave an absorption band at 903 cm−1 and that of longer V–O bonds appeared at 815 cm−1. In the FTIR spectra of MnV2O6/BiVO4 heterojunction samples with different molar ratios, some additional peaks were observed as the amount of MnV2O6 increased. This observation confirmed that no structural change happened in the synthesized heterojunction composite. Sharp peaks in the region 1500–1550 cm−1 were observed for all composites and correspond to interactions of two metals. A clear sharp band at 1700 cm−1 could be attributed to the metal–oxygen stretching vibrations. The O–H stretching vibrations of lattice water molecules were observed at 3750 cm−1. Change in the peak intensity of BiVO4 was noticed which might be due to the interactions of both the semiconductors through the formed interface. IR data of composites with different molar ratios confirmed that both BiVO4 and MnV2O6 semiconductors coexist in the composite.
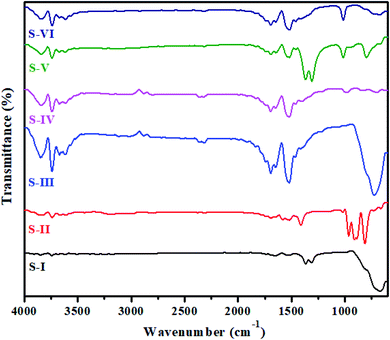 | ||
| Fig. 1 FTIR spectra of pure BiVO4 (S-I), MnV2O6 (S-II) and MnV2O6/BiVO4 heterojunction samples (S-III to S-VI). | ||
The XRD patterns of synthesized BiVO4, MnV2O6 and MnV2O6/BiVO4 heterojunctions are shown in Fig. 2. It can be noticed from the XRD pattern of BiVO4 semiconductor (Fig. 2S-I) that the diffraction peaks can be entirely indexed to a monoclinic phase (JCPDS card-96-901-3438). The corresponding peaks are displayed in the XRD spectrum of BiVO4 at 2θ = 18.7 (4.74 Å), 28.9 (3.07 Å), 30.7 (2.92 Å), 34.6 (2.59 Å), 35.3 (2.54 Å), 39.7 (2.26 Å), 42.3 (2.13 Å), 46.7 (1.94 Å), 50.3 (1.81 Å), 53.5 (1.71 Å), 58.7 (1.54 Å) and 59.6 (2.1 Å) with indices (110), (−221), (040), (200), (−202), (−311), (150), (240), (−402), (310), (−421) and (042), respectively. The XRD pattern of MnV2O6 semiconductor (Fig. 2S-II) clearly shows that the diffraction peaks match the provided data remarkably well (JCPDS card-96-711-9177), and no phase impurity was observed. The corresponding peaks are exhibited in the XRD spectra of MnV2O6 at 2θ = 9.5 (9.25 Å), 11.5 (7.65 Å), 17.5 (5.07 Å), 19.2 (3.45 Å), 25.8 (3.34 Å), 27.1 (3.29 Å), 28.3 (3.16 Å), 28.9 (2.13 Å), 31.5 (2.83 Å), 33.4 (2.67 Å), 35.0 (2.56 Å), 52.1 (1.75 Å) and 53.4 (1.71 Å) with indices (001), (100), (101), (002), (102), (011), (201), (003), (−112), (−302), (103), (401) and (−121), respectively. When a small amount of MnV2O6 was introduced, no diffraction peaks of MnV2O6 were observed in the XRD pattern of MnV2O6/BiVO4 heterojunction (Fig. 2S-II). With an increase of MnV2O6 content, S-III, S-IV, S-V and S-VI displayed diffraction peaks of both BiVO4 and MnV2O6, demonstrating the effective fabrication of MnV2O6/BiVO4 heterojunction. In order to find typical crystallite size of the samples, Debye–Scherer equation was employed.28,29 The crystallite sizes were observed as 32.7 (S-I), 34.01 (S-II), 31.35 (S-III), 25.49 (S-IV), 40.6 (S-V) and 40.1 nm (S-VI).
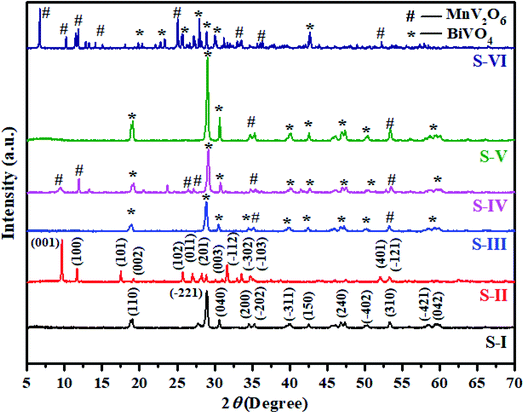 | ||
| Fig. 2 XRD patterns of pure BiVO4 (S-I), MnV2O6 (S-II) and MnV2O6/BiVO4 heterojunction samples (S-III to S-VI). | ||
FESEM was performed to explore the morphology of synthesized heterojunction (Fig. 3a and b). The MnV2O6/BiVO4 heterojunction demonstrated a belt-like morphology with a length of 8–10 μm (Fig. 3a). Fig. 3b illustrates that the MnV2O6/BiVO4 nano-belts were homogeneously mixed to form an interface between the two materials i.e., MnV2O6 and BiVO4, and the thickness of nanobelts was tens of nanometers. The FESEM micrograph of pure BiVO4 nanoparticles displayed a rod like structure with a high degree of homogeneity. Pure MnV2O6 nanoparticles have a globular structure with agglomeration (Fig. S2†). The morphology of the heterojunction was further explored by HRTEM (Fig. 3c and d). It was revealed that MnV2O6 particles were homogeneously mixed with BiVO4 nanoparticles, and particle size of the resulting composite material was in 35–45 nm range which is comparable to that obtained from the XRD results. The corresponding SAED pattern showed clear ring patterns confirming the formation of polycrystalline MnV2O6/BiVO4 heterojunction.
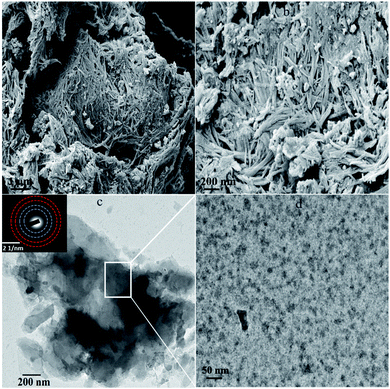 | ||
| Fig. 3 (a and b) FESEM images and (c and d) HRTEM images (inset SAED pattern) of MnV2O6/BiVO4 heterojunction. | ||
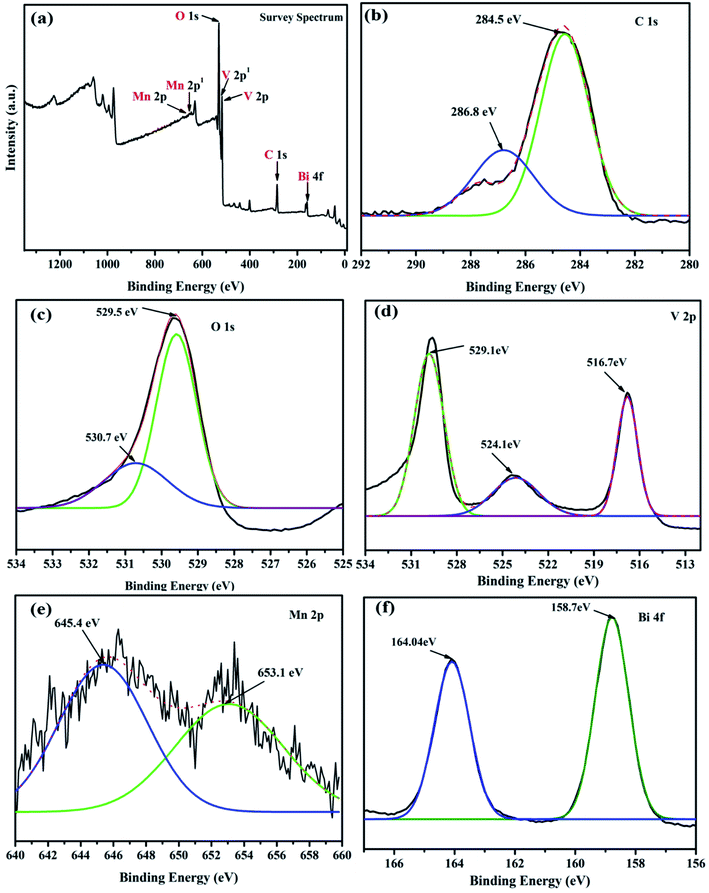
The chemical states of as-synthesized MnV2O6/BiVO4 heterojunction were examined by XPS. Elements C, Mn, V, O and Bi were confirmed by the survey scan of XPS spectra (Fig. 4a). The high-resolution C 1s spectrum of MnV2O6/BiVO4 heterojunction (Fig. 4b) may be deconvoluted into two dissimilar peaks at 286.8 eV and 284.5 eV which are attributed to epoxide C (O–C–O) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C sp2 hybridized material, respectively.30 Peaks at 529.5 and 530.78 eV in the O 1s spectrum (Fig. 4c) are ascribed to the oxygen bonded inside an oxide crystal (O2−) in the composite, and –OH groups adsorbed on the surface, respectively.31,32 For the V 2p orbital (Fig. 4d), binding energy peaks at 524.1 eV and 516.7 eV relate to V, 2p1/2 and V, 2p3/2 which originate from V5+.33 Mn 2p XPS spectrum (Fig. 4e) displayed two obvious peaks at 645.4 and 653.4 eV, related to Mn, 2p3/2 and Mn, 2p1/2, respectively, arising from Mn2+.25 In case of Bi (Fig. 4f), peak at 158.7 eV was attributed to the binding energy of Bi 4f7/2 whereas the peak at 164.04 eV was ascribed to Bi 4f5/2. The chemical states of as-synthesized MnV2O6 and BiVO4 nanoparticles were also examined by XPS. Elements C, Mn, V and O and C, V, Bi and O were confirmed by the survey scan of XPS spectra in MnV2O6 and BiVO4 nanomaterials, respectively (Fig. S3†).
C sp2 hybridized material, respectively.30 Peaks at 529.5 and 530.78 eV in the O 1s spectrum (Fig. 4c) are ascribed to the oxygen bonded inside an oxide crystal (O2−) in the composite, and –OH groups adsorbed on the surface, respectively.31,32 For the V 2p orbital (Fig. 4d), binding energy peaks at 524.1 eV and 516.7 eV relate to V, 2p1/2 and V, 2p3/2 which originate from V5+.33 Mn 2p XPS spectrum (Fig. 4e) displayed two obvious peaks at 645.4 and 653.4 eV, related to Mn, 2p3/2 and Mn, 2p1/2, respectively, arising from Mn2+.25 In case of Bi (Fig. 4f), peak at 158.7 eV was attributed to the binding energy of Bi 4f7/2 whereas the peak at 164.04 eV was ascribed to Bi 4f5/2. The chemical states of as-synthesized MnV2O6 and BiVO4 nanoparticles were also examined by XPS. Elements C, Mn, V and O and C, V, Bi and O were confirmed by the survey scan of XPS spectra in MnV2O6 and BiVO4 nanomaterials, respectively (Fig. S3†).
The porosity of MnV2O6/BiVO4 heterojunction was established by the N2 adsorption–desorption experiment. According to the IUPAC classification, this sort of isotherm is extremely close to the type II adsorption isotherm (Fig. 5). The specific surface area, total pore volume and micropore volume noticed from BET were 77.35 m2 g−1, 0.2331 cm3 g−1 and 0.00075 cm3 g−1, respectively. The mean pore diameter was determined to be 5.61 nm. Hence, MnV2O6/BiVO4 heterojunction has a high precision external zone and adequate pore structure which is extremely useful in surface interaction activities.
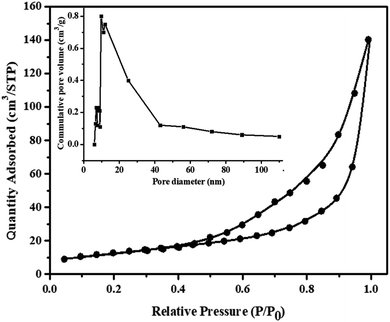 | ||
| Fig. 5 N2 adsorption–desorption isotherm curves of MnV2O6/BiVO4 heterojunction (S-V); inset: pore size distributions. | ||
Photochemical properties of semiconductors and their corresponding hybrid materials were studied with photoluminescence (PL) spectroscopy. The electrons and holes on excitation, started moving from the ground energy level to a higher energy level and recombined again.34,35 During return to the ground state, various emissions are generated, depending upon band gap energy values of the semiconductor. The intensity of these emissions can be recorded corresponding to their wavelength values using the PL technique, and based on their intensity, the rate of recombination of active charged species can be detected. The PL spectra of pure BiVO4, pure MnV2O6, and BiVO4/MnV2O6 heterojunction are shown in Fig. S4.† For the pure BiVO4 semiconductor material, excitation occurred at 325 nm wavelength and corresponding to this excited energy, a broad band in the region of 550–650 nm was produced in the emission spectrum. Pure MnV2O6 showed excitation at 450 nm and delivered a highly intense peak at 562 nm. The intensity of these pure compounds was much higher as compared to that of the hybrid BiVO4/MnV2O6 heterojunction photocatalyst which confirmed successful separation of active charged species (electron–hole pairs) and reduced recombination rate which favored high photocatalytic activity of the heterojunction.
3.1 Photocatalytic reduction of 4-nitrophenol over MnV2O6/BiVO4 heterojunction
The waste water disposed off by industries probably contains hazardous nitrophenols and their derivatives. The main sources of nitrophenols and their derivatives are insecticide, synthetic dye and herbicide manufacturing industries.36 Hence, the elimination of these hazardous chemicals from industrial effluents is vital before it is discharged into water bodies.However, it is hard to remove these compounds by regular microbial degradation due to their natural and artificial stability.37 Thus, it is essential to build up environment responsive strategies to remove such contaminants from waste effluents.38 UV-visible spectra during the reduction of 4-nitrophenol (4-NP) by NaBH4 using MnV2O6/BiVO4 heterojunction as a catalyst are given in Fig. 6. The absorption peak of the aqueous solution of yellow coloured 4-NP was observed at 317 nm. When an aqueous solution of NaBH4 was added, a red-shift was noticed at ∼400 nm owing to the generation of nitrophenolate anion (Fig. S5†). The absorption peak at 400 nm remained invariable for a prolonged period, suggesting that 4-nitrophenolate ions could not be reduced by sole NaBH4 in the absence of as-synthesized catalyst. Pure MnV2O6 and BiVO4 nanoparticles illustrated little activity and hence, both of them can't be considered worthwhile catalysts for 4-NP reduction. However, 4-NP was effortlessly reduced using both, NaBH4 and MnV2O6/BiVO4 heterojunction. The absorption peak corresponding to 4-NP at 400 nm progressively diminished and almost vanished after 40 min (Fig. 6a). Meanwhile, another absorption peak at ∼297 nm corresponding to 4-aminophenol (4-AP) with increasing intensity emerged. This outcome confirmed the comprehensive transformation of 4-NP to 4-AP without the production of intermediates as established in earlier reports also.39 Absorbance and concentration of the solution are proportionate to each other and hence, absorbance A0 (t = 0) corresponds to the initial concentration, and absorbance At corresponds to the concentration at time t (Ct). The rate constant (k) was evaluated from the plot of ln(Ct/C0) vs. time (min) and its values were determined to be 0.0118, 0.0120, 0.008, 0.030, 0.12 and 0.045 min−1 for S-I, II, III, IV, V and VI, respectively for reduction of 4-NP (Fig. 6b).
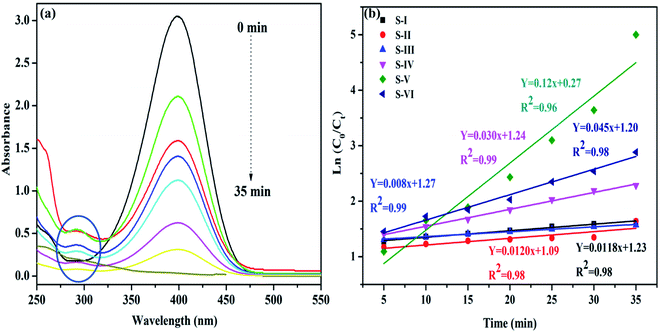 | ||
| Fig. 6 (a) Reduction of 4-nitrophenol with NaBH4 over MnV2O6/BiVO4 heterojunction shown by changes in UV-vis spectra; (b) graph of ln(C0/Ct) vs. time. | ||
3.2 Photocatalysis of dyes over MnV2O6/BiVO4 heterojunction
The photocatalytic activities of pure MnV2O6 and BiVO4 semiconductors as well as those of MnV2O6/BiVO4 heterojunctions were assessed by degrading MB and RhB dyes in solar light. The photocatalytic performance of MnV2O6/BiVO4 heterojunctions was optimized w.r.t. solution pH, varying photocatalyst dosage and lapse of time, to achieve maximum degradation.The degradation results were recorded over a wide range of photocatalyst amounts and pH. It was noticed that dye degradation performance varied as a function of the amount of MnV2O6/BiVO4 heterojunction photocatalyst. As expected, the amount of MnV2O6/BiVO4 heterojunction photocatalyst for the degradation of both MB and RhB dyes followed the order: 50 > 40 > 30 and 20 mg. The increase in photocatalyst amount from 20 mg to 50 mg leads to an increase in dye degradation from 25 to 98.1% and 23 to 96.2%, respectively, for MB and RhB dyes. Evidently, the enhancement of degradation proficiency with an increase in the amount of heterojunction is primarily attributed to the increased number of active sites on the surface of MnV2O6/BiVO4 photocatalyst for UV light absorption.
The pH of solution is another most essential factor in photocatalytic degradation. Fig. S6† presents the effect of pH (range 3–10) on the degradation of both the dyes over MnV2O6/BiVO4 heterojunction photocatalyst. It was observed that both the MB and RhB dyes degraded to a maximum extent at pH 7 compared to lower or higher pH values. This behavior might be due to the formation of Fenton's reagent at a lower pH and at a higher pH, MnV2O6/BiVO4 photocatalyst could leach into solution and form chemical sludge.40 Therefore, it was found that the as-synthesized photocatalyst was more efficient at pH 7.
The photocatalytic degradation of aqueous solutions of MB and RhB dyes over MnV2O6/BiVO4 heterojunction photocatalyst as a function of time was examined by UV-visible spectroscopy (Fig. S7†). A drastic decrease in absorption peak intensity with time was noticed, and the peak nearly disappeared within 6 and 35 min, respectively, for MB and RhB dyes.
It is clear from Fig. 7a that pure semiconductors BiVO4 (S-I) and MnV2O6 (S-II), and heterojunction photocatalysts, S-III, IV, V & VI have degraded 56, 45, 70, 86, 98 & 98.5% of MB dye after 6 min of sunlight irradiation. To endorse the self-photosensitization methodology, a blank experiment was likewise accomplished in the absence of catalyst under identical experimental conditions, and negligible degradation was noticed. When the catalyst was added to the dye solution, significant dye degradation was observed, indicating that the photocatalytic measure is largely responsible for dye degradation. To further investigate the photocatalytic efficiency of MnV2O6/BiVO4 heterojunction composite, the COD experiment was performed. The calculated COD value for MB and RhB solutions decreased from 160 to 40 mg L−1 and 115 to 37 mg L−1, respectively. These results showed that the mineralization yield of composite reached a value of 75% and 68%, respectively, for MB and RhB dyes, after irradiation with direct sunlight.
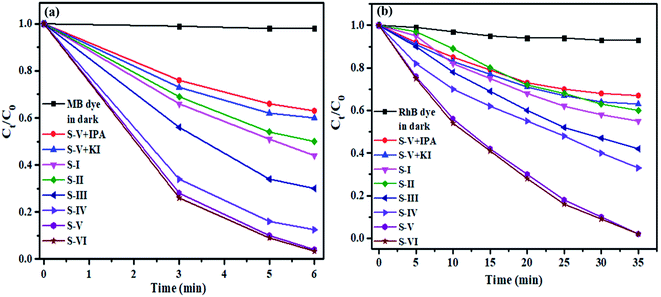 | ||
| Fig. 7 Plot illustrating the concentration change of the MB and RhB dyes as a function of time of irradiation. | ||
In addition, kinetic models were employed to comprehend the photocatalytic degradation process of MB dye. Similarly, a comparable performance for degradation of RhB dye under solar light in the presence of MnV2O6/BiVO4 heterojunction photocatalyst was observed (Fig. 7b). It was demonstrated from Fig. 7b that the intensity of absorption peak diminished appreciably with the passage of time, signifying the efficient disintegration of RhB dye using the MnV2O6/BiVO4 heterojunction photocatalyst. The debasement productivity of RhB dye over S-I, II, III, IV, V & VI was determined to be 47, 45, 58.7, 96 and 96.1%, respectively, after 35 min of sunlight irradiation.
In the heterogenous photocatalytic degradation process of organic pollutants, various active species comprising superoxide (˙O2−) anion radicals, hydroxide (˙OH) radicals, and photogenerated electrons (e−) and holes (h+) are created under appropriate light irradiation.41 To figure out the active species that assumes a significant role in dye photodegradation utilizing MnV2O6/BiVO4 heterojunction on irradiation by sunlight, different types of examinations on extinguishing active species were carried out by addition of separate scavengers in the reaction mixture. For this purpose, isopropyl alcohol (IPA), potassium iodide (KI) and benzoquinone (BQ) were employed for scavenging ˙OH, h+ and ˙O2− radicals, respectively. Due to extinguishing of active species, photocatalytic response is little restrained and prompts modest degradation of both the dyes. The degree of decline brought about by scavengers in degradation demonstrated the role of competing responsive species.
Fig. 7(a) and (b) illustrate that photodegradation of both the dyes over MnV2O6/BiVO4 heterojunction was considerably influenced on addition of scavengers. The degradation of dyes was significantly suppressed on addition of BQ (˙O2− scavenger) which indicated a crucial role of ˙O2− in the photodegradation procedure. The photodegradation activity of MnV2O6/BiVO4 only marginally decreased on introducing IPA and KI which suggested that both ˙OH and h+ have a minor but synergistic role in the degradation reaction.36,42
3.3 Plausible mechanism of photodegradation
In the light of results obtained, a tentative mechanism has been suggested to clarify the improved photocatalytic activity of MnV2O6/BiVO4 heterojunctions (Fig. 8). To perceive the band positions of MnV2O6/BiVO4 heterojunctions, the potentials at conduction band (CB) and valence band (VB) edges of MnV2O6 & BiVO4 semiconductors were designed using the equations below:23| ECB = χ − Ee − 0.5Eg | (1) |
| EVB = ECB + Eg | (2) |
| χ = [χ(A)aχ(B)b]1/[a+b] | (3) |
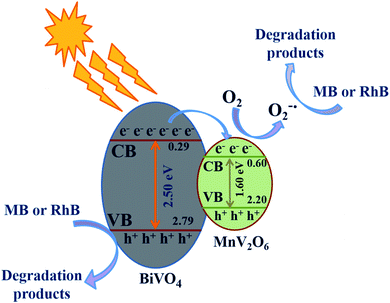 | ||
| Fig. 8 A plausible mechanism for organic pollutant removal over BiVO4/MnV2O6 heterojunction under direct sunlight illumination. | ||
The constants a and b denote the number of atoms in the compounds.44Eg, χ, ECB and EVB values for BiVO4 were found to be 2.50 eV, 6.04 eV, +0.29 and +2.79 eV/NHE, respectively and are comparable to the reported values.45,46 The values of Eg and χ for MnV2O6 are 1.60 eV and 5.90 eV, respectively. Consequently, ECB and EVB values for MnV2O6 were determined to be +0.60 and +2.20 eV/NHE.
In the light of above discussion and knowledge of active species involved, a potential mechanism for the degradation of organic dyes utilizing BiVO4/MnV2O6 heterojunction has been projected as follows and is displayed in Fig. 8. When sunlight was illuminated over BiVO4/MnV2O6 heterojunction, the photons approaching the photocatalyst were hopefully consumed by BiVO4 and MnV2O6 counterparts, prompting the production of a few electron–hole pairs. BiVO4 has a high negative flat band capability in comparison to MnV2O6. As a result, the electrons continue to move towards MnV2O6 from BiVO4 till the Fermi level stability of both is accomplished. Concurrently, OH˙ is generated by oxidation of adsorbed H2O molecules by the photoinduced holes of the VB of MnV2O6 and BiVO4 semiconductors. Simultaneously, the electrons gathered on the exterior of MnV2O6 interact with the adsorbed oxygen to generate ˙O2−. Hence, the produced active species like OH˙, h+ and ˙O2− efficiently break down the dye molecules to CO2, H2O and non-toxic inorganic products. A comparison of photocatalytic efficiency of some heterojunctions for degradation of organic contaminants is presented in Table 1.
| Sr. no. | Photocatalyst | Method of synthesis | Catalyst dosage (mg) | Pollutant/conc. | Source of light/time in min | Photocatalytic efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | ZnO/Ag2O | Photochemical route | 20 | MB/3.12 × 10−5 mol L−1 | 250 W UV, 500 W Xe lamp/4 | 99.5 | 47 |
| 2 | AgBr/Bi2WO6 | Hydrothermal | 200 | MB/10 mg L−1 | 500 W Xe lamp/30 min | 100 | 48 |
| 3 | Ag2O/TiO2 | Sol gel | 10 | 4-NP/200 ppm | Solar/210 s | 100 | 49 |
| 4 | Ni2P/Ni12P5 | Solvothermal | 1.5 | 4-NP/14 mg L−1 | Solar/8 min | 100 | 50 |
| 5 | CuO/ZnO | Hydrothermal | 30 | MB/5 mg L−1 | Solar radiation/210 min | 97 | 51 |
| 6 | CuSbSe2/TiO2 | Microwave method | 100 | RhB/MB/100 ppm | Long UV-A radiation/275 min | 75.93 (RhB), 42.72 (MB) | 52 |
| 7 | CuO/g-C3N4 | Ultrasonic | 10 | 4-NP/20 ppm | 35 W Xe lamp/100 min | 92 | 53 |
| 8 | Ag–CuO/g-C3N4 | Hydrothermal | 100 | 4-NP/100 ppm | Ni light irradiation/4 min | 97.8 | 54 |
| 9 | CeO2/CuO/Ag2CrO4 | Chemical precipitation | 125 | MB (5 mg L−1)/RhB (10 mg L−1) | LED lamp/80 min | 58.46 (MB), 84.79 (RhB) | 55 |
| 10 | Bi2Zr2O7/CdCuS | Hydrothermal | 50 | RhB/MB & 4-NP | Solar light/200 min | 84 (RhB), 90 (MB), 100 (4-NP) | 56 |
| 11 | WO3-BPNs | Co-precipitation | 50 | RhB/10 mg L−1 | 350 W Xe lamp/120 min | 92 | 57 |
| 12 | MOF/P–TiO2 | Self-assembly hydrothermal | 10 | RhB/10 ppm | 300 W Xe/25 min | 97.6 | 58 |
| 13 | MnV2O6/BiVO4 | One pot hydrothermal | 50 | 4-NP, MB & RhB (25 mg L−1) | Direct sunlight, 35 (4-NP), 6 (MB), 35 RhB | 100 (4-NP), 98 (MB), 96 (RhB) | Present work |
3.4 Photocatalytic degradation kinetics
Furthermore, pseudo first and second order models were used to explore the kinetics of dye degradation. The pseudo first order rate equation of Langmuir is given as: log(qe − qt) = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t, where qe and qt denote the concentration of dye adsorbed at equilibrium and at any time t, and the first order rate constant is represented by K1. The plot of ln(qe − qt) vs. t for pseudo first order kinetics of MB dye is shown in Fig. 9a. The calculated values of K1 and R2 are given in Table 2.
qe − k1t, where qe and qt denote the concentration of dye adsorbed at equilibrium and at any time t, and the first order rate constant is represented by K1. The plot of ln(qe − qt) vs. t for pseudo first order kinetics of MB dye is shown in Fig. 9a. The calculated values of K1 and R2 are given in Table 2.
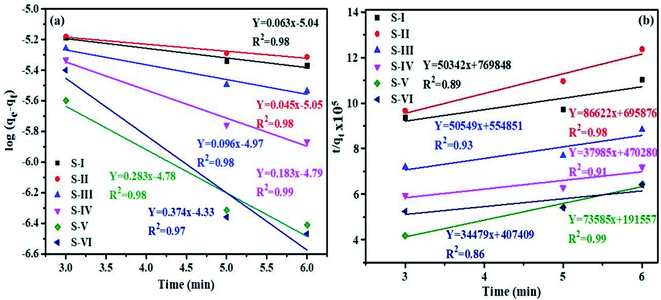 | ||
| Fig. 9 Graph of (a) pseudo first and (b) second order kinetics models for degradation of MB dye over MnV2O6/BiVO4 heterojunction. | ||
| Semiconductor/heterojunction | First order | Second order | ||
|---|---|---|---|---|
| K 1 | R 2 | K 2 | R 2 | |
| S-I | 0.063 | 0.98 | 3.2 × 103 | 0.89 |
| S-II | 0.045 | 0.98 | 11.3 × 103 | 0.98 |
| S-III | 0.096 | 0.98 | 4.6 × 103 | 0.93 |
| S-IV | 0.183 | 0.99 | 3.0 × 103 | 0.91 |
| S-V | 0.283 | 0.98 | 28.3 × 103 | 0.99 |
| S-VI | 0.374 | 0.97 | 2.9 × 103 | 0.86 |
The pseudo-second order rate equation was also applied to MB dye and is represented as: t/qt = 1/K2(qe)2 + t/qt, where K2 is the second order rate constant. Fig. 9b demonstrates the plot of (t/qt) vs. t for pseudo second order kinetics of MB dye and K2 and R2 values are given in Table 2.
A similar process was established for rhodamine B (RhB) for thorough comparison of kinetics. Fig. 10(a and b) presents both the kinetic models for the degradation of RhB dye.
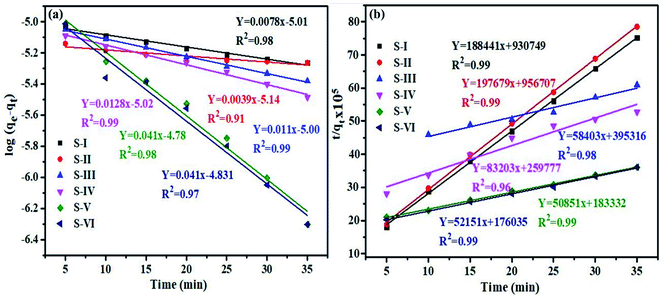 | ||
| Fig. 10 Graph (a) pseudo first and (b) second order kinetics models for degradation of RhB dye over the MnV2O6/BiVO4 heterojunction. | ||
The calculated K1, K2 and R2 values for RhB dye are given in Table 3. Furthermore, the model's applicability is examined using the R2 values of all photocatalyst samples.
| Semiconductor/heterojunction | First order | Second order | ||
|---|---|---|---|---|
| K 1 | R 2 | K 2 | R 2 | |
| S-I | 0.008 | 0.98 | 83.2 × 103 | 0.99 |
| S-II | 0.004 | 0.91 | 40.8 × 102 | 0.99 |
| S-III | 0.011 | 0.99 | 8.6 × 103 | 0.98 |
| S-IV | 0.013 | 0.99 | 26.6 × 103 | 0.96 |
| S-V | 0.041 | 0.98 | 14.1 × 103 | 0.99 |
| S-VI | 0.041 | 0.97 | 15.4 × 103 | 0.99 |
Interestingly, the results of kinetic models for the dyes are different. The R2 value for MB dye varies from 0.97 to 0.99, and 0.86 to 0.99 for pseudo first and second order kinetic models, respectively. For RhB dye, R2 varies from 0.91 to 0.99 and 0.96 to 0.99 for first and second order kinetic models, respectively. As a result, the data indicate that photocatalytic degradation of MB dye used a pseudo-first order process, whereas RhB dye used a pseudo-second order mechanism.
The cycling tests were performed to check the stability and reusability of MnV2O6/BiVO4 heterojunction (S-V) for photocatalytic degradation of MB and RhB dyes in solar light. The activity of the heterojunction was retained to a significant extent even after four consecutive cycles (Fig. 11a). The crystallinity and crystal structure of the photocatalyst were retained after four consecutive cyclic runs which is supported by the XRD pattern (Fig. 11b). The absence of leaching on the exterior throughout the photocatalytic response might be responsible for the insignificant drop in photocatalytic execution. These results indicate equitable stability and reusability of the synthesized heterojunction with extensive activity.
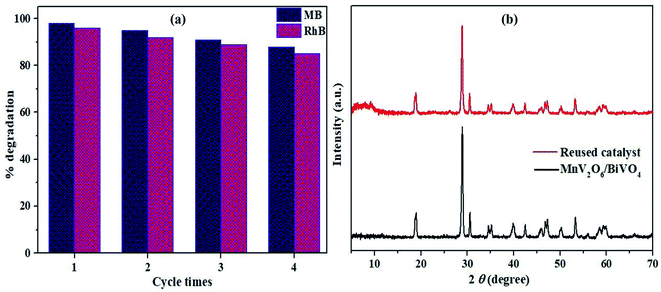 | ||
| Fig. 11 (a) Reusability of MnV2O6/BiVO4 catalyst for MB and RhB dye degradation; (b) XRD pattern of the unutilized and reused catalyst. | ||
4. Conclusions
MnV2O6/BiVO4 heterojunction samples were prepared employing a hydrothermal technique. Among the synthesized samples, MnV2O6/BiVO4 heterojunction sample (S-V) with a ratio of 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.00 (MnV2O6
1.00 (MnV2O6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BiVO4) showed the best performance under direct sunlight exposure for MB and RhB dye degradation. The active species playing the most significant role in dye photodegradation with MnV2O6/BiVO4 heterojunction was determined by employing isopropyl alcohol (IPA), potassium iodide (KI) and benzoquinone (BQ) as scavengers for ˙OH, h+ and ˙O2− radicals, respectively. The results revealed that degradation of dyes was significantly suppressed with BQ suggesting that ˙O2− played a key role in the photocatalytic degradation process. Furthermore, MnV2O6/BiVO4 heterojunction also successfully reduced 4-NP into 4-AP in a time span of 40 min without the production of any intermediates. This study provides an easy and speedy process for the degradation of toxic contaminants in waste water using direct sunlight.
BiVO4) showed the best performance under direct sunlight exposure for MB and RhB dye degradation. The active species playing the most significant role in dye photodegradation with MnV2O6/BiVO4 heterojunction was determined by employing isopropyl alcohol (IPA), potassium iodide (KI) and benzoquinone (BQ) as scavengers for ˙OH, h+ and ˙O2− radicals, respectively. The results revealed that degradation of dyes was significantly suppressed with BQ suggesting that ˙O2− played a key role in the photocatalytic degradation process. Furthermore, MnV2O6/BiVO4 heterojunction also successfully reduced 4-NP into 4-AP in a time span of 40 min without the production of any intermediates. This study provides an easy and speedy process for the degradation of toxic contaminants in waste water using direct sunlight.
Conflicts of interest
There are no conflicts to declare.References
- D. Shahidi, R. Roy and A. Azzouz, Advances in catalytic oxidation of organic pollutants − Prospects for thorough mineralization by natural clay catalysts, Appl. Catal., B, 2015, 174, 277–292 CrossRef.
- A. Chatterjee, S. Shamim, A. K. Jana and J. K. Basu, Insights into the competitive adsorption of pollutants on a mesoporous alumina–silica nanosorbent synthesized from coal fly ash and a waste aluminium foil, RSC Adv., 2020, 10, 15514–15522 RSC.
- N. Kaur, J. Kaur, R. Badru, S. Kaushal and P. P. Singh, BGO/AlFu MOF core shell nano-composite based bromide ion-selective electrode, J. Environ. Chem. Eng., 2020, 8, 104375 CrossRef CAS.
- C. Bradu, M. Magureanu and V. I. Parvulescu, Degradation of the chlorophenoxyacetic herbicide 2,4-D by plasma-ozonation system, J. Hazard. Mater., 2017, 336, 52–56 CrossRef CAS PubMed.
- M. Bilal and H. M. N. Iqbal, Microbial bioremediation as a robust process to mitigate pollutants of environmental concern, Case Studies in Chemical and Environmental Engineering, 2020, 2, 100011 CrossRef.
- M. N. Chong, B. Jin, C. W. K. Chow and C. Saint, Recent developments in photocatalytic water treatment technology: a review, Water Res., 2010, 44, 2997–3027 CrossRef CAS PubMed.
- M. Ni, M. K. H. Leung, D. Y. C. Leung and K. Sumathy, A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production, Renewable Sustainable Energy Rev., 2007, 11, 401–425 CrossRef CAS.
- M. Muuronen, S. M. Parker, E. Berardo, A. Le, M. A. Zwijnenburg and F. Furche, Mechanism of Photocatalytic Water Oxidation on Small TiO2 Nanoparticles, Chem. Sci., 2017, 8, 2179–2183 RSC.
- F. Lin, Z. Shao, P. Li, Z. Chen, X. Liu, M. Li, B. Zhang, J. Huang, G. Zhu and B. Dong, Low-cost dual cocatalysts BiVO4 for highly efficient visible photocatalytic oxidation, RSC Adv., 2017, 7, 15053 RSC.
- P. R. Paivaa and T. Noel, Application of Metal Oxide Semiconductors in Light-Driven Organic Transformations, Catal. Sci. Technol., 2019, 9, 5186–5232 RSC.
- H. Xu, S. Ouyang, L. Liu, P. Reunchan, N. Umezawaace and J. Ye, Recent advances in TiO2-based photocatalysis, J. Mater. Chem. A, 2014, 2, 12642 RSC.
- X. Shena, H. Shaoc, Y. Liua and Y. Zhai, Synthesis and photocatalytic performance of ZnO with flower-like structure from zinc oxide ore, J. Mater. Sci. Technol., 2020, 51, 1–7 CrossRef.
- M. Ganeshbabu, N. Kannan, P. S. Venkatesh, G. Paulraj, K. Jeganathan and D. M. Ali, Synthesis and characterization of BiVO4 nanoparticles for environmental applications, RSC Adv., 2020, 10, 18315 RSC.
- L. Suna, J. Suna, X. Yang, S. Bai, Y. Feng, R. Luo, D. Li and A. Chen, An integrating photoanode consisting of BiVO4, rGO and LDH for photoelectrochemical water splitting, Dalton Trans., 2019, 48, 16091–16098 RSC.
- H. Hirakawa, S. Shiota, Y. Shiraishi, H. Sakamoto, S. Ichikawa and T. Hirai, Au Nanoparticles Supported on BiVO4: Effective Inorganic Photocatalysts for H2O2 Production from Water and O2 under Visible Light, ACS Catal., 2016, 6, 4976–4982 CrossRef CAS.
- X. Xu, M. Du, T. Chen, S. Xiong, T. Wu, D. Zhao and Z. Fan, New insights into Ag-doped BiVO4 microspheres as visible light photocatalysts, RSC Adv., 2016, 6, 98788 RSC.
- J. H. Baek, T. M. Gill, H. Abroshan, S. Park, X. Shi, J. Nørskov, H. S. Jung, S. Siahrostami and X. Zheng, Selective and Efficient Gd-Doped BiVO4Photoanode for Two-Electron Water Oxidation to H2O2, ACS Energy Lett., 2019, 4, 720–728 CrossRef CAS.
- C. Regmi, T. H. Kim, S. K. Ray, T. Yamaguchi and S. W. Lee, Cobalt-doped BiVO4 (Co-BiVO4) as a visible-lightdriven photocatalyst for the degradation of malachitegreen and inactivation of harmful microorganisms in wastewater, Res. Chem. Intermed., 2017, 43, 5203–5216 CrossRef CAS.
- E. A. Ruiz, M. G. Galvan, P. Z. Robledo, J. C. B. Pacheco, J. V. Arenas, J. Peral and U. M. Garcia-Perez, Facile synthesis of visible-light-driven Cu2O/BiVO4 composites for the photomineralization of recalcitrant pesticides, RSC Adv., 2017, 7, 45885 RSC.
- J. Zhang, F. Ren, M. Deng and Y. Wang, Enhanced visible-light photocatalytic activity of a g-C3N4/BiVO4 nanocomposite: a first-principles study, Phys. Chem. Chem. Phys., 2015, 17, 10218 RSC.
- P. Ju, P. Wang, B. Li, H. Fan, S. Ai, D. Zhang and Y. Wang, A novel calcined Bi2WO6/BiVO4 heterojunction photocatalyst with highly enhanced photocatalytic activity, Chem. Eng. J., 2014, 236, 430–437 CrossRef CAS.
- B. Zoellner, E. Gordon and P. A. Maggard, A small bandgap semiconductor, p-type MnV2O6, active for photocatalytic hydrogen and oxygen production, Dalton Trans., 2017, 46, 10657–10664 RSC.
- S. Kaushal, P. Kurichh, K. Kaur and P. P. Singh, Novel 3D flower like ZnO/MnV2O6 heterojunction as an efficient adsorbent for the removal of imidacloprid and photocatalyst for degradation of organic dyes in waste water, Polyhedron, 2021, 201, 115161 CrossRef CAS.
- M. Nithya, S. Vidhya and K. Praveen, A Novel g-C3N4/MnV2O6 Heterojunction Photocatalyst for the Removal of Methylene Blue and Indigo Carmine, Chem. Phys. Lett., 2019, 737, 136832 CrossRef.
- X. Zhang, X. Lia, F. Jianga, W. Dua, C. Houa, Z. Xua, L. Zhua, Z. Wanga, H. Liua, W. Zhoua and H. Yuan, Improved electrochemical performance of 2D accordion-like MnV2O6 nanosheets as anode materials for Li-ion batteries, Dalton Trans., 2020, 49, 1794–1802 RSC.
- M. Yan, Y. Yan, Y. Wu, W. Shi and Y. Hua, Microwave-assisted synthesis of monoclinic–tetragonal BiVO4 heterojunctions with enhanced visible-light-driven photocatalytic degradation of Tetracycline, RSC Adv., 2015, 5, 90255 RSC.
- F. Guo, W. Shi, X. Lin and G. Che, Hydrothermal synthesis of graphitic carbon nitride–BiVO4 composites with enhanced visible light photocatalytic activities and the mechanism study, J. Phys. Chem. Solids, 2014, 75, 1217–1222 CrossRef CAS.
- Y. Singh, S. Kaushal and R. S. Sodhi, Biogenic synthesis of silver nanoparticles using cyanobacterium Leptolyngbya sp. WUC 59 cell-free extract and their effects on bacterial growth and seed germination, Nanoscale Adv., 2020, 2, 3972–3982 RSC.
- S. V. Kite, D. J. Sathe, A. N. Kadam, S. S. Chavan and K. M. Garadkar, Highly efficient photodegradation of 4-nitrophenol over the nano-TiO2 obtained from chemical bath deposition technique, Res. Chem. Intermed., 2020, 46, 1255–1282 CrossRef CAS.
- S. Kaushal, N. Kaur, M. Kaur and P. P. Singh, Dual-responsive pectin/graphene oxide (Pc/GO) nano-composite as an efficient adsorbent for Cr(III) ions and photocatalyst for degradation of organic dyes in waste water, J. Photochem. Photobiol., A, 2020, 403, 112841 CrossRef CAS.
- T. Palaniselvam, L. Shi, G. Mettela, D. H. Anjum, R. Li, K. P. Katuri, P. E. Saikaly and P. Wang, Vastly Enhanced BiVO4 Photocatalytic OER Performance by NiCoO2 as Cocatalyst, Adv. Mater. Interfaces, 2017, 4, 1700540 CrossRef.
- M. Long, W. Cai, J. Cai, B. Zhou, X. Chai and Y. Wu, Efficient Photocatalytic Degradation of Phenol over Co3O4/BiVO4 Composite under Visible Light Irradiation, J. Phys. Chem. B, 2006, 110, 20211–20216 CrossRef CAS PubMed.
- Z. Zhang, M. Wang, W. Cui and H. Sui, Synthesis and characterization of a core–shell BiVO4@g-C3N4 photo-catalyst with enhanced photocatalytic activity under visible light irradiation, RSC Adv., 2017, 7, 8167–8177 RSC.
- M. Guo, Y. Wang, Q. He, W. Wang, W. Wang, Z. Fu and H. Wang, Enhanced photocatalytic activity of S-doped BiVO4 photocatalysts, RSC Adv., 2015, 5, 58633 RSC.
- X. Lin, L. Yu, L. Yan, H. Li, Y. Yan, C. Liu and H. Zhai, Visible light photocatalytic activity of BiVO4 particles with different Morphologies, Solid State Sci., 2014, 32, 61–66 CrossRef CAS.
- E. Abroushan, S. Farhadi and A. Zabardasti, Ag3PO4/CoFe2O4 magnetic nanocomposite: synthesis, characterization and applications in catalytic reduction of nitrophenols and sunlight-assisted photocatalytic degradation of organic dye pollutants, RSC Adv., 2017, 7, 18293–18304 RSC.
- A. H. Abbar, A. H. Sulaymon and M. G. Jalhoom, Scale-up of a fixed bed electrochemical reactor consisting of parallel screen electrode used for p-aminophenol production, Electrochim. Acta, 2007, 53, 1671–1679 CrossRef CAS.
- M. Nasrollahzadeh, S. M. Sajadi, A. R. Vartooni, M. Alizadeh and M. Bagherzadeh, Green synthesis of the Pd nanoparticles supported on reduced graphene oxide using barberry fruit extract and its application as a recyclable and heterogeneous catalyst for the reduction of nitroarenes, J. Colloid Interface Sci., 2016, 466, 360–368 CrossRef CAS PubMed.
- Y. Liu, H. Xu and H. Yu, Synthesis of lignin-derived nitrogen-doped carbon as a novel catalyst for 4-NP reduction evaluation, Sci. Rep., 2020, 10, 20075 CrossRef CAS PubMed.
- K. Yu, S. Yang, C. Liu, H. Chen, H. Li, C. Sun and S. A. Boyd, Degradation of Organic Dyes via Bismuth Silver Oxide Initiated Direct Oxidation Coupled with Sodium Bismuthate Based Visible Light Photocatalysis, Environ. Sci. Technol., 2012, 46, 7318–7326 CrossRef CAS PubMed.
- Y. Ghaffari, N. K. Gupta, J. Bae and K. S. Kim, One-step fabrication of Fe2O3/Mn2O3 nanocomposite for rapid photodegradation of organic dyes at neutral pH, J. Mol. Liq., 2020, 315, 113691 CrossRef CAS.
- M. Mousavi, A. H. Yangjeh and M. Abitorabi, Fabrication of novel magnetically separable nanocomposites using graphitic carbon nitride, silver phosphate and silver chloride and their applications in photocatalytic removal of different pollutants using visible-light irradiation, J. Colloid Interface Sci., 2016, 480, 218–231 CrossRef CAS PubMed.
- S. R. Morrison, Electrochemistry at Semiconductor and Oxidized Metal Electrode, Plenum, New York, 1980 Search PubMed.
- Q. Yuan, L. Chen, M. Xiong, J. He, S. L. Luo, C. T. Au and S. F. Yin, Cu2O/BiVO4 heterostructures: synthesis and application in simultaneous photocatalytic oxidation of organic dyes and reduction of Cr(VI) under visible light, Chem. Eng. J., 2014, 255, 394–402 CrossRef CAS.
- M. Han, T. Sun, P. Y. Tan, X. Chen, O. K. Tan and M. S. Tse, m-BiVO4@γ-Bi2O3 core–shell p–n heterogeneous nanostructure for enhanced visible-light photocatalytic performance, RSC Adv., 2013, 3, 24964–24970 RSC.
- Parul, K. Kaur, R. Badru, P. Singh and S. Kaushal, Photodegradation of organic pollutants using heterojunctions: A review, J. Environ. Chem. Eng., 2020, 8, 103666 CrossRef CAS.
- S. Ma, J. Xue, Y. Zhou and Z. Zhang, Photochemical synthesis of ZnO/Ag2O heterostructures with enhanced ultraviolet and visible photocatalytic activity, J. Mater. Chem. A, 2014, 2, 7272 RSC.
- D. Wang, L. Guo, Y. Zhen, L. Yue, G. Xue and F. Fu, AgBr quantum dots decorated mesoporous Bi2WO6 architectures with enhanced photocatalytic activities for methylene blue, J. Mater. Chem. A, 2014, 2, 11716–11727 RSC.
- O. A. Zelekew and D. H. Kuo, A two-oxide nanodiode system made of double-layered p-type Ag2O@n-type TiO2 for rapid reduction of 4-nitrophenol, Phys. Chem. Chem. Phys., 2016, 18, 4405 RSC.
- F. Y. Tian, D. Hou, W. M. Zhang, X. Q. Qiao and D. S. Li, Synthesis of Ni2P/Ni12P5 bi-phase nanocomposite for efficient catalytic reduction of 4-nitrophenol based on the unique n-n heterojunction effects, Dalton Trans., 2017, 46, 14107–14113 RSC.
- Y. T. Prabhu, V. N. Rao, M. V. Shankar, B. Sreedhar and U. Pal, Facile hydrothermal synthesis of CuO@ZnO heterojunction nanostructures for enhanced photocatalytic hydrogen evolution, New J. Chem., 2019, 43, 6794–6805 RSC.
- A. S. Kshirsagar and P. K. Khanna, CuSbSe2/TiO2: novel type-II heterojunction nano-photocatalyst, Mater. Chem. Front., 2019, 3, 437–449 RSC.
- A. Verma, D. P. Jaihindh and Y. P. Fu, Photocatalytic 4-nitrophenol degradation and oxygen evolution reaction in CuO/g-C3N4 composites prepared by deep eutectic solvent assisted chlorine Doping, Dalton Trans., 2019, 48, 8594–8610 RSC.
- A. Verma, S. Kumar, W. K. Chang and Y. P. Fu, Bi-functional Ag-CuxO/g-C3N4 hybrid catalysts for the reduction of 4-nitrophenol and the electrochemical detection of dopamine, Dalton Trans., 2020, 49, 625–637 RSC.
- M. M. Sabzehmeidani, H. Karimi and M. Ghaedi, Enhanced visible light-active CeO2/CuO/Ag2CrO4 ternary heterostructures based on CeO2/CuO nanofiber heterojunctions for the simultaneous degradation of a binary mixture of dyes, New J. Chem., 2020, 44, 5033–5048 RSC.
- V. Jayaraman, C. Ayappan, B. Palanivel and A. Mani, Bridging and synergistic effect of the pyrochlore like Bi2Zr2O7 structure with robust CdCuS solid solution for durable photocatalytic removal of the organic pollutants, RSC Adv., 2020, 10, 8880 RSC.
- Q. Wang, B. Li, P. Zhang, W. Zhang, X. Hua and X. Li, 2D black phosphorus and tungsten trioxide heterojunction for enhancing photocatalytic performance in visible light, RSC Adv., 2020, 10, 27538 RSC.
- T. Zeng, D. Shi, Q. Cheng, G. Liao, H. Zhou and Z. Pan, Constructing of novel phosphonate-based MOF/P-TiO2 Heterojunction Photocatalysts: enhanced photocatalytic performance and mechanistic insight, Environ. Sci.: Nano, 2020, 7, 861–879 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1na00499a |
| This journal is © The Royal Society of Chemistry 2021 |