Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Porous intermetallic Ni2XAl (X = Ti or Zr) nanoparticles prepared from oxide precursors†
Yasukazu
Kobayashi
 *a,
Shohei
Tada
*a,
Shohei
Tada
 b and
Ryuji
Kikuchi
b and
Ryuji
Kikuchi
 c
c
aInterdisciplinary Research Center for Catalytic Chemistry, National Institute of Advanced Industrial Science and Technology (AIST), 1-1-1 Higashi, Tsukuba, Ibaraki 305-8565, Japan. E-mail: yasu-kobayashi@aist.go.jp
bDepartment of Materials Science and Engineering, Ibaraki University, 4-12-1 Nakanarusawacho, Hitachi, Ibaraki 316-8511, Japan
cDepartment of Chemical System Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan
First published on 22nd February 2021
Abstract
Porous intermetallic Ni2XAl (X = Ti or Zr) nanoparticles with small crystallite sizes (24–34 nm) and high Brunauer–Emmett–Teller (BET) surface areas (10–71 m2 g−1) were prepared from oxide precursors by a chemical route. CaH2 acted as a template to form the porous morphologies and assisted the reduction.
Heusler intermetallic compounds have attracted interest because of their applications in magnets, semiconductors, and thermoelectric materials.1–3 They are denoted as XYZ (half-Heusler structure) or X2YZ (full-Heusler structure), where X and Y are transition metals, and Z is a p-block metal. Some Heusler compounds such as Co2MnGe and Ni2TiAl have shown catalytic activities in hydrogenation and oxidation.4–6 Their catalytic activities can be tuned by substitution (e.g., X2YZ1−xZ′x), allowing for fine adjustments of the electronic state (ligand effect) and atomic arrangement (ensemble effect) on the surface where heterogeneous catalytic reactions occur. However, difficulty in obtaining Heusler intermetallic powders with high surface areas hinders their practical application as catalysts. Commonly, preparing high-surface-area intermetallics, including early transition metals such as La, Y, Ti, and Zr, is challenging because of the strong oxygen affinities of these metals.7–9 Therefore, they have been prepared by physical approaches in oxygen- and moisture-free conditions. Zr0.5Hf0.5CoSb0.8Sn0.2,10 Hf0.75Zr0.25NiSn0.99Sb0.01,11 and MCo1−xFexSnySb1−y (M = Ti, Zr, and Hf)12 have been prepared by mechanical ball milling. Vapor deposition-based approaches have been used to prepare Fe3Si13 and Co3Si.14 However, these methods are not practical for applications,15–17 whereas the chemical approach is more scalable but challenging. Chemical preparation of some Heusler nanoparticles, such as Fe3Si18 and Co2FeAl,19–23 has been reported, but chemically prepared Heusler nanoparticles involving early transition metals have not been reported. Besides, although multicomponent alloys with early transition metals have been studied, such as high-entropy PtPdRhRuCe for ammonia oxidation24 and high-entropy CoFeLaNiPt for electrocatalytic water splitting,25 it is necessary to develop simple and versatile techniques for the preparation of nano-sized multicomponent alloys involving hard-to-reduce metals.
Previously, we have reported the preparation of high-surface-area intermetallic nanoparticles, Ni3Al (13–27 m2 g−1)26,27 and NiAl (94–114 m2 g−1),27,28 by a chemical route. NiAl oxide precursors, NiO and NiAl2O4, were reduced to form porous intermetallic nanostructures in a molten LiCl–CaH2 system. In the molten LiCl at 600 °C, CaH2 works as a reducing as well as dehydration agent. Although pure Al tends to react with oxygen, molten LiCl prevents oxide formation and promotes the formation of NiAl and Ni3Al at 600 °C. The obtained intermetallic nanoparticles had high surface areas attributed to porous structures formed by CaO and CaH2, which acted as a template. The proposed preparation method was then applied to various intermetallic nanoparticles, such as YNi2Si2, LaNi2Si2,29 Pt2Y,30 and NiZn.31 In this study, we prepared high-surface-area Heusler nanoparticles comprising early transition metals. Ni2TiAl was selected because of its catalytic applications in steam reforming of methanol,6 whereas Ni2ZrAl was prepared to demonstrate the versatility of the method. This is the first report on the chemical preparation of Heusler nanoparticles involving early transition metals. Finally, the prepared nanoparticles were used for CO2 activation to evaluate their catalytic performances.
Intermetallic Ni2XAl (X = Ti or Zr) nanoparticles were prepared by reducing oxide precursors in a LiCl–CaH2 mixture at 600 °C.26–31 First, Ni(NO3)2·6H2O, X material (TiCl4 or ZrO(NO3)2·2H2O), Al(NO3)3·9H2O, and glycine were dissolved in distilled water in a molar ratio of Ni/X/Al/glycine = 2/1/1/4.8. The solution was then dried at 110 °C overnight, and the dried powder was finally heated at 500 °C in the air for 2 h to obtain the oxide precursor Ni2TiAl(Pre) or Ni2ZrAl(Pre). The oxide precursor CaH2 and LiCl were next mixed in a mortar in a weight ratio of precursor/CaH2/LiCl = 0.2/0.4/0.2. The mixed powder was then loaded in a stainless-steel reactor and heated at 600 °C for 2 h under Ar. Finally, the treated precursor was crushed in a mortar and rinsed with 0.1 M NH4Cl aqueous solution and distilled water, and the final powder, Ni2TiAl(RDT) or Ni2ZrAl(RDT), was obtained. Characterization and catalytic tests are described in ESI.†
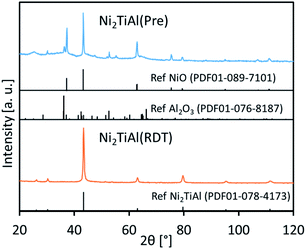
Fig. 1 shows the X-ray diffraction (XRD) patterns of Ni2TiAl(Pre) and Ni2TiAl(RDT). For Ni2TiAl(Pre), the peaks were assigned to NiO and Al2O3. Titanium-based oxides were not observed. Next, the precursor was reduced by the chemical approach, where the oxide precursor was reduced in a LiCl–CaH2 system at 600 °C. For the reduced powder of Ni2TiAl(RDT), the XRD peaks were mostly assigned to the intermetallic Ni2TiAl phase. The crystallite size of Ni2TiAl was estimated as 24 nm from the Scherrer equation (Table S1†).
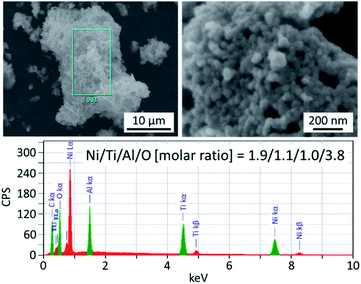
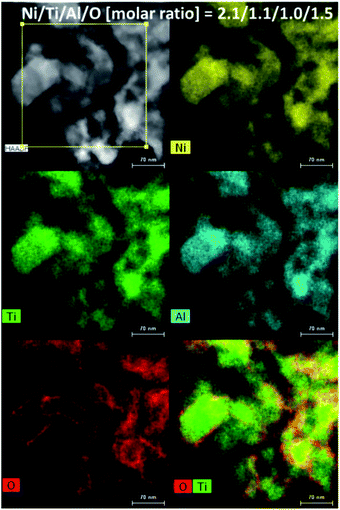
The morphology of Ni2TiAl(RDT) was then investigated by Scanning Electron Microscope (SEM) (Fig. 2 and S1–S3†) and (Scanning) Transmission Electron Microscope ((S)TEM) (Fig. 3 and S4–S8†) with energy-dispersive X-ray spectroscopy (EDS) analysis. Small particles of <100 nm interconnected to form porous structures. Moiré fringes indicate good crystallinity. Molar ratios of Ni, Ti, and Al in Ni2TiAl(RDT) calculated by SEM and TEM-EDS were consistent with the stoichiometric ratio of the intermetallic Ni2TiAl phase. On the elemental mappings, Ni, Ti, and Al are distributed evenly, and their positions are overlapped, indicating the homogeneity of the Ni2TiAl phase. Molar ratios of oxygen were relatively high in SEM and TEM-EDS, and the high-angle annular dark-field (HAADF)-STEM images show a high concentration of oxygen on the sample surface (Fig. 3, S7 and S8†). Because Ti and Al are readily oxidized in comparison with Ni, the sample surface was covered by titanium and aluminum oxides although they were not identified by XRD. Impurities such as Ca, Cl, and Li were not detected in Ni2TiAl(RDT), except for oxygen. Therefore, rinsing with NH4Cl removed these element-related species, such as CaO, CaH2, CaCl2, LiCl, and so on.
The surface area and porosity of Ni2TiAl(RDT) were examined by nitrogen adsorption/desorption. Fig. 4 shows the isotherm curves and the corresponding pore size distribution. The physical values calculated from the curves are summarized in Table S1.† Ni2TiAl(RDT) showed large nitrogen adsorption indicated by a high BET surface area (71 m2 g−1). Small hysteresis was observed between the adsorption and desorption curves, and a porous structure was suggested by a Barrett–Joyner–Halenda (BJH) method with mesopore sizes of <10 nm. A similar pore size distribution was observed in our previous reports for mesoporous NiAl,27,28 indicating that calcium species such as CaH2 and CaO worked as a template in molten LiCl. Table S2† summarizes BET surface areas and preparation methods of reported nickel-based aluminides. The BET surface area of Ni2TiAl powder prepared by arc melting is very small (0.13 m2 g−1). Thus, the Ni2TiAl powder prepared in this study had more than two orders of magnitude BET surface area compared to that prepared by arc melting. In addition, the reported BET surface areas of nickel aluminides prepared by physical and chemical methods are 15–27 m2 g−1. Our method provided remarkably high BET surface areas because of the porous structures. Enhanced catalyst durability and stable activity in ordered nanoporous alloys have been suggested due to their superior mechanical strength.32 Thus the prepared porous intermetallic nanoparticles could be promising catalysts from the practical point of view.
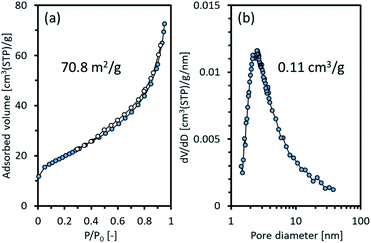 | ||
| Fig. 4 (a) Adsorption and desorption isotherms of nitrogen and (b) pore size distribution of Ni2TiAl(RDT). | ||
Next, surface states of Ni2TiAl(RDT) and the Ar+-etched sample, denoted by Ni2TiAl(RDT)-etch, were analyzed by X-ray Photoelectron Spectroscopy (XPS) measurements. Fig. 5 shows the XPS spectra corresponding to Ni 2p3/2, Ti 2p3/2, Al 2p, and Ni 3p. In Fig. 5(a), the Ni 2p3/2 spectrum of Ni2TiAl(RDT) had a peak at 856.1 eV (ref. 33) assigned to Ni3+. It is noteworthy that the spectrum of Ni2TiAl(RDT)-etch had an intense peak of metallic Ni at 852.8 eV (ref. 34) (Fig. 5(c)), where an intense peak of metallic Ni was also observed at 66.1 eV.35 In Fig. 5(b), the Ti 2p3/2 spectrum of Ni2TiAl(RDT) had a peak at 459.6 eV of Ti4+, whereas the spectrum of Ni2TiAl(RDT)-etch was separated into four peaks: Ti0, Ti2+, Ti3+, and Ti4+.36,37 The results suggest that the etching treatment partially removed the titanium oxide layer. In Fig. 5(c), the Al 2p spectrum of Ni2TiAl(RDT) had a peak at 74.8 eV corresponding to AlOx,38,39 confirming the formation of the surface oxide layer. The spectrum of Ni2TiAl(RDT)-etch had a clear peak corresponding to metallic Al at 72.3 eV.38,39 These results for Ni2TiAl(RDT) indicate the presence of the surface oxide layer, consistent with the results of STEM observations. Importantly, the oxide layer was thin enough to be mostly removed by etching, and the existence of Ni–Ti–Al alloy, which could be an intermetallic Ni2TiAl phase, below the oxide layer was confirmed by XPS measurements. Thus, porous Ni2TiAl nanoparticles are suitable to be used in catalytic applications.
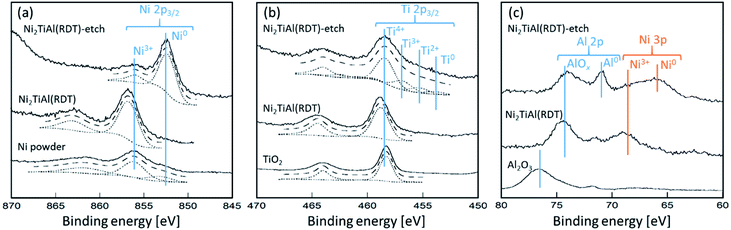 | ||
| Fig. 5 XPS spectra of (a) Ni 2p3/2, (b) Ti 2p3/2, and (c) Al 2p and Ni 3p for Ni2TiAl(RDT) and Ni2TiAl(RDT)-etch with references of Ni powder, TiO2, and Al2O3. | ||
Next, Ni2ZrAl(RDT) was prepared and characterized similarly to Ni2TiAl(RDT) to demonstrate the versatility of the method (Fig. S9–S17 and Table S1†). The Ni2ZrAl phase was obtained with a crystallite size of 34.4 nm and a BET surface area of 10.3 m2 g−1. Impurities such as NiAl, Ni10Zr7, and NiZr were observed, resulting in the lower surface area of Ni2ZrAl(RDT) compared with that of Ni2TiAl(RDT).
Finally, the catalytic performances of the prepared intermetallic compounds were evaluated in CO2 activation. The results are shown in Fig. 6. To evaluate the intrinsic catalytic performances, we calculated the turnover frequency (TOF) (Tables S3 and S4†). The TOF for CO2 activation, TOF(CO2), was in the order of Ni2ZrAl(RDT) > NiAl > Ni2TiAl(RDT) at 400 °C, indicating that Ni2ZrAl(RDT) activated CO2 more than did NiAl. In a lower reaction temperature range, TOF(CO2) and TOF(CH4) were in the order of NiAl > Ni2ZrAl(RDT) > Ni2TiAl(RDT). These trends indicated that the substitution of half of Al with Ti and Zr in NiAl harmed the CO2 activation for methane formation. The order of apparent activation energies (Ea) was given as Ni2ZrAl(RDT) (104 kJ mol−1) > NiAl (65 kJ mol−1) > Ni2TiAl(RDT) (61 kJ mol−1). Ni2ZrAl(RDT) gave a high Ea value close to 99 kJ mol−1 of the CeO2-supported Ni catalyst.40 On the other hand, NiAl and Ni2TiAl(RDT) showed low Ea values similar to 70 kJ mol−1 of the sponge Ni catalyst.40 Thus, the active sites on NiAl and Ni2TiAl(RDT) could be in the same state as that of sponge Ni to catalyze CO2 activation, suggesting that the non-supported metallic catalysts could decrease the energy barrier for CO2 activation.
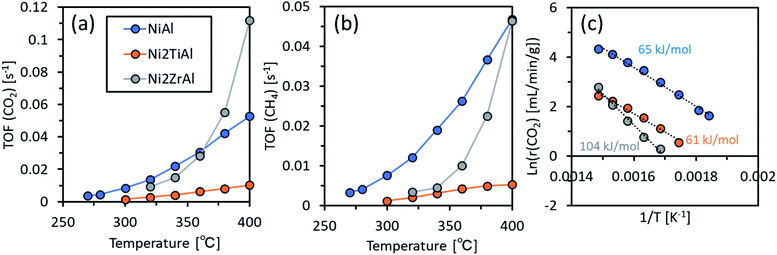 | ||
| Fig. 6 (a) TOF for CO2 activation and (b) TOF for CH4 production as functions of reaction temperature, and (c) the Arrhenius plots for NiAl, Ni2TiAl(RDT), and Ni2ZrAl(RDT). | ||
Conclusions
Porous intermetallic Ni2XAl (X = Ti or Zr) nanoparticles were prepared using CaH2 as a reducing agent in molten LiCl at 600 °C. The obtained BET surface areas were two orders of magnitude higher than those of powder samples prepared by arc melting. The versatility of our method allows for the preparation of porous intermetallic aluminide nanoparticles. The prepared Ni2ZrAl showed a higher TOF(CO2) at 400 °C than NiAl, whereas the Ni2TiAl activated CO2 with a lower activation energy than a reported supported catalyst. These findings suggested a potential advantageous application of the prepared nanoparticles in CO2 activation.Author contributions
YK carried out the experiments including synthesis and characterization. YK also conceptualized the project and supervised the research work. ST performed the experiments for XPS analysis and catalytic activity evaluation. RK discussed the results, helped to prepare the manuscript. All authors carried out the required revisions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Center for Instrumental Analysis at Ibaraki University is acknowledged.Notes and references
- F. Casper, T. Graf, S. Chadov, B. Balke and C. Felser, Semicond. Sci. Technol., 2012, 27, 063001 CrossRef.
- J.-W. G. Bos and R. A. Downie, J. Phys.: Condens. Matter, 2014, 26, 433201 CrossRef.
- M. Yin, J. Hasier and P. Nash, J. Mater. Sci., 2016, 51, 50 CrossRef CAS.
- T. Kojima, S. Kameoka and A.-P. Tsai, ACS Omega, 2017, 2, 147 CrossRef CAS.
- T. Kojima, S. Kameoka, S. Fujii, S. Ueda and A.-P. Tsai, Sci. Adv., 2018, 4, eaat6063 CrossRef CAS.
- T. Kojima, S. Kameoka and A.-P. Tsai, ACS Omega, 2019, 4, 21666 CrossRef CAS.
- M. Armbrüster, R. Schlögl and Y. Grin, Sci. Technol. Adv. Mater., 2014, 15, 034803 CrossRef.
- S. Furukawa and T. Komatsu, ACS Catal., 2017, 7, 735 CrossRef CAS.
- Y. Gong, J. Wu, M. Kitano, J. Wang, T.-N. Ye, J. Li, Y. Kobayashi, K. Kishida, H. Abe, Y. Niwa, H. Yang, T. Tada and H. Hosono, Nat. Catal., 2018, 1, 178 CrossRef CAS.
- X. Yan, G. Joshi, W. Liu, Y. Lan, H. Wang, S. Lee, J. W. Simonson, S. J. Poon, T. M. Tritt, G. Chen and Z. F. Ren, Nano Lett., 2011, 11, 556 CrossRef CAS.
- G. Joshi, X. Yan, H. Wang, W. Liu, G. Chen and Z. Ren, Adv. Energy Mater., 2011, 1, 643 CrossRef CAS.
- C. Wang, J. Meyer, N. Teichert, A. Auge, E. Rausch, B. Balke, A. Hütten, G. H. Fecher and C. Felser, J. Vac. Sci. Technol., B: Nanotechnol. Microelectron.: Mater., Process., Meas., Phenom., 2014, 32, 020802 Search PubMed.
- Y. Jing, Y. Xu and J.-P. Wang, J. Appl. Phys., 2009, 105, 07B520 CrossRef.
- K. Seo, S. Lee, H. Yoon, J. K. S. K. Varadwaj, Y. Jo, M.-H. Jung, J. Kim and B. Kim, ACS Nano, 2009, 3, 1145 CrossRef CAS.
- T. Ogawa, Y. Kobayashi, H. Mizoguchi, M. Kitano, H. Abe, T. Tada, Y. Toda, Y. Niwa and H. Hosono, J. Phys. Chem. C, 2018, 122, 10468 CrossRef CAS.
- Y. Lu, J. Li, T.-N. Ye, Y. Kobayashi, M. Sasase, M. Kitano and H. Hosono, ACS Catal., 2018, 8, 11054 CrossRef CAS.
- T.-N. Ye, Y. Lu, Y. Kobayashi, J. Li, S.-W. Park, M. Sasase, M. Kitano and H. Hosono, J. Phys. Chem. C, 2020, 124, 28589 CrossRef CAS.
- N. Dahal and V. Chikan, Chem. Mater., 2010, 22, 2892 CrossRef CAS.
- J. H. Du, Y. L. Zuo, Z. Wang, J. H. Ma and L. Xi, J. Mater. Sci. Technol., 2013, 29, 245 CrossRef CAS.
- C. H. Wang, Y. Z. Guo, F. Casper, B. Balke, G. H. Fecher, C. Felser and Y. Hwu, Appl. Phys. Lett., 2010, 97, 103106 CrossRef.
- C. Wang, L. Basit, Y. Khalavka, Y. Guo, F. Casper, T. Gasi, V. Ksenofontov, B. Balke, G. H. Fecher, C. Sönnichsen, Y.-K. Hwu, J.-J. Lee and C. Felser, Chem. Mater., 2010, 22, 6575 CrossRef CAS.
- C. Wang, F. Casper, Y. Guo, T. Gasi, V. Ksenofontov, B. Balke, G. H. Fecher, C. Felser, Y.-K. Hwu and J.-J. Lee, J. Appl. Phys., 2012, 112, 124314 CrossRef.
- C. Wang, F. Casper, T. Gasi, V. Ksenofontov, B. Balke, G. H. Fecher, C. Felser, Y.-K. Hwu and J.-J. Lee, J. Phys. D: Appl. Phys., 2012, 45, 295001 CrossRef.
- Y. Yao, Z. Huang, P. Xie, S. D. Lacey, R. J. Jacob, H. Xie, F. Chen, A. Nie, T. Pu, M. Rehwoldt, D. Yu, M. R. Zachariah, C. Wang, R. Shahbazian-Yassar, J. Li and L. Hu, Science, 2018, 359, 1489 CrossRef CAS.
- M. W. Glasscott, A. D. Pendergast, S. Goines, A. R. Bishop, A. T. Hoang, C. Renault and J. E. Dick, Nat. Commun., 2019, 10, 2650 CrossRef.
- Y. Kobayashi, Chem. Lett., 2019, 48, 1496 CrossRef CAS.
- Y. Kobayashi, S. Tada and R. Kikuchi, J. Chem. Eng. Jpn., 2020, 53, 562 CrossRef.
- Y. Kobayashi, S. Tada and R. Kikuchi, Chem. Lett., 2020, 49, 341 CrossRef CAS.
- Y. Kobayashi, S. Tada and R. Kikuchi, Mater. Trans., 2020, 61, 1037 CrossRef CAS.
- Y. Kobayashi, S. Tada and R. Kikuchi, Mater. Adv., 2020, 1, 2202 RSC.
- Y. Kobayashi, M. Sohmiya, S. Tada and R. Kikuchi, J. Jpn. Pet. Inst., 2020, 63, 380 CrossRef CAS.
- J. Li, Y. Zhang, C. Tian, H. Zhou, G. Hu and R. Xia, Microporous Mesoporous Mater., 2020, 295, 109955 CrossRef CAS.
- A. Liu, G. Liu, H. Zhu, B. Shin, E. Fortunato, R. Martins and F. Shan, Appl. Phys. Lett., 2016, 108, 233506 CrossRef.
- A. P. Grosvenor, M. C. Biesinger, R. St, C. Smart and N. S. Mclntyre, Surf. Sci., 2006, 600, 1771 CrossRef CAS.
- A. N. Mansour, Surf. Sci. Spectra, 1994, 3, 211 CrossRef CAS.
- V. Maurice, G. Despert, S. Zanna, P. Josso, M.-P. Bacos and P. Marcus, Acta Mater., 2007, 55, 3315 CrossRef CAS.
- T. Hanawa, J. Periodontal Implant Sci., 2011, 41, 263 CrossRef CAS.
- Q. Liu, H. Qin, J. A. Boscoboinik and G. Zhou, Langmuir, 2016, 32, 11414 CrossRef CAS.
- Y. Xu, M. Demura and T. Hirano, Appl. Surf. Sci., 2008, 254, 5413 CrossRef CAS.
- S. Tada, S. Ikeda, N. Shimoda, T. Honma, M. Takahashi, A. Nariyuki and S. Satokawa, Int. J. Hydrogen Energy, 2017, 42, 30126 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1na00047k |
| This journal is © The Royal Society of Chemistry 2021 |