Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
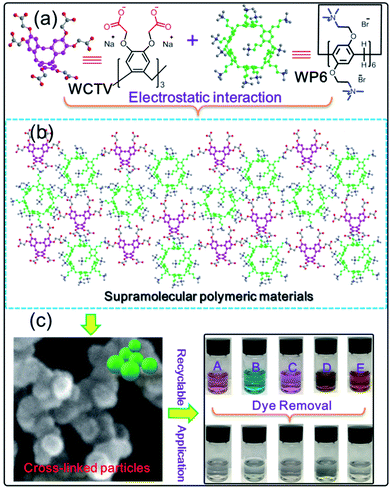
Pillar[6]arene-based supramolecular polymeric materials constructed via electrostatic interactions for rapid and efficient organic dye removal from water†
Xin
Yan‡
a,
Youyou
Huang‡
a,
Moupan
Cen
a,
Jin
Wang
a,
Jian
Shi
*b,
Bing
Lu
a,
Yang
Wang
 a and
Yong
Yao
a and
Yong
Yao
 *a
*a
aSchool of Chemistry and Chemical Engineering, Nantong University, Nantong, Jiangsu 226019, P. R. China. E-mail: yaoyong1986@ntu.edu.cn
bNantong University Analysis & Testing Center, Nantong, Jiangsu 226019, P. R. China. E-mail: shi.j1@ntu.edu.cn
First published on 15th February 2021
Abstract
The first pillar[6]arene-based supramolecular polymeric material constructed from electrostatic interactions was designed and prepared successfully. Importantly, it can adsorb and remove organic dye from water efficiently.
Organic dyes such as azophloxine, alcian blue, rhodamine B and so on are very important materials for the textile industry.1 However, the direct discharge of textile wastewater will cause serious water pollution and do harm to human health.2 To remove organic pollutants from water, adsorption with activated carbon (ATC) has been widely used due to its high efficiency and relatively low cost. But the regeneration of ATC, usually under air conditions with heating up to 500 °C, is energy intensive and will cause secondary pollution. Therefore, it is quite necessary to design and fabricate novel adsorbent materials with rapid adsorption and efficient uptake ability.3
Pillar[n]arenes,4 composed of hydroquinone derivative units and linked by –CH2– in the 2,5-positions, are a new type of emerging macro-cyclic host after crown ethers,5 cyclodextrins,6 calixarenes,7 and cucurbiturils.8 The facile syntheses, unique rigid pillar-like architectures, and π-electron rich cavities of pillar[n]arenes make them outstanding affinity hosts for selectively neutral and electron deficient guests.9 Considering the low preparation costs and the rich host–guest interactions, pillar[n]arene-based functional materials have been widely investigated and applied in various areas, such as drug delivery systems,10a molecular machines,10b trans-membrane channels10c and supramolecular polymers.10d Fortunately, adsorbent materials based on pillar[n]arene cross-linked polymers (AMPCPs) with rapid adsorption and efficient uptake ability have also been designed and fabricated in the past several years.11 For example, Prof. Ma and co-workers prepared a type of AMPCP by the reaction between tetrafluoroterephthalonitrile (TFTN) and hydroxyl units on pillar[n]arene for the efficient removal of organic pollutants from water.11a Prof. Huang and co-workers produced another type of AMPCP for the adsorption and removal of organic dyes from water by cross-linking carboxyl-derived pillar[5]arene and p-phenylenediamine.11b Prof. Coskun constructed a conjugated AMPCP incorporating pillar[5]arenes via a Pd-catalyzed Sonogashira–Hagihara cross-coupling reaction for propane/methane separation through host–guest interactions.11c
However, previous reported AMPCPs are all constructed by covalent bonds, and it is still a challenge to design and prepare AMPCPs from supramolecular interactions, such as electrostatic interactions, host–guest interactions and so on because supramolecular interactions are very weak and the AMPCPs from supramolecular interactions are not stable enough. Herein, we designed and constructed a novel AMPCP (WP6&WCTV) from quaternary ammonium modified water soluble pillar[6]arene (WP6) and carboxyl-derivatived water soluble cyclotriveratrylene (WCTV) via electrostatic interactions. The as-prepared WP6&WCTV polymeric materials were fully characterized by SEM, TEM, EDX mapping, solid-state 13C-NMR and elemental analysis. What's more, they can remove organic dye from water with a very fast adsorption rate and high adsorption capacity (Scheme 1).
To obtain the WP6&WCTV polymeric material, WP6 (10−4 M) and WCTV (2 × 10−4 M) were dissolved in water respectively. Then the solution of WP6 and WCTV was dropped into water with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
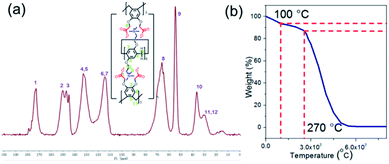
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 under vigorous stirring simultaneously. WP6&WCTV was obtained as gray powders after washing with distilled water and CH3OH twice. It was found that WP6&WCTV was completely insoluble in common organic solvents (Fig. S13†), indicating the formation of cross-linked structures. Fig. S6† shows the FT-IR spectra of WP6, WCTV and WP6&WCTV. The spectrum of WP6&WCTV showed the overlap of WP6 and WCTV, confirming the crosslinking between WP6 and WCTV. Then the obtained WP6&WCTV was verified with solid-state 13C-NMR. The 13C-NMR spectrum of WP6&WCTV showed resonances at 172.8 ppm, corresponding to carbonyl carbons. The resonance peaks at 150.2 ppm, 147.1 ppm, 144.9 ppm, 132.8 ppm, and 114.3 ppm correspond to the sp2 carbons of the phenyl rings. The peaks at 66.1 ppm, 54.7 ppm, 36.5 ppm and 30.2 ppm correspond to methylene carbons near carbonyl groups, methyl carbons near N atoms, and bridged methylene carbons, respectively (Fig. 1a). In addition, as shown in Fig. 1b, WP6&WCTV exhibited excellent stability up to 270 °C as investigated by thermogravimetric analysis (TGA).
2 under vigorous stirring simultaneously. WP6&WCTV was obtained as gray powders after washing with distilled water and CH3OH twice. It was found that WP6&WCTV was completely insoluble in common organic solvents (Fig. S13†), indicating the formation of cross-linked structures. Fig. S6† shows the FT-IR spectra of WP6, WCTV and WP6&WCTV. The spectrum of WP6&WCTV showed the overlap of WP6 and WCTV, confirming the crosslinking between WP6 and WCTV. Then the obtained WP6&WCTV was verified with solid-state 13C-NMR. The 13C-NMR spectrum of WP6&WCTV showed resonances at 172.8 ppm, corresponding to carbonyl carbons. The resonance peaks at 150.2 ppm, 147.1 ppm, 144.9 ppm, 132.8 ppm, and 114.3 ppm correspond to the sp2 carbons of the phenyl rings. The peaks at 66.1 ppm, 54.7 ppm, 36.5 ppm and 30.2 ppm correspond to methylene carbons near carbonyl groups, methyl carbons near N atoms, and bridged methylene carbons, respectively (Fig. 1a). In addition, as shown in Fig. 1b, WP6&WCTV exhibited excellent stability up to 270 °C as investigated by thermogravimetric analysis (TGA).
The morphology of 3D polymeric WP6&WCTV materials was then investigated by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). As shown in Fig. 2a, 3D cross-linked particles with a diameter of about 200 nm were observed, and this was also confirmed by the TEM image (Fig. 2b). Additionally, elemental analysis of WP6&WCTV showed that it consisted of 5.31% N, 63.49% C, and 7.18% H (Table S1†). Furthermore, energy dispersive X-ray analysis (EDX) mapping analysis (Fig. 2c) confirmed the homogeneous distributions of C, N, and O across an enlarged micro-particle; the analysis provided evidence that the polymeric materials were composed of WP6 and WCTV through electrostatic interactions with Br− and Na+ removed. Unfortunately, we failed to determine the surface area of WP6&WCTV due to its SBET being almost zero, which is in contrast to common porous materials. The extremely low surface area might due to the spherical morphology of WP6&WCTV. However, the cavity of pillar[5]arene incorporated into this 3D polymeric material provided an ideal platform to complex organic dye from water through host–guest interactions.
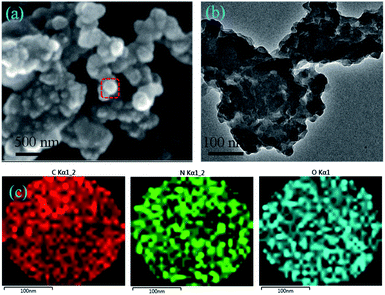 | ||
| Fig. 2 (a) SEM image of the WP6&WCTV materials. (b) TEM image of the WP6&WCTV materials. (c) EDX mapping of the WP6&WCTV materials (color code: C, red; N, green; O, cyan). | ||
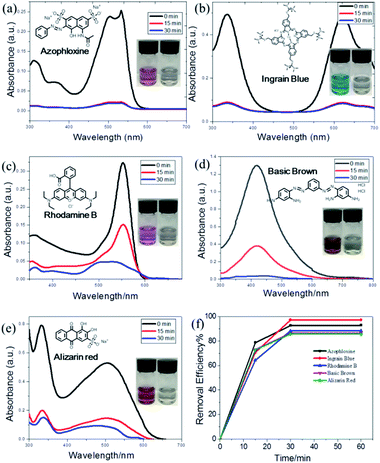
To test the performance of WP6&WCTV in organic pollutant removal, we evaluated five dyes causing harm to the environment: azophloxine (AX), ingrain blue (IB), rhodamine B (RhB), basic brown (BB) and alizarin red (AR). We submersed 5.00 mg WP6&WCTV materials into an aqueous solution of organic pollutants (0.100 mM, 5.00 mL) for 30 min and found that the dye solution became colorless (Fig. 3, inset). Then we used UV-Vis absorbance spectra to investigate the dye removal efficiency. As shown in Fig. 3, the characteristic peaks of each organic dye decreased sharply with absorbance time, indicating the dye concentration decreased substantially. It can be seen that the adsorption can reach equilibrium within 30 min, indicating a rapid adsorption process. All five dyes can be efficiently removed from water with uptakes greater than 85% (Table S2†). Importantly, WP6&WCTV materials can almost remove BB (97.3%) completely from water in 30 min (Fig. 3b). This is because the aniline groups on BB molecules well match the cavity of pillar[6]arene.12 These results confirmed that the WP6&WCTV polymeric material is an ideal platform for organic dye removal from water. Further 1H NMR investigation showed that the host–guest interactions between the host (WP6 or WCTV) and the organic dye caused this adsorption (Fig. S11–S15†).
Reusability is an important property of adsorbent materials. We also investigated the reuse ability of the obtained WP6&WCTV polymeric materials. The used WP6&WCTV was filtered and washed with water under ultrasonication and then dried under vacuum. The dried WP6&WCTV was added to the aqueous IB solution, which was then stirred for 30 min. As shown in Fig. 4, the IB removal efficiency was calculated to be 92% even after three cycles, indicating that WP6&WCTV had been regenerated successfully. With the low synthetic cost and good reusability, WP6&WCTV is economical and practical for waste-water treatment.
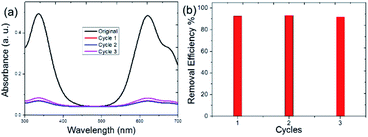 | ||
| Fig. 4 Evaluation of WP6&WCTV regeneration with IB as a model dye: (a) UV-Vis spectra of IB solutions and (b) removal efficiencies calculated on the basis of the decrease in absorption intensity. | ||
Conclusions
In conclusion, a new adsorbent material (WP6&WCTV) based on water soluble pillar[6]arene and cyclotriveratrylene was constructed successfully via electrostatic interactions. Its composition and 3D morphology were fully characterized by solid-state 13C-NMR, SEM, TEM and EDX mapping studies. Specifically, the obtained WP6&WCTV can be used as an adsorbent material to remove organic dye from water. In addition, this material can be regenerated easily by using a simple washing procedure with no loss in performance. These excellent findings demonstrate that this pillar[6]arene-based 3D network polymer can contribute to the removal of a wide range of micro-pollutants during water and waste-water treatment. We hope WP6&WCTV can be applied in real life fields, such as chemical separation and wastewater treatment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21801139, and 21871227), Natural Science Foundation of Jiangsu Province (BK20180942, and BK20190917), and Natural Science Foundation of the Higher Education Institutions of Jiangsu Province (19KJB150015). We also thank Duo Wei from Yangzhou University for helping us to perform solid-state 13C-NMR.Notes and references
- Y. Dong, Y. Qiu, D. Gao, K. Zhang, K. Zhou, H. Yin, G. Yi, J. Li, Z. Xia and Q. Fu, RSC Adv., 2019, 9, 33617 RSC.
- S. D. Richardson and T. A. Ternes, Anal. Chem., 2014, 86, 2813 CrossRef CAS PubMed.
- (a) E. K. Putra, R. Pranowo, J. Sunarso, N. Indraswati and S. Ismadji, Water Res., 2009, 43, 2419 CrossRef CAS PubMed; (b) A. Alsbaiee, B. J. Smith, L. Xiao, Y. Ling, D. E. Helbling and W. R. Dichtel, Nature, 2015, 529, 190 CrossRef PubMed.
- (a) T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022 CrossRef CAS PubMed; (b) P. Wang, B. Liang and D. Xia, Inorg. Chem., 2019, 58(4), 2252 CrossRef CAS PubMed; (c) J.-R. Wu and Y.-W. Yang, Chem. Commun., 2019, 55, 1533 RSC; (d) T. Xiao, L. Zhou, L. Xu, W. Zhong, W. Zhao, X.-Q. Sun and R. B. P. Elmes, Chin. Chem. Lett., 2019, 30, 271 CrossRef CAS; (e) S. Sun, D. Lu, Q. Huang, Q. Liu, Y. Yao and Y. Shi, J. Colloid Interf. Sci., 2019, 533, 42 CrossRef CAS PubMed; (f) W. Feng, M. Jin, K. Yang, Y. Pei and Z. Pei, Chem. Commun., 2018, 54, 13626 RSC; (g) Q. Lin, X.-W. Guan, S.-S. Song, H. Fan, H. Yao, Y.-M. Zhang and T.-B. Wei, Polym. Chem., 2019, 10, 253 RSC; (h) S. Lan, X. Yang, K. Shi, R. Fan and D. Ma, ChemCatChem, 2019, 11, 2864 CrossRef CAS; (i) C. Han, D. Zhao and S. Dong, Chem. Commun., 2018, 54, 13099 RSC; (j) S. Sun, M. Geng, L. Huang, Y. Chen, M. Cen, D. Lu, A. Wang, Y. Wang, Y. Shi and Y. Yao, Chem. Commun., 2018, 54, 13006 RSC; (k) Y. Sun, F. Zhang, J. Quan, F. Zhu, W. Hong, J. Ma, H. Pang, Y. Sun, D. Tian and H. Li, Nat. Commun., 2018, 9, 2617 CrossRef; (l) X. Zeng, H. Deng, X. Jia, L. Cui, J. Li, C. Li and J. Fang, Chem. Commun., 2018, 54, 11634 RSC; (m) Y. Han, L.-M. Xu, C.-Y. Nie, S. Jiang, J. Sun and C. G. Yan, Beilstein J. Org. Chem., 2018, 14, 1660 CrossRef CAS PubMed; (n) Y. Cai, Z. Zhang, Y. Ding, L. Hu, J. Wang, T. Chen and Y. Yao, Chin. Chem. Lett., 2020 DOI:10.1016/j.cclet.2020.10.036; (o) X. Sheng, E. Li, Y. Zhou, R. Zhao, W. Zhu and F. Huang, J. Am. Chem. Soc., 2020, 142, 6360 CrossRef CAS PubMed.
- M. M. Pyrch, J. M. Williams and T. Z. Forbes, Chem. Commun., 2019, 55, 9319 RSC.
- P.-Y. Li, Y. Chen, C.-H. Chen and Y. Liu, Chem. Commun., 2019, 55, 11790 RSC.
- Y. Cohen and S. Slovak, Org. Chem. Front., 2019, 6, 1705 RSC.
- C.-C. Zhang, Y. M. Zhang and Y. Liu, Chem. Commun., 2018, 54, 13591 RSC.
- (a) C. Li, L. Zhao, J. Li, X. Ding, S. Chen, Q. Zhang, Y. Yu and X. Jia, Chem. Commun., 2010, 46, 9016 RSC; (b) C. Li, Q. Xu, J. Li, F. Yao and X. Jia, Org. Biomol. Chem., 2010, 8, 1568 RSC.
- (a) G. Sun, Z. He, M. Hao, M. Zuo, Z. Xu, X.-Y. Hu, H.-J. Zhu and L. Wang, J. Mater. Chem. B, 2019, 7, 3944 RSC; (b) R. Zhang, C. Wang, R. Long, T. Chen, C. Yan and Y. Yao, Front. Chem., 2019, 7, 508 CrossRef CAS PubMed; (c) L. Chen, W. Si, L. Zhang, G. Tang, Z.-T. Li and J.-L. Hou, J. Am. Chem. Soc., 2013, 135, 2152 CrossRef CAS PubMed; (d) Q. Lin, P.-P. Mao, Y.-Q. Fan, L. Liu, J. Liu, Y.-M. Zhang, H. Yao and T.-B. Wei, Soft Matter, 2017, 13, 7085 RSC; (e) Y. Wu, J. Zhou, E. Li, M. Wang, K. Jie, H. Zhu and F. Huang, J. Am. Chem. Soc., 2020, 142, 19722 CrossRef CAS PubMed; (f) Y. Zhou, K. Jie, R. Zhao, E. Li and F. Huang, J. Am. Chem. Soc., 2020, 142, 6957 CrossRef CAS PubMed.
- (a) S. Lan, S. Zhan, J. Ding, J. Ma and D. Ma, J. Mater. Chem. A, 2017, 5, 2514 RSC; (b) B. Shi, H. Guan, L. Shangguan, H. Wang, D. Xia, X. Kong and F. Huang, J. Mater. Chem. A, 2017, 5, 24217 RSC; (c) S. N. Talapaneni, D. Kim, G. Barin, O. Buyukcakir, S. H. Je and A. Coskun, Chem. Mater., 2016, 28, 4460 CrossRef CAS.
- G. Yu, C. Han, Z. Zhang, J. Chen, X. Yan, B. Zheng, S. Liu and F. Huang, J. Am. Chem. Soc., 2012, 134, 8711 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0na00938e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |