Open Access Article
Open Access ArticleState-of-the-art developments in carbon-based metal nanocomposites as a catalyst: photocatalysis
Mohammad Ehtisham
Khan
 *
*
Department of Chemical Engineering Technology, College of Applied Industrial Technology (CAIT), Jazan University, Jazan, 45971, Kingdom of Saudi Arabia. E-mail: mekhan@jazanu.edu.sa; mehtishamkhan1@gmail.com
First published on 2nd March 2021
Abstract
The rapid progress of state-of-the-art carbon-based metals as a catalyst is playing a central role in the research area of chemical and materials engineering for effective visible-light-induced catalytic applications. Numerous admirable catalysts have been fabricated, but significant challenges persist to lower the cost and increase the action of catalysts. The development of carbon-based nanostructured materials (i.e., activated carbon, carbon nitride, graphite, fullerenes, carbon nanotubes, diamond, graphene, etc.) represents an admirable substitute to out-of-date catalysts. Significant efforts have been made by researchers toward the improvement of various carbon-based metal nanostructures as catalysts. Moreover, incredible development has been achieved in several fields of catalysis, such as visible-light-induced catalysis, electrochemical performance, energy storage, and conversion, etc. This review gives an overview of the up-to-date developments in the strategy of design and fabrication of carbon-based metal nanostructures as photo-catalysts by means of several methods within the green approach, including chemical synthesis, in situ growth, solution mixing, and hydrothermal approaches. Moreover, the photocatalytic effects of the resulting carbon-based nanostructure classifications are similarly deliberated relative to their eco-friendly applications, such as photocatalytic degradation of organic dye pollutants.
1. Introduction
The rapid upsurge in the world's population together with the speedy progress of different kinds of factories and industries has led to an energy crisis and environmental pollution. Among these, environmental pollution has become a serious concern around the globe, intimidating both industrialized and developing countries.1,2 Amongst the world's topmost eco-friendly concerns, water pollution is a foremost concern to human beings and as well as for aquatic life. Water is one of the most plentiful natural resources on Earth and covers about 70% of the Earth's surface, but only a small amount (3%) of water is in a drinkable form for human use.3 In the framework of the development of sustainable materials, carbon is the most plentiful component on the planet. Nature employs this component joined with hydrogen to deliver a source for the storage of renewable energy.4,5 Similarly, carbon-based schemes are progressively carrying out the most important part in incipient conversion technologies for renewable energy sources, such as energy storage devices, biofuels, catalysis, CO2 reduction and photocatalysis, etc.6–8 Carbon and carbon-based nanocomposites are broadly utilized in the cleansing of water, in the separation of gases, and as additives in soil. Moreover, the significance and future prospects of carbon-based nanostructured materials has been recognized in the present era by the highest and most renowned scientific awards, including the 1996 Nobel Prize in Chemistry (fullerenes), the Kavli Prize in Nanoscience in the year 2008 (carbon nanotubes), and the Nobel Prize in Physics (graphene) in the year 2010. As a significant focus of attention in the research area of carbon-based nanostructured materials, their possible utilization has exhibited speedy progress and signifies an imperative research topic in up-to-date research reports.9,10For a good influence, photocatalysts with considerable photon capturing nature, slow rate of recombination of photogenerated charge carriers and consumption of fast charge throughout redox reactions are the major requirement for complete degradation of organic model pollutants (dyes).11,12 The requirement of a probable photocatalyst for bulk scale and industrial requirements has led to the consideration of carbon, as it is cost-effective, eco-friendly and sustainable. Carbon has been extensively utilized in several forms in applications extending from solar devices to optical purposes, owing to its admirable physicochemical and electrochemical properties.13–17 Graphene, carbon nanotubes, conducting polymers and graphitic carbon nitride (g-C3N4) have been thoroughly explored in this field.18–20
During the past few years, the focus of research has been on heterogeneous photocatalysis, which has been investigated through the development of innovative photo-based catalysts, and reported to be effective under visible light irradiation and appropriate for organic combination.21 A special effect, called the plasmon effect, has been predicted in a new kind of effective metal nanostructured material that is effective in collecting photon energy for chemical-based fabrication, owing to their strong absorption in a wide range of the visible-light spectrum.22–26 The distinctive aspect of the plasmonic nanoparticles is their robust interface with incident photons by excitation of localized surface plasmon resonance. The photon energy of the incident photons can be increased by means of the electrons in the conduction band from the metal. The photo-based properties of the plasmon resonance depend on the size and morphology of the nanoparticles.27 This effective factor could possibly be applied for visible-light-induced photocatalysis to improve the photocatalytic performance in water splitting or degradation of organic model pollutants.28–30 In the year 2015–16, gold (Au) and AgNPs on graphene sheets displayed improved photocatalytic performance.31,32 Subsequently, metal NPs as both a photon absorber and a catalytic site act as potential materials that can be used for improved photocatalysis. This allows the coupling of photon collection and catalytic action, significantly extending the potential utilization of metal nanoparticles in photocatalysis.33 The environmental remediation applications of carbonaceous nanomaterials summarized in this review are both proactive (avoiding environmental degradation, refining public health) and retroactive (remediation, wastewater reuse, pollutant conversion).34 We start with a brief summary of carbonaceous nanomaterials and their comparable properties. We then review the foremost applications of carbon-based nanostructured materials in the field of photocatalysis (degradation of organic model pollutants).
It is well known that carbon-based nanostructures have outstanding properties in respect to their use in photo-based applications, including as visible light induced capacitive materials, and in hydrogen production, CO2 reduction water splitting, photo-electrochemical reactions, organic catalysts and photo-degradation of organic dyes. The attractive attributes of carbon-based materials include low cost, environmental friendliness, good chemical and thermal stability, ease of processing, and low framework density (Table 1).
| S. no. | Composition of catalyst | Used precursor | Visible-light applications | References |
|---|---|---|---|---|
| 1 | Graphitic-carbon nitride (g-C3N4-Fe3O4) | Melamine | Photodegradation | 35 |
| 2 | Alkalinized-C3N4-Fe | Melamine | Photodegradation | 36 |
| 3 | g-C3N4-AgBr | Melamine | Photodegradation | 37 |
| 4 | g-C3N4 nanofibers | Melamine | Photodegradation | 38 |
| 5 | g-C3N4-PNA | Melamine | Photodegradation | 39 |
| 6 | g-C3N4-Ag-TiO2 | Melamine | Photodegradation | 40 |
| 7 | Porous g-C3N4 | Dicyandiamide | Photodegradation | 41 |
| 8 | Porous g-C3N4 | Thiourea | Photodegradation | 42 |
| 9 | g-C3N4-bismuth-based oxide | Melamine or guanidine hydrochloride | Photodegradation | 43 |
| 10 | 3D porous g-C3N4 | Melamine | Photodegradation | 44 |
| 11 | Nanotube g-C3N4 | Melamine | Photodegradation | 45 |
| 12 | g-C3N4-ZIF | Urea | Photodegradation | 46 |
| 13 | Au-graphene | Graphene sheet | Photodegradation/photoelectrochemical | 31 |
| 14 | Ag-graphene | Graphene sheet | Photodegradation/photoelectrochemical | 32 |
| 15 | g-C3N4 | Cyanamide | Hydrogen production | 47 |
| 16 | g-C3N4 nano capsules | Cyanamide | Hydrogen production | 48 |
| 17 | g-C3N4-graphene | Dicyandiamide | Hydrogen production | 49 |
| 18 | g-C3N4-graphene-NiFe | Urea | Photoelectrochemical | 50 |
| 19 | g-C3N4 film | Melamine | Photoelectrochemical | 51 |
| 20 | g-C3N4-ZIF | Melamine | CO2 reduction | 52 |
| 21 | S-doped g-C3N4 | Thiourea and melamine | CO2 reduction | 53 |
| 22 | g-C3N4 nanoplatelets | Melamine | Water splitting | 54 |
| 23 | Sulfur-mediated g-C3N4 | Trithiocyanuric acid | Water oxidation | 55 |
| 24 | g-C3N4/Pd | Cyanamide | Organic catalyst | 56 |
| 25 | Oxidized g-C3N4 | Melamine | Organic synthesis | 57 |
In this review article, we discuss the advantageous appearance of carbon and its several forms as a conducting support structure in photocatalysis. The existing state of this precise research area, the challenges that persist, and future prospects are also presented. Carbon-based metal nanocomposites as a photocatalyst have not been broadly studied in photocatalytic processes, such as water splitting and organic model dye pollutant degradation, owing to the enormous challenges in their development. In conclusion, we deliver some explanation on the viewpoint, perspectives and future bearings of this impressive new research field.
2. Carbon-based nanostructured materials: concepts and properties
Carbon-based nanostructured materials have been examined and in use since the second half of the previous century as a catalytic support material in an extensive variety of chemical reactions used in manufacturing in industry.58 Activated carbon, graphite, and carbon black are the ones that have been used most regularly, followed in a smaller amount by glassy-based carbon, pyrolytic-based carbon and polymer-based carbon.59 Numerous advantages of carbon-based materials have been stated and their achievement has been enlightened in heterogeneous catalytic reactions, amongst which the following can be recorded: (i) chemical steadiness in both acid or basic medium, (ii) less corrosion capability, (iii) strong thermal constancy, (iv) hydrophobic behavior, (v) retrieval from the reaction mixture, and (vi) lower price.60–62Carbon plays an important part in the Earth's developments. It is able to form sturdy promises in diverse forms. The diamond and graphitic materials are the distinctive allotropes of carbon in nature. The C–C sp3 hybridization makes diamond the stiffest natural material, whereas graphite is a compact lubricant owing to the moveable interlamellar coupling between the sheets in the structure. During the last three decades, a lot of carbon-based nanostructures have been discovered, including fullerene, CNTs, graphene, mesoporous carbon, and so on. These carbon-based nanostructured materials display good prospects in the field of water treatment using visible light irradiation.62,63
2.1. Fullerene
Fullerene, commonly called as Buckminsterfullerene (C60), was the first revealed fullerene molecule in the carbon family. Fullerenes are now well-known owing to their notable chemical and physical properties due to their delocalized conjugated structure.63 Their structure, with a core shaped like a “bucky onion”, was self-sufficiently recognized by separate investigators in the early 1980s.63,64 The finding of fullerenes significantly extended the number of known allotropes of carbon, which were primarily restricted to graphitic materials and diamond. The inimitable chemical and physical properties of these novel forms of carbon stimulated many scientists to expect their applicable utilization. The C60 carbon clusters are able to work as electron acceptors, which are grounded on the conjugated polymer and fullerene offering high prospects as renewable energy sources.65,66The typical structure of a fullerene molecule has a cage-like fused-ring structure which is similar to a soccer ball.66 Fullerene comprises upwards of 20 carbon hexagons and 12 carbon pentagons with a bond formed laterally along each polygon edge. The bond between two hexagons could be considered a “double bond” and is shorter than the bond between a hexagon and a pentagon. The bond between two hexagons can be considered a double bond. C60 is tremendously stable at both high pressures and temperatures. The fullerenes are insoluble in water and moderately soluble in aromatic solvents such as toluene. The chemical reactivity of C60 is similar to that of an electron-lacking olefin, and it is able to react with nucleophiles (Fig. 1).67–69
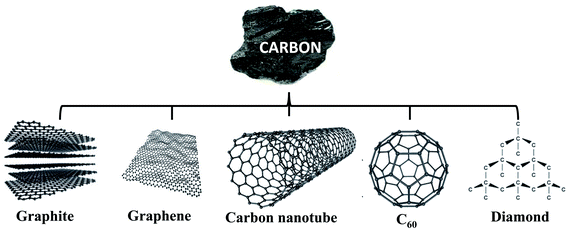 | ||
| Fig. 1 Schematic illustration of carbon structures, graphite, graphene, carbon nanotubes, C60 and diamond. | ||
2.2. Carbon nanotubes
Carbon nanotubes (CNTs) are a characteristic one-dimensional structure of carbon allotrope. Since their discovery in 1991, CNTs have been a hot topic in nanostructured material research, and they have received extensive attention both at the academic and industrial level owing to their notable characteristics and potential utilization in a wide variety of application areas.70 CNTs display advanced tensile strength as compared to steel and their strength arises from the sp2 bonds, which form between the specific carbon atoms. These bonds are more robust than the sp3 bonds that are initiated in the structure of diamond. At high pressure, the nanotubes are able to bond together and interchange some bonds for sp2 and sp3 hybridization states. The CNTs display fascinating thermal and electronic characteristics, which makes them a capable material that can be utilized in water treatment.71 For example, CNTs can play a role as either semiconducting or metallic materials, and this is completely reliant on their surface structures, and they also show excellent electron conductance.72 This is because the electrical properties of CNTs can increase the lifetime of the photogenerated charge carriers owing to the electron transfer from the conduction band of TiO2 to the structure of carbon, which is able to act as an effective electron sink. CNTs can also advance the photocatalytic performance of the TiO2 photocatalyst by providing an improved surface area (200 to 400 m2 g−1).73 The contact between TiO2 nanoparticles and CNTs allows rapid electron transfer from the conducting band of TiO2 to the CNT surface owing to their lower Fermi level.742.3. Graphene
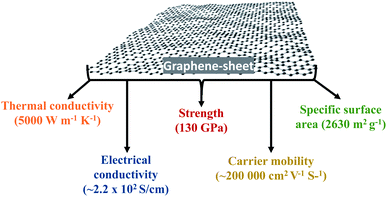
Graphene is a single atom thick, two-dimensional (2D) carbon-based structure that has attracted significant attention in the research community. Graphene is a sheet-like structured material that is composed of a thin layer of hybridized arrangements of carbon atoms (sp2) in the structure of a honeycomb crystal matrix.10,75,76 This material appeared to have potential as an innovative nanostructure for a variety of interesting purposes. The sheet-like structure of graphene revealed special photonic, electronic, and mechanical properties, such as fast charge-carrier mobility of 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1 at RT, good thermal conductivity of 5000 W m−1 K−1, an exceptional conductivity of up to 6000 S cm−1, and a wide and precise surface area of ∼2630 m2 g−1.48,49 Moreover, the sheet-like structure of graphene makes it extremely transparent, with an absorption ability of <2.3% toward visible light, and it has a Young's modulus of 1 TPa with a significant strength of ∼130 GPa.75 Accordingly, its inimitable structural features and excellent properties have fascinated massive research attention in both the scientific and engineering fields.
000 cm2 V−1 s−1 at RT, good thermal conductivity of 5000 W m−1 K−1, an exceptional conductivity of up to 6000 S cm−1, and a wide and precise surface area of ∼2630 m2 g−1.48,49 Moreover, the sheet-like structure of graphene makes it extremely transparent, with an absorption ability of <2.3% toward visible light, and it has a Young's modulus of 1 TPa with a significant strength of ∼130 GPa.75 Accordingly, its inimitable structural features and excellent properties have fascinated massive research attention in both the scientific and engineering fields.
The state-of-the-art catalytic and optoelectronic properties of the sheet-like structure of graphene based on hybridization with metal nanoparticles have received consideration. This is primarily owing to the sp2 characteristic of the carbon-bonds in the sheet-like structure of graphene, which permits electron delocalization.77,78 A graphene-sheet displays exceptional electronic conduction, and this passage of electrons through the sheet-like structure of graphene could be further enhanced by the integration of several metal nanoparticles (Fig. 2).78,79
Amongst several applications of graphene sheets, photocatalysis is one of the hopeful approaches to fabricate metal nanoparticle-decorated graphene nanostructures for water treatment applications.80,81 It is recognized that heterogeneous photocatalysis is an environmentally friendly and capable method that has established rising consideration throughout the research community. In the past few decades, intriguing developments have been achieved in the development of innovative metal nanoparticle-based photocatalysts and their possible utilization in many areas, such as H2 generation, water splitting, CO2 reduction and eco-friendly remediation.82–85 According to the present studies, it is very clear that metal-based graphene photocatalysts play a significant role in attaining exceedingly effective photocatalysis developments. However, an excessive number of photocatalysts display low quantum yield, applicable rate of visible light and stability, which has become an important challenge in developing metal-based photocatalysts for real utilization.86 Usefully, the amalgamation of graphene with metal nanoparticles delivers a simplistic approach to improve their performance.
2.4. Graphitic-carbon nitride (g-C3N4)
Graphitic carbon nitride (g-C3N4) is a polymeric material that contains carbon C, nitrogen N, and some contamination of H atoms, linked by tris-triazine-based patterns. In association with the superiority of carbon materials, it has electron rich properties, primitive surface functionalities and bonding themes due to the existence of N and H atoms.87 It is therefore observed as a probable choice to counterpart carbon-based utilization. g-C3N4 is not only the utmost constant allotrope of carbon nitrides under ambient atmosphere, but it also displays wide surface area properties that are striking for several applications, together with catalysis, owing to the existence of primary surface sites.88 The idyllic g-C3N4 uniquely contains an assemblage of C–N bonds deprived of electron localization in the π state.47 g-C3N4 is fabricated by a polycondensation process of cyanamide, which contains an insignificant amount of hydrogen in the form of primary and secondary amine groups on the terminating edges. The presence of hydrogen specifies that the actual g-C3N4 is moderately reduced and that a number of surface defects occur, which could be beneficial in catalysis, for instance, to increase electron delocalization on the surface, etc.47 Furthermore, its high thermal stability allows the material to function either in liquid or gaseous surroundings and at raised temperatures potentiating its extensive utilization in heterogeneous catalysis.47 The use of a sheet-like structure of g-C3N4 with a smaller band gap (Eg ∼ 2.7 eV) has attracted significant interest in recent years due to its metal-free nature, suitable visible-light activity, stimulating electronic band structure, and high physicochemical stability.89 As a result, the fabrication of g-C3N4 as a photocatalyst is low-cost and up-front.47,89 Certainly, it has been stated that g-C3N4 could be a useful catalyst for several reactions, including CO2 activation, oxygen reduction, hydrogen production, and photodegradation of organic dyes.47,90–92Fig. 3 displays the fundamental mechanism of g-C3N4 in photocatalysis. Due to its visible-light response by means of a band gap of ∼2.7 eV, the energy positions of the CB and VB are at −1.1 and 1.6 eV via a typical hydrogen electrode. Owing to these properties of g-C3N4, the utilization of g-C3N4 in water splitting, photo-reduction of CO2, purification of organic contaminants, organic catalytic synthesis, and fuel cells is more competent and operative.47,93
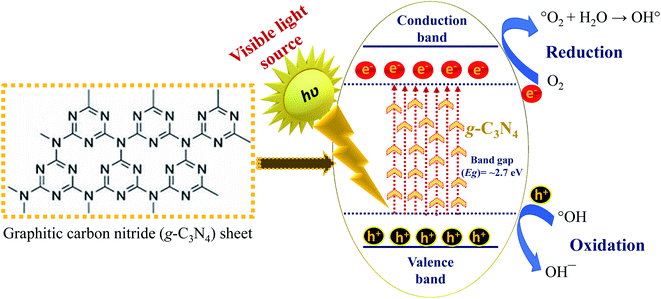 | ||
| Fig. 3 Graphitic-carbon nitride: fundamental steps involved in photocatalysis under visible light irradiation. | ||
3. Fabrication methods of carbon-based metal nanocomposites
The fabrication of carbon-based graphitic-carbon nitride is performed using precursors that are reactive, nitrogen-rich and free of oxygen components.94 The precursors comprise preboned C–N core structures, such as triazine and heptazine derivatives, most of which are unstable, difficult to obtain and highly volatile.95 The fabrication of single-phase sp3-hybridized carbon nitrides is a difficult task owing to their low thermodynamic stability.95 Unexpectedly, it appears that the defect-induced materials are much more valued than the idyllic one, particularly for catalysis, which involves surface defects. Consequently, the combination of g-C3N4 with defects is a fascinating topic, when the fabricated material is utilized in catalysis.95The pathways of condensation from cyanamide to dicyandiamide and from carbon/nitrogen rich constituents are modest and good synthetic routes to produce slightly defective polymeric materials.96 The precursor of cyanamide is finally converted to g-C3N4 at 550 °C, as proven from the reported literature.94 The fabrication process can be performed either in an inert atmosphere (e.g., N2, Ar) or in an air atmosphere with no substantial variations in the bulk structure, but modifications in the product yield, grade of condensation and specifically in the surface properties can be achieved.
In recent times, two-dimensional materials, sheet-like structures of graphene, graphitic-carbon nitride (g-C3N4), transition metals and hexagonal arrangements of boron nitride have displayed admirable properties that have been extensively utilized in electronic devices, chemical sensing, energy generation and eco-friendly remediation.95,97,98 In particular, g-C3N4 has fascinated rigorous consideration for its potential use in photocatalysis as a metal-free polymeric semiconductor with units of tri-s-triazine.98 g-C3N4 is an intermediate band gap semiconductor with a visible-light response up to (∼460 nm). This appropriate band gap with low-cost and simple fabrication, good chemical stability, and eco-friendly features is particularly appropriate for utilization in organic pollutant degradation, such as reduction of CO2, and organic and inorganic synthesis under visible light irradiation.7,97,99
Usually, the incorporation/intercalation of metallic impurities results in supplementary binding processes, which gives the doped-system inimitable photocatalytic properties by decreasing the value of the band gap and improving the visible-light absorption.100 In order to introduce metal ions into the context of carbon nitrides, a salt of equivalent solubility is constantly mixed with the g-C3N4 precursor. In this manner, a metal will be doped into the g-C3N4 framework through a thermal condensation procedure (Table 2).
| Doping element | Precursor | Synthesis | Band gap Eg (eV) | Application | Improved photocatalytic performance | References |
|---|---|---|---|---|---|---|
| Fe | Ferric chloride (Fe) with melamine (CN) | Impregnation | 2.56 | Degradation of RhB dye | 4–5 times improved compared to bare g-C3N4 nano-sheets | 100 |
| Cu | CuCl2 (Cu) with melamine (CN) | Thermal condensation | 2.25 | Degradation of MO dye | 90.2% degradation and 19.7% degradation (1 h) | 101 |
| Ce | Ce (SO4)2·4H2O(Ce) with melamine (CN) | Annealing | 2.57 | Degradation of RhB dye | 0.015 min−1/0.0073 min−1 (2.1 times) | 102 |
| Co | CoPc (Co) with melamine (CN) | Thermal condensation | 2.62 | H2 evolution | 28 mol h−1/9.5 mol h−1 (3 times) | 103 |
| Eu | Eu (NO3)3 (Eu) with melamine (CN) | Thermal condensation | 2.41 | Degradation of MB dye | 0.0121 min−1/0.0058 min−1 (2.1 times) | 104 |
| Mo | (NH4)6MO7O24·4H2O (Mo) with melamine (CN) | Thermal condensation | 1.45 | CO2 reduction | CO yield 887 mol g−1 | 105 |
| CH4 yield 123 mol g−1 | ||||||
| Zr | Zirconium nitrate (Zr) with urea (CN) | Thermal condensation | 2.55 | Degradation of RhB dye | 100% degradation/70% degradation (110 min) | 106 |
| Au | Chloroauric acid (HAuCl4·3H2O) with melamine (CN) | Thermal polycondensation method | 2.60 | Degradation of RhB dye | 100% degradation/25% degradation (120 min) | 107 |
| Ag | Silver nitrate (AgNO3) with urea (CN) | Single-strain biofilm fabrication | 2.40 | Degradation of RhB and MB dye | 100% MB degradation | 108 |
| 100% RhB degradation | ||||||
| 40% degradation within 210 and 240 min |
Separate from the doping of alkali-metals, the doping of other metals, such as Pd, Cu, Fe, W, Zr, and so forth, has also been generally applied to alter the electronic and optical properties of g-C3N4.106. The doping of metals could increase the absorption of light, decrease the band gap, increase the mobility of charge carriers, and extend the time period of charge carriers, which are all essential for noticeable photocatalytic performance.97,109 As a matter of fact, g-C3N4 can simply capture metal cations owing to the robust exchanges between the cations and the negatively charged atoms of nitrogen attributed to the lone pairs of electrons on the nitrogen edges of g-C3N4.109 Noble metals, such as platinum (Pt) and palladium (Pd), have been utilized to decorate the sheet-like structure of g-C3N4 with enhanced transporter mobility, improved separation of electron hole pairs, and narrowing of the band gap values.110 This article found and reported that the electronic and optical properties of g-C3N4 could be effortlessly adjusted by modification with metal atoms.110 The reported literature specified that metal-incorporation would improve the mobility of electrons, narrowing the bandgap value, thus promoting improved absorption and photocatalytic performance under visible-light irradiation.
Metal nanoparticles (M-NPs) as photocatalysts have attracted recent attention owing to their robust visible and UV light absorption. The photonic energy is captivated by the conduction electrons from the metal and the strong electric fields nearby, and twisted by the surface plasmon resonance (SPR) behavior, which has a significant effect of the activation of the molecules on the surface of the metal nanoparticles, and simplifies chemical alteration.88 Several synthesis routes of organic-based compounds utilize catalysts at higher temperatures to attain advanced competences.88,111 However, it will be specifically valued to initiate these reactions by visible-light illumination at ambient temperature, which will avoid unfavorable byproducts fabricated at higher temperatures. In the past, the area of heterogeneous structure-based photocatalysis has developed rapidly with the progress of novel photocatalysts that have shown the ability to work under visible-light irradiation. Furthermore, visible-light (sunlight) has attained much consideration, as it is a clean and plentiful source of energy. Solar light is a mixture of 5% UV light (wavelength 200–400 nm), 43% visible light (wavelength 400–800 nm), and 52% infrared (wavelength > 400 nm) energy that revealed higher performance with illumination in the solar spectrum, which is useful in photocatalysis.112
In brief, metal ions were broadly applied as dopants on a sheet-like structure of g-C3N4. Usually, the incorporation of metal ions could result in the creation of new energy levels in the band-gap, which extends the visible light response and possibly reduces the rate of recombination for electron–hole charges. Thus, several studies based on metal and/or transition metal-doped g-C3N4 have been reported (Fig. 4).
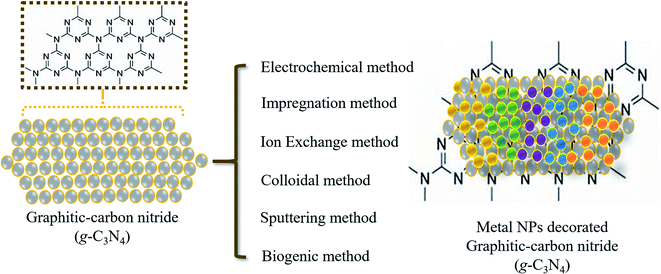 | ||
| Fig. 4 Schematic displaying the decoration of the sheet-like structure of graphitic-carbon nitride (g-C3N4) with different metal nanoparticles. | ||
4. Role of carbon-based metal nanocomposites as catalysts under visible light irradiation
Metal nanoparticles as a catalyst normally suffer from dissolution, sintering, and agglomeration issues. To lessen their catalyst degradation and expand their catalytic action and robustness, carbon-based metal NPs as a catalyst have been extensively established.113 A series of different carbon materials, such as activated carbon, porous carbon, carbon nanotubes, graphene and graphitic-carbon nitride, could possibly be used as catalyst supports, because of their precise features including:114(i) Immense stability in both acidic and basic media.
(ii) Large surface area and good dispersion as compared to other conventional catalyst supports (e.g., alumina, silica).
(iii) Potential to regulate the porosity and surface chemistry, and being preferred for the synthesis of metal materials and retrieval by burning of carbon/carbon-based materials.
Carbon based materials, especially carbon-based metal nanostructures, have fascinated consideration in the area of catalysis under visible-light irradiation owing to their intriguing properties and good tunability that can be modified by altering their morphology and compositions.115 Until now, many carbon-based photocatalysts with dissimilar structures and arrangements have been developed and a variety of well-designed fabrication approaches have been established.
4.1. Activated carbon (AC)
AC is a recognized highly porous carbonaceous material that is extensively utilized as an adsorbent to eliminate several organic compounds owing to its wide surface area, good physiochemical stability and simple fabrication process.116,117 Furthermore, AC can also be used as an effective support material for various metal-based nanocomposites as catalysts.118 The synergetic effects between the metal and AC can further improve the photocatalytic performance. In general, the active roles that AC can play in a variety of photocatalyst systems are as follows:(i) The ultrahigh surface area of AC makes the composite a photocatalyst with good capability to capture organic molecules in water as well as from air.
(ii) There is a high concentration of organic compounds around the surface of the photocatalyst, which is critical for the improvement of photo-based catalytic reaction sites.
(iii) The intermediates generated after photocatalysis can be efficiently adsorbed by AC for the next degradation cycle, so that the obtainability of the photocatalyst can be further enhanced.
(iv) The combination of AC with metal nanoparticles can moderately reduce the charge recombination of the photogenerated electron hole pairs, so that the photocatalytic performance of the nanocomposite system can be enhanced.
Accordingly, AC has been considered as a prospective support material for the design and combination of novel metal-based photocatalysts, and their different properties have been fabricated through various strategies.
4.2. Carbon nanotubes (CNTs)
CNTs are distinctive one-dimensional (1D) carbon nanostructures that exhibit the features of wide surface area, fascinating optical and electronic properties, inimitable physicochemical properties, and immense aspect ratio. Based on their special features, CNTs are potential candidates for the design and preparation of innovative photocatalysts.119 Several literature studies have been carried out on the strategy of preparing CNT-based metal nanocomposites as a catalyst, owing to the simple fabrication process and comparatively low-cost.120 Overall, CNTs primarily act as a support for several semiconductors to form composite photocatalysts. CNTs have attracted significant consideration owing to their distinct structure and high mechanical strength, which makes them potential candidates for innovative composite materials. They can be either semiconducting, semi-metallic or metallic, reliant on the helicity and the diameter of the tube structure.121 According to their shape and structure, CNTs are able to conduct electricity owing to the delocalization of the electrons of the pi bond. In addition, researchers have shown that CNTs are effective adsorbents owing to their bulky precise surface area, hollow and layered structures and the existence of pi bond electrons on the surface. Above and beyond that, more active sites could be formed on the nanotubes. Therefore, CNTs could be used as a useful material for the successful degradation of organic dyes using a CNT-based metal nanocomposite.122When CNT-based metal nanostructures are irradiated with photons that have energy equivalent to or higher than the value of the band gap energy, the incident photons excite the valence band (VB) electrons across the conduction band (CB), which leaves holes behind in the valence band. Accordingly, there must be at least two reactions existing at the same time: (a) oxidation from photogenerated holes, and (b) reduction from photogenerated electrons.122 The degradation rate could be improved by suppressing the rate of recombination of electron–hole pairs, which prevents particle accumulation and improves the adsorption capacity, as this is a significant progression in photocatalysis. In order to expand the photocatalytic efficiency, numerous procedures have been explored. These include:
(i) Improving the surface area of the used metal decoration on the CNT surface.
(ii) Creation of defects in the structures to induce space-charge separation and consequently lessen the charge recombination rate.
(iii) Variation in the CNT with metal nanostructures to prevent agglomeration.
4.3. Graphene
Graphene is a broadly considered two-dimensional (2D) carbon-based material with excessive potential for plentiful utilization. Primarily, graphene has an inimitable layered structure containing an sp2 bonded arrangement of carbon atoms in a hexagonal lattice, and the one-atom-thick carbon layer structure bestows it with desirable characteristics, such as admirable mechanical properties, captivating heat and electron conductivity, broad surface area, and robust physicochemical stability.123 The state-of-the-art catalytic and photon-based properties of the sheet-like structure of graphene based on hybridization with metal nanoparticles have attracted consideration. This is primarily owing to the sp2 hybridization structure of the carbon, which permits electron delocalization.75,123,124 Moreover, graphene is a semimetal with a minor degree of overlap between the VB and CB which makes it a potential candidate for application in photocatalysis.75 Metals, like Au, Ag, Pd and Pt,34,125,126 and combinations of these metals or with other carbon-based structures have also been evaluated for probable visible light-induced applications. The noble metal Au and Ag nanoparticles have fascinated intense consideration because of the possibility to advance their photocatalytic performance under visible light illumination, which provides an additional electron trap and suppresses the rate of recombination by increasing the interfacial charge transfer. Consequently, it has been determined that Au and Ag nanoparticles are capable of capturing and scattering photons with a comparatively high excitation cross-section in the visible light region. Subsequently, metal NPs are appropriate for improving the photo-based properties of carbon-based materials, such as graphene, which can be effectively used for photocatalysis.75Table 3 lists the performances of newly fabricated novel plasmonic nanocomposite with sheets of graphene as a support material. Several studies have preferred different kinds of organic pollutant dyes because the degradation procedure can be observed merely through the changes in the photo-absorption of the degrading solution.| Nanocomposite (metal–graphene) | Organic dyes | Source of photons | Outcome of degradation | References |
|---|---|---|---|---|
| a MB: methylene blue, CR: Congo red, RhB: rhodamine B, IC: indigo carmine. | ||||
| Au–Graphene | MB | Visible-light (λ > 420 nm) | 65% in 7 h | 31 |
| Ag–Graphene | MB and CR | (λ > 420 nm) | 65% and 90% in 6 and 5 h | 32 |
| Graphene–gold | MB, RhB and orange II | (λ > 420 nm) | 88.6%, 27.6% and 8.5% in 4 h | 127 |
| Pt–Pd–graphene | Basic fuchsin and IC dyes | (λ > 420 nm) | 70% and 65% in 50 min | 128 |
| Pt/graphene | RhB and MB | 8 W, halogen lamp | 70% and 82% in 180 min | 129 |
| Ag–Au on graphene sheets | 4-Nitrophenol | (λ > 420 nm) | 97.38% in 360 s | 130 |
| Metal nanocluster (Ag and Au)/graphene | 4-Nitrophenol | (λ > 420 nm) | 100% in 175 min | 131 |
| Ag–Au–rGO nanocomposite | 4-Nitrophenol | (λ > 420 nm) | 100% in 360 s | 130 |
4.4. Graphitic-carbon nitride (g-C3N4)
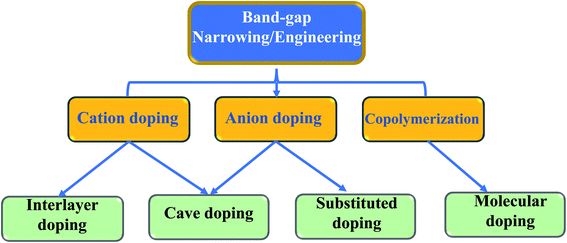
Recently, in the development of catalysts, graphitic-carbon nitride has become a novel research topic. Unlike out-of-date metal semiconductors, g-C3N4 is a polymeric semiconductor that contains carbon and nitrogen elements and has a graphite-like sheet/layered structure.132 This unique structure gives g-C3N4 fascinating properties, such as a narrow bandgap, good thermal stability, and facile fabrication, all of which make it a potential candidate for the creation of highly effective photocatalysts.133 Wang et al. initially employed this material for the visible-light photocatalytic production of H2, and many g-C3N4-based nanocomposites have been established.134 Zhang et al. described a facile and low-cost fabrication process to expand the photocatalytic degradation performance of g-C3N4. They utilized sacrificial templates to produce pores inside the g-C3N4 to increase its surface area and porosity, so that the mass transfer capability and photocatalytic performance of the engineered g-C3N4 could be further upgraded.135Overall, it is recognized that the idyllic bandgap value of a nanocomposite must be ∼2.0 eV, for which it is possible to harvest sufficient visible light to create the necessary electrons and holes with strong driving forces for photocatalytic reactions.136,137 However, the bandgap value of g-C3N4 is ∼2.7 eV, which makes it only able to absorb solar light at wavelengths below 460 nm. Consequently, in order to further improve the photon harvesting capability of g-C3N4, numerous band-gap approaches, together with atom-level and molecular-level doping, have been broadly attempted to attain improved photocatalytic performance (Fig. 5).138,139
5. Fundamentals of carbon-based nanostructures in photocatalysis
The carbon-based nano-structures have captivating properties, which is attributed to the recognition of several novel skills in the recent past.140 One of the utmost significant properties that makes them striking for photocatalytic utilization is their excellent stability in both acidic and basic conditions. This property makes their recovery easy and they can be recycled. This also makes them ecologically benign and perfect for utilization as a support material for catalysts. The integration of metal nanoparticles into carbon-based supports for photocatalysts serves dual purposes. On the one hand, it reduces the charge recombination rate of electron hole pairs, and on the other hand, it delivers a hydrophobic environment on the surface of the photocatalyst for localizing reactants close to the active sites.141 Amongst several carbon-based nanostructures, carbon nanotubes, graphene and graphitic-carbon nitride have exhibited good catalytic effectiveness. This is primarily ascribed to their wide-ranging specific surface areas and high electronic movement.141 Graphene and carbon nanotubes have been extensively examined for the production of metal nanocomposites as photocatalysts. The exceptional optical, electrical, and mechanical properties of carbon nanotubes could possibly expand the photo response of semiconductor photocatalysts.5.1. Activated carbon as a support material for photocatalysis
Generally, alumina, zeolite, clays, silica, and activated carbon are used as a support material for photocatalysts. Among these, activated carbon has a porous amorphous structure by means of permeability in the macro and microporous regime.140Some major necessities for a photocatalyst support are:
(i) Chemical interaction with the photocatalyst without affecting the photoactivity.
(ii) Strong photostability.
(iii) Strong chemical stability.
(iv) Economical and physical robustness.
Activated carbon as a support material plays an important role in the photocatalytic mechanism, due to the interaction between the support and the photocatalyst. The activated carbon is inert in nature, low-cost, and can be mass-produced by facile methods, making it an attractive finding with great profitable potential.140,141
5.2. Graphene as a support material for photocatalysis
Graphene has captivated the entire world with its fascinating properties since its discovery in 2004 by the great scientist Geim and coworkers.142 By electronic means, graphene is a semi-metal with a zero band-gap owing to the closely connected conduction band and valence band in the Brillouin zone, along with the sp2 hybridized carbon network. Graphene exhibits the highest carrier mobility at room temperature, as well as high optical absorptivity (2.3%), good thermal conductivity, and immense mechanical strength.143 These features make graphene sheets a potential photocatalyst support. The broad contact interface and strong interaction between the photocatalyst and graphene will permit effective transfer of electrons in photocatalytic composite materials. Remarkably, graphene can be made into an n-type or p-type semiconductor through functionalization. The essential mechanism of metal-graphene supported photocatalysis is based on the inimitable properties of the 2D sheet-like structure of graphene. Its chemical, physical, and mechanical properties deliver ideal performance for any catalytic reaction.143,144 The photocatalytic utilization of a few layers of graphene is of increasing consideration in present research. Aside from its large surface area, the immense adsorption capacity and better biocompatibility of graphene make it an ideal support material for anchoring metal nanoparticles and use in photocatalysis.143 By means of the deposition of metal nanoparticles onto the surface of a sheet-like structure of graphene, attractive properties arise owing to exchanges among the specific components, which have been oppressed for photocatalytic applications.143,145 The sheet-like structure of graphene has received considerable consideration in photocatalysis in comparison to other carbon-based materials. These photocatalysis systems can achieve the photodegradation of ecologically destructive organic dyes, hydrogen evolution, and the photo-synthesis of suitable chemicals.146 The basic mechanisms of semiconductor-based photocatalysis include photochemical progression of visible-light absorption, separation, electron–hole pair generation, and free charge carrier-induced redox reactions.147The photocatalytic degradation of colorful and colorless organic effluents or toxins occurs as soon as the catalytic metal nanoparticles are irradiated with photons whose photonic energy is equal to or greater than the band gap energy. An electron (e−-cb) is energized from the valence band (VB) to the conduction band (CB), which takes off a hole (h+-vb). The energized electrons and holes relocate to the surface of an alternative state.148 The charge recombination rate is regularly hindered by scavenging species or a carbon-based material, which could effectively trap the electrons or holes. Accordingly, more crystalline nanostructured materials with fewer defects usually minimize the trapping states and charge recombination sites, which results in enhanced effectiveness in the utilization of photoinduced transporters for the predicted photo-reactions.149 For effective and improved photocatalytic competence, the electron–hole pairs ought to be well separated and the charges must be moved quickly beyond the surface or interface to confine the rate of recombination. Graphene has a network of π–π conjugation and its unexpected conducting nature has proved it to be a competent electron acceptor.150 Metal nanoparticles have a tendency to absorb light and become excited. The energized electrons at the interface could possibly be moved to the sheet-like structure of graphene, which is stabilized by the network of conjugation and decreases the rate of recombination for improved photocatalytic performance.151
5.3. Graphitic-carbon nitride (g-C3N4) as a support material for photocatalysis
Graphitic-carbon nitride (g-C3N4) is a potential material for better photocatalytic utilization as a metal-free eco-friendly benign catalyst. g-C3N4 is a graphite analogue and exists as a constant allotrope of carbon nitride under ambient conditions.151 The constant structure of g-C3N4 comprises periodically associated tris-s-triazine units and the structure of g-C3N4 is very comparable to the network of graphitic nitride, where a few carbon atoms are substituted by atoms of nitrogen. The sp2 hybridized C and N atoms induce π conjugation in the planes of the graphitic structure. The basic electronic properties of this material are considerably influenced by the nitrogen lone pair and assigned mainly to the valence band. The carbon pz orbital contributes to the conduction band. g-C3N4 materials are known for their high rigidity, low-friction coefficient, and chemical inertness.152 Its stability, even at 600 °C, in air, is attractive for utilization in catalysis under extreme conditions. g-C3N4 has a tunable band-gap of 1.8–2.7 eV, which permits the harvesting of visible light at wavelengths of 460–698 nm, representing 13–49% of solar energy.152,153 The band-gap of g-C3N4 could possibly be reduced by doping with metallic elements. Metal decoration onto the sheet-like structure of g-C3N4 could possibly lower the band gap in the range of 2.67–1.58 eV by fine-tuning the electronic structure of g-C3N4 during synthesis. The light harvesting capability of carbon nitride could thus be meaningfully altered by incorporation of a minute amount of metal ions at the edges of the g-C3N4 nanosheets.Fig. 6 displays the photodegradation of silver nanoparticles and graphitic-carbon nitride under visible-light irradiation for the removal of organic pollutants.154
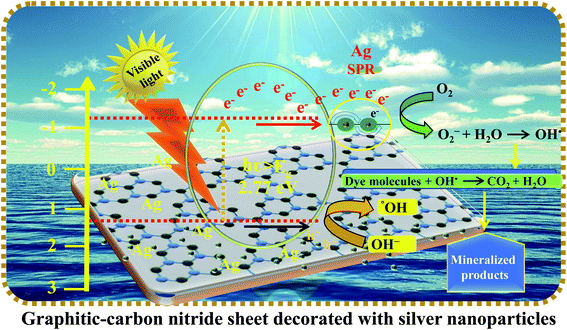 | ||
| Fig. 6 Schematic representation of the mechanism of charge transfer between the silver nanoparticles and graphitic-carbon nitride for photocatalysis. | ||
6. Conclusions and future perspectives
Carbon-based nanostructured materials have been broadly utilized as a support material for catalysis. First of all, this review highlighted the adaptability of carbon, as well as activated carbon, graphite, fullerenes, carbon nanotubes (CNTs), diamond, graphene (GN), and graphitic carbon nitride (g-C3N4) etc., with each nanostructured material offering inimitable characteristics and advantages. In addition, we highlighted their exceptional utilization in the research area of catalysis under visible-light irradiation, and their recent use in emerging diverse carbon-based catalysts, such as atom-doped carbon, carbon-based hybrids, catalysts supported on carbon, and so on. These innovative carbon-based catalysts present high catalytic activity in chemical, electro, or photocatalysis, and consequently they are abundantly established in several catalysis fields, as well as chemical synthesis, gas or oil de-sulfurization, biosensors, energy storage (e.g., batteries and supercapacitors) and conversion (e.g., fuel cells and solar cells), and organism-based photodegradation, etc.Though noteworthy developments have been achieved in the fabrication and utilization of carbon-based catalysts, there are still some challenges:
(i) Immense performance of carbon catalysts is vastly anticipated and remains an extensive challenge for the scientific community.
(ii) The catalysis mechanisms ought to be clarified for further enhancement of the catalytic performance.
(iii) Reasonable approaches for fabrication, manufacturing scalability, and commercial feasibility are desirable.
(iv) Novel methods using green and sustainable carbon-based catalysts with broader utilization must be established.
The recent progress in the combination of metal–carbonaceous material-based nanocomposites has mainly focused on the fabrication methods. The synergistic effects amongst the metal nanoparticles and carbonaceous nanostructures have led to their use in a variety of novel applications, extending from water treatment processes to the renewable energy sector. Several tasks need to be considered before actual industrial applications. In particular, the extensive production of carbon-based metal nanostructures with improved and uniform quality is still required. For the fabrication of carbon-based metal nanostructures concerning eco-friendly applications, carbonaceous material-based sheets as a support material have great potential as an electron acceptor with a decrease in the rate of charge recombination. Consequently, carbon-based metal nanostructures are of utmost importance for the degradation of various kinds of colorful and colorless harmful pollutants under visible light irradiation. This updated review could be helpful for better understanding the various properties related to carbon-based metal nanostructures, principally in photocatalysis or for water treatment applications.
These points are predicted to lead to the development of new applications:
(i) Abundant capacity to discover novel procedures for the facile fabrication of carbon-based sheet-like structures in increased amounts, which is essential to be cost-effective and ecologically friendly.
(ii) Proper understanding of the synergistic effect will definitely improve the potential utilization of carbon-based metal nanocomposites in several fields, together with biosensing and catalysis.
(iii) The precise fabrication of carbon-based metal nanocomposites, with precise shape, size, and crystallinity, which will not only avoid the accumulation of sheet like structures of carbonaceous materials, but also offer admirable patterns for doping/decoration with metal nanoparticles with improved photocatalytic utilization.
(iv) The combination and synergetic effects of these carbon-based metal nanostructures will significantly progress the photocatalytic degradation of organic dye pollutants under visible-light irradiation.
(v) Consequently, in the future, carbon-based metal nanostructures with inimitable properties and features will be fabricated and may resolve several waste-water treatment and eco-friendly related problems as well as energy matters.
This review article delivers a wide-ranging up-to-date analysis of the area, and will be supportive in manipulative innovative water treatment approaches by fabricating novel carbon-based metal nanostructures as a photocatalyst.
Conflicts of interest
The author declares no conflict of interest.Acknowledgements
Dr Mohammad Ehtisham Khan extends his appreciation to the Deputyship for Research & Innovation, Minister of Education in Saudi Arabia, Kingdom of Saudi Arabia – 45971.References
- D. Grey, D. Garrick, D. Blackmore, J. Kelman, M. Muller and C. Sadoff, Philos. Trans. R. Soc., A, 2013, 371, 20120406–20120416 CrossRef CAS.
- C. Frazzoli, O. E. Orisakwe, R. Dragone and A. Mantovani, Environ. Impact Assess. Rev., 2010, 30, 388–399 CrossRef.
- W. H. Organization and UNICEF, Global water supply and sanitation assessment 2000 report, World Health Organization (WHO), 2000, pp. 1–80 Search PubMed.
- A. Nikokavoura and C. Trapalis, Appl. Surf. Sci., 2018, 430, 18–52 CrossRef CAS.
- N. Linares, A. M. Silvestre-Albero, E. Serrano, J. Silvestre-Albero and J. García-Martínez, Chem. Soc. Rev., 2014, 43, 7681–7717 RSC.
- R. Ryoo, S. H. Joo, M. Kruk and M. Jaroniec, Adv. Mater., 2001, 13, 677–681 CrossRef CAS.
- W. Zhanghong, D. Shen, C. Wu and S. Gu, Green Chem., 2018, 20, 5031–5057 RSC.
- S. Ali, A. Razzaq and S.-I. In, Catal. Today, 2019, 335, 39–54 CrossRef CAS.
- H. Yang and D. Zhao, J. Mater. Chem., 2005, 15, 1217–1231 CAS.
- A. K. Geim and K. S. Novoselov, in Nanoscience and technology: a collection of reviews from nature journals, World Scientific, 2010, pp. 11–19 Search PubMed.
- J. Yu, J. Jin, B. Cheng and M. Jaroniec, J. Mater. Chem. A, 2014, 2, 3407–3416 RSC.
- A. Razzaq, C. A. Grimes and . S.-I, Carbon, 2016, 98, 537–544 CrossRef CAS.
- B. Kirubasankar, S. Vijayan and S. Angaiah, Sustainable Energy Fuels, 2019, 3, 467–477 RSC.
- D. Munoz-Rojas and X. Moya, Materials for Sustainable Energy Applications: Conversion, Storage, Transmission, and Consumption, CRC Press, 2017, vol. 37, pp. 1–826 Search PubMed.
- T. Chen and L. Dai, Mater. Today, 2013, 16, 272–280 CrossRef CAS.
- Z. Xiong, M. Fuji and J. Zhou, Sustainable Energy Fuels, 2019, 3, 2845–2858 RSC.
- Y. Luo, Y. Yan, S. Zheng, H. Xue and H. Pang, J. Mater. Chem. A, 2019, 7, 901–924 RSC.
- T. Di, B. Zhu, B. Cheng, J. Yu and J. Xu, J. Catal., 2017, 352, 532–541 CrossRef CAS.
- X.-H. Xia, Z.-J. Jia, Y. Yu, Y. Liang, Z. Wang and L.-L. Ma, Carbon, 2007, 45, 717–721 CrossRef CAS.
- J. Safaei, N. A. Mohamed, M. F. M. Noh, M. F. Soh, N. A. Ludin, M. A. Ibrahim, W. N. R. W. Isahak and M. A. M. Teridi, J. Mater. Chem. A, 2018, 6, 22346–22380 RSC.
- Z. Zuo, D. T. Ahneman, L. Chu, J. A. Terrett, A. G. Doyle and D. W. MacMillan, Science, 2014, 345, 437–440 CrossRef CAS.
- P. V. Kamat, J. Phys. Chem. B., 2002, 106(32), 7729–7744 CrossRef CAS.
- S. Linic, P. Christopher and D. B. Ingram, Nat. Mater., 2011, 10, 911–921 CrossRef CAS.
- L. Qiao, D. Wang, L. Zuo, Y. Ye, J. Qian, H. Chen and S. He, Appl. Energy, 2011, 88, 848–852 CrossRef CAS.
- M. Rycenga, C. M. Cobley, J. Zeng, W. Li, C. H. Moran, Q. Zhang, D. Qin and Y. Xia, Chem. Rev., 2011, 111, 3669–3712 CrossRef CAS.
- K. Watanabe, D. Menzel, N. Nilius and H.-J. Freund, Chem. Rev., 2006, 106, 4301–4320 CrossRef CAS.
- A. M. Schwartzberg and J. Z. Zhang, J. Phys. Chem. C, 2008, 112, 10323–10337 CrossRef CAS.
- I. Arabatzis, T. Stergiopoulos, D. Andreeva, S. Kitova, S. Neophytides and P. Falaras, J. Catal., 2003, 220, 127–135 CrossRef CAS.
- A. Patsoura, D. I. Kondarides and X. E. Verykios, Catal. Today, 2007, 124, 94–102 CrossRef CAS.
- V. Subramanian, E. Wolf and P. V. Kamat, J. Phys. Chem. B, 2001, 105, 11439–11446 CrossRef CAS.
- M. E. Khan, M. M. Khan and M. H. Cho, RSC Adv., 2015, 5, 26897–26904 RSC.
- M. E. Khan, M. M. Khan and M. H. Cho, New J. Chem., 2015, 39, 8121–8129 RSC.
- H. Zhu, X. Ke, X. Yang, S. Sarina and H. Liu, Angew. Chem., Int. Ed., 2010, 49, 9657–9661 CrossRef CAS.
- S. Sarina, E. R. Waclawik and H. Zhu, Green Chem., 2013, 15, 1814–1833 RSC.
- S. Kumar, B. Kumar, A. Baruah and V. Shanker, J. Phys. Chem. C, 2013, 117, 26135–26143 CrossRef CAS.
- Y. Li, S. Ouyang, H. Xu, X. Wang, Y. Bi, Y. Zhang and J. Ye, J. Am. Chem. Soc., 2016, 138, 13289–13297 CrossRef CAS.
- Y. Feng, J. Shen, Q. Cai, H. Yang and Q. Shen, New J. Chem., 2015, 39, 1132–1138 RSC.
- M. Tahir, C. Cao, N. Mahmood, F. K. Butt, A. Mahmood, F. Idrees, S. Hussain, M. Tanveer, Z. Ali and I. Aslam, ACS Appl. Mater. Interfaces, 2014, 6, 1258–1265 CrossRef CAS.
- Y. Guo, F. Kong, C. Wang, S. Chu, J. Yang, Y. Wang and Z. Zou, J. Mater. Chem. A, 2013, 1, 5142–5147 RSC.
- Y. Chen, W. Huang, D. He, Y. Situ and H. Huang, ACS Appl. Mater. Interfaces, 2014, 6, 14405–14414 CrossRef CAS.
- X. D. Sun, Y. Y. Li, J. Zhou, C. H. Ma, Y. Wang and J. H. Zhu, J. Colloid Interface Sci., 2015, 451, 108–116 CrossRef CAS.
- J. Xiao, Y. Xie, F. Nawaz, Y. Wang, P. Du and H. Cao, Appl. Catal., B, 2016, 183, 417–425 CrossRef CAS.
- W. Shan, Y. Hu, Z. Bai, M. Zheng and C. Wei, Appl. Catal., B, 2016, 188, 1–12 CrossRef CAS.
- X. Yuan, C. Zhou, Y. Jin, Q. Jing, Y. Yang, X. Shen, Q. Tang, Y. Mu and A.-K. Du, J. Colloid Interface Sci., 2016, 468, 211–219 CrossRef CAS.
- S. Wang, C. Li, T. Wang, P. Zhang, A. Li and J. Gong, J. Mater. Chem. A, 2014, 2, 2885–2890 RSC.
- S. Panneri, M. Thomas, P. Ganguly, B. N. Nair, A. P. Mohamed, K. Warrier and U. Hareesh, Catal. Sci. Technol., 2017, 7, 2118–2128 RSC.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS.
- Z. Tong, D. Yang, Z. Li, Y. Nan, F. Ding, Y. Shen and Z. Jiang, ACS Nano, 2017, 11, 1103–1112 CrossRef CAS.
- Y. Zheng, Y. Jiao, Y. Zhu, L. H. Li, Y. Han, Y. Chen, A. Du, M. Jaroniec and S. Z. Qiao, Nat. Commun., 2014, 5, 1–8 Search PubMed.
- Y. Hou, Z. Wen, S. Cui, X. Feng and J. Chen, Nano Lett., 2016, 16, 2268–2277 CrossRef CAS.
- J. Bian, Q. Li, C. Huang, J. Li, Y. Guo, M. Zaw and R.-Q. Zhang, Nano Energy, 2015, 15, 353–361 CrossRef CAS.
- L. Shi, T. Wang, H. Zhang, K. Chang and J. Ye, Adv. Funct. Mater., 2015, 25, 5360–5367 CrossRef CAS.
- K. Wang, Q. Li, B. Liu, B. Cheng, W. Ho and J. Yu, Appl. Catal., B, 2015, 176, 44–52 Search PubMed.
- Q. Han, F. Zhao, C. Hu, L. Lv, Z. Zhang, N. Chen and L. Qu, Nano Res., 2015, 8, 1718–1728 CrossRef CAS.
- J. Zhang, J. Sun, K. Maeda, K. Domen, P. Liu, M. Antonietti, X. Fu and X. Wang, Energy Environ. Sci., 2011, 4, 675–678 RSC.
- J. Sun, Y. Fu, G. He, X. Sun and X. Wang, Appl. Catal., B, 2015, 165, 661–667 CrossRef CAS.
- H. Wang, S. Jiang, S. Chen, D. Li, X. Zhang, W. Shao, X. Sun, J. Xie, Z. Zhao and Q. Zhang, Adv. Mater., 2016, 28, 6940–6945 CrossRef CAS.
- P. Serp and B. Machado, Nanostructured Carbon Materials for Catalysis, 2015, pp. 1–45 Search PubMed.
- F. Rodŕíguez-Reinoso and A. Seṕulveda-Escribano, Carbon Mater. Catal., John Wiley & Sons, 2008, pp. 131–155 Search PubMed.
- E. Pérez-Mayoral, V. Calvino-Casilda and E. Soriano, Catal. Sci. Technol., 2016, 6, 1265–1291 RSC.
- D. S. Su, S. Perathoner and G. Centi, Chem. Rev., 2013, 113, 5782–5816 CrossRef CAS.
- C. J. Shearer, A. Cherevan and D. Eder, in Carbon Nanotubes and Graphene, Elsevier, 2014, pp. 387–433 Search PubMed.
- P. Serp and B. Machado, Nanostructured carbon materials for catalysis, Royal Society of Chemistry, 2015 Search PubMed.
- Y. He, H.-Y. Chen, J. Hou and Y. Li, J. Am. Chem. Soc., 2010, 132, 1377–1382 CrossRef CAS.
- C. Li, Y. Chen, Y. Wang, Z. Iqbal, M. Chhowalla and S. Mitra, J. Mater. Chem., 2007, 17, 2406–2411 RSC.
- C.-Z. Li, H.-L. Yip and A. K.-Y. Jen, J. Mater. Chem., 2012, 22, 4161–4177 RSC.
- S.-E. Zhu, F. Li and G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7535–7570 RSC.
- L. Chen, W. Chen, H. Huang, H. L. Zhang, J. Yuhara and A. T. S. Wee, Adv. Mater., 2008, 20, 484–488 CrossRef CAS.
- Y. V. Vasil'ev, A. Hirsch, R. Taylor and T. Drewello, Chem. Commun., 2004, 15, 1752–1753 RSC.
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- A. T. Kuvarega and B. B. Mamba, Crit. Rev. Solid State Mater. Sci., 2017, 42, 295–346 CrossRef CAS.
- C. N. R. Rao, B. Satishkumar, A. Govindaraj and M. Nath, ChemPhysChem, 2001, 2, 78–105 CrossRef CAS.
- P. Serp, M. Corrias and P. Kalck, Appl. Catal., A, 2003, 253, 337–358 CrossRef CAS.
- Y. Yao, G. Li, S. Ciston, R. M. Lueptow and K. A. Gray, Environ. Sci. Technol., 2008, 42, 4952–4957 CrossRef CAS.
- M. E. Khan, M. M. Khan and M. H. Cho, Nanoscale, 2018, 10, 9427–9440 RSC.
- C. e. N. e. R. Rao, A. e. K. Sood, K. e. S. Subrahmanyam and A. Govindaraj, Angew. Chem., Int. Ed., 2009, 48, 7752–7777 CrossRef CAS.
- F. Zhang, C. Hou, Q. Zhang, H. Wang and Y. Li, Mater. Chem. Phys., 2012, 135, 826–831 CrossRef CAS.
- M. E. Khan, M. M. Khan and M. H. Cho, J. Phys. Chem. Solids, 2017, 104, 233–242 CrossRef CAS.
- X. Huang, X. Qi, F. Boey and H. Zhang, Chem. Soc. Rev., 2012, 41, 666–686 RSC.
- Q. Xiang, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2012, 41, 782–796 RSC.
- M.-Q. Yang, N. Zhang, M. Pagliaro and Y.-J. Xu, Chem. Soc. Rev., 2014, 43, 8240–8254 RSC.
- X. Chen, S. Shen, L. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS.
- F. E. Osterloh, Chem. Mater., 2008, 20, 35–54 CrossRef CAS.
- S. N. Habisreutinger, L. Schmidt-Mende and J. K. Stolarczyk, Angew. Chem., Int. Ed., 2013, 52, 7372–7408 CrossRef CAS.
- A. Di Paola, E. García-López, G. Marcì and L. Palmisano, J. Hazard. Mater., 2012, 211, 3–29 CrossRef.
- S. Zhang, P. Gu, R. Ma, C. Luo, T. Wen, G. Zhao, W. Cheng and X. Wang, Catal. Today, 2019, 335, 65–77 CrossRef CAS.
- X. Wang, S. Blechert and M. Antonietti, ACS Catal., 2012, 2, 1596–1606 CrossRef CAS.
- J. Zhu, Y. Wei, W. Chen, Z. Zhao and A. Thomas, Chem. Commun., 2010, 46, 6965–6967 RSC.
- M. E. Khan, M. M. Khan and M. H. Cho, RSC Adv., 2018, 8, 13898–13909 RSC.
- F. Goettmann, A. Thomas and M. Antonietti, Angew. Chem., Int. Ed., 2007, 46, 2717–2720 CrossRef CAS.
- X. Jin, V. V. Balasubramanian, S. T. Selvan, D. P. Sawant, M. A. Chari, G. e. Q. Lu and A. Vinu, Angew. Chem., Int. Ed., 2009, 48, 7884–7887 CrossRef CAS.
- X. Bai, L. Wang, R. Zong and Y. Zhu, J. Phys. Chem. C, 2013, 117, 9952–9961 CrossRef CAS.
- Y. Zhang, A. Thomas, M. Antonietti and X. Wang, J. Am. Chem. Soc., 2009, 131, 50–51 CrossRef CAS.
- L. Maya, D. R. Cole and E. W. Hagaman, J. Am. Ceram. Soc., 1991, 74, 1686–1688 CrossRef CAS.
- H. Wang, X. Yuan, Y. Wu, H. Huang, X. Peng, G. Zeng, H. Zhong, J. Liang and M. Ren, Adv. Colloid Interface Sci., 2013, 195, 19–40 CrossRef.
- L. Dai, Y. Xue, L. Qu, H.-J. Choi and J.-B. Baek, Chem. Rev., 2015, 115, 4823–4892 CrossRef CAS.
- W.-J. Ong, L.-L. Tan, Y. H. Ng, S.-T. Yong and S.-P. Chai, Chem. Rev., 2016, 116, 7159–7329 CrossRef CAS.
- X. Song, J. Hu and H. Zeng, J. Mater. Chem. C, 2013, 1, 2952–2969 RSC.
- Z. Zhao, Y. Sun and F. Dong, Nanoscale, 2015, 7, 15–37 RSC.
- S. Tonda, S. Kumar, S. Kandula and V. Shanker, J. Mater. Chem. A, 2014, 2, 6772–6780 RSC.
- S. Le, T. Jiang, Q. Zhao, X. Liu, Y. Li, B. Fang and M. Gong, RSC Adv., 2016, 6, 38811–38819 RSC.
- R. Jin, S. Hu, J. Gui and D. Liu, Bull. Korean Chem. Soc., 2015, 36, 17–23 CrossRef CAS.
- P.-W. Chen, K. Li, Y.-X. Yu and W.-D. Zhang, Appl. Surf. Sci., 2017, 392, 608–615 CrossRef CAS.
- D. Xu, X. Li, J. Liu and L. Huang, J. Rare Earths, 2013, 31, 1085–1091 CrossRef CAS.
- Y. Wang, Y. Xu, Y. Wang, H. Qin, X. Li, Y. Zuo, S. Kang and L. Cui, Catal. Commun., 2016, 74, 75–79 CrossRef CAS.
- Y. Wang, Y. Wang, Y. Li, H. Shi, Y. Xu, H. Qin, X. Li, Y. Zuo, S. Kang and L. Cui, Catal. Commun., 2015, 72, 24–28 CrossRef CAS.
- X. Huang, N. Zhu, F. Mao, Y. Ding, S. Zhang, F. Li, H. Liu and P. Wu, Sep. Purif. Technol., 2020, 252, 117485–117494 CrossRef CAS.
- M. E. Khan, T. H. Han, M. M. Khan, M. R. Karim and M. H. Cho, ACS Appl. Nano Mater., 2018, 1, 2912–2922 CrossRef CAS.
- H. Gao, S. Yan, J. Wang and Z. Zou, Dalton Trans., 2014, 43, 8178–8183 RSC.
- H. Pan, Y.-W. Zhang, V. B. Shenoy and H. Gao, ACS Catal., 2011, 1, 99–104 CrossRef CAS.
- X. Lang, X. Chen and J. Zhao, Chem. Soc. Rev., 2014, 43, 473–486 RSC.
- P. V. Kamat, J. Phys. Chem. B, 2002, 106, 7729–7744 CrossRef CAS.
- T. Matsumoto, T. Komatsu, K. Arai, T. Yamazaki, M. Kijima, H. Shimizu, Y. Takasawa and J. Nakamura, Chem. Commun., 2004, 840–841, 10.1039/b400607k.
- H. T. Chung, J. H. Won and P. Zelenay, Nat. Commun., 2013, 4, 1922–1933 CrossRef.
- K. R. Reddy, C. H. V. Reddy, M. N. Nadagouda, N. P. Shetti, S. Jaesool and T. M. Aminabhavi, J. Environ. Manage., 2019, 238, 25–40 CrossRef CAS.
- V. K. Gupta, J. Environ. Manage., 2009, 90, 2313–2342 CrossRef CAS.
- A. Dąbrowski, P. Podkościelny, Z. Hubicki and M. Barczak, Chemosphere, 2005, 58, 1049–1070 CrossRef.
- Z. Jiang, B. Huang, Z. Lou, Z. Wang, X. Meng, Y. Liu, X. Qin, X. Zhang and Y. Dai, Dalton Trans., 2014, 43, 8170–8173 RSC.
- H. M. Yang and S.-J. Park, Mater. Chem. Phys., 2017, 186, 261–270 CrossRef CAS.
- W. Wang, P. Serp, P. Kalck and J. L. Faria, J. Mol. Catal. A: Chem., 2005, 235, 194–199 CrossRef CAS.
- A. Chinnappan, C. Baskar, H. Kim and S. Ramakrishna, J. Mater. Chem. A, 2016, 4, 9347–9361 RSC.
- R. Saito, R. Matsuo, T. Kimura, G. Dresselhaus and M. S. Dresselhaus, Chem. Phys. Lett., 2001, 348, 187–193 CrossRef CAS.
- A. K. Geim and K. S. Novoselov, Nanosci. Technol., 2010, 6, 11–19 Search PubMed.
- X. Li, J. Yu, S. Wageh, A. A. Al-Ghamdi and J. Xie, Small, 2016, 12, 6640–6696 CrossRef CAS.
- B. Qiu, M. Xing and J. Zhang, Chem. Soc. Rev., 2018, 47, 2165–2216 RSC.
- P. V. Kamat, J. Phys. Chem. C, 2007, 111, 2834–2860 CrossRef CAS.
- Z. Xiong, L. L. Zhang, J. Ma and X. S. Zhao, Chem. Commun., 2010, 46, 6099–6101 RSC.
- B. Z. Kurt, Z. Durmus and A. Durmus, Solid State Sci., 2016, 51, 51–58 CrossRef CAS.
- K. Ullah, S. Ye, L. Zhu, Z.-D. Meng, S. Sarkar and W.-C. Oh, Mater. Sci. Eng., B, 2014, 180, 20–26 CrossRef CAS.
- K. Hareesh, R. P. Joshi, D. V. Sunitha, V. N. Bhoraskar and S. D. Dhole, Appl. Surf. Sci., 2016, 389, 1050–1055 CrossRef.
- M. A. Koklioti, T. Skaltsas, Y. Sato, K. Suenaga, A. Stergiou and N. Tagmatarchis, Nanoscale, 2017, 9, 9685–9692 RSC.
- A. Thomas, A. Fischer, F. Goettmann, M. Antonietti, J.-O. Müller, R. Schlögl and J. M. Carlsson, J. Mater. Chem., 2008, 18, 4893–4908 RSC.
- P. Niu, L. Zhang, G. Liu and H.-M. Cheng, Adv. Funct. Mater., 2012, 22, 4763–4770 CrossRef CAS.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS.
- X. Wang, K. Maeda, X. Chen, K. Takanabe, K. Domen, Y. Hou, X. Fu and M. Antonietti, J. Am. Chem. Soc., 2009, 131, 1680–1681 CrossRef CAS.
- X. Li, J. Yu, J. Low, Y. Fang, J. Xiao and X. Chen, J. Mater. Chem. A, 2015, 3, 2485–2534 RSC.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- S. Cao, J. Low, J. Yu and M. Jaroniec, Adv. Mater., 2015, 27, 2150–2176 CrossRef CAS.
- Y. Zheng, L. Lin, B. Wang and X. Wang, Angew. Chem., Int. Ed., 2015, 54, 12868–12884 CrossRef CAS.
- L. Dai, Carbon nanotechnology: recent developments in chemistry, physics, materials science and device applications, Elsevier, 2006 Search PubMed.
- V. Kumaravel and S. Somasundaram, in Advanced Nanostructured Materials for Environmental Remediation, Springer, 2019, pp. 279–319 Search PubMed.
- Z. Zhen and H. Zhu, in Graphene, Elsevier, 2018, pp. 1–12 Search PubMed.
- D. Haag and H. H. Kung, Top. Catal., 2014, 57, 762–773 CrossRef CAS.
- A. Fujishima and K. Honda, nature, 1972, 238, 37–38 CrossRef CAS.
- D. Xu, B. Cheng, S. Cao and J. Yu, Appl. Catal., B, 2015, 164, 380–388 CrossRef CAS.
- X. Zheng and L. Zhang, Energy Environ. Sci., 2016, 9, 2511–2532 RSC.
- K. Hashimoto, H. Irie and A. Fujishima, Jpn. J. Appl. Phys., 2005, 44, 8269–8285 CrossRef CAS.
- J. Yu, T. Ma, G. Liu and B. Cheng, Dalton Trans., 2011, 40, 6635–6644 RSC.
- N. Zhang, R. Ciriminna, M. Pagliaro and Y.-J. Xu, Chem. Soc. Rev., 2014, 43, 5276–5287 RSC.
- H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li, Z. Wang, J. Liu and X. Wang, Chem. Soc. Rev., 2014, 43, 5234–5244 RSC.
- J. Zhu, P. Xiao, H. Li and S. A. Carabineiro, ACS Appl. Mater. Interfaces, 2014, 6, 16449–16465 CrossRef CAS.
- Y. Zheng, Y. Jiao, J. Chen, J. Liu, J. Liang, A. Du, W. Zhang, Z. Zhu, S. C. Smith and M. Jaroniec, J. Am. Chem. Soc., 2011, 133, 20116–20119 CrossRef CAS.
- D. Geng, Y. Chen, Y. Chen, Y. Li, R. Li, X. Sun, S. Ye and S. Knights, Energy Environ. Sci., 2011, 4, 760–764 RSC.
- M. E. Khan, T. H. Han, M. M. Khan, M. R. Karim and M. H. Cho, ACS Appl. Nano Mater., 2018, 1, 2912–2922 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |