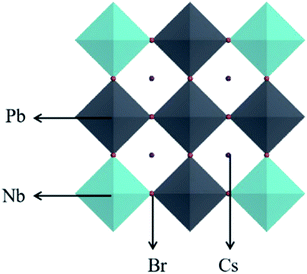
Open Access Article
Open Access ArticleTheoretical study of the influence of doped niobium on the electronic properties of CsPbBr3
Xingyou
Liang
a,
Xuefeng
Ren
 *c,
Shuzhang
Yang
d,
Lizhao
Liu
*b,
Wei
Xiong
f,
Li
Cheng
b,
Tingli
Ma
*c,
Shuzhang
Yang
d,
Lizhao
Liu
*b,
Wei
Xiong
f,
Li
Cheng
b,
Tingli
Ma
 de and
Anmin
Liu
de and
Anmin
Liu
 *a
*a
aState Key Laboratory of Fine Chemicals, School of Chemical Engineering, Dalian University of Technology, China. E-mail: anmin0127@163.com; liuanmin@dlut.edu.cn
bKey Laboratory of Materials Modification by Laser, Ion and Electron Beams (Dalian University of Technology), Ministry of Education, Dalian 116024, China. E-mail: lizhao_liu@dlut.edu.cn
cSchool of Ocean Science and Technology, Dalian University of Technology, Panjin, 124221, China. E-mail: renxuefeng@dlut.edu.cn
dGraduate School of Life Science and Systems Engineering, Kyushu Institute of Technology, 2-4 Hibikino, Wakamatsu, Kitakyushu, Fukuoka 808-0196, Japan
eDepartment of Materials Science and Engineering, China Jiliang University, Hangzhou, 310018, China
fKey Laboratory of Industrial Ecology and Environmental Engineering (Ministry of Education), School of Environmental Sciences and Technology, Dalian University of Technology, Dalian 116024, China
First published on 23rd February 2021
Abstract
In the family of inorganic perovskite solar cells (PSCs), CsPbBr3 has attracted widespread attention due to its excellent stability under high humidity and high temperature conditions. However, power conversion efficiency (PCE) improvement of CsPbBr3-based PSCs is markedly limited by the large optical absorption loss coming from the wide band gap and serious charge recombination at interfaces and/or within the perovskite film. In this work, using density functional theory calculations, we systemically studied the electronic properties of niobium (Nb)-doped CsPbBr3 with different concentration ratios. As a result, it is found that doped CsPbBr3 compounds are metallic at high Nb doping concentration but semiconducting at low Nb doping concentration. The calculated electronic density of states shows that the conduction band is predominantly constructed of doped Nb. These characteristics make them very suitable for solar cell and energy storage applications.
1. Introduction
Organic–inorganic hybrid perovskite solar cells (PSCs) have favourable properties including appropriate band gap, high absorption coefficient, low cost and easy fabrication process, and show great potential for photovoltaic devices.1–6 Their power conversion efficiency (PCE) has been increased dramatically from 3.8% to a certified 25.5% by various strategies such as composition engineering, interface engineering, construction engineering, and preparation techniques.7–14 Although the rapid increase of PCE has been realized, the intrinsic stability of hybrid perovskites under high humidity and high temperature is still a key issue for meeting the commercial requirements.15,16To reduce the instability issues, the use of all-inorganic cesium lead halides (CsPbX3), which contain inorganic cesium (Cs) rather than organic cations as in organic–inorganic perovskites, has been demonstrated to be an effective strategy for improving the stability of PSCs.17–19 Thus, currently, CsPbX3 has attracted much attention. It is divided into different components by tuning the halide anions, mainly focusing on CsPbI3,20,21 CsPbI2Br,22,23 CsPbIBr2,24,25 and CsPbBr3.26,27 From the family of all-inorganic PSCs, CsPbBr3 exhibits superior stability under high humidity and high temperature conditions, which is significant to the practical application of PSC devices. However, the PCE is limited by large optical absorption loss and serious charge recombination. At present, the best PCE of CsPbBr3-based PSCs is just around 11%.28 Therefore, it is urgent to know how to improve the PCE and reduce the cost of CsPbBr3 PSCs.
To date, several strategies have been applied to enhance the PCE and simultaneously prolong the thermal stability of inorganic PSCs. For example, Chu and co-workers fabricated an amorphous Nb2O5 film as the ETL of inorganic planar CsPbBr3 by the magnetron sputtering method, and 5.74% PCE was obtained due to the suitable surface work function and high transmittance of amorphous Nb2O5 and low charge recombination at the amorphous Nb2O5/CsPbBr3 interface.29 Yu et al. demonstrated an interesting method by face-down liquid-space restriction to prepare high-quality CsPbBr3 perovskite films on compact TiO2 layers. This novel method can obtain uniform, smooth and high-quality perovskite films, which greatly increased the PCE and Voc in planar all-inorganic CsPbBr3 PSCs.30 Zhong et al. developed a one-step solution-processing method to fabricate CsPbBr3 films using precursor engineering and applied it for a CsPbBr3-based device. A PCE of 7.37% has been achieved with high stability over 1500 h under an ambient atmosphere with 30–35% relative humidity.31 Liao et al. developed a modified multistep spin-coating strategy for CsPbBr3 film preparation, and the optimized PCE was improved to 8.12%. This might be mainly attributed to the high crystallinity and reduced density of trap states of the CsPbBr3 film.32 They also selected a novel antisolvent-washing strategy for highly efficient carbon-based CsPbBr3 PSCs, increasing the PCE to 8.55%.33 Tang et al. reported a Lewis-base polymer, polyvinyl acetate (PVAc), to modify the CsPbBr3 film. A maximum PCE of up to 9.53% with an excellent Voc of 1.553 V was achieved for the CsPbBr3-based PSC.34 Yang et al. applied a vapor-assisted solution technique to prepare a uniform and pure CsPbBr3 film. The optimized CsPbBr3 PSC showed a PCE of 10.45% and outstanding stability for over 90 days under harsh conditions (80% RH, 85 °C).35 Qi et al. proposed a phase transition-induced method to produce high-quality CsPbBr3 thin films. A PCE of 10.91% was obtained for n–i–p structured PSCs with metal electrodes, and the carbon electrode-based devices exhibited excellent long-term stability and retained 80% of the initial efficiency in ambient air for more than 2000 h without any encapsulation.28
Although there are many ways to improve the efficiency of the device, few studies were reported to fundamentally improve the absorption capacity of this kind of material, and the current efficiency is far from its ultimate efficiency. Theoretical calculations are an effective method to design materials and study the mechanism.36–40
In this study, we systematically study the electronic properties of niobium (Nb)-doped CsPbBr3 with different concentration ratios using first-principles calculations. This work will provide a good theoretical basis for understanding the structure and electronic properties of these compounds. Through the analysis of these results, we propose that Nb-doped CsPbBr3 may be a promising solar cell absorber with good band gap and light absorption properties, which provides a feasible method for improving the efficiency of the device.
2. DFT calculation details
The Vienna Ab initio Simulation Package (VASP) based on the plane-wave pseudopotential technique was employed, using the Perdew–Burke–Ernzerhof (PBE) functional for the exchange-correlation interaction and the projector augmented wave (PAW) pseudopotential for the ion–electron interaction.41–44 A kinetic energy cutoff of 350 eV was chosen to ensure good convergence of the total energy and stress. The well-tested Monkhorst–Pack k grid of a uniform spacing of 0.02 Å−1 was adopted to sample the first Brillouin zone. All the structures were fully relaxed using the conjugate gradient algorithm until the force on each atom was less than 0.001 eV Å−1 and the energy was converged to 10−4 eV.We calculate the doping energy (Edoping) of five kinds of supercell structures doped with CsPbBr3 at different Nb concentrations, and the formula of Edoping is given by
| Edoping = Edop + EPb − Epure − ENb | (1) |
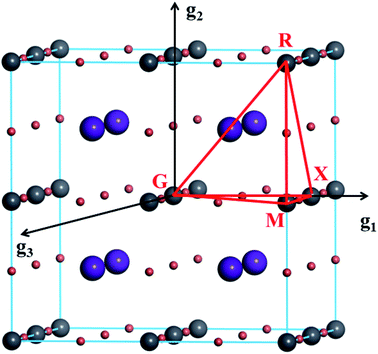 | ||
| Fig. 1 Visualization of k-point paths in the 1st Brillouin zone; g1, g2, and g3 are vectors for the reciprocal lattice. | ||
3. Results and discussion
In order to explore the effect of Nb doping of CsPbBr3 on its absorption capacity, we doped Nb into CsPbBr3 by expanding cells, that is, doping one Nb atom in each supercell, as shown in Fig. 2. We constructed Nb-doped CsPbBr3 with 50%, 25%, 12.5%, 6.25%, and 3.125% concentration ratios, and the corresponding supercells and chemical formulae are shown in Table 1. The original CsPbBr3 structure of five different supercells and the crystal structure after doping with different Nb concentrations are shown in Fig. 3.| Supercell | Chemical formula | Doping concentration |
|---|---|---|
| 2 × 1 × 1 | Br6NbCs2Pb | 50% |
| 2 × 2 × 1 | Br12NbCs4Pb3 | 25% |
| 2 × 2 × 2 | Br24NbCs8Pb7 | 12.5% |
| 2 × 2 × 4 | Br48NbCs16Pb15 | 6.25% |
| 2 × 4 × 4 | Br96NbCs32Pb31 | 3.125% |
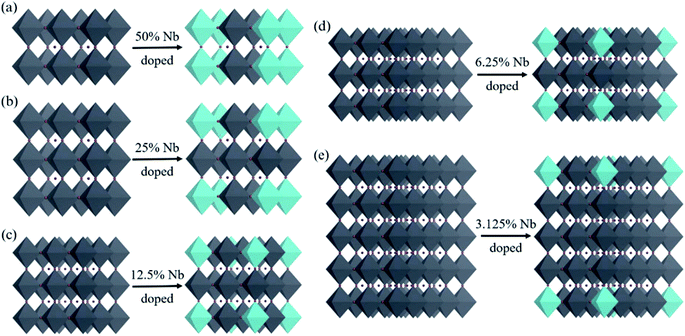 | ||
| Fig. 3 Schematic diagrams of the pristine and Nb-doped CsPbBr3 crystal structures with different concentration ratios. | ||
The raw data used to calculate the doping energy are shown in Table 2. According to formula (1), we calculated the doping energies of Nb atoms doped in five supercell structures. The detailed calculation results are shown in Table 3. The results show that as the doping concentration increases, the doping energy gradually increases. When the doping concentration of Nb reaches 12.5%, the doping energy reaches the maximum value of −2.77 eV. However, when the doping concentration of Nb atoms is increased, the doping energy will gradually decrease.
| Structures | Pristine (eV) | Doped (eV) |
|---|---|---|
| 2 × 1 × 1 supercell | −31.7945 | −35.2799 |
| 2 × 2 × 1 supercell | −63.5882 | −66.9168 |
| 2 × 2 × 2 supercell | −127.177 | −130.491 |
| 2 × 2 × 4 supercell | −254.354 | −257.757 |
| 2 × 4 × 4 supercell | −508.72 | −512.19 |
| Pb atom | −0.095720351 | |
| Nb atom | −0.64194424 | |
| Supercell | Chemical formula | Doping concentration | k-points | Gap (eV) | Doping energy (eV) |
|---|---|---|---|---|---|
| 2 × 1 × 1 | Cs2Pb2Br6 | 0 | 4 × 8 × 8 | 2.51 | — |
| 2 × 1 × 1 | Cs2PbNbBr6 | 50% | 4 × 8 × 8 | 0 (metallic) | −2.94 |
| 2 × 2 × 1 | Cs4Pb3NbBr12 | 25% | 4 × 4 × 8 | 0 (metallic) | −2.78 |
| 2 × 2 × 2 | Cs8Pb7NbBr24 | 12.5% | 4 × 4 × 2 | 1.94 | −2.77 |
| 2 × 2 × 4 | Cs16Pb15NbBr48 | 6.25% | 4 × 4 × 2 | 1.89 | −2.87 |
| 2 × 4 × 4 | Cs32Pb31NbBr96 | 3.125% | 4 × 2 × 2 | 1.84 | −2.92 |
The optimization results of lattice parameters for these pristine and differently Nb doped CsPbBr3 are shown in Table 4. Since Nb is significantly smaller than the atomic radius of Pb, when Nb is doped to replace the Pb atom in CsPbBr3, the lattice constant will be significantly reduced.
| Lattice parameters | Supercells | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 × 1 × 1 | 2 × 2 × 1 | 2 × 2 × 2 | 2 × 2 × 4 | 2 × 4 × 4 | ||||||
| Pristine | 50% Nb doped | Pristine | 25% Nb doped | Pristine | 12.5% Nb doped | Pristine | 6.25% Nb doped | Pristine | 3.125% Nb doped | |
| a (Å) | 12.001 | 11.426 | 11.986 | 11.758 | 11.986 | 11.881 | 11.982 | 11.936 | 11.980 | 11.956 |
| b (Å) | 6.005 | 5.713 | 11.986 | 11.758 | 11.986 | 11.881 | 11.982 | 11.936 | 23.960 | 23.908 |
| c (Å) | 6.005 | 5.713 | 5.993 | 5.8796 | 11.986 | 11.881 | 23.964 | 23.873 | 23.960 | 23.908 |
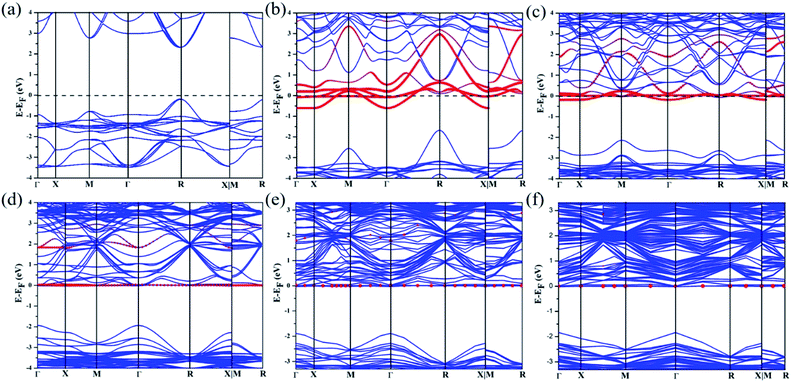
Based on the relaxed structures, we studied the electronic properties of these compounds, including the band structures and density of states, which will be discussed below. Fig. 4 shows the band structures of the undoped system calculated by the PBE method and the Nb-doped system with different concentrations. Through comparison, it is found that the Fermi level of CsPbBr3 after doping with Nb is closer to the conduction band. As shown in Fig. 4a, the energy band of CsPbBr3 undoped with Nb shows a direct band gap. The highest point of the valence band (VBM) and the lowest point of the conduction band (CBM) are located at point R in the first Brillouin zone, respectively. Moreover, all undoped structures have the same band gap. When the doping concentration of Nb is 50% or 25%, both structures show the properties of metals, as shown in Fig. 4b and c. Furthermore, when the doping concentration of Nb is 12.5%, 6.25% or 3.125%, the Fermi level is closer to the conduction band, which indicates an n-type semiconductor. There is a direct band gap at the Γ point, and the forbidden band widths are 1.94 eV, 1.89 eV, and 1.84 eV, which correspond to Fig. 4d, e and f, respectively.
The properties of the electronic band gap energy of different concentration ratios of Nb doping in CsPbBr3 are further explained by the density of states. The density of states (DOS) explains the properties of these materials, as shown in Fig. 5. It can be clearly seen that the materials in Fig. 5a and b have crossed the Fermi level, which proves that when the doping concentration of Nb in CsPbBr3 is 50% or 25%, the material becomes a metallic conductor. In Fig. 5c–e, none of them cross the Fermi level, which proves that these materials are semiconductors.
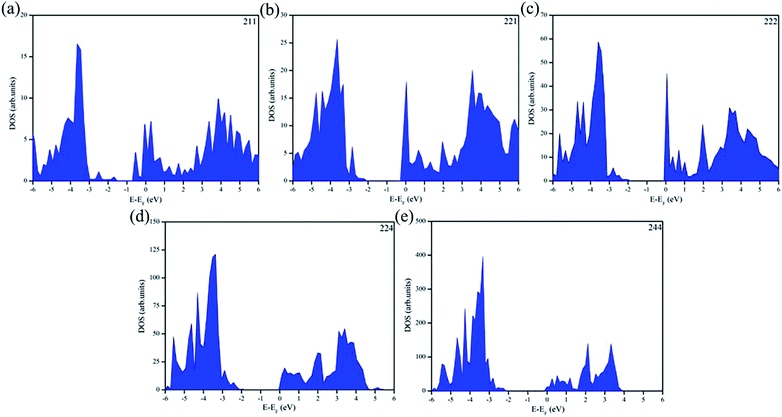 | ||
| Fig. 5 Density of states (DOS) of Nb-doped CsPbBr3 with different concentration ratios. (a) 50%, (b) 25%, (c) 12.5%, (d) 6.25%, and (e) 3.125%. | ||
4. Conclusion
In summary, CsPbBr3 is an excellent material for improving the stability of PSCs. Doping Nb into the material is considered to be an effective method to improve the PCE. Through DFT calculations, we systematically studied the electronic properties of CsPbBr3 doped with different Nb concentration ratios, including the band structure and density of states. The results show that when the doping concentration of Nb is 3.125%, 6.25% or 12.5%, all three compounds have semiconductor properties, the band gaps are direct, and the basic band gaps occur at Γ symmetry points. As the doping concentration increases, the band gap width gradually increases. When the doping concentration of Nb is too high (25%, 50%), the compounds are all metallic conductors, which do not have the properties of semiconductors. From this work, we can expect that the performance of PCE and long-term stability of doped CsPbBr3 at a certain Nb concentration will be greatly improved.Conflicts of interest
The authors declare no competing interests.Acknowledgements
Support of the National Natural Science Foundation of China (21902021, 21908017, 51972293, and 51772039), the Open Foundation of Key Laboratory of Industrial Ecology and Environmental Engineering, MOE(KLIEEE-20-01), the Fundamental Research Funds for the Central Universities (DUT20RC(4)020, DUT20RC(4)018, and DUT20LAB304), and the Supercomputing Center of Dalian University of Technology for this work is gratefully acknowledged.References
- M. Z. Liu, M. B. Johnston and H. J. Snaith, Efficient planar heterojunction perovskite solar cells by vapour deposition, Nature, 2013, 501(7467), 395–398 CrossRef CAS PubMed.
- W. Y. Nie, H. H. Tsai, R. Asadpour, J. C. Blancon, A. J. Neukirch, G. Gupta, J. J. Crochet, M. Chhowalla, S. Tretiak, M. A. Alam, H. L. Wang and A. D. Mohite, High-efficiency solution-processed perovskite solar cells with millimeter-scale grains, Science, 2015, 347(6221), 522–525 CrossRef CAS PubMed.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. I. Seok, Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells, Nat. Mater., 2014, 13(9), 897–903 CrossRef CAS PubMed.
- S. D. Stranks, P. K. Nayak, W. Zhang, T. Stergiopoulos and H. J. Snaith, Formation of Thin Films of Organic–Inorganic Perovskites for High-Efficiency Solar Cells, Angew. Chem., Int. Ed., 2015, 54(11), 3240–3248 CrossRef CAS PubMed.
- W. J. Yin, J. H. Yang, J. Kang, Y. F. Yan and S. H. Wei, Halide perovskite materials for solar cells: a theoretical review, J. Mater. Chem. A, 2015, 3(17), 8926–8942 RSC.
- L. Meng, J. B. You, T. F. Guo and Y. Yang, Recent Advances in the Inverted Planar Structure of Perovskite Solar Cells, Acc. Chem. Res., 2016, 49(1), 155–165 CrossRef CAS PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells, J. Am. Chem. Soc., 2009, 131(17), 6050–6051 CrossRef CAS PubMed.
- Laboratory, N. R. E., Best Research-Cell Efficiency Chart. 2020 Search PubMed.
- P. Kumar and A. K. Chauhan, Highly efficient flexible perovskite solar cells and their photo-stability, J. Phys. D: Appl. Phys., 2020, 53(3), 035101 CrossRef CAS.
- Y. H. Xu, C. Gao, S. W. Tang, J. Zhang, Y. Q. Chen, Y. J. Zhu and Z. Y. Hu, Comprehensive understanding of TiCl4 treatment on the compact TiO2 layer in planar perovskite solar cells with efficiencies over 20%, J. Alloys Compd., 2019, 787, 1082–1088 CrossRef CAS.
- D. S. Mann, Y. H. Seo, S. N. Kwon and S. I. Na, Efficient and stable planar perovskite solar cells with a PEDOT:PSS/SrGO hole interfacial layer, J. Alloys Compd., 2020, 812 Search PubMed.
- E. Gonzalez-Juarez, D. Gonzalez-Quijano, D. F. Garcia-Gutierrez, D. I. Garcia-Gutierrez, J. Ibarra-Rodriguez and E. Sanchez, Improving performance of perovskites solar cells using solvent engineering, via Lewis adduct of MAI-DMSO-PbI2 and incorporation of imidazolium cation, J. Alloys Compd., 2020, 817 Search PubMed.
- T. T. Ngo, S. Masi, P. F. Mendez, M. Kazes, D. Oron and I. M. Sero, PbS quantum dots as additives in methylammonium halide perovskite solar cells: the effect of quantum dot capping, Nanoscale Adv., 2019, 1(10), 4109–4118 RSC.
- Z. Liu, S. Z. Wu, X. J. Yang, Y. J. Zhou, J. R. Jin, J. M. Sun, L. Zhao and S. M. Wang, The dual interfacial modification of 2D g-C3N4 for high-efficiency and stable planar perovskite solar cells, Nanoscale Adv., 2020, 2(11), 5396–5402 RSC.
- S. Z. Yang, X. Z. Song, L. G. Gao, N. Wang, X. G. Ding, S. F. Wang and T. L. Ma, Seamless Interfacial Formation by Solution-Processed Amorphous Hydroxide Semiconductor for Highly Efficient Electron Transport, ACS Appl. Energy Mater., 2018, 1(9), 4564–4571 CrossRef CAS.
- Y. Wang, T. Y. Zhang, F. Xu, Y. H. Li and Y. X. Zhao, A Facile Low Temperature Fabrication of High Performance CsPbI2Br All-Inorganic Perovskite Solar Cells, Sol. RRL, 2018, 2(1), 1700180 CrossRef.
- J. R. Zhang, G. Hodes, Z. W. Jin and S. Z. Liu, All-Inorganic CsPbX3 Perovskite Solar Cells: Progress and Prospects, Angew. Chem., Int. Ed., 2019, 58(44), 15596–15618 CrossRef CAS PubMed.
- S. Liu, Y. J. Guan, Y. S. Sheng, Y. Hu, Y. G. Rong, A. Y. Mei and H. W. Han, A Review on Additives for Halide Perovskite Solar Cells, Adv. Energy Mater., 2020, 10(13), 1902492 CrossRef CAS.
- G. Q. Tong, L. K. Ono and Y. B. Qi, Recent Progress of All-Bromide Inorganic Perovskite Solar Cells, Energy Technol., 2020, 8(4), 1900961 CrossRef CAS.
- P. Y. Wang, X. W. Zhang, Y. Q. Zhou, Q. Jiang, Q. F. Ye, Z. M. Chu, X. X. Li, X. L. Yang, Z. G. Yin and J. B. You, Solvent-controlled growth of inorganic perovskite films in dry environment for efficient and stable solar cells, Nat. Commun., 2018, 9, 2225 CrossRef PubMed.
- G. E. Eperon, G. M. Paterno, R. J. Sutton, A. Zampetti, A. A. Haghighirad, F. Cacialli and H. J. Snaith, Inorganic caesium lead iodide perovskite solar cells, J. Mater. Chem. A, 2015, 3(39), 19688–19695 RSC.
- C. Liu, W. Z. Li, C. L. Zhang, Y. P. Ma, J. D. Fan and Y. H. Mai, All-Inorganic CsPbI2Br Perovskite Solar Cells with High Efficiency Exceeding 13%, J. Am. Chem. Soc., 2018, 140(11), 3825–3828 CrossRef CAS PubMed.
- L. Yan, Q. F. Xue, M. Y. Liu, Z. L. Zhu, J. J. Tian, Z. C. Li, Z. Chen, Z. M. Chen, H. Yan, H. L. Yip and Y. Cao, Interface Engineering for All-Inorganic CsPbI2Br Perovskite Solar Cells with Efficiency over 14%, Adv. Mater., 2018, 30(33), 1802509 CrossRef PubMed.
- S. Z. Yang, Z. L. Guo, L. G. Gao, F. Y. Yu, C. Zhang, M. Q. Fan, G. Y. Wei and T. L. Ma, Bifunctional Dye Molecule in All-Inorganic CsPbIBr2 Perovskite Solar Cells with Efficiency Exceeding 10%, Sol. RRL, 2019, 3(9), 1900212 CrossRef.
- C. Liu, W. Z. Li, J. H. Chen, J. D. Fan, Y. H. Mai and R. E. I. Schropp, Ultra-thin MoOx as cathode buffer layer for the improvement of all-inorganic CsPbIBr2 perovskite solar cells, Nano Energy, 2017, 41, 75–83 CrossRef CAS.
- J. L. Duan, Y. Y. Zhao, B. L. He and Q. W. Tang, High-Purity Inorganic Perovskite Films for Solar Cells with 9.72% Efficiency, Angew. Chem., Int. Ed., 2018, 57(14), 3787–3791 CrossRef CAS PubMed.
- J. L. Duan, Y. Y. Zhao, X. Y. Yang, Y. D. Wang, B. L. He and Q. W. Tang, Lanthanide Ions Doped CsPbBr3 Halides for HTM-Free 10.14%-Efficiency Inorganic Perovskite Solar Cell with an Ultrahigh Open-Circuit Voltage of 1.594 V, Adv. Energy Mater., 2018, 8(31), 1802346 CrossRef.
- G. Q. Tong, T. T. Chen, H. Li, L. B. Qiu, Z. H. Liu, Y. Y. Dang, W. T. Song, L. K. Ono, Y. Jiang and Y. B. Qi, Phase transition induced recrystallization and low surface potential barrier leading to 10.91%-efficient CsPbBr3 perovskite solar cells, Nano Energy, 2019, 65, 104015 CrossRef CAS.
- F. Zhao, Y. X. Guo, X. Wang, J. H. Tao, J. C. Jiang, Z. G. Hu and J. H. Chu, Enhanced performance of carbon-based planar CsPbBr3 perovskite solar cells with room-temperature sputtered Nb2O5 electron transport layer, Sol. Energy, 2019, 191, 263–271 CrossRef CAS.
- P. P. Teng, X. P. Han, J. W. Li, Y. Xu, L. Kang, Y. R. Q. Wang, Y. Yang and T. Yu, Elegant Face-Down Liquid-Space-Restricted Deposition of CsPbBr3 Films for Efficient Carbon-Based All-Inorganic Planar Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2018, 10(11), 9541–9546 CrossRef CAS PubMed.
- D. W. Huang, P. F. Xie, Z. X. Pan, H. S. Rao and X. H. Zhong, One-step solution deposition of CsPbBr3 based on precursor engineering for efficient all-inorganic perovskite solar cells, J. Mater. Chem. A, 2019, 7(39), 22420–22428 RSC.
- X. Y. Liu, X. H. Tan, Z. Y. Liu, H. B. Ye, B. Sun, T. L. Shi, Z. R. Tang and G. L. Liao, Boosting the efficiency of carbon-based planar CsPbBr3 perovskite solar cells by a modified multistep spin-coating technique and interface engineering, Nano Energy, 2019, 56, 184–195 CrossRef CAS.
- X. Y. Liu, Z. Y. Liu, X. H. Tan, H. B. Ye, B. Sun, S. Xi, T. L. Shi, Z. R. Tang and G. L. Liao, Novel antisolvent-washing strategy for highly efficient carbon-based planar CsPbBr3 perovskite solar cells, J. Power Sources, 2019, 439, 227092 CrossRef CAS.
- Y. Ding, B. L. He, J. W. Zhu, W. Y. Zhang, G. D. Su, J. L. Duan, Y. Y. Zhao, H. Y. Chen and Q. W. Tang, Advanced Modification of Perovskite Surfaces for Defect Passivation and Efficient Charge Extraction in Air-Stable CsPbBr3 Perovskite Solar Cells, ACS Sustainable Chem. Eng., 2019, 7(23), 19286–19294 CrossRef CAS.
- X. Li, Y. Tan, H. Lai, S. P. Li, Y. Chen, S. W. Li, P. Xu and J. Y. Yang, All-Inorganic CsPbBr3 Perovskite Solar Cells with 10.45% Efficiency by Evaporation-Assisted Deposition and Setting Intermediate Energy Levels, ACS Appl. Mater. Interfaces, 2019, 11(33), 29746–29752 CrossRef CAS PubMed.
- Y.-F. Ding, Z.-L. Yu, P.-B. He, Q. Wan, B. Liu, J.-L. Yang and M.-Q. Cai, High-performance Photodetector Based on InSe/Cs2XI2Cl2 (X = Pb, Sn, and Ge) Heterostructures, Phys. Rev. Appl., 2020, 13(6), 064053 CrossRef CAS.
- L.-Y. Pan, Y.-F. Ding, Z.-L. Yu, Q. Wan, B. Liu and M.-Q. Cai, Layer-dependent optoelectronic property for all-inorganic two-dimensional mixed halide perovskite Cs2PbI2Cl2 with a Ruddlesden-Popper structure, J. Power Sources, 2020, 451 Search PubMed.
- T. Ruan, B. Wang, Y. Yang, X. Zhang, R. Song, Y. Ning, Z. Wang, H. Yu, Y. Zhou, D. Wang, H. Liu and S. Dou, Interfacial and Electronic Modulation via Localized Sulfurization for Boosting Lithium Storage Kinetics, Adv. Mater., 2020, 32(17), 2000151 CrossRef CAS PubMed.
- D.-N. Yan, C.-S. Liao, Y.-Q. Zhao, B. Liu, J.-L. Yang and M.-Q. Cai, Theoretical prediction of double perovskite Cs2AgxCu1−xInyTb1−yCl6 for infrared detection, J. Phys. D: Appl. Phys., 2020, 53(26), 265302 CrossRef CAS.
- B. Wang, T. Liu, A. Liu, G. Liu, L. Wang, T. Gao, D. Wang and X. S. Zhao, A Hierarchical Porous C@LiFePO4/Carbon Nanotubes Microsphere Composite for High-Rate Lithium-Ion Batteries: Combined Experimental and Theoretical Study, Adv. Energy Mater., 2016, 6(16), 1600426 CrossRef.
- G. Kresse and J. Furthmuller, Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set, Comput. Mater. Sci., 1996, 6(1), 15–50 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77(18), 3865–3868 CrossRef CAS PubMed.
- P. E. Blochl, Projector Augmented-Wave Method, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50(24), 17953–17979 CrossRef PubMed.
- G. Kresse and D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59(3), 1758–1775 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |