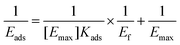A mechanistic study of cellulase adsorption onto lignin
Lan
Yao†
 ab,
Haitao
Yang†
c,
Chang Geun
Yoo
ab,
Haitao
Yang†
c,
Chang Geun
Yoo
 d,
Congxin
Chen
e,
Xianzhi
Meng
d,
Congxin
Chen
e,
Xianzhi
Meng
 b,
Jun
Dai
a,
Chunlei
Yang
f,
Jun
Yu
f,
Arthur J.
Ragauskas
b,
Jun
Dai
a,
Chunlei
Yang
f,
Jun
Yu
f,
Arthur J.
Ragauskas
 *bgh and
Xiong
Chen
*a
*bgh and
Xiong
Chen
*a
aKey Laboratory of Fermentation Engineering (Ministry of Education), College of Bioengineering, Hubei University of Technology, 28th of Nanli Road, Wuhan 430068, China. E-mail: cx163_qx@163.com; Tel: +86 13308639592
bDepartment of Chemical and Biomolecular Engineering, The University of Tennessee, Knoxville, TN 37996-2200, USA. E-mail: aragausk@utk.edu
cHubei Provincial Key Laboratory of Green Materials for Light Industry, Hubei University of Technology, Wuhan 430068, China
dDepartment of Chemical Engineering, State University of New York College of Environmental Science and Forestry, Syracuse, NY 13210, USA
eChina Tobacco Hubei Industrial Cigarette Materials, LLC, Wuhan 430050, China
fTobacco Research Institute of Hubei Province, 6 Baofeng Road, Qiaokou District, Wuhan 430030, China
gJoint Institute for Biological Sciences, Biosciences Division, Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA
hDepartment of Forestry, Wildlife, and Fisheries, Center for Renewable Carbon, The University of Tennessee, Institute of Agriculture, Knoxville, TN 37996-2200, USA
First published on 28th October 2020
Abstract
To explore the effect of lignin composition on cellulase adsorption, dehydrogenation polymers (DHPs) were prepared from p-glucocoumaryl alcohol/coniferin/syringin, giving rise to H-DHP, G-DHP, and S-DHP, respectively. The structures of DHPs were thoroughly characterized and compared by GPC and NMR techniques, and the Langmuir isotherm protocol was applied to determine the cellulase adsorption behaviors of these different types of DHPs. The adsorption study indicated that the binding strength between the DHPs and cellulase varied in the following order: G-DHP > H-DHP > S-DHP. The inhibition of different types of DHPs on enzymatic hydrolysis of cellulose was in the same order as the cellulase adsorption, indicating that non-productive adsorption was the main way to influence cellulase. The correlation analysis results showed a positive association between the phenolic hydroxyl group content in DHPs and their maximum adsorption capacity toward enzymes. A negative correlation between the PDI and binding strength was also observed. It was also found that the adsorbed cellulase could be desorbed and retained normal enzyme activity, and so it was presumed that DHPs and cellulase were mainly linked by physisorption such as hydrogen bonding. This study clearly showed that the composition of lignin had a great impact on cellulase, and that G-type lignin exhibited the most detrimental effect. The results could provide useful information on the mechanism of cellulase adsorption onto lignin using DHPs as lignin model compounds.
Introduction
Lignin is the second-largest biopolymer in plant cell walls.1 It is mainly composed of guaiacyl (G), syringyl (S) and p-hydroxyphenyl (H) units linked through C–C (e.g., β-β, β-5, and β-4, 5-5′) and C–O interunit (e.g., β-O-4, 4-O-5, and α-O-4) linkages, with their content vary among species.2 Lignin has been reported as an important factor affecting the recalcitrance of biomass due to its adverse effect on the enzymatic hydrolysis process.3 In the past decades, various pretreatment methods have been studied and employed to decrease the content of lignin or alter the characteristics of lignin to maximize the fermentable sugar production with minimal enzyme loading.4Lignin-derived inhibition has been proposed to occur in a few ways: (I) lignin could act as a physical barrier by covering the reactive carbohydrate surface and/or forming lignin–carbohydrate complexes (LCC), (II) non-productive binding to enzymes, and (III) deactivation of enzymes with soluble lignin fragments.5 Several studies have shown that there is a strong correlation between lignin structural features and non-productive binding of lignin with cellulase.5–7 Our previous studies have also reported the influences of lignin subunits, phenolic hydroxyls, condensed aromatics, molecular weight, and polydispersity index (PDI) on cellulase binding.8–10
A recent study indicated that lignin S/G is an important factor influencing the cellulase hydrolysis and ethanol fermentation processes.11 Similarly, the results from numerous studies indicated that the composition of lignin showed significant effects on the cellulase hydrolysis process.7,10,12 Nakagame et al. reported that softwood lignin showed the highest binding strength with cellulase among the lignins isolated from softwood, hardwood, and grass due to the abundance of G-type units in softwood lignin.13 In contrast, Tan and her coworkers reported that S-type lignin had stronger cellulase adsorption than G-type lignin.12 Guo et al. also tested the cellulase binding to lignins using different lignin resources from aspen, pine, corn stover, and kenaf, and their results concluded that lignin with lower S/G could adsorb more cellulase,7 which has also been confirmed recently by Mou and his coworkers.14 As can be seen, much of the literature reports conflicting trends on the effects of lignin composition on cellulase adsorption behaviors. The lack of consensus across literature studies could be significantly attributed to the fact that lignin–enzyme interaction is a complicated multi-variant and multi-scale problem. It is quite challenging to observe the impact of any single factor, such as lignin composition on enzyme adsorption. While most lignin studies do report their negative effects on cellulose hydrolysis,6,15 some exhibited either no effect or even a positive effect on enzymatic hydrolysis occasionally.16 The lack of consensus can be attributed to several issues. First, lignin has multiple properties including hydroxyl group content, molecular weight, aromatic composition, the abundance of inter-unit linkages, and others depending on its feedstock species, extraction method, and even environments. Although the aforementioned previous studies tried to minimize the variable factors in their studies, the tested lignins showed variations in several properties. When the effects of multiple factors offset and/or one factor is dominant over others, it is challenging to evaluate them properly. In this study, we tried to minimize the variable factors by testing with DHPs composed of one type of aromatic unit. Second, the nature of lignin used was believed to be a critical factor. For example, native lignin (e.g., CEL) extracted from the plant cell wall of various origins or technical lignins (e.g., organosolv lignin) isolated from the pretreated biomass or during the biomass pretreatment were typically used as the feedstock in those studies. These lignins suffer unavoidably from various modifications or degradation effects as well as impurities, including residual polysaccharides or proteins.17 Moving forward, a useful strategy to overcome these challenges will include using model compounds.
A mechanistic study of well-representative lignin model compounds can provide a better picture of the targeted factor by minimizing other variations. Freudenberg and Neish proposed a dehydrogenation polymer as the natural lignin model for the first time.18 Terashima also synthesized G-type lignin from coniferin using β-glucosidase, glucose oxidase, and peroxidase.19 The structure of lignin synthesized from coniferin was similar to that of milled wood lignin (MWL) compared to the one synthesized from coniferyl alcohol.19 The lignin model compounds solely composed of S or H-type lignin could also be synthesized similarly. These compounds offer promising opportunities for a direct comparison of each type of lignin based on cellulase adsorption without the interference of other factors. In 2011, dehydrogenation polymers (DHPs) of monolignols were synthesized by Nakagame et al. from coniferyl alcohol (CA) and ferulic acid (FA) to study the effect of the carboxylic acid content of lignin on non-productive binding of cellulase,20 confirming the feasibility of this method. In this study, three dehydrogenation polymers (DHPs) were solely synthesized from coniferin/syringin/p-glucocoumaryl alcohol, and the cellulase adsorption and desorption onto and from each of these DHPs were subsequently investigated. For the fundamental understanding of their structural characteristics, gel permeation chromatography (GPC) and nuclear magnetic resonance (NMR) were used. The influence of lignin composition on cellulase adsorption could be elucidated by correlation analysis. The present study could provide information on lignin modification to decrease the binding between lignin and cellulase.
Materials and methods
Synthesis of DHPs
First, p-glucocoumaryl alcohol, coniferin, and syringin were synthesized from the starting compounds p-hydroxybenzaldehyde, vanillin, and syringaldehyde, respectively. Then p-glucocoumaryl alcohol, coniferin, and syringin were used to obtain their corresponding DHPs by the following method, which was called discontinuous dehydrogenation method (Zulauf, ZL). In brief, 500 mg of p-glucocoumaryl alcohol/coniferin/syringin was loaded in sodium acetate buffer (pH = 4.8) with 94.5 U β-glucosidase, 198.4 U glucose oxidase and 316 U peroxidase at 30 °C as described in Yang's study.21 An additional 92.61 U β-glucosidase, 297.65 U glucose oxidase, and 206 U peroxidase were supplemented after 9 h. The product was separated by centrifugation at 8000 rpm after 98 h of reaction, washed with distilled water, and then vacuum-dried to obtain crude DHPs. The crude DHPs were purified by dissolving in 5 ml of dichloroethane/ethanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) followed by dropping into 50 ml of ether with agitation. The precipitates were obtained by centrifugation, washed three times with ether, and finally vacuum-dried to obtain H-DHP, G-DHP, and S-DHP. The synthesis yields of H-DHP, G-DHP, and S-DHP were 35.94%, 45.42% and 5.3%, respectively.
1, v/v) followed by dropping into 50 ml of ether with agitation. The precipitates were obtained by centrifugation, washed three times with ether, and finally vacuum-dried to obtain H-DHP, G-DHP, and S-DHP. The synthesis yields of H-DHP, G-DHP, and S-DHP were 35.94%, 45.42% and 5.3%, respectively.
Analytical methods
The molecular weight and chemical structures of DHPs were analyzed by gel permeation chromatography (GPC) and nuclear magnetic resonance (NMR). GPC analysis of each lignin sample was performed after being acetylated and was then analyzed using a Shimadzu HPLC system (Shimadzu, Kyoto, Japan) by employing shim-pack GPC-800P columns. Tetrahydrofuran (THF) was used as an eluent, and the flow rate was 1.0 ml min−1.8About 50 mg of DHPs in DMSO-d6 (0.5 mL) was characterized using a Bruker Avance III 400 MHz spectrometer at 298 K to obtain the two-dimensional (2D) 1H–13C heteronuclear single quantum coherence (HSQC) spectrum. 31P NMR spectra were also acquired after derivatization of DHPs with 2-chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane (TMDP).22
Non-productive binding of cellulase to DHPs
Cellulase from Trichoderma reesei was purchased from Sigma-Aldrich. The Langmuir isotherm protocol was applied to measure the binding of cellulase to the DHPs. Different concentrations of cellulase (0.5 ml) were mixed with 10 mg of DHPs. The mixture was kept at 50 °C until the adsorption was constant. The following equation was used to determine the maximum adsorption capacity (Emax) and the equilibrium constant (Kads).where Ef (mg ml−1) is the free protein concentration, Eads (mg g−1) is the amount of protein adsorbed by the DHP, Kads is the Langmuir adsorption constant, and [Emax] is the maximum amount of adsorbed protein.
After adsorption, the supernatant and precipitate were separated by centrifugation. The precipitate (called P0) was used to study the desorption of cellulase.
Desorption of cellulase
At room temperature, 0.5 ml of acetic acid/sodium acetate buffer solution (pH 4.8) was added to P0, the supernatant and precipitate were separated by centrifugation to obtain S1 and P1. S1 was used for cellobiohydrolase activity analysis. P1 was further washed with buffer to obtain S2 and P2. This process was continued until there was no cellobiohydrolase activity in the Sx. DHP with buffer was set as the control.The influence of DHP on cellulase hydrolysis of phosphoric acid swelling cellulose (PASC)
Phosphoric acid swelling cellulose (PASC) was prepared according to the procedure described in a previous study.23 Enzymatic hydrolysis of the PASC was performed in sodium citrate buffer (50 mM, pH 4.8) at 50 °C and 150 rpm with 2% (w/v) substrate and cellulase loading of 25 FPU g−1 glucan for 72 h. To investigate the effects of each DHP on enzymatic hydrolysis, 20 mg of DHP was added to 80 mg of PASC. PASC without DHP was also tested as the control. The glucose concentration in the hydrolysate was determined by high-performance liquid chromatography (HPLC, Shimadzu, Kyoto, Japan) with a refractive index detector (Shimadzu) on an Aminex HPX-87P column (Bio-Rad, Hercules, CA, USA) running at a flow rate of 0.6 mL min−1 at 65 °C, with water as the eluent. All experiments were carried out in duplicate.Results and discussion
Molecular weight determination
The number average molecular weights (Mn), weight average molecular weights (Mw) and polydispersity indexes (PDI) of DHPs are shown in Table 1. The weight average molecular weights of H-DHP, G-DHP, and S-DHP were 3456, 2032, and 1834 g mol−1, respectively, which are comparable to the published literatures.24,25 Earlier research indicated that methoxyl groups’ steric hindrance on benzene rings limits or precludes reactivity at some sites.26 That could be the reason for the higher molecular weight of H-DHP. All the three DHPs showed narrow molecular weight distribution as the PDIs were all lower than 2.| M n (g mol−1) | M w (g mol−1) | PDI | |
|---|---|---|---|
| H-DHP | 2004 | 3456 | 1.7 |
| G-DHP | 1281 | 2032 | 1.6 |
| S-DHP | 912 | 1834 | 2.0 |
HSQC determination
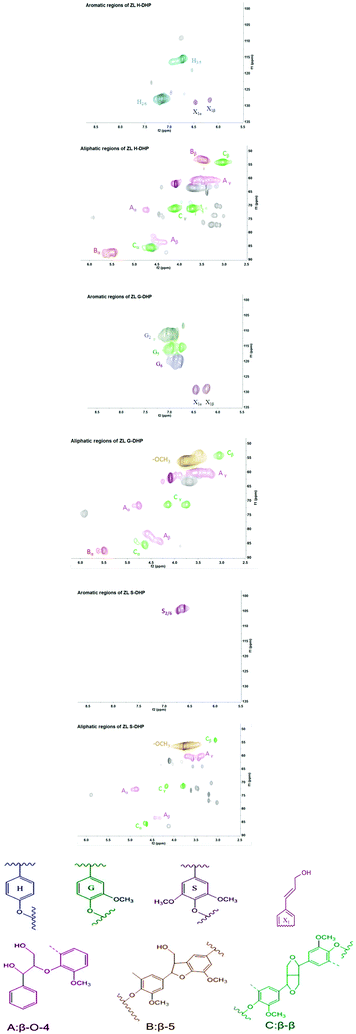
The 2D-HSQC spectra of DHPs are shown in Fig. 1. The major signals were assigned according to previous publications,3,8,27 and the contents of different interlinkages are shown in Table 2. The aromatic region (100–135/5.5–8.5 ppm) mainly offers information about lignin monolignol compositions. The cross peak for S2/6 was present in S-DHP at δC/δH 103.8/6.7 ppm. The C–H correlations of G2, G5, and G6 were observed in G-DHP at 110.9/7.0, 115.8/6.8, and 119.1/6.7 ppm, respectively. Furthermore, cross signals from H2/6 and H3/5 were observed in H-DHP at δC/δH 127.7/7.1 and 115.5/6.7 ppm, respectively. In addition, signals from the side chains of cinnamyl alcohol end groups were also found in H-DHP and G-DHP at δC/δH 129.2/6.44 (X1α) and 128.9/6.24 ppm (X1β), respectively. The difference in the end groups of different DHPs is primarily due to the endwise coupling of monolignols. It was confirmed that the synthesized DHPs were composed of p-hydroxyphenyl/guaiacyl/syringyl units as expected.Methoxyl and major lignin interunit linkages including β-O-4 aryl ether (A), phenylcoumaran (β-5) (B), and resinol (β-β) (C) were found in the aliphatic region (50–90/2.5–6.0 ppm) of the HSQC spectra. The results showed that H-DHP and G-DHP contained all three major lignin interunit linkages, while only two (β-O-4 and β-β) were detected in S-DHP, as a methoxyl group occupied the C5 position of S. The quantitative information of the interunit linkages is presented in Table 2. It was indicated that phenylcoumaran (β-5) was the major interunit linkage in H-DHP, accounting for nearly 46.8% of the total linkages. These results were in accordance with earlier studies, which indicated that H-DHP tends to form branched linkages like β-β and β-5.28 As no methoxy substituents on the ring of mono-lignol forming by H lignin subunit, the unpaired electron density is greatest on the carbon nuclei, which could result in C–C reactions preferably.29 For S-DHPs, β-O-4 is the most dominant type of interlinkage, which suggested that this DHP might form a linear type of structure.28 It has been revealed that more methoxylation could result in a higher degree of unpaired electron density on the phenolic oxygen and subsequently, many C–O linkages.30 The contents of β-O-4 in DHPs were relatively lower than those in MWL,25 while the β-5 and β-β linkages were predominant instead of the β-O-4 linkage.
Determination of hydroxyl groups by 31P NMR
Table 3 presents the contents of the hydroxyl groups in each DHP. Aliphatic OH was the main OH group in all DHPs, and it was 2.9, 3.5, and 3.4 mmol g−1 DHP in H-DHP, G-DHP, and S-DHP, respectively. Phenolic hydroxyl group contents were 1.1, 1.4, and 0.3 mmol g−1 DHP in H-DHP, G-DHP, and S-DHPs, respectively. S-DHP contained the lowest phenolic hydroxyl group content, which was in accordance with the HSQC results. It could be explained by the abundance of the β-O-4 linkage in S-DHP. Similarly, the phenolic OH content of H-DHP was lower than that of G-DHP, as more β-5 was found in H-DHP. Carboxylic acid OH was 0.66, 0.43, and 0.30 mmol g−1 DHP in H-DHP, G-DHP, and S-DHP, respectively. The total hydroxyl group content varied in the following order: G-DHP (5.3 mmol g−1 DHP) > H-DHP (4.7 mmol g−1 DHP) > S-DHP (4.0 mmol g−1 DHP).| DHP (mmol g−1) | Aliphatic OH | Syringyl OH | Guaiacyl OH | p-Hydroxyphenyl OH | Carboxylic acid OH | Total OH |
|---|---|---|---|---|---|---|
| H-DHP | 2.9 | — | — | 1.1 | 0.66 | 4.7 |
| G-DHP | 3.5 | — | 1.4 | — | 0.43 | 5.3 |
| S-DHP | 3.4 | 0.3 | — | — | 0.30 | 4.0 |
The impact of DHPs on the enzymatic digestibility of PASC
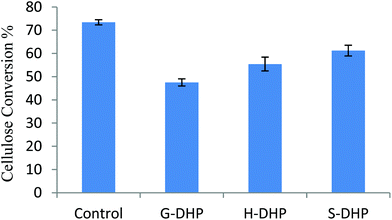
The influence of lignin composition on cellulase hydrolysis was tested with PASC and the synthesized DHPs (Fig. 2). Upon addition of different DHPs, the enzymatic digestibility of PASC was decreased to various extents. The conversion of cellulose was 73.4% without DHP loading, but it decreased to 47.5%, 55.4%, and 61.3% upon addition of G-DHP, H-DHP, and S-DHP, respectively. Previous research indicated that cellulase deactivation occurred with lignin during the hydrolysis process.15,31 It was suggested that G-DHP showed the most inhibitory effect on cellulose conversion, followed by H-DHP and S-DHP. The inhibitory effect might be due to the non-productive adsorption of cellulase onto DHP, which is discussed later.Combined with the structural features of DHP, it was found that hydroxyl groups, especially phenolic hydroxyl groups, played an important role in cellulase hydrolysis of PASC. For example, a negative effect of the content of phenolic OH (y = −15.71x + 70.38, R2 = 0.90) from DHP on cellulose conversion was found, which was also shown in a previous study.6 It was proposed that phenolic OH mainly reacts with the amino group in cellulase by electrostatic and hydrogen bonds.32
Cellulase adsorption onto DHPs
The binding ability between cellulase and DHPs was further assessed to elucidate the effect of lignin composition on cellulase adsorption (Table 4). The maximum cellulase adsorption capacity was found with G-DHP (31.95 mg g−1), followed by H-DHP and S-DHP. Binding strength is a parameter to estimate the amounts of enzymes onto DHP by incorporating both the maximum adsorption capacity and equilibrium constant. The results showed that the binding strengths of DHP with cellulase were 285.7, 162.1, and 37.9 ml g−1 for G-DHP, H-DHP, and S-DHP, respectively. The difference between the DHPs in cellulase adsorption was due to their different monolignol compositions and structural features. In particular, the lignin composition was found to have a significant effect on cellulase adsorption, which was the most in G-type lignin and the least in S-type lignin. The trend of the binding strength and bonding ability of each DHP was consistent with the effect of DHP on cellulase hydrolysis, as the DHP that adsorbed the most cellulase showed the strongest inhibitory effect on cellulase hydrolysis. A strong negative correlation was found between the 72 h cellulose conversion and the binding strength (y = −17.83x + 1138, r2 = 0.99). It was suggested that non-productive binding was the leading cause of deactivation of cellulase by DHP.| E max (mg g−1) | K ads (ml mg−1) | Binding strength (ml g−1) | |
|---|---|---|---|
| H-DHP | 24.39 | 6.64 | 162.07 |
| G-DHP | 31.95 | 8.94 | 285.71 |
| S-DHP | 8.40 | 4.51 | 37.88 |
In this study, the structural properties of each DHP were investigated to explain the difference in the affinity of DHP to cellulase. The three DHPs had different abundance of the inter-unit linkages. G-DHP and H-DHP contained more of β-β and β-5 than S-DHP. Earlier studies showed that C–C coupling of monolignols normally result in a highly branched polymer, which was more inhibitory to cell wall degradation.33 However, even though H-DHP had the highest C–C linkage contents, it did not exhibit the highest inhibitory effect on cellulase. The amount of C–O interunit (e.g., β-O-4) also varied among the three DHPs. S-DHP had the most and H-DHP contained the least. Our previous studies showed that a decrease of β-O-4 in residual lignin was beneficial for the enzymatic hydrolysis of pretreated wheat straw.3 Non-productive binding sites of β-O-4 with cellobiohydrolase I has been recently reported.34 Previous studies on down-regulated alfalfa plants showed an improved fermentable sugar yield, which was rich in H-lignin.35 Later, Pu and his coworkers proposed that reducing β-O-4 ether linkage in H rich plants could reduce LCC linking, which might facilitate the accessibility of cellulase to cellulose, thus decreasing the recalcitrance of biomass and improving cell wall enzymatic degradability.36 However, the correlation of β-O-4 amount in DHP with cellulase was not found in the present study. These results indicated that all kinds of inter-unit linkages showed an adverse impact on cellulase, but which one is more detrimental than the other needs further investigation.
It was interesting to note that the cinnamyl alcohol end groups were only found in G-DHP and H-DHP, which was 21.0% and 12.0%, respectively, expressed as a fraction of S + G + H. The disappearance of the signal in S-DHP might be due to different endwise coupling of monolignols, as less reaction sites were available in S-DHP. In a previous study, cinnamyl alcohol dehydrogenase (CAD), which catalyzes the last step in the biosynthesis of lignin monomers (cinnamaldehyde to cinnamyl alcohol), was downregulated.37 It resulted in the decrease of cinnamyl alcohol and improved the digestibility of the forage crop and saccharification efficiency in switchgrass.37,38 The positive effect of the cinnamyl alcohol content in DHP on the binding strength with cellulase was found in the present study(y = 11.72x + 32.93, R2 = 0.99). Similarly, the inhibitory effect of the cinnamyl alcohol content on cellulose conversion was observed (y = −0.65x + 61.86, R2 = 0.97). These results indicated that the cinnamyl alcohol end groups in lignin had an adverse impact on cellulase.
Furthermore, the phenolic hydroxyl groups were positively correlated with the maximum adsorption capacity (y = 21.26x + 1.92, R2 = 0.99) and binding strength (y = 210.85x − 34.91, R2 = 0.94). It was indicated that there was hydrogen bonding between cellulase and the hydroxyl groups of DHP. Many studies have confirmed that hydrogen bonding occurred between the amide groups in cellulase and the aromatic phenolic OH groups in lignin,6,32 which would block the catalytic tunnel of cellulase. Later, modified lignin with less aromatic phenolic OH showed less inhibitory effect on cellulase.39 Hydrogen bonding is also a recognized reason for cellulase denaturation by phenolic compounds,40,41 which can change the cellulase structure by destroying the α-helix structure, increasing the β-sheet and random coil contents in cellulase.40 The correlation between the PDI of DHP and adsorption parameters was found to be negative (y = −58.02x + 124.27, R2 = 0.99 for Emax; and y = −572.13x + 1172.6, R2 = 0.92 for binding strength between DHP and cellulase). The negative correlation between the lignin PDI and cellulase adsorption was also found in our previous study and by other researchers.7,9 A more uniform fragment size is favorable for the interaction between lignin and proteins. These results were in accordance with the impact of DHPs on the enzymatic digestibility of PASC, which indicated that the main reason for the inhibitory effect of DHP on cellulase was non-productive adsorption.
Desorption of cellulase from DHPs
The DHP adsorbed cellulase was washed with buffer and centrifuged to remove free cellulase and desorb the cellulase. Cellobiohydrolases comprise approximately 60% of cellulase mixture from Trichoderma reesei.42 In the present study, the supernatants were incubated with p-nitrophenol-D-cellobioside (pNPC) to analyze the cellobiohydrolase activity. Table 5 shows that the adsorption values for the first two washing times were higher because they contained free cellulase and part of desorbed cellulase. The cellobiohydrolase activity of the desorbed cellulase from G-DHP was the highest, followed by H-DHP and S-DHP with the same number of washing steps. After washing 4 times, the cellobiohydrolase activity was not detected in the supernatant of S-DHP, while H-DHP and G-DHP needed washing 6 and 8 times. These results indicated that the adsorbed cellulase could retain its activity and the adsorption of cellulase onto DHP was reversible.| H-DHP | G-DHP | S-DHP | |
|---|---|---|---|
| 1st | 3.321 | 3.797 | 2.255 |
| 2nd | 0.971 | 1.193 | 0.631 |
| 3rd | 0.322 | 0.504 | 0.267 |
| 4th | 0.204 | 0.341 | 0 |
| 5th | 0.097 | 0.230 | — |
| 6th | 0 | 0.131 | — |
| 7th | — | 0.022 | — |
| 8th | — | 0 | — |
Conclusion
Dehydrogenation polymers (DHPs) composed only of guaiacyl/syringyl/p-hydroxyphenyl were synthesized and used in the mechanism study of the effect of lignin composition on cellulase. The results showed that the lignin composition had a significant influence on cellulase adsorption and the cellulase hydrolysis process, and that G-DHP showed the maximum cellulase adsorption capacity and the most significant adverse impact on cellulase, followed by H-DHP and S-DHP. The difference between the DHPs in their affinity to cellulase might be due to various inter-unit linkages. Furthermore, the hydroxyl group content, especially the phenolic hydroxyl group and PDI, exhibited a distinct effect on cellulase. The desorbed cellulase retained its activity, indicating that the adsorption of cellulase onto DHP was reversible. These results will lead to the establishment of a fundamental theory for the structural alteration of lignin to decrease the unfavorable binding.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the support from the National Natural Science Foundation of China (No. 21978074 and 31871789), the China Scholarship Council (No. 2011842330 and 201508420257) and Key Laboratory of Pulp and Paper Science & Technology of Ministry of Education of China (No. KF201611 and KF201719), Key Project of Hubei Provincial Department of Education (No. D20161402), the Foundation of Hubei Provincial Key Laboratory of Green Materials for Light Industry (No. 201611B01 and 201806A02), and the Natural Science Foundation of Hubei Provincial Department of Education (No. B2016046).References
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, K. A. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344, 709–718 CrossRef CAS.
- J. Ralph, M. Bunzel, J. M. Marita, R. D. Hatfeld, F. Lu, H. Kim, P. F. Schatz, J. H. Grabber and H. Steinhart, Phytochem. Rev., 2004, 3, 79–96 CrossRef CAS.
- H. Yang, Y. Xie, X. Zheng, Y. Pu, F. Huang, X. Meng, W. Wu, A. Ragauskas and L. Yao, Bioresour. Technol., 2016, 207, 361–369 CrossRef CAS.
- H. Yang, C. Yoo, X. Meng, Y. Pu, W. Muchero, G. A. Tuskan, T. J. Tschaplinski, A. J. Ragauskas and L. Yao, Bioresour. Technol., 2020, 295, 122240–122246 CrossRef CAS.
- C. Yoo, M. Li, X. Meng, Y. Pu and A. Ragauskas, Green Chem., 2017, 19, 2006–2016 RSC.
- S. Sun, Y. Huang, R. Sun and M. Tu, Green Chem., 2016, 18, 4276–4286 RSC.
- F. Guo, W. Shi, W. Sun, X. Li, F. Wang, J. Zhao and Y. Qu, Biotechnol. Biofuels, 2014, 7, 38–47 CrossRef.
- L. Yao, H. Yang, C. Yoo, X. Meng, M. Li, Y. Pu, A. Ragauskas and R. Sykes, Bioresour. Technol., 2017, 244, 957–962 CrossRef CAS.
- L. Yao, C. Yoo, X. Meng, M. Li, Y. Pu, A. Ragauskas and H. Yang, Biotechnol. Biofuels, 2018, 11, 96–106 CrossRef.
- L. Yao, H. Yang, C. Yoo, Y. Pu, X. Meng, Y. Pu, N. Hao and A. Ragauskas, Front. Energy Res., 2018, 6, 1–9 CrossRef.
- C. Yoo, A. Dumitrache, W. Muchero, J. Natzke, H. Akinosho, M. Li, R. Sykes, S. Brown, B. Davison, G. Tuskan, Y. Pu and A. Ragauskas, ACS Sustainable Chem. Eng., 2018, 6, 2162–2168 CrossRef CAS.
- L. Tan, W. Sun, X. Li, J. Zhao, Y. Qu, Y. Choo and S. K. Loh, Biotechnol. J., 2015, 10, 915–925 CrossRef CAS.
- S. Nakagame, R. Chandra and J. Saddler, Biotechnol. Bioeng., 2010, 105, 871–879 CAS.
- H. Mou, J. Huang, W. Li, X. Wu, Y. Liu and H. Fan, Int. J. Biol. Macromol., 2020, 149, 794–800 CrossRef.
- J. L. Rahikainen, R. Martin-Sampedro, H. Heikkinen, S. Rovio, K. Marjamaa, T. Tamminen, O. J. Rojas and K. Kruus, Bioresour. Technol., 2013, 133, 270–278 CrossRef CAS.
- C. Lai, B. Yang, Z. Lin, Y. Jia, C. Huang, X. Li, X. Song and Q. Yong, Biotechnol. Biofuels, 2019, 12, 57–68 CrossRef.
- H. Palonen, F. Tjerneld, G. Zacchi and M. Tenkanen, J. Biotechnol., 2004, 107, 65–72 CrossRef CAS.
- K. Freudenberg and A. C. Neish, Q. Rev. Biol., 1968, 46, 171–175 Search PubMed.
- N. Terashima, Holzforschung, 1996, 50, 9–14 CrossRef CAS.
- S. Nakagame, R. P. Chandra, J. F. Kadla and J. N. Saddler, Biotechnol. Bioeng., 2011, 108, 538–548 CrossRef CAS.
- H. Yang, Doctoral thesis, South China University of Technology, 2007.
- X. Meng, C. Crestini, H. Ben, N. Hao, Y. Pu and A. Ragauskas, Nat. Protocols, 2019, 14, 2627–2647 CAS.
- Y. H. Zhang, J. Cui, L. R. Lynd and L. Kuang, Biomacromolecules, 2006, 7, 644–648 CrossRef CAS.
- B. Cathala, B. Saake, O. Faix and B. Monties, Polym. Degrad. Stab., 1998, 59, 65–69 CrossRef CAS.
- B. Saake, D. S. Argyropoulos, O. Beinhoff and O. Faix, Phytochemistry, 1996, 43, 499–507 CrossRef CAS.
- A. K. Sangha, B. H. Davison, R. F. Standaert, M. F. Davis, J. C. Smith and J. M. Parks, J. Phys. Chem. B, 2014, 118, 164–170 CrossRef CAS.
- J. Zeng, G. L. Helms, X. Gao and S. Chen, J. Agric. Food Chem., 2013, 61, 10848–10857 CrossRef CAS.
- A. E. Harman-Ware, R. M. Happs, B. H. Davison and M. F. Davis, Biotechnol. Biofuels, 2017, 10, 281–291 CrossRef.
- A. Ziebell, K. Gracom, R. Katahira, F. Chen, Y. Pu, A. Ragauskas, R. Dixon and M. Davis, J. Biol. Chem., 2010, 285, 38961–38968 CrossRef CAS.
- W. Russell, M. Burkitt, L. Scobbie and A. Chesson, Biomacromolecules, 2006, 7, 268–273 CrossRef CAS.
- Z. Yu, K. S. Gwak, T. Treasure, H. Jameel, H. M. Chang and S. Park, ChemSusChem, 2014, 7, 1942–1950 CrossRef CAS.
- X. Pan, J. Biobased Mater. Bioenergy, 2008, 2, 25–32 CrossRef.
- J. Grabber, Crop Sci., 2005, 45, 820–831 CrossRef CAS.
- Y. Tokunaga, T. Nagata, K. Kondo, M. Katahira and T. Watanabe, Biotechnol. Biofuels, 2020, 13, 164 CrossRef CAS.
- F. Chen and R. A. Dixon, Nat. Biotechnol., 2007, 25, 759–761 CrossRef CAS.
- Y. Pu, F. Chen, A. Ziebell, B. Davison and A. Ragauskas, Bioenergy Res., 2009, 2, 198–208 CrossRef.
- M. Baucher, M. Bernard-Vailhé, B. Chabbert, J. Besle, C. Opsomer, M. Montagu and J. Botterman, Plant Mol. Biol., 1999, 39, 437–447 CrossRef CAS.
- C. Fu, X. Xiao, Y. Xi, Y. Ge, F. Chen, J. Bouton, R. Dixon and Z. Wang, Bioenergy Res., 2011, 4, 153–164 CrossRef.
- Q. Yang and X. Pan, Biotechnol. Bioeng., 2016, 113, 1213–1223 CrossRef CAS.
- Y. Tian, Y. Jiang and S. Ou, Food Chem., 2013, 138, 1022–1027 CrossRef CAS.
- M. Michelin, E. Ximenes, M. de Moraes and M. R. Ladisch, Bioresour. Technol., 2016, 199, 275–278 CrossRef CAS.
- C. Divne, J. Ståhlberg, T. Teeri and T. Jones, J. Mol. Biol., 1998, 275, 309–325 CrossRef CAS.
Footnote |
| † The two authors contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2021 |