Chocolate as a food matrix reduces the bioavailability of galloylated catechins from green tea in healthy women
Rie
Mukai
 *ab,
Takashi
Fukuda
a,
Asami
Ohnishi
a,
Takeshi
Nikawa
*ab,
Takashi
Fukuda
a,
Asami
Ohnishi
a,
Takeshi
Nikawa
 c,
Mutsuki
Furusawa
a and
Junji
Terao
c,
Mutsuki
Furusawa
a and
Junji
Terao
 a
a
aDepartment of Food Science, Graduate School of Biomedical Sciences, Tokushima University, Tokushima, Japan
bDepartment of Food Science, Graduate School of Technology, Industrial and Social Sciences, Tokushima University, 2-1, Minamijosan-jima, Tokushima 770-8513, Japan. E-mail: rmukai@tokushima-.ac.jp; Fax: +81-88-656-8029; Tel: +81-88-656-9917
cDepartment of Nutritional Physiology, Institute of Medical Nutrition, Tokushima University Graduate School, Tokushima, Japan
First published on 9th December 2020
Abstract
In this study, we evaluated the food matrix effects of chocolate on the absorption of green tea catechins (GTCs), (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECg), and (−)-epigallocatechin gallate (EGCg), in five healthy 22-year-old women. In the single-intake experiment, the plasma concentrations of ECg (P < 0.05, at 1.5 h) and EGCg (P < 0.05, at 6 h) but not those of EC and EGC were reduced by the chocolate matrix. Regardless of the chocolate matrix, ECg and EGCg were mainly present as their aglycones in the plasma, whereas EGC and EC were found mostly as conjugated metabolites. After daily intake of GTCs mixed with chocolate for 14 days followed by overnight fasting, ECg but not EGCg was detected in the plasma. To compare the plasma profiles of ECg and EGCg, a mixture containing approximately equal amounts of ECg and EGCg was administered to nine rats for 14 days. Following treatment and overnight food deprivation, the plasma content of ECg was higher than that of EGCg. After a single injection of the same mixture in seven rats, ECg levels were higher than those of EGCg, and a greater amount of conjugated metabolites of ECg than those of EGCg was detected in the plasma 10 h after administration. In conclusion, the chocolate matrix affects the plasma profiles of GTCs, particularly ECg. ECg appears to persist in the plasma for a longer period, regardless of the chocolate matrix.
Introduction
Catechins in green tea exert anti-inflammatory and anti-obesity effects in humans through their antioxidative action.1–3 The four major green tea catechins (GTCs) are (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECg), and (−)-epigallocatechin gallate (EGCg) (Fig. 1).4 EGCg, which has the most potent antioxidant potential, possesses numerous health-promoting properties, such as anti-obesity and anti-cardiovascular-disease1,3 properties, whereas EC and ECg exert cardioprotective effects.5–8 | ||
| Fig. 1 Chemical structures of the catechins assessed in this study. EC: (−)-epicatechin; EGC: (−)-epigallocatechin; ECg: (−)-epicatechin gallate; EGCg: (−)-epigallocatechin gallate. | ||
The bioavailability of GTCs has been demonstrated in animal and human studies. After being consumed, GTCs undergo conjugation or methylation in the intestinal mucosa and liver,9 with their plasma concentrations peaking within 90 to 180 min. These metabolites pass into enterohepatic circulation.10,11 In addition, GTCs reaching the large intestine are converted into several species of organic acids by the gut microflora.11 Pharmacokinetic studies have found that all catechin components are cleared from the plasma within 24 h and excreted in the urine within 48 h of consumption.10,12 Thus, to experience the beneficial effects of GTCs, it may be necessary to ingest them regularly. GTCs are mainly ingested by beverages, but since the stability of GTCs in liquids is reported as low,13 we considered using solid foods as the food matrix to GTCs. If it could be ingested GTCs in food other than beverages, more people can achieve the health benefits of catechins. For example, it can be supplied for elderly people with chronic disease at risk of aspiration, and astronauts who exposing oxidative stress during space flight because of the portability.14 Chocolate is eaten all over the world, and since it can suppress the bitterness of catechins, it was applied as the food matrix in the current study. In addition, since chocolate contains EC oligomers (procyanidins) that would affect the bioavailability of GTEs, in this study, we examined the effect of cacao polyphenols on the bioavailability of GTCs.
We aimed to investigate the impact of chocolate as a food matrix on the bioavailability of GTCs; in particular, plasma profiles reflecting conjugation metabolism and dynamic changes to GTC plasma concentrations were assessed.
Experimental
Materials
The GTC mixture was obtained from Taiyo Kagaku (Sunphenon BG-5 for humans and Sunphenon 20 ECg-OP for rats; Mie, Japan). The weight percentages of each catechin in the mixture were as follows: Sunphenon BG-5 (quantified by our laboratory): EC (9.1%), EGC (28.7%), ECg (2.9%), EGCg (27.0%), and other catechins (5.2%); Sunphenon 20ECg-OP (quantified by Taiyo Kagaku): EC (3.6%), EGC (2.3%), ECg (33.8%), EGCg (27.9%), and other catechins (4.4%). Milk chocolate and milk chocolate-mixed Sunphenon BG-5 were provided by LOTTE Co. Ltd (Tokyo, Japan). The milk chocolate contained 7.6% protein, 33.8% fat, and 56% carbohydrate. Ethyl gallate and ascorbic acid were purchased from Wako Chemicals (Osaka, Japan). β-Glucuronidase, EC, and EGCg were obtained from Sigma (St Louis, MO), while ECg and EGC were purchased from Kurita Kogyo Co. (Tokyo, Japan). All other reagents used in the study were commercially available and of analytical and high-performance liquid chromatography (HPLC) grade.Human study
This study was conducted according to the guidelines of the Declaration of Helsinki, and all procedures involving human subjects were approved by the ethical committee of Tokushima University (Tokushima, Japan; approval number 346). All subjects gave their informed verbal consent for inclusion before they participated in the study.The single-intake study (Exp. I) and continuous-intake study (Exp. II) were carried out separately.
Exp. I Healthy female volunteers aged 22 years (BMI = 16.2–20.2) were enrolled (n = 5). Three kinds of test meals were prepared: 375 mg of Sunphenon BG-5 (BG-5) in 200 mL of water, 20 g of chocolate (Cho), or 20 g of chocolate with 375 mg BG-5 (Cho + BG-5). The catechin composition of each test meal is listed in Table 1. All subjects took the same test meal at the same time. The experimental order was Cho + BG-5, Cho, and BG-5. The washout period between the test meal was 7–8 weeks. The protocol of Exp. I was described in a previous report.15 Briefly, all subjects were asked to avoid catechin-rich foods and drinks for one day before the experiment. After an overnight food fast, blood samples were collected into heparinised tubes just before test meal intake, then 1.5 and 6 h after consumption.
| BG-5 | BG-5 + Cho | Cho | |
|---|---|---|---|
| BG-5, Sunphenon BG-5: tea catechin mix for human experiments. Cho, chocolate | |||
| (−)-Epicatechin (EC) | 117 | 132 | 14 |
| (−)-Epicatechin gallate (ECg) | 25 | 25 | n.d. |
| (−)-Epigallocatechin (EGC) | 351 | 351 | n.d. |
| (−)-Epigallocatechin gallate (EGCg) | 221 | 221 | n.d. |
Exp. II Healthy female volunteers aged 22 years (BMI = 16.2–21.6) were enrolled (n = 5). Four of them were the same women as Exp. I, one of them was changed to another woman (there was no health problem, but personal reason). The study subjects ingested test meal once a day at 12:00 pm for 14 days. All subjects took the same test meal at the same time. The experimental order was Cho + BG-5, and Cho. The washout period between the test meal was 5 weeks. All subjects were instructed to consume their usual diet, except for catechin-rich foods and drinks, during the experimental period. Fasting blood samples were collected into heparinised tubes at day 0 (just before the first intake), 15 (24 h after final intake), and 22 (after a seven-day washout period) after overnight fasting. To control the baseline plasma levels of catechins, the same feeding restrictions as in Exp. I was applied the day before each blood collection.
The plasma was separated by centrifugation at 9000g for 10 min at 4 °C, then stored at −30 °C until analysis.
Animal experiments
All animal experiments were performed in accordance with the guidelines for the care and use of laboratory animals set by Tokushima University, Japan (approval number 12144). The protocol was approved by the Committee on Animal Experiments of Tokushima University. All surgeries were performed under anaesthesia, and efforts were made to minimise animal suffering.Male Wistar rats (Japan SLC) were housed in a room maintained at 23 ± 1 °C with a 12 h light–dark cycle. The animals were allowed free access to a commercial diet (AIN-93 M, Oriental Yeast Co., Ltd Tokyo, Japan) and water. Before test meal administration, they were deprived of food for 24 h but had free access to water. Nine rats (three 15-, 19-, and 27-week-old, respectively) were included in the daily injection study (Exp. III). The catechin composition of each test substance is listed in Table 2. Sunphenon 20 ECg-OP (ECg-OP) was dissolved in water and administered to the rats once a day for 14 days through a gastric feeding tube (100 mg per kg body weight). Blood samples were collected on day 0 (just before the first dose) and 14, following 24 h food deprivation. Seven rats (three eight- and four 19-week-old) were included in the single-injection study (Exp. IV). ECg-OP was dissolved in water and administered to the rats via a gastric feeding tube (200 mg per kg body weight). Blood samples were drawn just before injection, then at 1, 4, 8, 10, 18, and 24 h after injection. The plasma was separated by centrifugation at 9000g for 10 min at 4 °C, then stored at −30 °C until analysis.
| Experiment III (100 mg Sunphenon 20 ECg-OP per kg body weight) | Experiment IV (200 mg Sunphenon 20 ECg-OP per kg body weight) | |
|---|---|---|
| (−)-Epicatechin (EC) | 12.4 | 24.8 |
| (−)-Epicatechin gallate (ECg) | 76.4 | 152.8 |
| (−)-Epigallocatechin (EGC) | 7.5 | 15.0 |
| (−)-Epigallocatechin gallate (EGCg) | 60.9 | 121.8 |
Sample preparation for HPLC analysis
The sample preparation method was described previously.15 Total plasma concentration of each catechin (including aglycones and their conjugated metabolites) was determined using HPLC after deconjugation treatment. Plasma was incubated with β-glucuronidase (1 U μl−1; Sigma) in 0.1 M sodium acetate buffer (pH 5.0) and 50 mM ascorbic acid for 30 min. To measure the aglycone concentration, plasma without deconjugation treatment was analysed. Ethyl gallate was added to the samples as an internal standard. The liberated aglycones were extracted with ethyl acetate (Kanto Chemical Co., Inc., Tokyo, Japan) and evaporated using a centrifugal evaporator, then dissolved in the mobile phase (50 mM lithium acetate, 13% methanol, 2% acetic acid). Finally, the samples were injected into the HPLC detection system.HPLC analysis
The concentration of catechin in human plasma was determined using HPLC with an electrochemical detector at 300 mV application voltage (COULOCHEM III, Esa), equipped with a TSK-gel ODS-80Ts HPLC column (150 × 4.6 mm; Tosoh, Tokyo, Japan). The mobile phase was 50 mM lithium acetate, 13% methanol, and 2% acetic acid, and the flow rate was 0.9 mL min−1.The same detection system, equipped with a Cadenza CD-C18 column (75 × 4.6 mm; Imtakt, Kyoto, Japan) was used for rat plasma. The mobile phase was 1% acetic acid and acetonitrile (90/10, v/v) and the flow rate was 1.0 mL min−1.
Statistical analyses
Data are expressed as the mean ± standard error (SE). Differences between three or more groups were analysed using one-way analysis of variance with Bonferroni post hoc test for multiple comparisons, whereas differences between two groups were assessed using a two-sided Student's t-test. Differences with P < 0.05 were considered statistically significant, and the significance levels quoted were two-sided. All statistical analyses were performed using Excel Tokei Ver.7.0 for Windows (ESUMI Co., Ltd, Tokyo, Japan).Results and discussion
Plasma catechin concentration after a single intake or continuous intake of GTCs mixed with chocolate
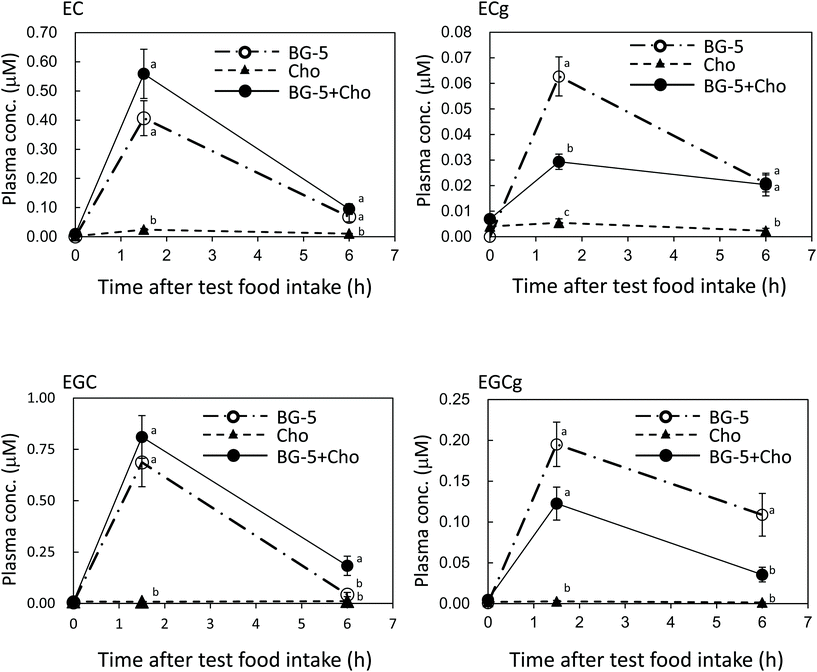
Changes in the plasma concentration of each catechin after a single test food intake are shown in Fig. 2. Catechins with a galloyl moiety (i.e., EGCg and ECg) were more poorly absorbed than those without a galloyl moiety (i.e., EC and EGC), with ECg displaying the lowest plasma levels of all measured catechins. At 1.5 h after test food intake, the plasma concentration of ECg was significantly lower in the BG-5 + Cho group than in the BG-5 group (P = 0.003) and remained fairly constant until the 6 h mark. Similarly, the plasma concentration of EGCg at 1.5 h was lower in women consuming BG-5 + Cho than in those consuming BG-5 alone; however, the difference was not statistically significant (P = 0.112). Unlike ECg, the plasma content of EGCg dropped between 1.5. and 6 h after the test food intake. Chocolate did not influence the plasma concentration of EC or EGC.In the single-intake experiment, the plasma concentrations of ECg and EGCg were lowered by mixing them with chocolate. The absorption of GTCs is affected by the food matrix.16–18 The bioavailability of EGCg and ECg, but not that of EC and EGC, is reduced by skim milk, caseinase, and soy protein.16 The proteins contained in these foods are suggested to impair the absorption of EGCg and ECg. The chocolate used in this study contained 7.6% protein, which may have inhibited intestinal absorption of ECg and EGCg. The fat content of chocolate (33.8% in the current study) also seems to affect the intestinal absorption of GTCs. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values (a measure of hydrophobicity) of EGC, EC, EGCg, and ECg were −0.50, +0.11, +0.39 and +1.06, respectively.19 The gallate moiety on the C ring increases the hydrophobicity of the molecule. It is assumed that the higher the hydrophobicity of the catechin, the slower its release from the chocolate matrix. Thus, EGC and EC, which are hydrophilic, may be more easily released from the food matrix and enter the digestive juice more readily than hydrophobic EGCg and ECg.
P values (a measure of hydrophobicity) of EGC, EC, EGCg, and ECg were −0.50, +0.11, +0.39 and +1.06, respectively.19 The gallate moiety on the C ring increases the hydrophobicity of the molecule. It is assumed that the higher the hydrophobicity of the catechin, the slower its release from the chocolate matrix. Thus, EGC and EC, which are hydrophilic, may be more easily released from the food matrix and enter the digestive juice more readily than hydrophobic EGCg and ECg.
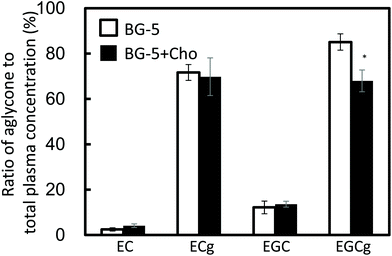
EGCg and ECg mostly existed as aglycones in human plasma (Fig. 3). The ratio of aglycone of EGCg to total EGCg was significantly lower after BG-5 + Cho consumption than after BG-5 consumption (P = 0.033).
According to previous reports, GTCs with a galloyl moiety at the 3-position mostly exist as aglycones in the plasma.20,21 Our results obtained 1.5 h after test food intake may reflect conjugation metabolism in enterocytes and hepatocytes (Fig. 2). Chocolate increased the levels of conjugation metabolites of EGCg (Fig. 3). To our knowledge, this is the first report to demonstrate that food components can enhance the conjugation metabolism of ingested GTCs.
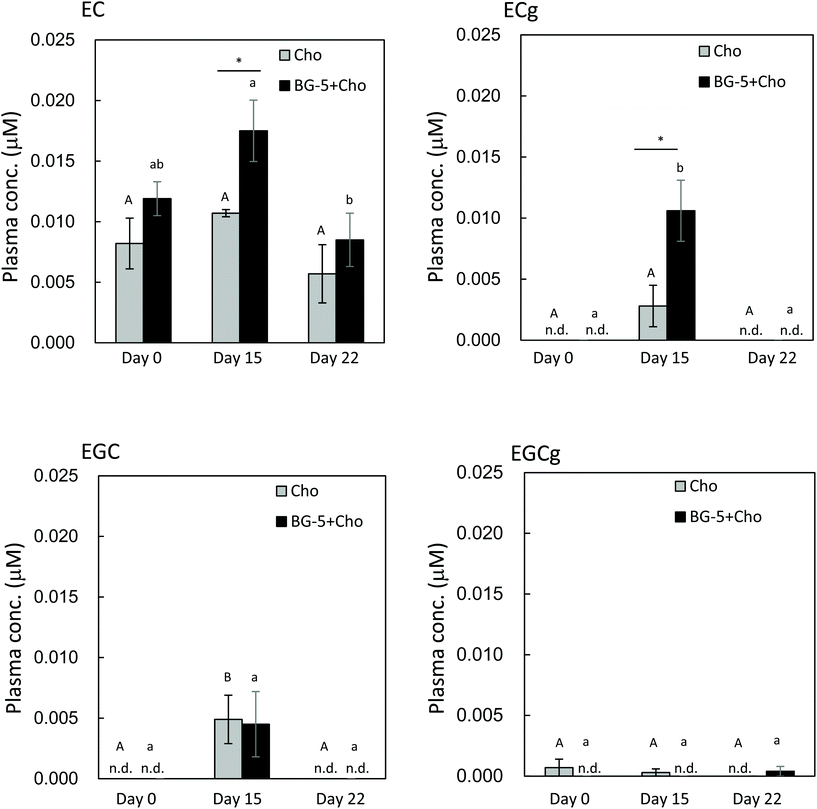
After 14 days of daily test food intake, the plasma concentration of ECg was increased in women consuming BG-5 + Cho, and it was significantly higher than in those consuming chocolate alone (P = 0.034) (Fig. 4). EC present in chocolate increased plasma EC levels in the Cho group. However, since the EC content of BG5 was 10 times higher than that of chocolate, the plasma EC concentration was higher in the Cho + BG5 group than in the Cho group on day 15. Chocolate had no effect on the plasma concentration of EGC. The plasma content of EGCg was markedly lower than that of ECg, and it did not change significantly during the study period. Plasma levels of all catechins returned to baseline after a seven-day washout period (day 22).
The results of our human experiment imply that excessive accumulation of ECg resulting from daily intake can be avoided because plasma ECg levels returned to baseline after a seven-day washout period (day 22). Our data provide evidence that daily consumption of GTCs is required to achieve the desired health-promoting effects, such as anti-atherosclerotic,8 antioxidative,22,23 and nephroprotective24 effects. As reported by Kawai et al.8 ECg was detected in foamy macrophages in human atherosclerotic aorta by using specific antibodies to ECg, where it was shown to inhibit the expression of CD36, a class B scavenger receptor implicated in the development of atherosclerosis. Maintenance of an appropriate level of ECg in the blood through daily consumption of GTCs could facilitate ECg transport to atherosclerotic lesions.
The results of our single-intake experiment in women showed that 1.5 h after test food consumption, the maximum plasma levels of ECg and EGCg were lowered by chocolate, whereas those of EC and EGC were comparable between the BG-5 and BG-5 + Cho groups. In contrast, after 14 days of daily test food intake, the plasma concentration of ECg was higher in women ingesting BG-5 + Cho than in those consuming BG-5 alone. These data suggest that chocolate delays intestinal absorption of ECg after a single intake, without reducing the total amount of absorbed ECg. Although the plasma content of ECg was much lower than that of EGCg at 1.5 h after a single BG-5 intake (Fig. 2), after 14 days of daily BG-5 intake followed by an 18 h fast, ECg concentration exceeded that of EGCg (Fig. 4). These data suggest that ECg could be a preferred food factor that exerts beneficial effects on vascular endothelium function upon daily intake of tea catechins. Previous studies on catechin bioavailability demonstrated that the clearance of ingested ECg from plasma is slower than that of EGC and EGCg.9,22 In the study by Van Amelsvoort et al., the plasma concentrations of EGC and EGCg returned to basal levels within 24 h of test meal ingestion, whereas that of ECg remained.22 Therefore, ECg may be eliminated more slowly and persist in the blood for a longer period than EGCg.
Approximately 15% of the polyphenols in chocolate are procyanidins, such as procyanidin B2 [epicatechin-(4β-8)-epicatechin], procyanidin C1 (trimer), and cinnamtannin A2 (tetramer).25 The chocolate used in our clinical investigation contained 144 mg polyphenols per 20 g (determined using the Folin-Ciocalteu method; data not shown), thus, it was estimated to contain approximately 20 mg procyanidins per 20 g. Among them, procyanidin B2 is partly absorbed as EC and its conjugated/methylated metabolites.26,27 Therefore, EC in the plasma of women consuming chocolate-mixed BG-5 or chocolate alone may partly originate from procyanidins, such as procyanidin B2. However, the impact of procyanidins on the absorption of monomeric catechins is not yet fully understood. Further studies are required to clarify the interactions between catechins and procyanidins, and the effects of procyanidins on GTC absorption when chocolate is used as the food matrix.
In these clinical studies, it is necessary to monitor the ingestion during the clinical test, so we had to employ students who belonged to the university as volunteers. This was why only 22-year-old women were selected as the study subjects, and it was the limit of this study. However, the effect of chocolate on the bioavailability of GTCs was properly analyzed because all subjects ingested their respective test meals reliably. In the future study, it will be necessary to employ subjects with different genders, ages, races, health conditions, and eating habits, and to increase the number of subjects to clarify the food matrix effect of chocolate.
Pharmacokinetic properties of ECg and EGCg
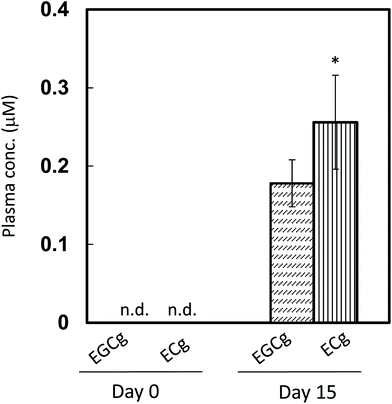
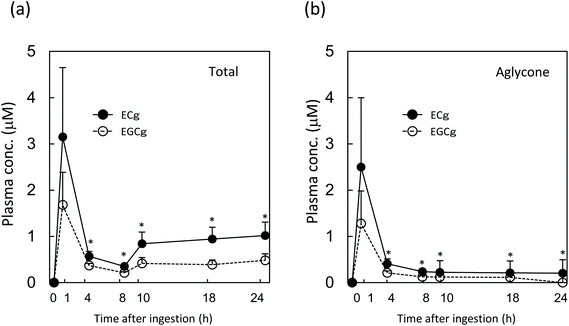
The pharmacokinetic properties of ECg and EGCg were assessed in rats to study how the two catechins accumulate in the plasma. After 14 days of ECg-OP ingestion, both ECg and EGCg accumulated in the plasma, and the level of ECg was significantly higher than that of EGCg (Fig. 5). These results were in accordance with those of the human study.After a single gastric injection of ECg-OP, the maximum plasma concentration of total ECg (conjugates and aglycones combined) was higher than that of total EGCg (Fig. 6). The aglycone data followed the same trend until 8 h after ingestion. The concentration of ECg was significantly higher than that of EGCg between 4 and 24 h after injection. From 10 h after injection onwards, the plasma content of total ECg and total EGCg increased, whereas that of their respective aglycones decreased over time.
The plasma concentration of total ECg and EGCg peaked at 1.5 h after test substance injection, before steadily decreasing until the 4 h mark. Between 4 and 8 h after ingestion, both ECg and EGCg entered various peripheral tissues, such as the liver, re-entered the duodenum via enterohepatic circulation, or were eliminated by the kidneys. From 10 h after ingestion onwards, the plasma level of total ECg (aglycone and conjugated metabolites combined) but not that of the aglycone of ECg increased again, and its concentration was significantly higher than that of EGCg. Therefore, most ECg was conjugated metabolites, which were likely reabsorbed via enterohepatic circulation and/or re-entered circulation from peripheral tissues. During the latter process, ECg may undergo conjugation metabolism several times, and the metabolite profile of ECg present in blood may become more complex, according to previous studies.9 In contrast, the EGCg level did not change appreciably after the 10 h mark. ECg in the body was responsible for its elevated plasma levels after 14 days of daily GTC intake in women. ECg seems to undergo more efficient enterohepatic recycling than EGCg, which likely underlies its longer persistence in the plasma during regular intake.In addition, chocolate may influence the plasma ECg profile of humans.
To avoid stress from blood sampling several times in humans, rats were applied to a time-course study to confirm the longer persistence of ECg in the blood. Comparing the metabolite profile of tea catechins between rats and humans, glucuronides mainly existed in rat plasma, however not only glucuronides but also sulfates existed in human plasma.28,29 In this study, the conjugated metabolites were hydrolysed by the deconjugation reaction, and then the total concentration (subtotal of aglycone and conjugated metabolites) were mainly analyzed. Therefore, it was not possible to confirm the effect of the difference in metabolite profile on the plasma concentration of each catechin. In the pharmacokinetics study of tea catechins, common results are known to be obtained from both rats and humans. For example, in both cases, the maximum concentration of ingested catechins appears in 1–2 hours, disappears from the blood in about 24 hours, and most of the gallate-type catechins existed as aglycones.9,12,30,31 Therefore, the plasma concentration of each catechin in this study can be extrapolated pharmacokinetics appropriately.
Conclusions
This study showed that the absorption of catechins with a gallate moiety (e.g., ECg and EGCg) is reduced by the chocolate matrix following a single intake of GTCs in women. However, daily intake of chocolate-mixed GTCs increased the plasma concentration of ECg but not of EGCg. Regardless of the chocolate matrix, ECg persisted for a longer period in the plasma than EGCg after continuous GTC intake.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Prof. Yasuo Tsutsumi (Tokushima University Graduate School) provided experimental support. The test meals (chocolate and chocolate-mixed GTCs) were kindly gifted by Mr Hiroaki Ashitani and Ms Yoko Usamikrank of LOTTE Co., Ltd, Central Laboratory.This study was funded by the Commission of Space Science and Technology Promotion, MEXT (2013) (J. T. and T. N.), and LOTTE Co., Ltd (Tokyo, Japan, 2012-2014) (J. T. and T. N.). These financial supports did not have a grant number. All funders played no role in the design, analysis, or writing of this article.
References
- S. Wang, N. Moustaid-Moussa, L. Chen, H. Mo, A. Shastri, R. Su, P. Bapat, I. Kwun and C.-L. Shen, Novel insights of dietary polyphenols and obesity, J. Nutr. Biochem., 2014, 25, 1–18 CrossRef CAS.
- T. Ohishi, S. Goto, P. Monira, M. Isemura and Y. Nakamura, Anti-inflammatory Action of Green Tea, Antiinflamm. Antiallergy Agents Med. Chem., 2016, 15, 74–90 CrossRef CAS.
- Q. Y. Eng, P. V. Thanikachalam and S. Ramamurthy, Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases, J. Ethnopharmacol., 2018, 210, 296–310 CrossRef CAS.
- P. Svoboda, H. Vlčková and L. Nováková, Development and validation of UHPLC-MS/MS method for determination of eight naturally occurring catechin derivatives in various tea samples and the role of matrix effects, J. Pharm. Biomed. Anal., 2015, 114, 62–70 CrossRef CAS.
- I. Bernatova, Biological activities of (-)-epicatechin and (-)-epicatechin-containing foods: Focus on cardiovascular and neuropsychological health, Biotechnol. Adv., 2018, 36, 666–681 CrossRef CAS.
- J. Terao, M. Piskula and Q. Yao, Protective effect of epicatechin, epicatechin gallate, and quercetin on lipid peroxidation in phospholipid bilayers, Arch. Biochem. Biophys., 1994, 308, 278–284 CrossRef CAS.
- J.-H. Chen, M.-S. Lee, C.-P. Wang, C.-C. Hsu and H.-H. Lin, Autophagic effects of Hibiscus sabdariffa leaf polyphenols and epicatechin gallate (ECG) against oxidized LDL-induced injury of human endothelial cells, Eur. J. Nutr., 2017, 56, 1963–1981 CrossRef CAS.
- Y. Kawai, H. Tanaka, K. Murota, M. Naito and J. Terao, (-)-Epicatechin gallate accumulates in foamy macrophages in human atherosclerotic aorta: implication in the anti-atherosclerotic actions of tea catechins, Biochem. Biophys. Res. Commun., 2008, 374, 527–532 CrossRef CAS.
- M. N. Clifford, J. J. J. van der Hooft and A. Crozier, Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols, Am. J. Clin. Nutr., 2013, 98, 1619S–1630S CrossRef CAS.
- G. Borges, J. I. Ottaviani, J. J. J. van der Hooft, H. Schroeter and A. Crozier, Absorption, metabolism, distribution and excretion of (-)-epicatechin: A review of recent findings, Mol. Aspects Med., 2018, 61, 18–30 CrossRef CAS.
- A. Scalbert and G. Williamson, Dietary intake and bioavailability of polyphenols, J. Nutr., 2000, 130, 2073S–2085S CrossRef CAS.
- U. Ullmann, J. Haller, J. P. Decourt, N. Girault, J. Girault, A. S. Richard-Caudron, B. Pineau and P. Weber, A single ascending dose study of epigallocatechin gallate in healthy volunteers, J. Int. Med. Res., 2003, 31, 88–101 CrossRef CAS.
- O. Krupkova, S. J. Ferguson and K. Wuertz-Kozak, , Stability of (-)-epigallocatechin gallate and its activity in liquid formulations and delivery systems, J. Nutr. Biochem., 2016, 37, 1–12 CrossRef CAS.
- J. G. Steller, J. R. Alberts and A. E. Ronca, Oxidative Stress as Cause, Consequence, or Biomarker of Altered Female Reproduction and Development in the Space Environment, Int. J. Mol. Sci., 2018, 19, 3729–3754 CrossRef.
- K. Murota, N. Matsuda, Y. Kashino, Y. Fujikura, T. Nakamura, Y. Kato, R. Shimizu, S. Okuyama, H. Tanaka, T. Koda, K. Sekido and J. Terao, alpha-Oligoglucosylation of a sugar moiety enhances the bioavailability of quercetin glucosides in humans, Arch. Biochem. Biophys., 2010, 501, 91–97 CrossRef CAS.
- S. Egert, J. Tereszczuk, S. Wein, M. J. Muller, J. Frank, G. Rimbach and S. Wolffram, Simultaneous ingestion of dietary proteins reduces the bioavailability of galloylated catechins from green tea in humans, Eur. J. Nutr., 2013, 52, 281–288 CrossRef CAS.
- N. Naumovski, B. L. Blades and P. D. Roach, Food Inhibits the Oral Bioavailability of the Major Green Tea Antioxidant Epigallocatechin Gallate in Humans, Antioxidants, 2015, 4, 373–393 CrossRef CAS.
- K. H. van het Hof, G. A. Kivits, J. A. Weststrate and L. B. Tijburg, Bioavailability of catechins from tea: the effect of milk, Eur. J. Clin. Nutr., 1998, 52, 356–359 CrossRef CAS.
- A. Shoji, A. Yanagida, H. Shindo and Y. Shibusawa, Comparison of elution behavior of catechins in high-performance liquid chromatography with that on high-speed countercurrent chromatography, Jpn. Anal., 2004, 53, 953–958 CrossRef CAS.
- S. Roowi, A. Stalmach, W. Mullen, M. E. J. Lean, C. A. Edwards and A. Crozier, Green tea flavan-3-ols: colonic degradation and urinary excretion of catabolites by humans, J. Agric. Food Chem., 2010, 58, 1296–1304 CrossRef CAS.
- M.-J. Lee, P. Maliakal, L. Chen, X. Meng, F. Y. Bondoc, S. Prabhu, G. Lambert, S. Mohr and C. S. Yang, Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability, Cancer Epidemiol. Biomarkers Prev., 2002, 11, 1025–1032 CAS.
- J. M. Van Amelsvoort, K. H. Van Hof, J. N. Mathot, T. P. Mulder, A. Wiersma and L. B. Tijburg, Plasma concentrations of individual tea catechins after a single oral dose in humans, Xenobiotica, 2001, 31, 891–901 CrossRef CAS.
- C.-C. Huang, W.-B. Wu, J.-Y. Fang, H.-S. Chiang, S.-K. Chen, B.-H. Chen, Y.-T. Chen and C.-F. Hung, (-)-Epicatechin-3-gallate, a green tea polyphenol is a potent agent against UVB-induced damage in HaCaT keratinocytes, Molecules, 2007, 12, 1845–1858 CrossRef CAS.
- S. Malik, K. Suchal, J. Bhatia, N. Gamad, A. K. Dinda, Y. K. Gupta and D. S. Arya, Molecular mechanisms underlying attenuation of cisplatin-induced acute kidney injury by epicatechin gallate, Lab. Invest., 2016, 96, 853–861 CrossRef CAS.
- M. Natsume, N. Osakabe, M. Yamagishi, T. Takizawa, T. Nakamura, H. Miyatake, T. Hatano and T. Yoshida, Analyses of polyphenols in cacao liquor, cocoa, and chocolate by normal-phase and reversed-phase HPLC, Biosci. Biotechnol. Biochem., 2000, 64, 2581–2587 CrossRef CAS.
- S. Baba, N. Osakabe, M. Natsume and J. Terao, Absorption and urinary excretion of procyanidin B2 [epicatechin-(4beta-8)-epicatechin] in rats, Free Radicals Biol. Med., 2002, 33, 142–148 CrossRef CAS.
- J. P. Spencer, H. Schroeter, B. Shenoy, S. K. Srai, E. S. Debnam and C. Rice-Evans, Epicatechin is the primary bioavailable form of the procyanidin dimers B2 and B5 after transfer across the small intestine, Biochem. Biophys. Res. Commun., 2001, 285, 588–593 CrossRef CAS.
- J. I. Ottaviani, G. Borges, T. Y. Momma, J. P. Spencer, C. L. Keen, A. Crozier and H. Schroeter, The metabolome of [2-(14)C](-)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives, Sci. Rep., 2016, 6, 29034 CrossRef CAS.
- G. Borges, J. J. J. van der Hooft and A. Crozier, A comprehensive evaluation of the [2-(14)C](-)-epicatechin metabolome in rats, Free Radicals Biol. Med., 2016, 99, 128–138 CrossRef CAS.
- A. Stalmach, S. Troufflard, M. Serafini and A. Crozier, Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans, Mol. Nutr. Food Res., 2009, 53(Suppl 1), S44–53 CrossRef.
- S. Baba, N. Osakabe, M. Natsume, Y. Muto, T. Takizawa and J. Terao, Absorption and urinary excretion of (-)-epicatechin after administration of different levels of cocoa powder or (-)-epicatechin in rats, J. Agric. Food Chem., 2001, 49, 6050–6056 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |