Discovery of hCES2A inhibitors from Glycyrrhiza inflata via combination of docking-based virtual screening and fluorescence-based inhibition assays†
Yun-Qing
Song‡
a,
Xiao-Qing
Guan‡
a,
Zi-Miao
Weng‡
b,
Jun-Ling
Liu
a,
Jing
Chen
a,
Lu
Wang
a,
Long-Tao
Cui
c,
Sheng-Quan
Fang
d,
Jie
Hou
 *b and
Guang-Bo
Ge
*b and
Guang-Bo
Ge
 *ad
*ad
aInstitute of Interdisciplinary Integrative Medicine Research, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, China. E-mail: geguangbo@dicp.ac.cn
bDepartment of Biotechnology, College of Basic Medical Sciences, Dalian Medical University, Dalian 116044, China. E-mail: houjie@dlmedu.edu.cn
cBasic Medical College, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, China
dYueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, 200473, China
First published on 18th November 2020
Abstract
Human carboxylesterase 2 (hCES2A) is a key target to ameliorate the intestinal toxicity triggered by irinotecan that causes severe diarrhea in 50%–80% of patients receiving this anticancer agent. Herbal medicines are frequently used for the prevention and treatment of the intestinal toxicity of irinotecan, but it is very hard to find strong hCES2A inhibitors from herbal medicines in an efficient way. Herein, an integrated strategy via combination of chemical profiling, docking-based virtual screening and fluorescence-based high-throughput inhibitor screening assays was utilized. Following the screening of a total of 73 herbal products, licorice (the dried root of Glycyrrhiza species) was found with the most potent hCES2A inhibition activity. Further investigation revealed that the chalcones and several flavonols in licorice displayed strong hCES2A inhibition activities, while isoliquiritigenin, echinatin, naringenin, gancaonin I and glycycoumarin exhibited moderate inhibition of hCES2A. Inhibition kinetic analysis demonstrated that licochalcone A, licochalcone C, licochalcone D and isolicoflavonol potently inhibited hCES2A-mediated fluorescein diacetate hydrolysis in a reversible and mixed inhibition manner, with Ki values less than 1.0 μM. Further investigations demonstrated that licochalcone C, the most potent hCES2A inhibitor identified from licorice, dose-dependently inhibited intracellular hCES2A in living HepG2 cells. In summary, this study proposed an integrated strategy to find hCES2A inhibitors from herbal medicines, and our findings suggested that the chalcones and isolicoflavonol in licorice were the key ingredients responsible for hCES2A inhibition, which would be very helpful to develop new herbal remedies or drugs for ameliorating hCES2A-associated drug toxicity.
1. Introduction
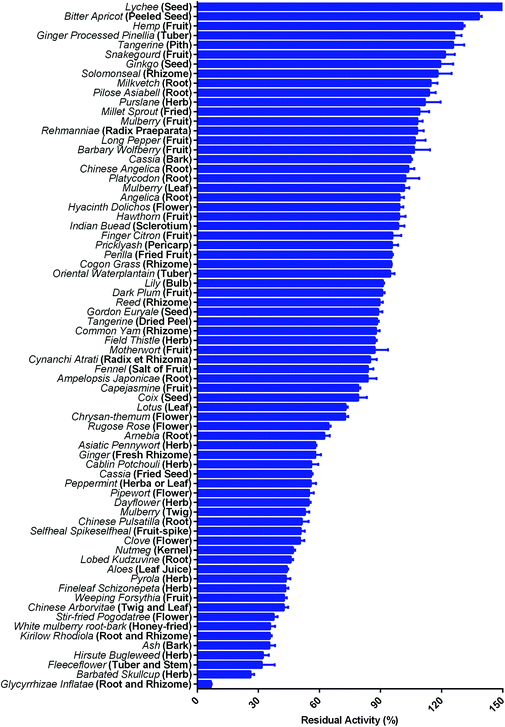
Mammalian carboxylesterases (CES), an important class of α/β-hydrolases, catalyze the hydrolysis of numerous endogenous substances and xenobiotic substrates bearing ester or amide bond(s).1–3 In humans, the majority of known CES fall into carboxylesterase 1 (hCES1A) and carboxylesterase 2 (hCES2A) subfamilies, and their substrate spectra and biological functions have been extensively studied.4,5 As one of the most abundant hydrolases distributed in the intestinal epithelial cells, hCES2A plays a crucial role in hydrolytic metabolism of a range of therapeutic agents bearing ester or amide bond(s), such as irinotecan, capecitabine and flutamide.6–8 Notably, intestinal hCES2A is the predominant contributor to hydrolyze irinotecan into its cytotoxic metabolite SN-38, which may induce severe delayed diarrhea when SN-38 is over-accumulated in the intestinal tract. Accumulating evidence has indicated that co-administration with an hCES2A inhibitor can ameliorate the intestinal toxicity of some important hCES2A-substrate drugs, such as irinotecan.9 At present, loperamide (LPA), a known anti-diarrhea agent with hCES2A inhibition activity, has been used to ameliorate the intestinal toxicity triggered by irinotecan.10,11 However, the moderate hCES2A inhibition potency and some side effects of LPA (such as cardiac dysrhythmia, nausea, drowsiness and headache) strongly limit the long-term use of this agent.12–14 Thus, it is desirable to find more efficacious hCES2A inhibitors as novel anti-diarrhea agents to ameliorate irinotecan-induced intestinal toxicity.Over the past decade, with the help of isoform-specific optical substrates for hCES isoenzymes,15 a variety of herbal constituents with diverse skeletons (such as flavonoids, triterpenoids and tanshinones) have been found with strong to moderate hCES2A inhibition activity,16–18 which inspired us to find more efficacious hCES2A inhibitors from herbal medicines. Recently, a large-scale screening campaign has been set up by us to assess the inhibition potential of commercially available herbal products against hCES2A. Following the screening of more than seventy concentrated herbal granules, licorice (the dried root of Glycyrrhiza species) granules have been found with the most potent hCES2A inhibition activity (Fig. 1), suggesting that some constituents in this herbal product may act as strong hCES2A inhibitors. As one of the oldest and most commonly used herbal products in both Western and Eastern countries, licorice is used to make sweets and add flavors in foods owing to its sweet taste and high safety profiles.19 Licorice is also the most frequently used material for preparing dietary supplements and herbal medicines, such as Huangqin-Tang, Naolejing oral liquid, compound liquorice tablets, mistura glycyrrhizae composita and Huoxiang Zhengqi tincture.20 Notably, licorice-containing herbal medicines (such as Huangqin-Tang and Banxia Xiexin decoction) have been used to ameliorate the intestinal toxicity triggered by irinotecan in clinical settings. However, the key ingredients in licorice responsible for hCES2A inhibition and their inhibitory mechanisms have not been revealed yet.
It is well known that licorice and licorice-containing herbal products are widely used as adjuvant therapeutics in cancer chemotherapy,21 indicating that licorice-related herbal products are more likely co-administered with irinotecan in the clinic. In these cases, it is necessary to investigate whether licorice constituents ameliorate irinotecan-induced intestinal toxicity via inhibition of hCES2A. Herein, an integrated strategy via combination of chemical profiling, docking-based virtual screening and fluorescence-based inhibition assays was used to identify the naturally occurring hCES2A inhibitors in this herbal product. Furthermore, both inhibition kinetic analyses and in silico investigations were performed to gain deeper insights into the inhibitory mechanisms of the identified hCES2A inhibitors. All of these studies will be very helpful for deciphering the key ingredients in licorice responsible for hCES2A inhibition and their inhibitory mechanisms, as well as be useful for the development of novel hCES2A inhibitors to alleviate hCES2A-associated drug toxicity.
2. Materials and experimental
2.1 Chemicals and reagents
Seventy-two kinds of herbal granules were obtained from Tianjiang Pharmaceutical Co., Ltd (Jiangsu, China). The seventeen constituents isolated from licorice including isoliquiritigenin (ILG), licochalcone A (LCA), echinatin (EC), naringenin (NG), licochalcone D (LCD), isolicoflavonol (ILF), licochalcone B (LCB), licoflavonol (LF), gancaonin I (GCI), glycycoumarin (GCM), neoliquiritin (NL), liquiritin apioside (LA), liquiritin (LQ), licochalcone C (LCC), liquiritigenin (LG), formononetin (FMNT) and glycyrrhizic acid (GA) were purchased from Chengdu Pufei Biotech Co., Ltd (Chengdu, China). The chemical structures of fifty-three compounds identified from licorice are shown in Table 1. Loperamide (LPA) and fluorescein diacetate (FD) were purchased from TCI (Shanghai, China). The purities of all compounds used in this study were higher than 98%. Human liver microsomes (HLM, Lot No. X008067) were used as the enzyme source for hCES inhibition assays, while HepG2 cells were used to validate the inhibition potency of LCC against intracellular hCES2A. The ethics statement of HLM and the gene test report of HepG2 cells are presented in S1 and S2.† LC grade DMSO, methanol and acetonitrile were obtained from Tedia (Fairfield, OH, USA).| No. | Formula | Identification | Structure | tR (min) | [M − H]− (m/z) | MS/MS spectra | Ref. |
|---|---|---|---|---|---|---|---|
| a These compounds are validated using authentic standards. | |||||||
| 1 | C32H40O18 | Glucoliquiritin apioside |
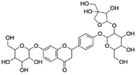
|
2.05 | 711.2153 | 549.1605, 429.1281, 255.0661, 135.0083 | 45 |
| 2 | C26H28O14 | Schaftoside |
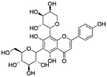
|
3.76 | 563.1440 | 545.1295, 503.1194, 473.1082, 443.0979, 383.0769, 353.0665 | 45 |
| 3 | C27H32O14 | Glucoliquiritin |
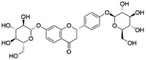
|
4.06 | 579.1716 | 506.0005, 417.1183, 255.0662, 135.0085, 119.0500 | 37 |
| 4 | C21H22O9 | Neoliquiritina |
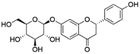
|
5.51 | 417.1184 | 255.0678, 241.0747, 152.0117, 135.0090, 119.0505 | 39 |
| 5 | C27H32O14 | Glucoisoliquiritin |
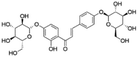
|
5.56 | 579.1716 | 506.0005, 417.1183, 255.0662, 135.0085, 119.0500 | 37 |
| 6 | C26H30O13 | Liquiritin apiosidea |
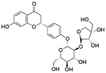
|
5.88 | 549.1610 | 429.1177, 357.0957, 255.0664, 135.0089, 119.0503 | 33 |
| 7 | C21H22O9 | Liquiritina |
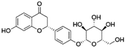
|
5.96 | 417.1185 | 255.0663, 254.0576, 213.0564, 135.0089 | 39 |
| 8 | C21H22O10 | 5-Hydroxyliquiritin |
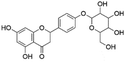
|
9.63 | 433.1138 | 271.0619, 177.0188, 151.0037, 119.0501 | 33 |
| 9 | C26H30O13 | Isoliquiritin apioside |
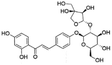
|
13.43 | 549.1618 | 429.1263, 399.1044, 255.0669, 135.0082, 119.0500 | 33 |
| 10 | C21H22O9 | Isoliquiritin |
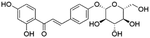
|
13.92 | 417.1192 | 255.0663, 254.0576, 213.0564, 135.0089 | 39 |
| 11 | C15H12O4 | Liquiritigenina |
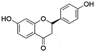
|
14.07 | 255.0667 | 135.0092, 119.0504, 117.0338 | 36 |
| 12 | C16H14O5 | Licochalcone Ba |
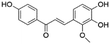
|
14.92 | 285.0406 | 285.0418, 270.0530, 269.0467, 229.0528 | 37 |
| 13 | C21H22O9 | Neoisoliquiritin |
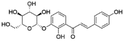
|
15.03 | 417.1186 | 297.0764, 269.0839, 255.0671, 237.0610, 148.0172 | 39 |
| 14 | C35H36O15 | Licorice glycoside B |
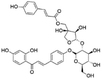
|
16.69 | 695.1995 | 549.1612, 531.1500, 399.1086, 255.0666, 135.0085 | 36 |
| 15 | C36H38O16 | Licorice glycoside A |
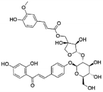
|
17.00 | 725.2103 | 549.1610, 531.1506, 255.0668, 399.1088, 135.0091 | 37 |
| 16 | C54H84O24 | Licorice saponin O4 |
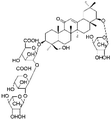
|
20.34 | 1115.5296 | 1116.5313, 793.4405, 616.3322, 497.1138, 339.0915 | 33 |
| 17 | C15H12O5 | Naringenina |
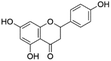
|
20.51 | 271.0614 | 253.0574, 227.0712, 151.0035, 119.0500, 107.0134 | 45 |
| 18 | C15H10O5 | Genistein |
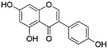
|
21.31 | 269.0821 | 241.0518, 223.0377, 213.0549, 133.0286, 105.0353 | 39 |
| 19 | C16H14O4 | Echinatina |
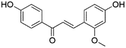
|
22.43 | 269.0821 | 269.0825, 253.0501, 254.0570, 237.0558, 161.0247, 133.0295, 120.0212 | 45 |
| 20 | C42H64O16 | Licorice saponin J2 |
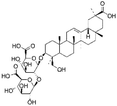
|
24.27 | 823.4136 | 824.4166, 761.4131, 647.3801, 351.0573, 333.0461, 289.0570, 193.0363 | 39 |
| 21 | C48H72O22 | 24-Hydroxy-licorice-saponin A3 |
|
25.79 | 999.4461 | 1000.4500, 837.3927, 819.3859, 661.3605, 497.1151 | 35 |
| 22 | C42H62O18 | 22-Hydroxy-licorice-saponin G2 |
|
26.15 | 853.3877 | 791.3867, 677.3556, 501.3215, 289.0569, 193.0344 | 35 and 38 |
| 23 | C15H12O4 | Isoliquiritigenina |
|
27.22 | 255.0664 | 135.0091, 119.0502, 117.0343 | 36 |
| 24 | C48H74O19 | Licorice saponin M3 |
|
27.43 | 953.0000 | 891.4772, 777.4400, 351.0568, 289.0575 | 33 |
| 25 | C16H12O4 | Formononetina |
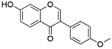
|
27.61 | 267.0662 | 252.0434, 208.0529, 195.0452, 180.0579, 167.0490, 132.0216, | 39 |
| 26 | C44H64O18 | 22β-Acetoxylglycyrrhizic acid |
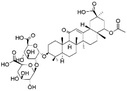
|
29.20 | 879.4028 | 861.3891, 817.4073, 703.3718, 527.3408, 351.0546, 289.0552 | 36 |
| 27 | C42H62O17 | 23-Hydroxyl licorice saponin E 2 |
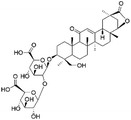
|
29.29 | 837.3931 | 775.3928, 661.3601, 485.3267, 351.0568 | 45 |
| 28 | C42H62O17 | Licorice saponin G 2 |
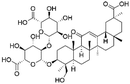
|
32.34 | 837.3928 | 837.3199, 775.3919, 661.3579, 485.3161, 351.0562 | 45 |
| 29 | C44H66O18 | 22β-Acetoxyl licorice saponin J 2 |
|
33.02 | 879.4028 | 861.3891, 817.4073, 703.3718, 527.3408, 351.0546, 289.0552 | 36 |
| 30 | C42H62O16 | Glycyrrhizic acida |
|
34.62 | 821.3979 | 351.0565, 193.0355, 113.0241 | 39 |
| 31 | C48H72O21 | Licorice saponin A3 |
|
34.62 | 983.4470 | 821.3973, 759.3981, 645.3643, 469.3325, 351.0562 | 33 |
| 32 | C21H22O5 | Licochalcone Da |
|
35.16 | 353.1388 | 338.1185, 323.1016, 283.0672, 227.0705 | 37, 42 and 44 |
| 33 | C20H18O6 | Gancaonin L |
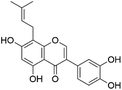
|
35.18 | 351.0871 | 352.0809, 321.0413, 283.0974, 205.0662 | 40 and 41 |
| 34 | C42H64O15 | Licorice saponin b2 |
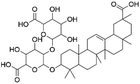
|
36.24 | 807.4175 | 745.4179, 631.3832 | 33 |
| 35 | C42H62O16 | Licorice saponin H2 |
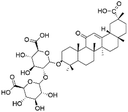
|
36.54 | 821.3982 | 759.3981, 583.3657, 351.0562, 193.0358 | 39 |
| 36 | C21H20O5 | Gancaonin M |
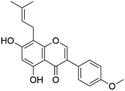
|
37.15 | 351.0870 | 335.0560, 321.0402, 297.0394 | 40 and 41 |
| 37 | C21H20O6 | Glycycoumarina |
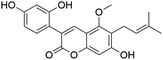
|
37.16 | 367.1183 | 337.0725, 309.0409, 297.0413, 281.0459, 253.0512 | 39, 43 and 45 |
| 38 | C20H20O6 | Licoagrodione |
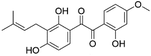
|
37.65 | 355.1544 | 323.1285, 283.0609, 254.0577, 233.1182 | 44 |
| 39 | C42H62O16 | Licorice saponin K2 |
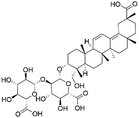
|
38.10 | 821.3979 | 645.3654, 351.0566, 193.0357, 113.0244 | 39 |
| 40 | C41H62O14 | Apioglycyrrhizin |
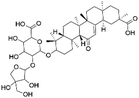
|
38.24 | 777.4077 | 778.4104, 715.4045, 627.3550, 583.3620, 537.3591, 469.3313 | 39 |
| 41 | C20H18O6 | Gancaonin C |
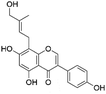
|
38.8 | 353.1025 | 353.1999, 323.0564, 297.0403, 284.0322 | 37, 42 and 44 |
| 42 | C21H22O4 | Licochalcone Ca |
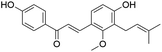
|
39.76 | 337.1077 | 279.0741, 243.1098, 159.0498 | 37 |
| 43 | C42H62O15 | Licorice saponin C2 |
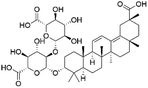
|
40.09 | 805.4024 | 787.3927, 743.4026, 629.3693, 351.0574 | 33 |
| 44 | C21H22O4 | Licochalcone Aa |
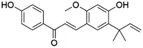
|
41.62 | 337.1077 | 293.0450, 281.0453, 253.0520, 268.0396 | 37 |
| 45 | C20H18O6 | Isolicoflavonola |
|
41.69 | 353.1386 | 338.1163, 295.0956, 283.0602, 267.0655 | 37, 42 and 44 |
| 46 | C20H18O6 | Licoflavonola |
|
41.73 | 353.1030 | 338.0808, 285.0329, 267.1027, 243.1033 | 37, 42 and 44 |
| 47 | C21H18O6 | Isoglycyrol |
|
41.88 | 365.1024 | 307.0253, 295.0251, 251.0352, 207.0453 | 39 |
| 48 | C36H54O10 | Glycyrrhetinic acid 3-O-glucuronide |
|
42.67 | 645.3644 | 583.3627, 569.3463, 523.3408, 497.3347, 469.3318, 425.3411, 113.0242 | 46 |
| 49 | C21H20O5 | Gancaonin G |
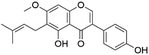
|
44.46 | 351.0872 | 335.0567, 319.0608, 283.0975, | 40 and 41 |
| 50 | C22H26O5 | Kanzonol R |
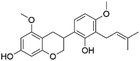
|
48.16 | 369.1702 | 339.1237, 311.0931, 279.0671, 217.0874, 135.0454 | 42 |
| 51 | C20H20O6 | Licofuranone |
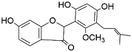
|
49.76 | 355.1545 | 323.1285, 233.1182, 135.0448, 203.0721 | 44 |
| 52 | C21H22O5 | Gancaonin Ia |
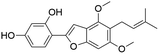
|
51.21 | 353.1377 | 338.1146, 323.0916, 295.0610, 267.0652 | 37, 42 and 44 |
| 53 | C30H47O4 | Glycyrrhetinic acid |
|
54.05 | 469.3316 | 467.8349, 425.3416, 409.3107, 355.2670 | 34 |
2.2 Chemical profiling of the constituents in licorice by LC-TOF-MS/MS
The chemical constituents in licorice were carefully identified using an UPLC system (Agilent, California, USA) coupled with a Triple TOF 4600 Mass Spectrometer system (AB SCIEX, Foster City, USA) equipped with an electrospray ionization (ESI) source. Mass detection was performed in both positive and negative ion modes with the MS parameters as follows: the m/z range was set at m/z 50 to m/z 1700, ion spray voltages were set at 5 kV/−4.5 kV, both ion source gas 1 and gas 2 were set at 50 psi, the curtain gas was set at 35 psi, the ion source temperature was set at 500 °C, the declustering potential was 100 eV and the collision energy was set at ±40 eV.2.3 Docking-based virtual screening
The structures of the fifty-three constituents in licorice were generated using Discovery Studio Visualizer (BIOVIA Discovery Studio 3.0, Dassault Systèmes, San Diego, USA), followed by the Prepare Ligands protocol to enumerate the isomers and canonical tautomer, generating 3D conformations. Homology modeling of hCES2A was performed using SWISS-MODEL to obtain its three-dimensional structure based on the crystal structure of hCES1A (PBD ID:1MX5), which was described in our previous work.16 The docking grid was generated centered at the catalytic triad (Ser228, Glu354, and His457). Next, docking-based virtual screening of the 53 compounds was conducted using precise docking modules in Discovery Studio 3.0 (including binding energy calculation and CDOCKER analyses).22,23 The binding energies were calculated using the method in the Scoring module based on the predicted ligand binding poses from LibDock.24 Then, the ligands with negative binding energies were further re-scored by CDOCKER analysis using the standard protocol.2.4 Enzyme inhibition assays
The procedure and details of hCES inhibition assays have been reported previously.25 In short, DME, a highly specific hCES1A substrate, was used to test the residual activities of hCES1A in the presence of each herbal extract or licorice ingredient and nevadensin as a positive control.26,27 To assay the inhibition potential of the herbal medicines or licorice ingredients against hCES2A, LPA was applied as the positive inhibitor while FD was used as a highly specific substrate.28,292.5 Inhibition kinetic analyses
The inhibition constant (Ki) and the inhibition mode of LCA, LCC, LCD and ILF against hCES2A were investigated by performing a series of kinetic assays for FD hydrolysis in the presence of increasing concentrations of each tested compound (LCA, LCC, LCD or ILF) in which four different concentrations of FD were used. As described in previous reports,27,30 the hydrolytic rates of hCES2A in the presence of LCA, LCC, LCD and ILF at increasing dosages were measured to draw the inhibition kinetic plots and to calculate the Ki value.2.6 Inhibition of hCES2A by LCC in living cells
The inhibition potential of LCC (the most potent chalcone-type hCES2A inhibitor identified from licorice) was investigated in living cells. Herein, NCEN was used as the isoform-specific fluorescent substrate for sensing the residual hCES2A activities in living HepG-2 cells, according to the previously reported procedure.31 In brief, increasing concentrations of LCC were added in 48-well plates with HepG-2 cells (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per 300 μL per well) and then incubated at 37 °C for 1 h. After that, NCEN (10 μM, final concentration) was added into 48-well plates and incubated at 37 °C for another 45 min. An equal volume of ice-cold acetonitrile was added to terminate the reaction. The medium were transferred to Eppendorf tubes and then centrifuged at 20
000 cells per 300 μL per well) and then incubated at 37 °C for 1 h. After that, NCEN (10 μM, final concentration) was added into 48-well plates and incubated at 37 °C for another 45 min. An equal volume of ice-cold acetonitrile was added to terminate the reaction. The medium were transferred to Eppendorf tubes and then centrifuged at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 20 min at 4 °C. Subsequently, the supernatant was taken for further LC-FD analysis. The detection conditions for the quantification of the hydrolytic metabolite of NCEN have been reported previously.32
000g for 20 min at 4 °C. Subsequently, the supernatant was taken for further LC-FD analysis. The detection conditions for the quantification of the hydrolytic metabolite of NCEN have been reported previously.32
2.7 Molecular dynamics simulations
Whole-protein molecular docking was performed using AutoDock vina 1.1.2 to identify the binding sites of LCC on the target enzymes (hCES2A) as described previously.30 The protein–ligand complexes with the lowest energy values were selected as the starting conformation for molecular dynamics simulations, using the Gromacs (Version 2016.5) software with the CHARMM36 all atom force field. The protein was solvated with SPC water in a cubic box. Sodium ions were added to neutralize the system charge. After that, the systems were energy minimized using the steepest descent method and then equilibrated under NVT followed by NPT conditions. The calculations were performed at 310 K for 50 ns. The integration step was set to 2 fs. The visualizations of the trajectory were carried out using PyMOL (Version 2.3) and the root-mean-square deviation values were calculated.2.8 Statistical analysis
All data are shown as mean ± SD of triplicate assays. GraphPad Prism 6.0 was used to determine the inhibition constants (including IC50 and Ki values).3. Results and discussion
3.1 Inhibitory effects of Chinese medicine granules on hCES2A
Firstly, the inhibition potential of seventy-two kinds of herbal granules against hCES2A was preliminarily assayed. As shown in Fig. 1, licorice granules (prepared from the extract from the dried root of Glycyrrhiza species) exhibited the most potent inhibitory effects on hCES2A-catalyzed FD hydrolysis at a dose of 250 μg mL−1. Next, the dose–inhibition curve of this herbal product against hCES2A was determined using increasing doses. As shown in Fig. S1,†Glycyrrhiza inflata dose-dependently inhibited hCES2A-mediated FD hydrolysis in HLM, with a calculated IC50 value of 44.64 μg mL−1. Meanwhile, the IC50 value of Glycyrrhiza inflata granules against hCES1A-catalyzed DME hydrolysis was also assayed in HLM, with a calculated IC50 value of 476.1 μg mL−1. These results demonstrated that Glycyrrhiza inflata displayed relatively strong inhibition of hCES2A (the most abundant intestinal hydrolase), while the inhibition potency against hCES1A (the most abundant hepatic hydrolase) by this herbal product is relatively weak, which inspired us to further identify the bioactive constituents in licorice responsible for hCES2A inhibition.3.2 Chemical profiling of the constituents in licorice by LC-TOF-MS/MS
To identify the naturally occurring hCES2A inhibitors from Glycyrrhiza inflata in a more efficient way, an integrated strategy via combination of chemical profiling, docking-based virtual screening and fluorescence-based inhibition assays was used in this study. Herein, the constituents in licorice were carefully characterized by LC-TOF-MS/MS, with the help of the commercially available standards and previously reported literature.33–46 The results are shown in Table 1 and Fig. S2.† A total of fifty-three constituents were identified from licorice granules (prepared from the extract from the dried root of Glycyrrhiza species) by LC-TOF-MS/MS, while nineteen constituents were confirmed by comparison of the retention times (tR) and UV and MS/MS spectra of the standards.3.3 Virtual screening of the fifty-three constituents in licorice
Next, docking-based virtual screening was used to predict the potential of the fifty-three constituents in licorice as strong hCES2A inhibitors. To assess the inhibition potential of these constituents in licorice against hCES2A, the predicted binding energy values and CDOCKER interaction energy values of the fifty-three ligands were calculated and are presented in Table S1.† Meanwhile, these parameters of the positive hCES2A inhibitor (LPA) on hCES2A were also calculated under some conditions. As shown in Table S1,† in contrast to LPA, a total of seventeen constituents displayed relatively low binding energy values and CDOCKER interaction energy values, implying that these seventeen constituents may stably bind with hCES2A. These observations encouraged us to test the inhibitory effects and to determine the IC50 values of these licorice constituents against hCES2A.3.4 Inhibition of hCES2A by licorice constituents
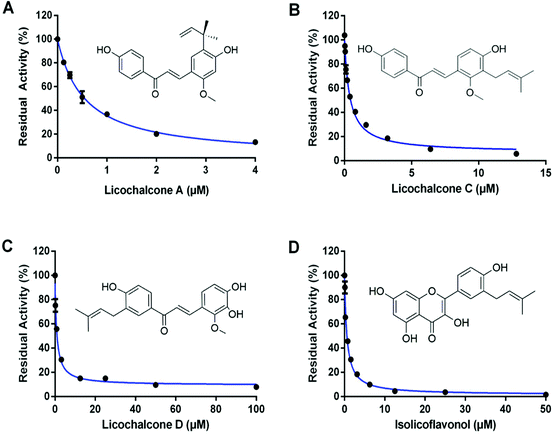
Subsequently, seventeen commercially available licorice constituents (including eleven constituents selected by docking-based virtual screening, along with another six abundant licorice constituents) were collected, while their hCES2A inhibition activities were routinely assayed using FD hydrolysis as the probe reaction. As shown in Fig. 2 and Table 2, the preliminary screening results demonstrated that the eleven constituents selected by docking-based virtual screening could dose-dependently inhibit hCES2A. By contrast, the six abundant licorice constituents that were not selected by docking-based virtual screening displayed very weak hCES2A inhibition activity. After that, the dose–response curves of these licorice constituents against hCES2A were obtained (Fig. 3 & Fig. S3†), while LPA was used as a positive inhibitor (a known hCES2A inhibitor). Among all the tested licorice constituents, LCA, LCC, LCD and ILF displayed the most potent inhibition of hCES2A (IC50 < 1 μM), while other seven licorice constituents isolated from licorice exhibited moderate hCES2A inhibition activities, with IC50 values ranging from 1.28 μM to 11.73 μM (Table 2). Meanwhile, the IC50 value of the positive inhibitor (LPA) against hCES2A was also determined as 1.22 μM (Fig. S3†). These results showed that LCA, LCC, LCD and IFL displayed potent inhibitory effects on hCES2A, which inspired us to further investigate their inhibition mechanisms against hCES2A.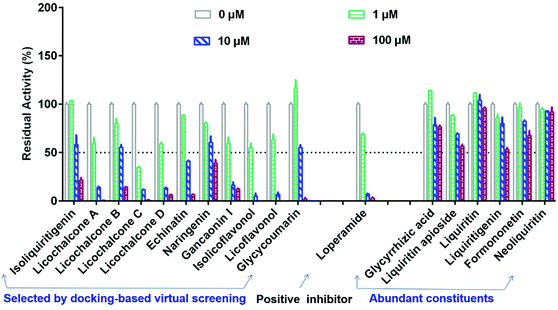 | ||
| Fig. 2 The residual activities of hCES2A upon addition of seventeen constituents from licorice and the positive inhibitor loperamide. All data are shown as mean ± SD of triplicate assays. | ||
| Class | No. | Compound name | CAS no. | MW | IC50 (μM) |
|---|---|---|---|---|---|
| Chalcones | 1 | Isoliquiritigenin (ILG) | 961-29-5 | 256.257 | 10.72 ± 1.60 |
| 2 | Licochalcone A (LCA) | 58749-22-7 | 338.403 | 0.54 ± 0.06 | |
| 3 | Licochalcone B (LCB) | 58749-23-8 | 286.283 | 11.73 ± 1.42 | |
| 4 | Licochalcone C (LCC) | 144506-14-9 | 338.403 | 0.39 ± 0.04 | |
| 5 | Licochalcone D (LCD) | 144506-15-0 | 354.402 | 0.94 ± 0.11 | |
| 6 | Echinatin (EC) | 34221-41-5 | 270.284 | 3.91 ± 0.31 | |
| 7 | Naringenin (NG) | 67604-48-2 | 272.256 | 10.75 ± 2.03 | |
| Flavonols | 8 | Isolicoflavonol (ILF) | 94805-83-1 | 354.11 | 0.60 ± 0.08 |
| 9 | Licoflavonol (LF) | 60197-60-6 | 354.35 | 1.28 ± 0.09 | |
| Isoflavones | 10 | Gancaonin I (GCI) | 126716-36-7 | 354.40 | 1.72 ± 0.24 |
| Coumarins | 11 | Glycycoumarin (GCM) | 94805-82-0 | 368.37 | 6.75 ± 0.88 |
| Positive control | — | Loperamide (LPA) | 34552-83-5 | 513.503 | 1.22 ± 0.09 |
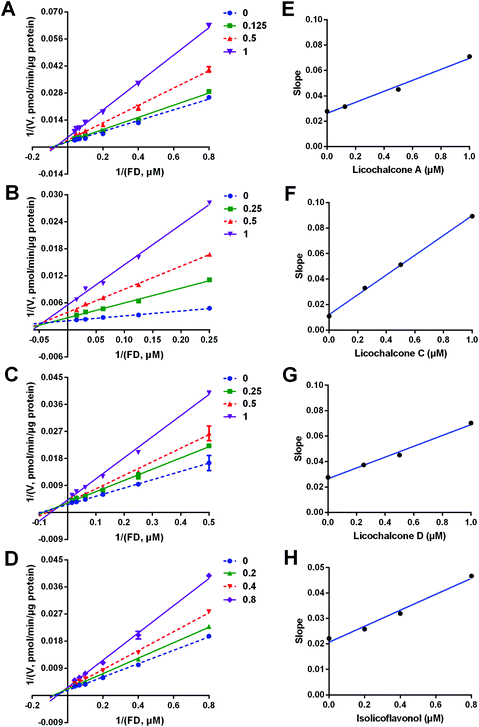
3.5 Inhibition kinetics of LCA, LCC, LCD and IFL against hCES2A
Next, the inhibition kinetics and the inhibition modes of LCA, LCC, LCD and IFL (the most potent hCES2A inhibitors in licorice) against hCES2A-mediated FD hydrolysis were carefully investigated in HLM. As illustrated in Fig. 4, S4, S5† and Table 3, the Lineweaver–Burk plots clearly showed that LCA, LCC, LCD and IFL could potently inhibit hCES2A-catalyzed FD hydrolysis in HLM in a mixed-inhibition manner. Meanwhile, the Ki values of these four natural compounds against hCES2A were also determined as 0.56 μM, 0.13 μM, 0.90 μM and 0.76 μM for LCA, LCC, LCD and IFL, respectively. These findings suggest that LCA, LCC, LCD and IFL are potent and reversible inhibitors against hCES2A-mediated FD hydrolysis, with Ki values less than 1 μM.| Enzyme source | Inhibitor | IC50 (μM) | K i (μM) | Inhibition modes | Goodness of fit (R2) |
|---|---|---|---|---|---|
| HLM | LCA | 0.54 ± 0.06 | 0.56 ± 0.08 | Mixed | 0.99 |
| LCC | 0.39 ± 0.04 | 0.13 ± 0.01 | Mixed | 0.99 | |
| LCD | 0.94 ± 0.11 | 0.90 ± 0.16 | Mixed | 0.99 | |
| ILF | 0.60 ± 0.08 | 0.76 ± 0.08 | Mixed | 0.99 |
3.6 Inhibition of intracellular hCES2A by LCC
As illustrated in Fig. S6,† LCC dose-dependently inhibited intracellular hCES2A in living cells, with an estimated IC50 value of 1.04 μM. This inhibition potency was even more stronger than that of LPA against hCES2A in HLM.47,48 This finding demonstrated that LCC was a cell-permeable natural compound and could strongly inhibit the hydrolytic activity of intracellular hCES2A in living cells, which can be used as a promising lead compound to develop more efficacious hCES2A inhibitors for alleviating hCES2A-associated drug toxicity.3.7 Molecular dynamics simulations of LCC in hCES2A
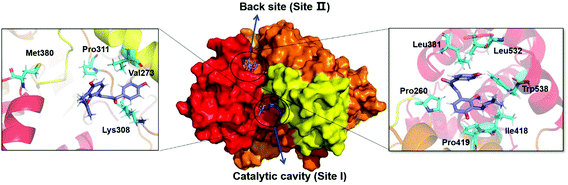
To better understand the interactions between LCC and hCES2A at the molecular level, both docking simulations and molecular dynamics simulations were carefully performed. As shown in Table S2,† the binding affinity of LCC docked with hCES2A was −8.2 kcal mol−1 and −8.0 kcal mol−1 on two distinct ligand-binding sites of hCES2A (Fig. 5). Compared to the substrate FD, one was located in the catalytic cavity (site I) which acted as a competitive inhibitor, and the other one (back site, site II) acted as a non-competitive inhibitor. Then we conducted molecular dynamics simulations to study its binding stability; the results demonstrated that LCC could stably bind at both the catalytic cavity (site I) and the back site (site II) of hCES2A during 50 ns simulations (Fig. 6). Equilibrium complex structures are shown in Fig. 5; the hydrophobic interactions contributed the most in binding, especially for Met 380, Pro311, Val273 and Lys308 of the activity site and Leu381, Leu532, Trp538, Pro260, Pro419 and Ile418 of the back site. These results and observations were highly consistent with the experimental findings, in which LCC was found to be a potent inhibitor against hCES2A-mediated FD hydrolysis in a mixed-inhibition manner.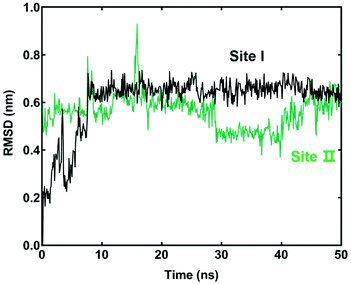 | ||
| Fig. 6 Root mean square deviation (RMSD) analysis of the ligand (licochalcone C) bound with hCES2A at the catalytic cavity (site I) or the back site (site II). | ||
As one of the most popular used herbal materials, licorice extract has been widely used for preparing dietary supplements and herbal medicines in both Eastern and Western countries. Licorice has been listed in Chinese Pharmacopoeia and the List of Herbal Materials that can be used for health foods in accordance with the law on food hygiene by the China Food and Drug Administration, and the daily recommended dose is up to 60 g day−1.49 In China and other Asian nations, licorice appears in more than half of herbal formulas or Chinese medicine prescriptions, including some famous and commonly used prescriptions, such as Huoxiang Zhengqi tincture, Gegen-Qinlian decoction (GQD) and Huangqin-Tang (PHY906). Notably, licorice is the major material for preparing Huangqin-Tang, one of the most frequently used Chinese herbal prescriptions, which has been reported to be capable of ameliorating the gastrointestinal toxicity induced by CPT-11.21 Lam et al. have reported that Huangqin-Tang could counteract the intestinal toxicity triggered by CPT-11 via reduction of intestinal inflammation and other inflammation associated pathways, but they did not investigate the inhibitory effects of the constituents from Huangqin-Tang on intestinal hCES2A. As mentioned above, our preliminary screening results demonstrated that licorice (the dried root of Glycyrrhiza species) granules displayed the most potent hCES2A inhibition activity (Fig. 1), which encouraged us to find the naturally occurring hCES2A inhibitors from this herbal product.
In this study, to identify the naturally occurring hCES2A inhibitors from licorice (the dried root of Glycyrrhiza species) granules in a more efficient way, an integrated strategy via combination of chemical profiling, docking-based virtual screening and fluorescence-based inhibition assays was utilized. Our results clearly demonstrated that more than ten constituents in licorice displayed strong to moderate hCES2A inhibition activities, while LCA, LCC, LCD and IFL were potent hCES2A inhibitors, with IC50 and Ki values of less than 1 μM. It is well known that licorice and licorice-related herbal products are frequently used via oral administration; the local exposure of these licorice constituents in the intestinal tract will be much higher than that in other deep tissues (such as the liver and kidneys), implying that licorice constituents are more likely to regulate intestinal metabolizing enzymes but hardly regulate hepatic metabolizing enzymes. Considering that intestinal hCES2A is one of the key targets for ameliorating CPT-11 induced life-threatening diarrhea, while the chalcones and some flavonols in licorice display strong hCES2A inhibition activities, it is readily conceivable that licorice or licorice-related dietary supplements may partially block the over-accumulation of SN-38 in the small intestine and then ameliorate CPT-11 associated life-threatening diarrhea via targeting intestinal hCES2A.
Over the past ten years, with the help of isoform-specific optical substrates for two hCES enzymes,15 numerous natural compounds with diverse skeletons (such as flavonoids, chalcones, triterpenoids and tanshinones) have been found with hCES2A inhibition activity16–18 which provide useful hints for chemists to develop more potent hCES2A inhibitors with good drug-likeness and high safety profiles. In this study, our results demonstrate that the chalcones (such as LCA and LCC) and some flavonols (such as IFL) in licorice display strong hCES2A inhibition activities, but their inhibition potential and drug-likeness properties could be significantly improved when these compounds are separately used in vivo. From the viewpoints of medicinal chemists, the natural chalcones isolated from licorice are α,β-unsaturated ketones, and these compounds are chemically unstable at room temperature or under physiological conditions.50 Meanwhile, these natural chalcones bearing several phenolic groups could be readily metabolized by phase II drug-metabolizing enzymes in metabolic organs.51,52 In these cases, it is necessary to design and develop more chemically stable and metabolically stable chalcone derivatives as hCES2A inhibitors. Notably, chalcones and their derivatives could be readily obtained from natural pharmaceutical resources or via chemical synthesis,53 which strongly enables chemists to synthesize structurally diverse chalcone analogues for further investigations on the chemical stabilities and drug-likeness properties of chalcone analogues, as well as the structure–activity relationships of chalcone derivatives as hCES2A inhibitors. In the near future, more detailed studies on chemical modification, structure–activity relationship analysis and drug-likeness evaluation of chalcones as hCES2A inhibitors should be performed, which will be very helpful to develop chalcone-type hCES2A inhibitors as novel anti-diarrhea drugs for alleviating hCES2A-associated drug toxicity.
4. Conclusions
In summary, this study revealed that the chalcones and some flavonols in licorice were the key ingredients responsible for hCES2A inhibition by using an integrated strategy via combination of chemical profiling, docking-based virtual screening and fluorescence-based inhibition assays. Following the screening of a total of 73 herbal products, licorice was found with the most potent hCES2A inhibition activity, with an IC50 value of 44.64 μg mL−1. After that, fifty-three constituents were identified from licorice by LC-TOF-MS/MS, while virtual screening showed that a total of seventeen constituents could stably bind with hCES2A. Subsequently, fluorescence-based high-throughput inhibitor screening demonstrated that LCA, LCC, LCD and ILF displayed the most potent inhibition of hCES2A (IC50 < 1 μM). Further investigations showed that LCA, LCC, LCD and IFL potently inhibited hCES2A-mediated FD hydrolysis in a mixed-inhibition manner, while LCC (the most potent hCES2A inhibitor identified from licorice) could potently inhibit intracellular hCES2A in living cells. Collectively, this study proposed an integrated strategy to find hCES2A inhibitors from herbal medicines, while the key findings present here were very useful for a deeper understanding of the interactions between licorice constituents and hCES2A, as well as helpful for the development of new herbal remedies or drugs to alleviate hCES2A-associated drug toxicity.Conflicts of interest
The authors have no conflicts of interest.Acknowledgements
We thank Professor Min Ye from the School of Pharmacy, Peking University, and Professor Xin-miao Liang from the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, for providing some commercially unavailable licorice constituents. We also thank Professor Wei-dong Zhang from the School of Pharmacy, the Second Military Medical University for the technical support. This work was financially supported by the NSF of China (81922070, 81973286, 81773687, 81973168, 82003847), the Natural Science Foundation of Liaoning Province (20180530025), the Three-year Action Plan of Shanghai TCM Development [ZY-(2018-2020)-CCCX-5001], the Program of Shanghai Academic/Technology Research Leader (18XD1403600), the Shanghai Talent Development Fund (2019093) and the Innovative Entrepreneurship Program of High-level Talents in Dalian [2017RQ121].References
- S. P. Sanghani, S. K. Quinney, T. B. Fredenburg, Z. J. Sun, W. I. Davis, D. J. Murry, O. W. Cummings, D. E. Seitz and W. F. Bosron, Carboxylesterases expressed in human colon tumor tissue and their role in CPT-11 hydrolysis, Clin. Cancer Res., 2003, 9, 4983–4991 CAS.
- S. Tetsuo and H. Masakiyo, Structure, function and regulation of carboxylesterases, Chem.-Biol. Interact., 2006, 162, 195–211 CrossRef.
- S. P. Sanghani, P. C. Sanghani, M. A. Schiel and W. F. Bosron, Human carboxylesterases: an update on CES1, CES2 and CES3, Protein Pept. Lett., 2009, 16, 1207–1214 CrossRef CAS.
- D. Yang, R. E. Pearce, X. Wang, R. Gaedigk, Y. J. Y. Wan and B. Yan, Human carboxylesterases HCE1 and HCE2: ontogenic expression, inter-individual variability and differential hydrolysis of oseltamivir, aspirin, deltamethrin and permethrin, Biochem. Pharmacol., 2009, 77, 238–247 CrossRef CAS.
- C. C. Wong, K. W. Cheng, G. Xie, D. Zhou, C. H. Zhu, P. P. Constantinides and B. Rigas, Carboxylesterases 1 and 2 hydrolyze phospho-nonsteroidal anti-inflammatory drugs: relevance to their pharmacological activity, J. Pharmacol. Exp. Ther., 2012, 340, 422–432 CrossRef CAS.
- P. M. Potter, J. S. Wolverton, C. L. Morton, M. Wierdl and M. K. Danks, Cellular localization domains of a rabbit and a human carboxylesterase: influence on irinotecan (CPT-11) metabolism by the rabbit enzyme, Cancer Res., 1998, 58, 3627–3632 CAS.
- S. K. Quinney, S. P. Sanghani, W. I. Davis, T. D. Hurley, Z. Sun, D. J. Murry and W. F. Bosron, Hydrolysis of capecitabine to 5′-deoxy-5-fluorocytidine by human carboxylesterases and inhibition by loperamide, J. Pharmacol. Exp. Ther., 2005, 313, 1011–1016 CrossRef CAS.
- T. Fukami, M. Kariya, T. Kurokawa, A. Iida and M. Nakajima, Comparison of substrate specificity among human arylacetamide deacetylase and carboxylesterases, Eur. J. Pharm. Sci., 2015, 78, 47–53 CrossRef CAS.
- U. Swami, S. Goel and S. Mani, Therapeutic targeting of CPT-11 induced diarrhea: a case for prophylaxis, Curr. Drug Targets, 2013, 14, 777–797 CrossRef CAS.
- M. L. Rothenberg, J. R. Eckardt, J. G. Kuhn, H. Burris, J. Nelson, S. G. Hilsenbeck, G. I. Rodriguez, A. M. Thurman and L. S. Smith, Phase II trial of irinotecan in patients with progressive or rapidly recurrent colorectal cancer, J. Clin. Oncol., 1996, 14, 1128–1135 CrossRef CAS.
- H. C. Pitot, D. B. Wender, M. J. O'Connell, G. Schroeder, R. M. Goldberg, J. Rubin, J. A. Mailliard, J. A. Knost, C. Ghosh and R. J. Kirschling, Phase II trial of irinotecan in patients with metastatic colorectal carcinoma, J. Clin. Oncol., 1997, 15, 2910–2919 CrossRef CAS.
- D. E. Baker, Loperamide: a pharmacological review, Rev. Gastroenterol. Disord., 2007, S11–S18 Search PubMed.
- S. B. Hanauer, The role of loperamide in gastrointestinal disorders, Rev. Gastroenterol. Disord., 2008, 8, 15–20 Search PubMed.
- W. Eggleston, K. H. Clark and J. M. Marraffa, Loperamide Abuse Associated With Cardiac Dysrhythmia and Death, Ann. Emerg. Med, 2016, 83–86 Search PubMed.
- D. D. Wang, J. Ning, X. Lv and G. B. Ge, Research Progress in Specific Probe Substrates for Drug Metabolizing Enzymes, Prog. Pharm. Sci., 2017, 41, 110–123 Search PubMed.
- L. W. Zou, Y. G. Li, P. Wang, K. Zhou, J. Hou, Q. Jin, D. C. Hao, G. B. Ge and L. Yang, Design, synthesis, and structure-activity relationship study of glycyrrhetinic acid derivatives as potent and selective inhibitors against human carboxylesterase 2, Eur. J. Med. Chem., 2016, 112, 280–288 CrossRef CAS.
- L. W. Zou, Y. G. Li, P. Wang, K. Zhou, J. Hou and Q. Jin, Carboxylesterase inhibitors: an update, Curr. Med. Chem., 2018, 25, 1627–1649 CrossRef CAS.
- Z. M. Weng, G. B. Ge, T. Y. Dou, P. Wang, P. K. Liu, X. H. Tian, N. Qiao, Y. Yu, L. W. Zou, Q. Zhou, W. D. Zhang and J. Hou, Characterization and structure-activity relationship studies of flavonoids as inhibitors against human carboxylesterase 2, Bioorg. Chem., 2018, 77, 320–329 CrossRef CAS.
- T. C. Kao, C. H. Wu and G. C. Yen, Bioactivity and Potential Health Benefits of Licorice, J. Agric. Food Chem., 2014, 62, 542–553 CrossRef CAS.
- B. G. El-Saber, B. A. Magdy, A. El-Mleeh, M. M. Abdel-Daim and D. H. Prasad, Glycyrrhiza glabra Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of L. (Fabaceae), Biomolecules, 2020, 10, 352 CrossRef.
- W. Lam, S. Bussom, F. Guan, Z. Jiang, W. Zhang, E. A. Gullen, S. H. Liu and Y. C. Cheng, The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity, Sci. Transl. Med., 2010, 2, 45ra59 Search PubMed.
- D. J. Diller and K. M. Merz, High throughput docking for library design and library prioritization, Proteins: Struct., Funct., Bioinf., 2015, 43, 113–124 CrossRef.
- G. Wu, D. H. Robertson, C. L. B. Iii and M. Vieth, Detailed analysis of grid-based molecular docking: A case study of CDOCKER-A CHARMm-based MD docking algorithm, J. Comput. Chem., 2010, 24, 1549–1562 CrossRef.
- J. Tirado-Rives and W. L. Jorgensen, Contribution of Conformer Focusing to the Uncertainty in Predicting Free Energies for Protein Ligand Binding, J. Med. Chem., 2006, 49, 5880–5884 CrossRef CAS.
- Y. Q. Song, Z. M. Weng, T. Y. Dou, M. Finel, Y. Q. Wang, L. L. Ding, Q. Jin, D. D. Wang, S. Q. Fang, Y. F. Cao, J. Hou and G. B. Ge, Inhibition of human carboxylesterases by magnolol: Kinetic analyses and mechanism, Chem.-Biol. Interact., 2019, 308, 339–349 CrossRef CAS.
- D. D. Wang, Q. Jin, L. W. Zou, J. Hou, X. Lv, W. Lei, H. L. Cheng, G. B. Ge and L. Yang, A bioluminescent sensor for highly selective and sensitive detection of human carboxylesterase 1 in complex biological samples, Chem. Commun., 2016, 52, 3183–3186 RSC.
- Y. Q. Wang, Z. M. Weng, T. Y. Dou, J. Hou, D. D. Wang, L. L. Ding, L. W. Zou, Y. Yu, J. Chen, H. Tang and G. B. Ge, Nevadensin is a naturally occurring selective inhibitor of human carboxylesterase 1, Int. J. Biol. Macromol., 2018, 120, 1944–1954 CrossRef CAS.
- J. Wang, E. T. Williams, J. Bourgea, Y. N. Wong and C. J. Patten, Characterization of recombinant human carboxylesterases: fluorescein diacetate as a probe substrate for human carboxylesterase 2, Drug Metab. Dispos., 2011, 39, 1329–1333 CrossRef CAS.
- C. Jewell, C. Ackermann, N. A. Payne, G. Fate, R. Voorman and F. M. Williams, Specificity of procaine and ester hydrolysis by human, minipig, and rat skin and liver, Drug Metab. Dispos., 2007, 35, 2015–2022 CrossRef CAS.
- Y. Q. Song, X. Q. Guan, Z. M. Weng, Y. Q. Wang, J. Chen, Q. Jin, S. Q. Fang, B. Fan, Y. F. Cao, J. Hou and G. B. Ge, Discovery of a highly specific and efficacious inhibitor of human carboxylesterase 2 by large-scale screening, Int. J. Biol. Macromol., 2019, 137, 261–269 CrossRef CAS.
- Q. Jin, L. Feng, D. D. Wang, Z. R. Dai, P. Wang, L. W. Zou, Z. H. Liu, J. Y. Wang, Y. Yu, G. B. Ge, J. N. Cui and L. Yang, A two-photon ratiometric fluorescent probe for imaging carboxylesterase 2 in living cells and tissues, ACS Appl. Mater. Interfaces, 2015, 7, 28474–28481 CrossRef CAS.
- L. J. Xue, X. K. Qian, Q. Jin, Y. D. Zhu, X. Y. Wang, D. D. Wang, G. B. Ge and L. Yang, Construction and application of a high-content analysis for identifying human carboxylesterase 2 inhibitors in living cell system, Anal. Bioanal. Chem., 2020, 412, 2645–2654 CrossRef CAS.
- L. Montero, E. Ibanez, M. Russo, R. Sanzo, L. Rastrelli, A. L. Piccinelli, R. Celano, A. Cifuentes and M. Herrero, Metabolite profiling of licorice (Glycyrrhiza glabra) from different locations using comprehensive two-dimensional liquid chromatography coupled to diode array and tandem mass spectrometry detection, Anal. Chim. Acta, 2016, 913, 145–159 CrossRef CAS.
- S. Ji, Q. Wang, X. Qiao, H. C. Guo, X. D. Ma, C. T. Liu, X. Y. Chen, Q. F. Miao, B. C. Guan and S. W. Su, New triterpene saponins from the roots of Glycyrrhiza yunnanensis and their rapid screening by LC/MS/MS, J. Pharm. Biomed. Anal., 2014, 90, 15–26 CrossRef CAS.
- Y. F. Zheng, L. W. Qi, J. L. Zhou and P. Li, Structural characterization and identification of oleanane-type triterpene saponins in Glycyrrhiza uralensis Fischer by rapid-resolution liquid chromatography coupled with time-of-flight mass spectrometry, Rapid Commun. Mass Spectrom., 2010, 24, 3261–3270 CrossRef CAS.
- J. Z. Zhang, W. Y. Gao, Y. Gao, D. L. Liu and L. Q. Huang, Analysis of influences of spaceflight on chemical constituents in licorice by HPLC–ESI-MS/MS, Acta Physiol. Plant., 2011, 33, 2511–2520 CrossRef CAS.
- M. A. Farag, A. Porzel and L. A. Wessjohann, Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC–MS, LC–MS and 1D NMR techniques, Phytochemistry, 2012, 76, 60–72 CrossRef CAS.
- A. Shakeri, M. Masullo and G. D'Urso, In depth chemical investigation of Glycyrrhiza triphylla Fisch roots guided by a preliminary HPLC-ESIMS n profiling, Food Chem., 2018, 248, 128–136 CrossRef CAS.
- T. X. Duan, C. H. Ma, W. Q. Wang, Y. H. Yan, S. L. Wei and S. Li, Identification of the Fingerprints of Licorice by LC-MS, Chin. Pharm., 2009, 12, 414–417 CAS.
- T. Fukai, Q. H. Wang and M. Takayama, Structures of five new prenylated flavonoids, Gancaonin L, M, N, O, and P from aerial parts of Glycyrrhiza uralensis, Heterocycles, 1990, 30, 373–382 Search PubMed.
- T. Nomura, T. Fukai and Q. H. Wang, Structures of six isoprenoid-substituted flavonoids, Gancaonins F, G, R, I, Glycyrol, and isoglycyrol from Xibei Licorice, Heterocycles, 1989, 29, 1761–1772 CrossRef.
- Y. M. Zhao, S. X. Liu and C. X. Zhang, Analysis on chemical constituents from dlycyrrhizae oadix by HPLC-Q-TOF-MS, Chin. Tradit. Herb. Drugs, 2016, 47, 2061–2068 Search PubMed.
- J. M. Sun and H. Zhang, Analysis of Components in the Aqueous of Glycyrrhiza uralensis Fisch. And Glycyrrhiza uralensis Fisch. Preparated with honey by SPE-HPLC-ESI/MS/MS, Chin. Soc. Sci. Technol., 2009, 5, 29–33 Search PubMed.
- P. D. Chen, X. Zhou and A. W. Ding, Constituents in aqueous extract of honey fried licorice by HPLC-MS, Chin. Tradit. Pat. Med., 2014, 36, 2115–2120 CAS.
- L. L. Shan, N. Yang, Y. W. Zhao, X. Sheng, S. Yang and Y. Li, A rapid classification and identification method applied to analysis of glycosides in Bupleuri radix and Liquorice by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry, J. Sep. Sci., 2018, 41, 19 CrossRef.
- L. C. Guo, W. Katiyo and L. S. Lu, Glucuronates-Glucuronides; Study Results from Jiangnan University Provide New Insights into Glucuronides [Glycyrrhetic Acid 3-O-Mono-beta-D-glucuronide (GAMG): An Innovative High-Potency Sweetener with Improved Biological Activities], Food Weekly News, 2018 Search PubMed.
- Y. S. Zhao, X. K. Qian, X. Q. Guan, P. F. Song, Y. Q. Song, R. J. He, M. R. Sun, Y. X. Wang, L. W. Zou and G. B. Ge, Discovery of natural alkaloids as potent and selective inhibitors against human carboxylesterase 2, Bioorg. Chem., 2020, 105, 104367 CrossRef CAS.
- Y. S. Zhao, H. L. Ruan, X. Y. Wang, C. Chen, P. F. Song, C. W. Lu and L. W. Zou, Catalyst-free visible-light-induced condensation to synthesize bis (indolyl) methanes and biological activity evaluation of them as potent human carboxylesterase 2 inhibitors, RSC Adv., 2019, 9, 40168–40175 RSC.
- H. A. Sigurjonsdottir, L. Franzson, K. Manhem, J. Ragnarsson, G. Sigurdsson and S. Wallerstedt, Liquorice-induced rise in blood pressure: a linear dose-response relationship, J. Hum. Hypertens., 2001, 15, 549–552 CAS.
- M. Funakoshi-Tago, K. Nakamura, R. Tsuruya, M. Hatanaka, T. Mashino, Y. Sonoda and T. Kasahara, The fixed structure of Licochalcone A by alpha, beta-unsaturated ketone is necessary for anti-inflammatory activity through the inhibition of NF-kappaB activation, Int. Immunopharmacol., 2010, 10, 562–571 CrossRef CAS.
- H. Xin, X. Y. Qi, J. J. Wu, X. X. Wang, Y. Li, J. Y. Hong, W. He, W. Xu, G. B. Ge and L. Yang, Assessment of the inhibition potential of Licochalcone A against human UDP-glucuronosyltransferases, Food Chem. Toxicol., 2016, 90, 112–122 CrossRef CAS.
- X. Qiao, S. Ji, S. W. Yu, X. H. Lin, H. W. Jin, Y. K. Duan, L. R. Zhang, D. A. Guo and M. Ye, Identification of key licorice constituents which interact with cytochrome P450: evaluation by LC/MS/MS cocktail assay and metabolic profiling, AAPS J., 2014, 16, 101–113 CrossRef CAS.
- X. Qiao, S. Ji, S. W. Yu, X. H. Lin, H. W. Jin, Y. K. Duan, L. R. Zhang, D. A. Guo and M. Ye, A chalcone derivative, 1m-6, exhibits atheroprotective effects by increasing cholesterol efflux and reducing inflammation-induced endothelial dysfunction, Br. J. Pharmacol., 2020, 177, 5375–5392 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0fo02140g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |