The co-effect of ampicillin and multi-walled carbon nanotubes on activated sludge in sequencing batch reactors: microbial status, microbial community structure and ARGs propagation†
Wenlin
Zhou
,
Yan
Wang
 *,
Min
Wang
,
Binghong
Qian
,
Li
Li
*,
Min
Wang
,
Binghong
Qian
,
Li
Li
 and
Baoyu
Gao
and
Baoyu
Gao

Shandong Key Laboratory of Water Pollution Control and Resource Reuse, School of Environmental Science and Engineering, Shandong University, Jinan 250100, P.R. China. E-mail: wangyan_sdjn@aliyun.com; Fax: +86 531 88364513; Tel: +86 531 88361812
First published on 27th November 2020
Abstract
The abuse of antibiotics and the release of nanoparticles (NPs) into the environment are of grave concern; however, their co-effect on the environment is not yet clear. In this study, the response of activated sludge exposed to ampicillin (AMP) and multi-walled carbon nanotubes (MWCNTs) was investigated in a sequencing batch reactor (SBR). The results showed that mixed liquor volatile suspended solid (MLVSS) values exposed to AMP and MWCNTs decreased to 83.48–90.53%, but the specific oxygen utilization rate (SOUR) was even higher with respect to the control for 24 h exposure. Compared to individual AMP, the co-occurrences of AMP and MWCNTs showed lower cytotoxicity to activated sludge, supported by a lower membrane permeability and higher cell metabolic viability. The microbial community structure changed when exposed to AMP and MWCNTs. Firmicutes phylum emerged in the top 20 phyla under the co-effect of AMP and MWCNTs, but not for AMP alone. It is worth noting that the relative abundances of antibiotic resistance genes (ARGs) under the co-effect of AMP and MWCNTs were significantly increased, especially blaDHA. These results provide useful information for understanding the risk of the co-occurrences of antibiotics and CNTs, and can aid the sustainable and widespread application of novel carbon materials.
Environmental significanceBoth carbon nanotubes and antibiotics could be discharged into wastewater treatment plants. However, their co-effect on the environment is not yet clear. Consequently, this study investigated the response of activated sludge exposed to ampicillin and multi-walled carbon nanotubes in a sequencing batch reactor. The co-occurrences of ampicillin and multi-walled carbon nanotubes showed a lower cytotoxicity to activated sludge compared to the individual materials. However, the relative abundance of antibiotic resistance genes increased after co-exposure to ampicillin and multi-walled carbon nanotubes. These results provided new insight into the potential risk of the co-occurrences of antibiotics and carbon nanotubes. |
1. Introduction
Carbon nanotubes (CNTs) are recognized as the “king of nanomaterials” due to their excellent mechanical, electrical and chemical properties.1 However, the potential ecological and health risks of CNTs have raised extensive concerns along with their widespread applications. CNTs are reported to show some toxic effects on fish, invertebrates, algae and microorganisms in the aquatic environment.2–4 For example, CNTs could produce a higher microbial oxidative stress response that may damage the integrity of cell membranes.5 This results in the change in the microbial community structure and a decrease in the denitrification efficiency of microorganism.6,7In recent years, it has been observed that carbon nanomaterials could interact with coexisting pollutants and could change their mobility, toxicity and bioavailability.8 CNTs into riverine sediment were found to significantly increase the adsorption amount of 2,4-dichlorophenol in sediment from 0.541 to 1.44 mg g−1, and slightly inhibited the dehydrogenase activity of microorganisms in the sediment.9 Other studies showed that the presence of CNTs could protect microorganisms from the cytotoxicity of phenol in wastewater but reduced the bacterial diversity in sequencing batch reactors.10 Also, it was found that carbon nanomaterials could lead to a significant increment in the intracellular iron concentration in anaerobic wastewater treatment.11 Although many valuable results have been obtained, efforts are still required to advance the understanding of the environmental risks of CNTs with coexisting pollutants.
Antibiotics residues have become a critical environmental and public health issue globally because they could result in the propagation of antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARGs).12–14 Recently, it was recognized that the co-occurrences of antibiotics and engineering nanoparticles (ENPs) present synergistic effects on microorganisms.15–17 Deng et al. found that tetracycline can bind silver nanoparticles to form a tetracycline-nano Ag complex, which can present a synergistic growth inhibition against Salmonella bacteria through strong interaction with the cells and a temporal high concentration of Ag+ near the bacteria cell wall.18 Moreover, it has been found that nano-ZnO and nano-Al2O3 could promote the horizontal transfer of ARGs within or across genera in pure cultures or indigenous aquatic microbiota systems by damaging cell membranes.19–21
According to material flow analysis, both CNTs and antibiotics can be discharged into wastewater and may eventually enter wastewater treatment plants (WWTPs).22,23 Theoretically, the co-occurrences of CNTs and antibiotics are bound to affect the status of activated sludge in WWTPs. This would pose an environmental risk and even a dissemination risk of ARB and ARGs. However, to the best of our knowledge, there are few reports about this issue in the literature.
Therefore, multi-walled carbon nanotubes (MWCNTs) and ampicillin (AMP), which is widely used in aquaculture, husbandry, and health care due to its low cost and high performance,24 were chosen in this study to explore the co-effect of CNTs and antibiotics on activated sludge in a sequencing batch reactor (SBR). The operating state of the SBR was investigated through analysing mixed liquor volatile suspended solids and the specific oxygen utilization rates. The physiological status of the microorganisms was assessed by membrane permeabilization, cell metabolic viability and cell death mode. Furthermore, the microbial community structure and resistance genes were identified by high-throughput sequencing and fluorescence quantitative PCR. Based on our findings, this study can serve as a reference for a better understanding of the environmental risks of carbon nanomaterials.
2. Materials and methods
2.1 Multi-walled carbon nanotubes stock suspension
In this study, the MWCNTs were purchased from Hengqiu Nano Technology Co. (Suzhou, China). The MWCNTs were more than 95% pure by dry weight, had specific surface areas greater than 874.93 m2 g−1, inner diameters of 3–5 nm, outer diameters of 8–15 nm and were 3–12 μm in length. The MWCNTs stock suspension was prepared by dispersing MWCNTs in deionized water for 30 min in an ultrasonic bath followed by 60 min in an ultrasonic cell disruptor (Ningbo Scientz Biotechnology Co., China). The suspension was sonicated again in the ultrasonic bath for 30 min before use to ensure a homogeneous mixture was obtained.25 The size distribution and morphology of the MWCNTs in the stock suspension (0.1 mg L−1) were analysed by dynamic light scattering (DLS) (Malvern Zetasizer Nano ZS90, Malvern Instruments, UK) and transmission electron microscopy (TEM, Tecnai G2 F20, USA), the details of which are depicted in ESI† S1. MWCNTs are thought to be a good potential adsorbent, so an adsorption experiment, described in ESI† S2, was performed to verify their adsorption amount of AMP.2.2 Exposure experiments
In this study, six SBRs were operated to investigate the co-effect of MWCNTs and AMP. A schematic diagram of them is shown in Fig. S1.† Each bioreactor was a cylinder with an internal diameter of 25 cm and an effective working volume of 10 L. The seed sludge of the SBR was taken from the aeration tank of WWTPs in Qingdao, Shandong. The mixed liquid suspended solids (MLSS) concentration in each reactor was 3500 ± 100 mg L−1 in the initial operation period. The synthetic wastewater was prepared according to ref. 5, 10 and 11 to simulate domestic sewage, and its preparation method and composition are described in ESI† S3 and Table S1. The operating parameters of SBR are described in Table S2.† The whole experimental period was 15 days, which included five days of domestication and ten days of short-term exposure experimentation.In order to avoid the interaction between the MWCNTs and AMP before entering the bioreactor, the MWCNTs stock suspension was directly fed into the bioreactor in one go as a mixture of sludge after domestication, while AMP was fed as a contaminant in the synthetic wastewater. Although the concentrations of MWCNTs in the wastewater as a contaminant was predicted to be between 6.6 ng L−1 and 18 ng L−1 according to their fade models,26 MWCNTs could accumulate in the sludge due to their high hydrophobic nature.27 Considering the significantly biotoxicity of high doses of MWCNTs in soil and the aquatic environment,3 the MWCNTs concentration in SBR was set from 20 mg L−1 to 100 mg L−1 in this study. The concentration of AMP in the synthetic wastewater used was 50 μg L−1 based on its content of 16–150 μg L−1 in the actual wastewater.28 The concentrations of AMP and MWCNTs, beside the labels of each bioreactors, are shown in Table 1.
| No. | Name of reactor | Total mass of MWCNTs (mg) | Concentration of MWCNTs (mg L−1) | Concentration of AMP (μg L−1) |
|---|---|---|---|---|
| 1 | R CP | 0 | 0 | 0 |
| 2 | R CNT | 1000 | 100 | 0 |
| 3 | R AMP | 0 | 0 | 50 |
| 4 | R D20 | 200 | 20 | 50 |
| 5 | R D50 | 500 | 50 | 50 |
| 6 | R D100 | 1000 | 100 | 50 |
In order to avoid the fluctuation error of SBR, the whole exposure experiments were repeated three times. During each exposure experiment, mixed liquor volatile suspended solid (MLVSS) and the specific oxygen utilization rate (SOUR) of each SBR were measured according to the standard for the sludge stabilization treatment of municipal wastewater treatment plants (CJ/T510-2017). Total organic carbon (TOC) and the concentration of AMP in the effluent of each bioreactor were analysed using the TOC-L analyser (Shimadzu Co., Kyoto, Japan) and high-performance liquid chromatography (HPLC) (Thermo Fisher Scientific, USA), respectively. Sludge samples were homogeneously harvested to be evaluated from each bioreactor at 24 h and at the end of the exposure experiments (240 h).
2.3 Physiological status of the microorganisms
The physiological status of the microorganisms in activated sludge was assessed by various staining procedures, as shown in Fig. 1. The microorganisms in the activated sludge were prepared as single-cells through dilution and sonication according to a previous method.29 A PI staining (Invitrogen, Carlsbad, CA, USA) and Live/Dead cell kit (Calcein-AM and PI, Invitrogen, Carlsbad, CA, USA) were used to evaluate the content of the intact cells in the sludge samples based on the membrane integrity and membrane permeability of the microorganisms.30,31 The death modes of the microorganisms, including apoptosis and necrosis, were analysed using an Annexin V-FITC/PI apoptosis kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions.32,33 The Alamar Blue assay (AB, China) was used to visually estimate microbial activity according to cellular proliferation. The fluorescence intensity of the PI staining, Live/Dead cell kit and Annexin V-FITC/PI apoptosis kit were tested by Image StreamX Mark II flow cytometry (Amnis, USA), and the Spark multifunctional microplate reader (Tecan, Austria) was used to measure the fluorescence intensity of the Alamar Blue assay. Details of the extraction and analysis process are available in ESI† S4–S7.2.4 Microbial community and ARGs analysis
Sludge samples harvested at the end of the exposure experiments (240 h) were immediately stored at −20 °C until their DNA was extracted (ESI† S8). 16S-rDNA gene high-throughput sequencing of the activated sludge samples was performed at the Personalbio Genomics Institute (Shanghai, China) using the Illumina Hiseq platform. ARGs, as well as mobile genetic elements (intI1 and intI2), were quantified by fluorescence quantitative PCR (TIB 8600, China) at Sunny Biotechnology Co., Ltd (Shanghai, China). All the information on the primers is given in Table S3.†34–37 Also, the details of the microbial community and ARGs analysis are available in ESI† S9.2.5 Other analytical procedures
Extracellular polymeric substances (EPSs) from the activated sludge samples were extracted by a heat method according to the previous method (ESI† S10).38 The content of polysaccharides in EPS were obtained by anthrone-sulfate colorimetry.39The degree of intracellular reactive oxygen species (ROS) accumulation (including O2−, H2O2, HO2˙ and ˙OH) in the activated sludge was determined using a fluorescence probe 2′, 7′-dichlorofluoresceindiacetate (DCFH-DA; Sigma-Aldrich, Saint Louis, MO, USA), according to the manufacturer's instructions.40,41 The details are in ESI† S11.
2.6 Statistical analysis
Each test in every exposure experiment was conducted in at least triplicate, and an independent sample t-test using SPSS 23.0 (IBM-SPSS, Chicago, IL, USA) by the Personalbio Genomics Institute (Shanghai, China) was used to determine whether there was statistical evidence that the set of test values were significantly different for the evaluation of the microbial community structures. A value of P < 0.05 was noted to be significant, and a value of P < 0.01 was noted to be very significant.3. Results and discussion
3.1 MWCNTs characteristics in the SBR
The toxicity of MWCNTs largely depends on their dispersed state (such as aggregate diameter and morphology) in an aquatic environment.15,42 Herein, the preliminary characterizations of the MWCNTs were analysed as they were dispersed in the synthetic wastewater and with the activated sludge in the SBR (Fig. S2–S4†). It was found that the aggregate diameter of MWCNTs fell in the range of 10 to 100 nm as they were added into the wastewater samples in the first 10 min after ultrasound treatment, and then increased to the range of 40 to 200 nm after 240 h. This agreed with the fact that MWCNTs gradually aggregate as they disperse into an aquatic environment.10 The dispersed morphology of the MWCNTs supported the fact that they did not totally stretch out as polyline but got tangled like a loose ball of string with diffusing ends as they dispersed into the wastewater (Fig. S3†). The MWCNTs adsorbed and intertwined on the sludge particles as they were dispersed with the activated sludge in the SBR (Fig. S4†). However, it should be pointed out that the morphology of the MWCNTs did not change significantly with the increasing dispersal time. This indicated that steric repulsion impacted the adsorption of the organic matter in the wastewater and kept the initial morphology of the MWCNTs. This was in agreement with other studies.43 Besides, the MWCNTs showed some adsorbed ability onto antibiotics in aquatic environment. The concentration of AMP in the water (10 mg L−1) decreased to 4.627 mg L−1 as the MWCNTs (1 g L−1) were applied as adsorbents (Fig. S5†).3.2 Operating state of the SBR
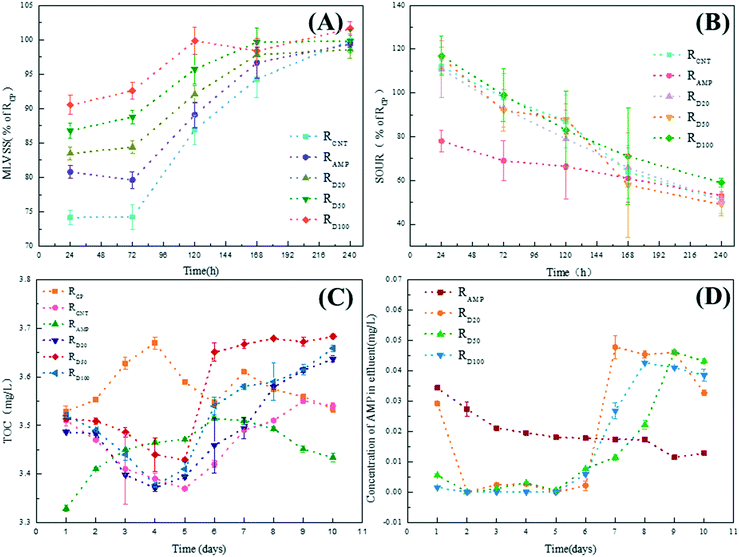
The number of microorganisms, respiration intensity, and effluent quality are important indicators for the operating state of the sewage treatment; therefore, the MLVSS, SOUR, effluent TOC and AMP concentration of various SBR were determined and are shown in Fig. 2.It can be found in Fig. 2A that the MLVSS values in RCNT, RAMP and RD were lower than 100% of RCP for the 24 h exposure case. This may be due to the cytotoxicity of AMP and MWCNTs. AMP can induce cell shrinkage, apoptosis and necrocytosis through inhibiting cell wall synthesis and cell granulation, and MWCNTs exhibit a strong antimicrobial activity via oxidative stress and membrane perturbation.5,44 However, the MLVSS values of various RD were larger than those of RCNT and RAMP, and increased with the increase in the concentration of MWCNTs. This suggests that compared with exposure to individual AMP or MWCNTs, higher amounts of original microorganisms could resist the co-occurrences of AMP and MWCNTs. A similar phenomenon was found in other reports; whereby an antagonistic effect could be observed between the toxicity of carbon nanoparticles and toxic pollutant (e.g. phenol and Cu2+).45 This was mainly due to the occupation of the functional groups of the AMP and MWCNTs in the adsorption. MWCNTs could bind AMP in wastewater through –OH, –COOH and so on. The adsorbed AMP would twist its functional groups and then could hardly bind to penicillin binding proteins,46 which is a key step to inhibiting the bacterial cell wall synthesis of AMP.47 Thus, the effect of AMP on bacteria would be weakened. Meanwhile, the adsorption reaction between AMP and MWCNTs would result in the occupation of the functional groups of MWCNTs, which has been proved to be related with the biotoxicity of CNTs. Gao et al. found that the surface chemistry modification on MWCNTs can cause less immune perturbations in mice and in macrophages through altering the receptor recognition.48 Thus, the effect of MWCNTs on bacteria also would be restrained. After 240 h exposure, the surviving microorganisms adapted to the co-occurrences, whether MWCNTs, AMP or AMP combined with MWCNTs, and this was supported by the MLVSS values of the various SBRs, which were nearly recovered to the level of RCP (98.6–101.6%).
Besides the number of active microorganisms, microbial respiration was influenced by AMP and MWCNTs based on the SOUR analysis (Fig. 2B). The SOUR of RCNT was slightly improved to 112% of RCP, while the SOUR of RAMP evidently declined to 78% of SRCP after 24 h exposure. This indicated that the MWCNTs could enhance microbial respiration, although it resulted in the death of some microorganisms. In agreement with other reports, it was found that CNTs could increase the activities of some microorganisms and enzymes through promotion of the generation, transfer and consumption of electrons.49 The co-effect of AMP and MWCNTs on the respiration intensity of microorganisms was similar to that of individual MWCNTs for 24 h exposure. Moreover, the SOUR of RD increased with the increase in the concentration of MWCNTs. The microbial respiration of RD was even slightly higher than that of RCNT when the concentration of MWCNTs was over 50 mg L−1. Although the surviving microorganisms adapted to the presence of AMP, MWCNTs, or AMP combined with MWCNTs in the 240 h exposure case, the SOUR values of RCNT, RAMP or RD decreased to 50–60% of RCP.
The TOC values of the effluent fluctuated from between 3.3 and 3.7 mg L−1 during the whole exposure experiment, in which the AMP concentrations showed a decreasing trend and then an upward trend with the operation time, especially for RD (Fig. 2CD and ). Compared to Fig. 2B, it could be found that the AMP efficiency of wastewater treatment in SBR is closely related to the microbial respiration. The effluent quality would gradually deteriorate with SOUR decreasing. Also, it should be noted that in the first five days, the AMP concentrations in the effluent of RD were lower than that of RAMP. This may be due to the adsorption of MWCNTs and the high metabolic viability of the bacteria. The MWCNTs showed a high adsorption ability for AMP (Fig. S5†), which would lead to a decrease in the concentration of AMP in RD with the increase in the concentrations of MWCNTs within the first five days. Moreover, the boosted metabolic viability of the aerobic heterotrophic bacteria in RD (the highest SOUR) also contributed to the lower AMP concentration in the effluent. Besides, the highest content of polysaccharide, whose adsorption is the primary path for the removal of antibiotics in activated sludge process,50–52 in RD was one of the contributors to the low concentration of AMP in the effluent (Fig. S6†). However, the quality of the effluent for RD sharply deteriorated after seven days of exposure due to the decrease in SOUR and the adsorption saturation of the MWCNTs, especially for concentrations of MWCNTs of 20 mg L−1 and 50 mg L−1.
3.3 Physiological status of the microorganisms
The operational efficiencies of SBR are related to the physiological status of the microorganisms, including intact, necrotic and lysed cells, which is directly influenced by the cytotoxicity of the AMP and MWCNTs.5,34,53,54 In this study, the physiological status of the microorganisms in the SBR was evaluated according to the scheme shown in Fig. 1, and the results are shown in Fig. 3–5. | ||
| Fig. 5 Microbial cell apoptosis and/or necrosis in RCP, RCNT, RAMP and RD: (A) after 24 h exposure and (B) after 240 h exposure. (a) RCP; (b) RCNT; (c) RAMP; (d) RD20; (e) RD50; (f) RD100. | ||
Membrane permeability, which can be determined through PI staining, is associated with the integrity of the cell membrane.55–57 FCM analysis showed that the membrane permeability was increased in RAMP and RCNT (as evidenced by the 3.92- and 3.43-fold increase after 24 h exposure, respectively). This agreed with other studies that found that CNTs could damage the cell membrane by oxidative stress and membrane agitation, while AMP could result in cell debris due to DNA degradation or cell granulation.5 In contrast, the membrane permeability in RD was significantly lower than that in RAMP and RCNT and decreased with the increasing concentration of MWCNTs. This suggests that the presence of enough MWCNTs would protect microbes from the inflammatory effect of AMP. Because more microbes could maintain their integrated cell membrane to AMP combined with MWCNTs, the MLVSS values of RD were larger than those of RCNT and RAMP for the 24 h exposure case.
However, cell membrane integrity is thought not to fully represent cell activity because some of the cellular functions would cease before the membrane integrity is lost.58 Thus, the cell metabolic and proliferative viability were analysed based on the fluorescence-based readout of the Alamar Blue (AB) assay, which is highly sensitive to the proliferation of microorganisms.59 As shown in Fig. 4, it could be found that the cell metabolic viability in RAMP, RCNT and RD decreased slightly compared with that of RCP after 24 h exposure based on the fluorescent signal produced by microbial proliferation, except when the concentration of MWCNTs was 100 mg L−1. For 240 h exposure, the metabolic viability in RAMP, RCNT and RD was further reduced. Compared with RAMP, RD showed a weaker inhibition of the metabolic viability, irrespective of whether it was 24 h or 240 h exposure. Furthermore, the metabolic viability of the activated sludge increased relatively with the increase in the concentration of the MWCNTs. The metabolic viability in RD100 was even higher than that of RCP after 24 h exposure. This higher metabolic viability of the activated sludge contributed to a higher SOUR of RD for the 24 h exposure case (Fig. 2B).
Generally, cell metabolic and proliferative viability are closely related to cellular reactive oxygen species (ROS) generation, which are natural by-products during the process of normal aerobic metabolism, especially when the cells interact with exogenous pollutants.60 Excessive ROS generation could destroy the dynamic equilibrium between the oxidant and anti-oxidant processes, and damage DNA, protein, and lipids in microbial cells.53 This causes a cumulative inhibition effect on the microbial respiratory activity, which then leads to irreparable metabolic dysfunction.53,61RAMP showed the highest ROS production (3–6-fold that of RCP) in this study (Fig. S7†). However, the cellular ROS level (1.5–2-fold that of RCP) was significantly lower than that of RAMP, and similar to that of RCNT (1.5–1.8-fold that of RCP) as AMP and MWCNTs co-existed in the SBR. Thus, this may be one of the reasons why the proportion of cell metabolic and proliferative viability in RD was higher than that in RAMP.
Beside the ROS, the cell metabolic and proliferative viability were found to be associated with extracellular polysaccharide as well. As a complex high-molecular-weight mixture of polymers produced by microorganisms,62 extracellular polysaccharide can play an essential role in the removal of trace contaminants through absorbing many organic pollutants, including antibiotics, through hydrophobic regions.63,64 Furthermore, extracellular polysaccharide could provide a formidable protective defence against any interference from the external environment.65,66 Previous studies have proved that NPs could be prevented by extracellular polysaccharide from piercing through the membrane; thereby averting cytotoxicity to microorganisms.67 As AMP and MWCNTs co-existed in the SBR in our experiment, the largest amount of extracellular polysaccharide would be observed in RD (Fig. S6†).
This would reduce the cytotoxicity of MWCNTs to the microorganisms, which may be another reason for the higher cell metabolic and proliferative viability in the SBR in the co-presence of AMP and MWCNTs.
Excessive ROS generation ultimately contributed to a higher number of dead bacteria in RCNT, RAMP and RD compared to RCP (Fig. S8†). However, the production of the largest amount of extracellular polysaccharide contributed to the higher number of viable bacteria with an intact membrane and a lower number of dead bacteria in RD compared to RAMP and RCNT (Fig. S8†). Combining the effect of the ROS and extracellular polysaccharide, the co-occurrence of AMP and MWCNTs showed a lower cytotoxicity compared with the individual AMP or MWCNTs. This contributed to higher MLVSS values of RD than RAMP and RCNT for the 24 h exposure (Fig. 2).
In order to further investigate the co-effect of AMP and MWCNTs, the cell death modes were adopted. The cell death modes within activated sludge were divided into: early apoptotic cells, late apoptotic cells, and necrotic cells attributed to cell shrinkage, phosphatidylserine (PS) exposure and DNA fragmentation.32,33,68 These were quantified based on AV-FITC/PI staining, and the results are presented in Fig. 5, which shows the live cells (AV− PI−), early apoptotic cells (AV+ PI−), late apoptotic cells (AV+ PI+) and necrotic cells (AV− PI+) distributions.
For the 24 h exposure, the proportions of late apoptotic and necrotic cells increased dramatically in RAMP, and that of the early apoptotic cells increased in RCNT compared to RCP. The higher proportions of early apoptotic cells, late apoptotic cells and necrotic cells in RCNT and RAMP contributed to a decrease in the MLVSS values for the 24 h exposure. Furthermore, the proportion of necrotic cells in RD was significantly higher than that in RCP, indicating that the co-occurrences of AMP and MWCNTs would mainly produce cytotoxicity and genotoxicity based on DNA breakage and fragmentation. However, the proportion of necrotic cells in RD decreased gradually with the increasing concentration of MWCNTs for the 24 h exposure (Fig. 5A). For example, when the concentration of MWCNTs increased to 100 mg L−1, the proportion of necrotic cells in RD was similar to that in RCP.
The results for the physiological status of the microorganisms showed that the co-occurrence of AMP and MWCNTs had lower cytotoxicity compared with the individual AMP or MWCNTs after 24 h exposure. For 240 h exposure, the proportion of necrotic cells in RD increased dramatically (from 2.00- to 3.29-fold), and the proportions of both apoptotic and necrotic cells increased sharply in RCNT compared to those in RCP (Fig. 5B). This was also supported by the higher number of dead bacteria in RCNT, RAMP and RD with the increase in exposure time (Fig. S8†). The large amount of apoptotic and necrotic cells in RCNT, RAMP and RD inhibited the cell metabolic viability in the activated sludge, which led to a sharp decrease in SOUR for the 240 h exposure.
3.4 Microbial community structure and ARGs analysis
The system stability and wastewater treatment performance of activated sludge exposed to antibiotics depend mostly on the microbial community structure.69 The microbial community structure in RAMP, RCNT and RD was visualized by UPGMA clustering tree, histogram and a heatmap of the classified genera (Fig. 6A–C). The UPGMA clustering tree showed that the microbial community structure in RAMP, RCNT and RD deviated from the RCP due to the effect of the AMP and MWCNTs (Fig. 6A). The community structure in RCNT was obviously different from that of RAMP and RD. RAMP showed almost the same community structure as RD when the concentration of MWCNTs was 20 mg L−1. However, the community structures of RD gradually deviated from RAMP as the concentration of MWCNTs increased to 100 mg L−1.Analysis of the taxonomic composition at the phyla level, as shown in Fig. 6B, indicated the presence of Proteobacteria, Acidobacteria, Bacteroidetes, Chloroflexi, Planctomycetes, Actinobacteria and Gemmatimonadetes, which are commonly found in wastewater treatment systems,70 and these were the dominant phyla in RCP. Acidobacteria, Chloroflexi, Actinobacteria and Nitrospirae tended to increase, whereas Proteobacteria, Bacteroidetes, Planctomycetes and Gemmatimonadetes decreased, while Patescibacteria phylum emerged after 240 h exposure to MWCNTs. The exposure to AMP did not result in a decrease in Proteobacteria. The taxonomic composition in RD was similar to that in RAMP when the concentration of MWCNTs was 20 mg L−1, and it tended to be similar to that of RCNT with increasing concentrations of MWCNTs. However, it is worth noting that Firmicutes phylum emerged in the top 20 phyla in RD50 and RD100, but not in the other exposures.
To further illustrate the response of the microbial compositions in the co-occurrences of AMP and MWCNTs, the populations at the genus level are identified in Fig. 6C. Exposure to individual MWCNTs greatly increased the abundance of Dechloromonas, Zoogloea, Legionella and Bdellovibrio, while AMP led to a significant increase in the abundance of Defluviimonas and Chlorobi bacterium OLB5. The genera with great increases in abundance after exposure to the co-occurrences of AMP and MWCNTs were Bacteroide, Alloprevotella, Thauera, etc., especially in RD50 and RD100. The different microbial community structures induced by the exposure of AMP and MWCNTs would be another reason for the cell metabolic and proliferative viability in the SBR.
It has been reported that the host bacteria contribute to the dominant gene type, and also, the resistant microorganism fates.71,72 Herein, the relative abundance (defined as the absolute copy number of genes normalized to the absolute copy number of ambient 16S-rRNA genes) was used in this study to compare the differences in three AmpC β-lactamase genes (blaFOX, blaDHA and Lak) and two integron (intI1 and intI2) acquired in RCP, RAMP and RD after 240 h exposure, and the results are shown in Fig. 6D. Both RAMP and RD showed enrichment in all detected genes compared to RCP due to the presence of antibiotics. Also, the relative abundances of blaDHA, Lak, intI1 and intI2 in RD were significantly (p < 0.05) higher than those detected in RAMP. This indicated that the co-occurrences of AMP and MWCNTs would further increase the abundance of ARGs in the activated sludge. In addition, a high relative abundance of intI1 and intI2, which are considered as potential indicators for ARGs horizontal transfer,73 in RD meant that the MWCNTs showed a synergistic effect on ARGs horizontal transfer when they were combined with antibiotics.
Non-susceptible bacteria may survive exposure to antibiotics, with a consequence on the abundance of resistance genes during wastewater treatment.74 In the meanwhile, bacteria without ARGs could convert into ARB through ARGs horizontal transfer.75 The ARGs horizontal transfer is thought to be the main reason for the increase in the abundance of ARGs, and this could co-occur with the removal and accumulation of antibiotics during the wastewater treatment process.76 The process of ARGs transfer, replication and formation could not continue unless the antibiotics concentrations in the cell could be reduced below the threshold concentration.77 The concentrations of AMP in RD were significantly lower than those in RAMP for 24 h exposure (Fig. 2D). Consequently, there were more opportunities for those bacteria without ARGs to survive and acquire resistance genes in the initial exposure period. In addition, MWCNTs would still increase ARGs conjugative transfer frequency as well as facilitate the microbial uptake of naked resistance plasmids based on a modest rise in membrane permeability, like nano-ZnO and nano-Al2O3 in previous reports.19–21
It should be pointed out that our research was specific to AMP and MWCNTs, and whether the relative mechanism on antibiotics with other NPs are acceptable is unknown. In addition, considering the toxicity of NPs in natural and engineered aquatic systems is inseparable from considering the environmental variables, including natural organic matter, ionic strength and aggregation state. These are all important to deeper research on antibiotics and NPs to explore their potential mechanisms in the future.
4. Conclusions
This study investigated the microbial physiological status, microbial community structure and resistance genes in SBRs in order to elaborate the co-effects of AMP and MWCNTs on activated sludge in WWTPs. The co-occurrences of AMP and MWCNTs showed a lower cytotoxicity to activated sludge in SBRs compared to individual ones. The microorganisms in RD showed a lower membrane permeability and higher cell metabolic viability than that in RAMP and RCNT. This resulted in lower AMP concentrations and TOC values of the effluent of RD in the initial exposure period with a higher MLVSS and SOUR values compared with RAMP and RCNT. After 240 h exposure, the quality of effluent for RD sharply deteriorated, although the surviving microorganisms adapted to the exposure to AMP and MWCNTs. Also, it should be noted that the relative abundance of ARGs in RD was significantly higher than that in RAMP after 240 h exposure. These conclusions indicated that the long-term exposure to AMP and MWCNTs would magnify the dissemination risk of ARB and ARGs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by Shandong Provincial Natural Science Foundation (no. ZR2019MEE006) and the grants from Tai Shan Scholar Foundation (no. ts 201511003). We would like to thank Zhifeng Li, Jingyao Qu from State Key Laboratory of Microbial Technology of Shandong University for assistance in ImageStreamX Mark II flow cytometry (Amnis, USA). We also thank TopEdit (www.topeditsci.com) for its linguistic assistance during the preparation of this manuscript.Notes and references
- R. H. Baughman, A. A. Zakhidov and W. A. de Heer, Carbon nanotubes - the route toward applications, Science, 2002, 297, 787–792 CrossRef CAS PubMed.
- F. Icoglu Aksakal, A. Ciltas and N. Simsek Ozek, A holistic study on potential toxic effects of carboxylated multi-walled carbon nanotubes (MWCNTs-COOH) on zebrafish (Danio rerio) embryos/larvae, Chemosphere, 2019, 225, 820–828 CrossRef CAS PubMed.
- X. Xin, F. Zhao, H. Zhao, S. L. Goodrich, M. R. Hill, B. S. Sumerlin, P. J. Stoffella, A. L. Wright and Z. He, Comparative assessment of polymeric and other nanoparticles impacts on soil microbial and biochemical properties, Geoderma, 2020, 367, 114278 CrossRef CAS.
- C. Sun, W. Li, Y. Xu, N. Hu, J. Ma, W. Cao, S. Sun, C. Hu, Y. Zhao and Q. Huang, Effects of carbon nanotubes on the toxicities of copper, cadmium and zinc toward the freshwater microalgae Scenedesmus obliquus, Aquat. Toxicol., 2020, 224, 105504 CrossRef CAS PubMed.
- M. Gao, F. Gao, B. Ma, N. Yu, Z. She, C. Zhao, L. Guo, Y. Zhao, S. Li and C. Jin, Insights into long-term effects of amino-functionalized multi-walled carbon nanotubes (MWCNTs-NH2) on the performance, enzymatic activity and microbial community of sequencing batch reactor, Environ. Pollut., 2019, 254, 113118 CrossRef CAS PubMed.
- S. Kang, M. S. Mauter and M. Elimelech, Microbial Cytotoxicity of Carbon-Based Nanomaterials: Implications for River Water and Wastewater Effluent, Environ. Sci. Technol., 2009, 43, 2648–2653 CrossRef CAS PubMed.
- Z. Tong, M. Bischoff, L. F. Nies, P. Myer, B. Applegate and R. F. Turco, Response of soil microorganisms to As-produced and functionalized single-wall carbon nanotubes (SWNTs), Environ. Sci. Technol., 2012, 46, 13471–13479 CrossRef CAS PubMed.
- X. Fan, J. Xu, M. Lavoie, W. Peijnenburg, Y. Zhu, T. Lu, Z. Fu, T. Zhu and H. Qian, Multiwall carbon nanotubes modulate paraquat toxicity in Arabidopsis thaliana, Environ. Pollut., 2018, 233, 633–641 CrossRef CAS PubMed.
- B. Song, J. Gong, W. Tang, G. Zeng, M. Chen, P. Xu, M. Shen, S. Ye, H. Feng, C. Zhou and Y. Yang, Influence of multi-walled carbon nanotubes on the microbial biomass, enzyme activity, and bacterial community structure in 2,4-dichlorophenol-contaminated sediment, Sci. Total Environ., 2020, 713, 136645 CrossRef CAS PubMed.
- Y. Qu, Q. Ma, J. Deng, W. Shen, X. Zhang, Z. He, J. D. Van Nostrand, J. Zhou and J. Zhou, Responses of microbial communities to single-walled carbon nanotubes in phenol wastewater treatment systems, Environ. Sci. Technol., 2015, 49, 4627–4635 CrossRef CAS PubMed.
- M. Jiang, L. Feng, X. Zheng and Y. Chen, Bio-denitrification performance enhanced by graphene-facilitated iron acquisition, Water Res., 2020, 180, 115916 CrossRef CAS PubMed.
- Y. Xiao, H. Chang, A. Jia and J. Hu, Trace analysis of quinolone and fluoroquinolone antibiotics from wastewaters by liquid chromatography-electrospray tandem mass spectrometry, J. Chromatogr. A, 2008, 1214, 100–108 CrossRef CAS PubMed.
- A. J. Watkinson, E. J. Murby and S. D. Costanzo, Removal of antibiotics in conventional and advanced wastewater treatment: implications for environmental discharge and wastewater recycling, Water Res., 2007, 41, 4164–4176 CrossRef CAS PubMed.
- M. S. Diaz-Cruz, M. J. Garcia-Galan and D. Barcelo, Highly sensitive simultaneous determination of sulfonamide antibiotics and one metabolite in environmental waters by liquid chromatography-quadrupole linear ion trap-mass spectrometry, J. Chromatogr. A, 2008, 1193, 50–59 CrossRef CAS PubMed.
- E. J. Petersen, L. Zhang, N. T. Mattison, D. M. O'Carroll, A. J. Whelton, N. Uddin, T. Nguyen, Q. Huang, T. B. Henry, R. D. Holbrook and K. L. Chen, Potential release pathways, environmental fate, and ecological risks of carbon nanotubes, Environ. Sci. Technol., 2011, 45, 9837–9856 CrossRef CAS PubMed.
- P. A. Neale, A. K. Jamting, B. I. Escher and J. Herrmann, A review of the detection, fate and effects of engineered nanomaterials in wastewater treatment plants, Water Sci. Technol., 2013, 68, 1440–1453 CrossRef CAS PubMed.
- S. J. Sarma, I. Bhattacharya, S. K. Brar, R. D. Tyagi and R. Y. Surampalli, Carbon Nanotube—Bioaccumulation and Recent Advances in Environmental Monitoring, Critical Reviews in, Environ. Sci. Technol., 2015, 45, 905–938 CAS.
- H. Deng, D. McShan, Y. Zhang, S. S. Sinha, Z. Arslan, P. C. Ray and H. Yu, Mechanistic Study of the Synergistic Antibacterial Activity of Combined Silver Nanoparticles and Common Antibiotics, Environ. Sci. Technol., 2016, 50, 8840–8848 CrossRef CAS PubMed.
- X. Wang, F. Yang, J. Zhao, Y. Xu, D. Mao, X. Zhu, Y. Luo and P. J. J. Alvarez, Bacterial exposure to ZnO nanoparticles facilitates horizontal transfer of antibiotic resistance genes, NanoImpact, 2018, 10, 61–67 CrossRef.
- A. Kumar, A. K. Pandey, S. S. Singh, R. Shanker and A. Dhawan, Engineered ZnO and TiO(2) nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli, Free Radical Biol. Med., 2011, 51, 1872–1881 CrossRef CAS PubMed.
- C. Ding, J. Pan, M. Jin, D. Yang, Z. Shen, J. Wang, B. Zhang, W. Liu, J. Fu, X. Guo, D. Wang, Z. Chen, J. Yin, Z. Qiu and J. Li, Enhanced uptake of antibiotic resistance genes in the presence of nanoalumina, Nanotoxicology, 2016, 10, 1051–1060 CrossRef CAS PubMed.
- V. K. Upadhyayula, S. Deng, M. C. Mitchell and G. B. Smith, Application of carbon nanotube technology for removal of contaminants in drinking water: a review, Sci. Total Environ., 2009, 408, 1–13 CrossRef CAS PubMed.
- A. Gobel, A. Thomsen, C. S. McArdell, A. Joss and W. Giger, Occurrence and sorption behavior of sulfonamides, macrolides, and trimethoprim in activated sludge treatment, Environ. Sci. Technol., 2005, 39, 3981–3989 CrossRef PubMed.
- C. Zhang, S. You, J. Zhang, W. Qi, R. Su and Z. He, An effective in-situ method for laccase immobilization: Excellent activity, effective antibiotic removal rate and low potential ecological risk for degradation products, Bioresour. Technol., 2020, 308, 123271 CrossRef CAS PubMed.
- L. L. Li, Z. H. Tong, C. Y. Fang, J. Chu and H. Q. Yu, Response of anaerobic granular sludge to single-wall carbon nanotube exposure, Water Res., 2015, 70, 1–8 CrossRef PubMed.
- F. Gottschalk, T. Sonderer, R. W. Scholz and B. Nowack, Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions, Environ. Sci. Technol., 2009, 43, 9216–9222 CrossRef CAS PubMed.
- S. K. Brar, M. Verma, R. D. Tyagi and R. Y. Surampalli, Engineered nanoparticles in wastewater and wastewater sludge--evidence and impacts, Waste Manage., 2010, 30, 504–520 CrossRef CAS PubMed.
- S. Kim and D. S. Aga, Potential ecological and human health impacts of antibiotics and antibiotic-resistant bacteria from wastewater treatment plants, J. Toxicol. Environ. Health, Part B, 2007, 10, 559–573 CAS.
- P. Foladori, B. Laura, A. Gianni and Z. Giuliano, Effects of sonication on bacteria viability in wastewater treatment plants evaluated by flow cytometry--fecal indicators, wastewater and activated sludge, Water Res., 2007, 41, 235–243 CrossRef CAS PubMed.
- D. Kennedy, U. P. Cronin, A. Piterina and M. G. Wilkinson, Heat and Chemical Treatments Affect the Viability, Morphology, and Physiology of Staphylococcus aureus and Its Subsequent Antibody Labeling for Flow Cytometric Analysis, Appl. Environ. Microbiol., 2019, 85(17), e01006 CrossRef CAS PubMed.
- X. Chen, A. Kis, A. Zettl and C. R. Bertozzi, A cell nanoinjector based on carbon nanotubes, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8218–8222 CrossRef CAS PubMed.
- J. Yun, E. R. Woo and D. G. Lee, Effect of isoquercitrin on membrane dynamics and apoptosis-like death in Escherichia coli, Biochim. Biophys. Acta, Biomembr., 2018, 1860, 357–363 CrossRef CAS PubMed.
- D. J. Dwyer, D. M. Camacho, M. A. Kohanski, J. M. Callura and J. J. Collins, Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis, Mol. Cell, 2012, 46, 561–572 CrossRef CAS PubMed.
- X. He, Y. Xu, J. Chen, J. Ling, Y. Li, L. Huang, X. Zhou, L. Zheng and G. Xie, Evolution of corresponding resistance genes in the water of fish tanks with multiple stresses of antibiotics and heavy metals, Water Res., 2017, 124, 39–48 CrossRef CAS PubMed.
- H. Volkmann, T. Schwartz, P. Bischoff, S. Kirchen and U. Obst, Detection of clinically relevant antibiotic-resistance genes in municipal wastewater using real-time PCR (TaqMan), J. Microbiol. Methods, 2004, 56, 277–286 CrossRef CAS PubMed.
- A. Naghoni, G. Emtiazi, M. A. Amoozegar, M. S. Cretoiu, L. J. Stal, Z. Etemadifar, S. A. S. Fazeli and H. Bolhuis, Microbial diversity in the hypersaline Lake Meyghan, Iran, Sci. Rep., 2017, 7, 11522 CrossRef PubMed.
- N. Rayamajhi, S. G. Kang, M. L. Kang, H. S. Lee, K. Y. Park and H. S. Yoo, Assessment of Antibiotic Resistance Phenotype and Integrons in Salmonella enterica serovar Typhimurium Isolated from Swine, J. Vet. Med. Sci., 2008, 70, 1133–1137 CrossRef CAS PubMed.
- C. Feng, T. Lotti, Y. Lin and F. Malpei, Extracellular polymeric substances extraction and recovery from anammox granules: Evaluation of methods and protocol development, Chem. Eng. J., 2019, 374, 112–122 CrossRef CAS.
- R.-Q. Song, T.-G. Nan, Y. Yuan, Y. Jin, Q. Yang, M. Zhang and K.-Y. Hu, Study on polysaccharide content and monosaccharide composition of Polyporus umbellatus from different production areas, Zhongguo Zhongyao Zazhi, 2019, 44, 3608–3614 Search PubMed.
- E. Lopez, C. Arce, M. J. Oset-Gasque, S. Canadas and M. P. Gonzalez, Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture, Free Radical Biol. Med., 2006, 40, 940–951 CrossRef CAS PubMed.
- Z. Huang, K. He, Z. Song, G. Zeng, A. Chen, L. Yuan, H. Li, L. Hu, Z. Guo and G. Chen, Antioxidative response of Phanerochaete chrysosporium against silver nanoparticle-induced toxicity and its potential mechanism, Chemosphere, 2018, 211, 573–583 CrossRef CAS.
- L. M. Pasquini, R. C. Sekol, A. D. Taylor, L. D. Pfefferle and J. B. Zimmerman, Realizing comparable oxidative and cytotoxic potential of single- and multiwalled carbon nanotubes through annealing, Environ. Sci. Technol., 2013, 47, 8775–8783 CAS.
- N. B. Saleh, L. D. Pfefferle and M. Elimelech, Influence of Biomacromolecules and Humic Acid on the Aggregation Kinetics of Single-Walled Carbon Nanotubes, Environ. Sci. Technol., 2010, 44, 2412–2418 CrossRef CAS.
- D. Nathwani and M. J. Wood, Penicillins: A current review of their clinical pharmacology and therapeutic use, Drugs, 1993, 45, 866–894 CrossRef CAS PubMed.
- J. Zhao, F. Ning, X. Cao, H. Yao, Z. Wang and B. Xing, Photo-transformation of graphene oxide in the presence of co-existing metal ions regulated its toxicity to freshwater algae, Water Res., 2020, 176, 115735 CrossRef CAS PubMed.
- S. Turk, O. Verlaine, T. Gerards, M. Zivec, J. Humljan, I. Sosic, A. Amoroso, A. Zervosen, A. Luxen, B. Joris and S. Gobec, New noncovalent inhibitors of penicillin-binding proteins from penicillin-resistant bacteria, PLoS One, 2011, 6, e19418 CrossRef CAS.
- S. L. Chopra, D. G. Dale and A. G. Blackwood, Effect of Ampicillin on E. Coli of Swine Origin, Can. J. Comp. Med. Vet. Sci., 1963, 27, 223–227 CAS.
- N. Gao, Q. Zhang, Q. Mu, Y. Bai, L. Li, H. Zhou, E. R. Butch, T. B. Powell, S. E. Snyder, G. Jiang and B. Yan, Steering carbon nanotubes to scavenger receptor recognition by nanotube surface chemistry modification partially alleviates NFκB activation and reduces its immunotoxicity, ACS Nano, 2011, 5, 4581–4591 CrossRef CAS PubMed.
- J. J. Ambuchi, Z. Zhang, L. Shan, D. Liang, P. Zhang and Y. Feng, Response of anaerobic granular sludge to iron oxide nanoparticles and multi-wall carbon nanotubes during beet sugar industrial wastewater treatment, Water Res., 2017, 117, 87–94 CrossRef CAS.
- Z. Liu, P. Sun, S. G. Pavlostathis, X. Zhou and Y. Zhang, Adsorption, inhibition, and biotransformation of ciprofloxacin under aerobic conditions, Bioresour. Technol., 2013, 144, 644–651 CrossRef CAS PubMed.
- H. Zhang, S. Song, Y. Jia, D. Wu and H. Lu, Stress-responses of activated sludge and anaerobic sulfate-reducing bacteria sludge under long-term ciprofloxacin exposure, Water Res., 2019, 164, 114964 CrossRef CAS PubMed.
- L. Wang, Y. Li, L. Wang, M. Zhu, X. Zhu, C. Qian and W. Li, Responses of biofilm microorganisms from moving bed biofilm reactor to antibiotics exposure: Protective role of extracellular polymeric substances, Bioresour. Technol., 2018, 254, 268–277 CrossRef CAS PubMed.
- A. Helland, P. Wick, A. Koehler, K. Schmid and C. Som, Reviewing the environmental and human health knowledge base of carbon nanotubes, Environ. Health Perspect., 2007, 115, 1125–1131 CrossRef CAS.
- S. Kang, M. Pinault, L. D. Pfefferle and M. Elimelech, Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity, Langmuir, 2007, 23, 8670–8673 CrossRef CAS.
- Y. Zhang, A. Z. Gu, T. Cen, X. Li, M. He, D. Li and J. Chen, Sub-inhibitory concentrations of heavy metals facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes in water environment, Environ. Pollut., 2018, 237, 74–82 CrossRef CAS PubMed.
- X. Xiao, Z.-Y. Han, Y.-X. Chen, X.-q. Liang, H. Li and Y.-C. Qian, Optimization of FDA–PI method using flow cytometry to measure metabolic activity of the cyanobacteria, Microcystis aeruginosa, Phys. Chem. Earth, 2011, 36, 424–429 CrossRef.
- C. C. Otto, T. M. Cunningham, M. R. Hansen and S. E. Haydel, Effects of antibacterial mineral leachates on the cellular ultrastructure, morphology, and membrane integrity of Escherichia coli and methicillin-resistant Staphylococcus aureus, Ann. Clin. Microbiol. Antimicrob., 2010, 9, 26 CrossRef PubMed.
- J. T. Lisle, B. H. Pyle and G. A. Mcfeters, The use of multiple indices of physiological activity to access viability in chlorine disinfected Escherichia coli O157:H7, Lett. Appl. Microbiol., 1999, 29, 42–47 CrossRef CAS PubMed.
- B. Pimentel, C. C. de Foggi, P. A. Barbugli, R. C. de Oliveira, E. D. de Avila, E. Longo and C. E. Vergani, Antifungal activity and biocompatibility of alpha-AgVO3 microcrystals: A promising material against oral Candida disease, Mater. Sci. Eng., C, 2020, 108, 110405 CrossRef CAS.
- Q. Zhang, M. Wang, C. Gu and C. Zhang, Water disinfection processes change the cytotoxicity of C60 fullerene: Reactions at the nano-bio interface, Water Res., 2019, 163, 114867 CrossRef CAS PubMed.
- G. M. Zeng, A. W. Chen, G. Q. Chen, X. J. Hu, S. Guan, C. Shang, L. H. Lu and Z. J. Zou, Responses of Phanerochaete chrysosporium to toxic pollutants: physiological flux, oxidative stress, and detoxification, Environ. Sci. Technol., 2012, 46, 7818–7825 CrossRef CAS PubMed.
- G. P. Sheng, H. Q. Yu and X. Y. Li, Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review, Biotechnol. Adv., 2010, 28, 882–894 CrossRef CAS.
- G.-P. Sheng, M.-L. Zhang and H.-Q. Yu, Characterization of adsorption properties of extracellular polymeric substances (EPS) extracted from sludge, Colloids Surf., B, 2008, 62, 83–90 CrossRef CAS.
- J. Wingender, T. R. Neu and H. C. Flemming, Microbial Extracellular Polymeric Substances, Springer, Berlin Heidelberg, 1999 Search PubMed.
- H. C. Flemming, J. Wingender, U. Szewzyk, P. Steinberg, S. A. Rice and S. Kjelleberg, Biofilms: an emergent form of bacterial life, Nat. Rev. Microbiol., 2016, 14, 563–575 CrossRef CAS PubMed.
- Z. Xue, V. R. Sendamangalam, C. L. Gruden and Y. Seo, Multiple roles of extracellular polymeric substances on resistance of biofilm and detached clusters, Environ. Sci. Technol., 2012, 46, 13212–13219 CrossRef CAS PubMed.
- A. Panacek, L. Kvitek, M. Smekalova, R. Vecerova, M. Kolar, M. Roderova, F. Dycka, M. Sebela, R. Prucek, O. Tomanec and R. Zboril, Bacterial resistance to silver nanoparticles and how to overcome it, Nat. Nanotechnol., 2018, 13, 65–71 CrossRef CAS.
- A. Chen, G. Zeng, G. Chen, L. Liu, C. Shang, X. Hu, L. Lu, M. Chen, Y. Zhou and Q. Zhang, Plasma membrane behavior, oxidative damage, and defense mechanism in Phanerochaete chrysosporium under cadmium stress, Process Biochem., 2014, 49, 589–598 CrossRef CAS.
- L. Yang, Q. Wen, Y. Zhao, Z. Chen, Q. Wang and H. Burgmann, New insight into effect of antibiotics concentration and process configuration on the removal of antibiotics and relevant antibiotic resistance genes, J. Hazard. Mater., 2019, 373, 60–66 CrossRef CAS PubMed.
- P. Wang, G. You, J. Hou, C. Wang, Y. Xu, L. Miao, T. Feng and F. Zhang, Responses of wastewater biofilms to chronic CeO2 nanoparticles exposure: Structural, physicochemical and microbial properties and potential mechanism, Water Res., 2018, 133, 208–217 CrossRef CAS PubMed.
- R. Zhao, J. Feng, J. Liu, W. Fu, X. Li and B. Li, Deciphering of microbial community and antibiotic resistance genes in activated sludge reactors under high selective pressure of different antibiotics, Water Res., 2019, 151, 388–402 CrossRef CAS.
- W. Yan, N. Shen, Y. Xiao, Y. Chen, F. Sun, V. K. Tyagi and Y. Zhou, The role of conductive materials in the start-up period of thermophilic anaerobic system, Bioresour. Technol., 2017, 239, 336–344 CrossRef CAS PubMed.
- M. S. Wright, C. Baker-Austin, A. H. Lindell, R. Stepanauskas, H. W. Stokes and J. V. McArthur, Influence of industrial contamination on mobile genetic elements: class 1 integron abundance and gene cassette structure in aquatic bacterial communities, ISME J., 2008, 2, 417–428 CrossRef CAS PubMed.
- T. Fernandes, I. Vaz-Moreira and C. M. Manaia, Neighbor urban wastewater treatment plants display distinct profiles of bacterial community and antibiotic resistance genes, Environ. Sci. Pollut. Res., 2019, 26, 11269–11278 CrossRef CAS PubMed.
- T. U. Berendonk, C. M. Manaia, C. Merlin, D. Fatta-Kassinos, E. Cytryn, F. Walsh, H. Burgmann, H. Sorum, M. Norstrom, M. N. Pons, N. Kreuzinger, P. Huovinen, S. Stefani, T. Schwartz, V. Kisand, F. Baquero and J. L. Martinez, Tackling antibiotic resistance: the environmental framework, Nat. Rev. Microbiol., 2015, 13, 310–317 CrossRef CAS PubMed.
- H. Li, H. Xu, Y.-L. Yang, X.-L. Yang, Y. Wu, S. Zhang and H.-L. Song, Effects of graphite and Mn ore media on electro-active bacteria enrichment and fate of antibiotic and corresponding resistance gene in up flow microbial fuel cell constructed wetland, Water Res., 2019, 165, 114988 CrossRef CAS PubMed.
- S. Nolivos, J. Cayron, A. Dedieu, A. Page, F. Delolme and C. Lesterlin, Role of AcrAB-TolC multidrug efflux pump in drug-resistance acquisition by plasmid transfer, Science, 2019, 364, 778–782 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0en00668h |
| This journal is © The Royal Society of Chemistry 2021 |