The synergistic photocatalytic effects of surface-modified g-C3N4 in simple and complex pollution systems based on a macro-thermodynamic model†
Kexin
Li
 ab,
Dongxiao
Zhao
ab,
Yawen
Li
ab,
Shenglian
Luo
*ab and
Zhentao
Zhou
ab
ab,
Dongxiao
Zhao
ab,
Yawen
Li
ab,
Shenglian
Luo
*ab and
Zhentao
Zhou
ab
aKey Laboratory of Jiangxi Province for Persistent Pollutants Control and Resources Recycle, NanChang Hangkong University, NanChang 330063, People's Republic of China. E-mail: sllou@hnu.edu.cn
bNational-Local Joint Engineering Research Center of Heavy Metals Pollutants Control and Resource Utilization, NanChang Hangkong University, NanChang 330063, People's Republic of China
First published on 16th December 2020
Abstract
In this article, three types of surface-modified g-C3N4 (H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4) with unique photocatalytic properties have been successfully fabricated by simple and low-cost strategies. The morphology, elemental composition, textural properties, structural information, and photoelectric properties of the as-prepared materials are systematically characterized. Subsequently, the as-prepared materials are applied to Cr(VI) reduction, 4-FP degradation, and simultaneous Cr(VI) reduction with 4-FP degradation photocatalytic systems under normal and anoxic conditions. Based on the photocatalytic test results, we successfully constructed a macro-thermodynamic model to reveal the essential laws of synergistic photocatalytic effects. The constructed macro-thermodynamic model is suitable for complex photocatalytic systems and is of great significance for the application of photocatalytic technology in real wastewater treatment with multiple pollutants.
Environmental significanceIn the field of photocatalytic wastewater treatment, traditional research models focus too much on the micro-kinetics of photocatalytic reactions, such as the degradation routes of target pollutants and hydroxyl radicals generated by photocatalysts, but ignore the macro-thermodynamics of photocatalytic reactions, which is not conducive to the practical application of photocatalytic technology for wastewater treatment. In this article, we establish a macro-thermodynamic model to discuss the synergistic photocatalytic effects of various components in photocatalysts or multi-component pollution systems. Compared with the complex micro-kinetics model, this simple macro-thermodynamic model is more conducive to the industrial development of photocatalytic wastewater treatment. |
1. Introduction
Heavy metal ions and organic pollutants in wastewater have caused long-term pollution to the natural environment with the continuous development of the world's industry.1,2 Photocatalytic technology is an effective strategy for removing heavy metal ions and organic pollutants from wastewater based on the concept of sustainable development.3,4 On the one hand, heavy metal ions in wastewater, such as highly toxic hexavalent chromium [Cr(VI)], can be reduced to low toxic trivalent chromium [Cr(III)] by photocatalytic reduction.5–7 On the other hand, organic pollutants in wastewater can be decomposed into H2O, CO2 and other inorganic small molecules through photocatalytic oxidation.8–10 In the past 40 years, humans have made some breakthrough progress in the field of environmental photocatalysis.11–16 However, until now, the practical application of photocatalytic technology for wastewater treatment has not seen hope. We believe that there are some problems with the current research strategies on photocatalytic wastewater treatment and therefore need to be improved. In the study of photocatalyst development, many researchers tend to synthesize new photocatalytic materials and evaluate the activity of as-prepared materials by photo-generated carrier (e−–h+) separation efficiency while ignoring the connection between photocatalysts and target pollutants.17–20 In the study of photocatalytic performance, the traditional research model only considers the removal rate and conversion process of target pollutants in the ideal single-component pollution system and ignores the interactions between various pollutant components in the multi-component pollution system.21–24 Therefore, the research thoughts on photocatalyst development and photocatalytic performance study should be reconsidered in order to realize the application of photocatalytic technology for wastewater treatment.Synergy, a ubiquitous natural law, is a relationship that inhibits or promotes each other among components in a complex system and is divided into internal and external synergy. Internal synergy is a global effect due to the collaboration between components in the system, and individual components may have no effect. External synergy is an additive effect due to the collaboration between components in the system, and individual components also have a certain effect. We believe that synergy in a photocatalytic system, that is, synergistic photocatalytic effect, is universal in nature. According to the meaning of internal synergy, the photocatalytic reduction of aqueous Cr(VI) under acidic conditions takes advantage of the internal synergy between a semiconductor photocatalyst and hydrogen ions (H+) because an individual semiconductor photocatalyst (such as g-C3N4) or H+ has no effect on the photocatalytic reduction of aqueous Cr(VI).25–28 According to the meaning of external synergy, the construction of a heterojunction photocatalyst takes advantage of external synergy between two semiconductor components because each semiconductor component in the heterojunction photocatalyst usually has its own photocatalytic properties.29–32 However, no systematic research has been conducted on the synergistic photocatalytic effect so far. The systematic study of synergistic photocatalytic effect in the photocatalytic system is beneficial to the application of photocatalytic technology for wastewater treatment.
In our previous work, we found that there is an external synergy in the reaction solution between oxidizing and reducing pollutants and clarified its mechanism.33,34 That is, the electron transport effect of a photocatalyst accelerates the redox reaction between oxidizing and reducing pollutants, so the removal efficiencies of various pollutant components in a multi-component pollution system are simultaneously improved compared with that of a single-component pollution system. Some researchers have also found that there is a synergistic photocatalytic effect in the combined pollution system containing both Cr(VI) and organic pollutants, but they have not given the essential laws about the synergistic photocatalytic effect.35–38 Whether a perfect research model could be established to clarify the synergistic photocatalytic effects between photocatalyst components, between photocatalyst and pollutant, and between pollutant components in a photocatalytic system is a very interesting scientific question and has great significance for the application of photocatalytic technology in wastewater treatment.
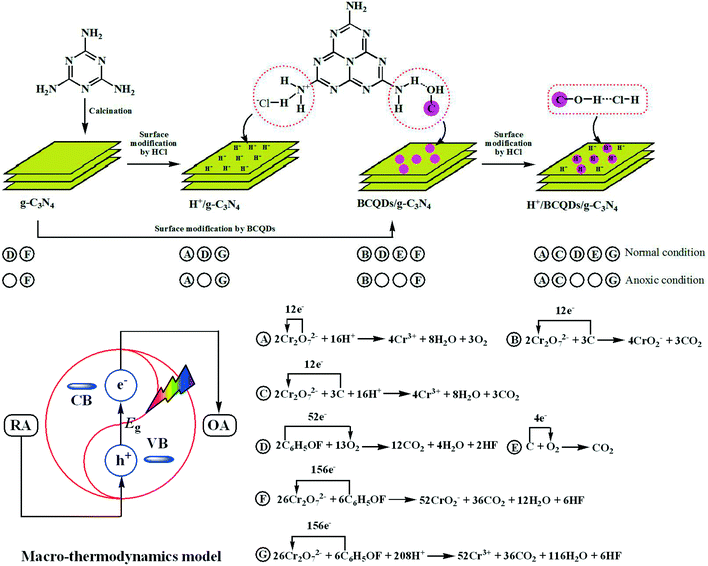
Construction of g-C3N4-based composites by surface modification is a simple and effective method to improve the photocatalytic performance of g-C3N4, which has been widely reported by many researchers in recent years.39–41 For example, Dong et al. reported that a perylene imide (PI)-modified g-C3N4 was used to remove nitric oxide under visible-light conditions,42 Zhang et al. fabricated a surface hydroxyl-modified g-C3N4 with enhanced photocatalytic oxidation activity,43 and Wang et al. synthesized an oxygen-containing ultrathin porous carbon quantum dots/g-C3N4 composite by a template-free method with superior photocatalytic activity for PPCPs remediation.44 In this article, we successfully fabricated three surface-modified g-C3N4 photocatalysts (H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4) by simple and low-cost strategies and investigated the performance of as-prepared materials in single- and multi-component pollution systems under normal and anoxic conditions. Based on the photocatalytic test results, we successfully constructed a macro-thermodynamic model to reveal the essential laws of synergistic photocatalytic effects. The basic idea of the macro-thermodynamic model is that the elementary reactions in the photocatalytic process can be ignored, and the photo-generated carriers are the key to start the redox reaction in the photocatalytic system. Finally, the electron transport effect of the photocatalyst accelerates the electronic transmission between the oxidizing and the reducing agent. This macro-thermodynamic model is generally applicable to complex photocatalytic systems and is of great significance for the application of photocatalytic technology in wastewater treatment.
2. Experimental
2.1. Chemicals and reagents
Urea (CH4N2O, AR grade) and hydrochloric acid (HCl, 36–38%, AR grade) were purchased from Shanghai Xilong Chemical Co., Ltd. Waste Camellia oleifera shells (abbreviated WCOSs) were collected from Jiangxi Green Sea Oil Co., Ltd, China. Phloroglucinol (C6H6O3, ≥99.0%) was purchased from Shanghai Macklin Biochemical Co., Ltd. Potassium dichromate (K2Cr2O7, AR grade) and 4-fluorophenol (C6H5OF, 99%, abbreviated 4-FP) were procured from Aladdin Chemistry Co., Ltd. Diphenylcarbazide (C13H14N4O, AR grade) was bought from Sinopharm Chemical Reagent Co., Ltd. All chemicals were used without further purification. Ultra-pure water was used in the high-performance liquid chromatography analysis. Deionized water was used in photocatalyst preparation.2.2. Preparation
Bio-based carbon quantum dot (BCQD) aqueous precursor was prepared directly from WCOSs based on our previous work.45 In a typical synthesis, 1.6 g of WCOS powder and 0.4 g of phloroglucinol were uniformly dispersed in 60 mL of water by using a 500 W ultrasonic crasher for 1 h. The resulting suspension was stirred for 6 h at room temperature and then hydrothermally treated at 230 °C for 12 h at a heating rate of 1 °C min−1. After hydrothermal treatment, the black suspension was filtered using an organic filter with a pore size of 0.15 μm, and the obtained brown filtrate was BCQD aqueous precursor.Pure g-C3N4 was prepared by directly calcining urea in air. Typically, 30 g of urea powder was put into a 100 mL alumina crucible with a cover. The crucible was heated to 550 °C from room temperature in a muffle furnace at a heating rate of 10 °C min−1. After keeping the temperature at 550 °C for 2 h, the yellow g-C3N4 was obtained by natural cooling.
For the preparation of H+ surface-modified g-C3N4 (H+/g-C3N4), in a typical synthesis, 200 mg of g-C3N4 was uniformly dispersed in 100 mL of HCl diluted with a double volume of water and then stirred at room temperature for 3 h. After centrifugation, the obtained solid sample was washed several times with water. The yellow H+/g-C3N4 was obtained after drying at 60 °C for 12 h.
For the preparation of BCQD surface-modified g-C3N4 (BCQDs/g-C3N4), in a typical synthesis, 500 mg of g-C3N4 was uniformly dispersed in 100 mL of BCQD aqueous precursor by ultrasonic crashing for 1 h. The obtained suspension was stirred at 80 °C for 4 h and then separated by filtration. The brown BCQDs/g-C3N4 was obtained after fixing at 160 °C for 3 h in a vacuum.
For the preparation of H+ and BCQD surface-modified g-C3N4 (H+/BCQDs/g-C3N4), the preparation steps of H+/g-C3N4 were repeated but g-C3N4 was replaced with BCQDs/g-C3N4 to obtain brown H+/BCQDs/g-C3N4.
2.3. Characterization
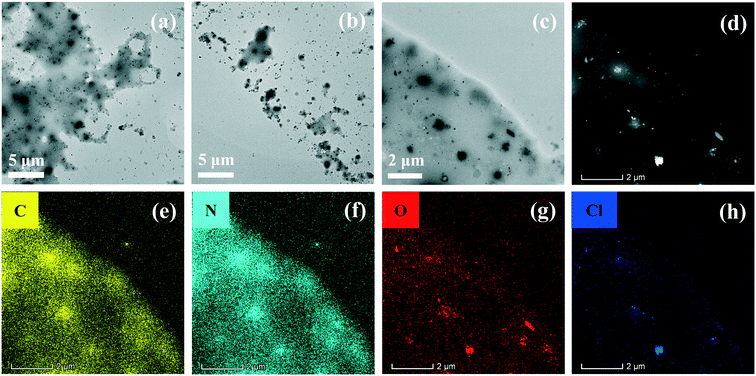
Transmission electron microscopy (TEM) and scanning transmission electron microscopy-high angle annular dark field (STEM-HAADF) images were recorded on a FEI Talos F200X field emission transmission electron microscope. The elemental mappings of the samples were determined using an energy-dispersive X-ray (EDX) spectrometer equipped on the TEM. Nitrogen gas porosimetry measurements were performed on a Quantachrome NOVA 2000e surface area and porosity analyzer after the samples were outgassed under a vacuum at 70 °C for 20 min and 120 °C for 6 h. X-ray diffraction (XRD) patterns were obtained using a D8ADVANCE diffractometer via Cu-Kα radiation with the 2θ angle scanning range of 10–80°. Fourier transform infrared (FTIR) spectra were recorded on a Nicolet iS5 FTIR apparatus. X-ray photoelectron spectroscopy (XPS) was performed using an Axis Ultra DLD instrument with a monochromated Al-Kα source at a residual gas pressure of less than 10−8 Pa. All the binding energies were referenced to the C 1s peak at 284.8 eV of the surface adventitious carbon. Steady-state photoluminescence (PL) and time-resolved photoluminescence (TR-PL) measurements were carried out on an Edinburgh FS5 spectrofluorometer. Electrochemical impendence spectra (EIS), transient photocurrents, and Mott–Schottky plots were tested on a BioLogic VSP electrochemical workstation (Claix, France). Zeta potential measurements were performed using a Malvern MPT-2 automatic titrator. UV-vis/diffuse reflectance spectroscopy (UV-vis/DRS) was conducted using a Lambda 750S UV/VIS/NIR spectrometer.2.4. Photocatalytic tests
A PLS-SXE 300 Xe lamp (300 W, Beijing PerfectLight Co., Ltd., China) with an output wavelength (λ) >320 nm served as the simulated sunlight source. Visible-light irradiation was obtained by removing UV irradiation from the lamp using a 420 nm cutoff filter, which could control λ >420 nm. Then, 30 mg of photocatalyst and 30 mL of reaction solution were poured into a 100 ml beaker with a quartz cover. For the single-component pollution systems, the initial concentrations of Cr(VI) and 4-FP were 10 mg L−1. For the multi-component pollution systems, 30 mL of Cr(VI)/4-FP reaction solution was prepared by mixing 15 mL Cr(VI) and 4-FP solution at a concentration of 20 mg L−1. The reaction solution was stirred in the dark until the adsorption–desorption equilibrium was reached. Subsequently, the light source was switched on, and fixed amounts of reaction solution were taken out at predetermined time intervals during irradiation. Changes in the Cr(VI) concentrations were analyzed via a diphenylcarbazide method by using a Lambda 750S UV/VIS/NIR spectrometer at λ = 540 nm. Changes in the 4-FP concentrations were examined using an Agilent 1100 series high-performance liquid chromatography (HPLC) system with a C18 column and a UV detector (λ = 280 nm). Acetonitrile/water (60/40 v/v) was used as a mobile phase at a flow rate of 1.0 mL min−1. The anoxic photocatalytic tests were realized by continuously passing argon gas into the reaction solution, and other steps were the same as above.3. Results and discussion
3.1. Characterization
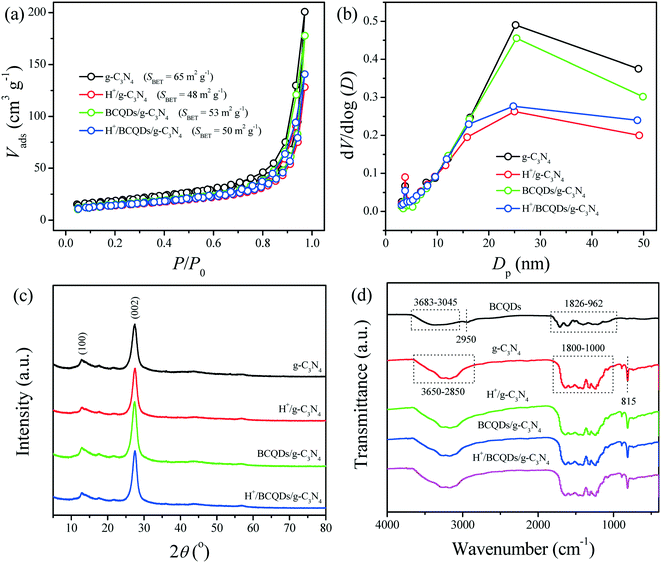 | ||
| Fig. 2 Nitrogen gas adsorption–desorption isotherms (a), pore-size distribution curves (b), XRD patterns (c), and FTIR spectra (d) of as-prepared g-C3N4, H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4. | ||
The phase structures of as-prepared g-C3N4, H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4 were characterized by XRD. As shown in Fig. 2c, all the tested materials exhibit a typical interlayer stacking (002) peak at 27.5° which corresponds to an interlayer distance of d = 0.33 nm, while the (100) peak at 12.6° represents an in-plane structural packing motif with a period of 0.675 nm. The similar XRD patterns of the tested materials indicate that the phase structure of g-C3N4 is not affected by the surface modification.
The chemical structures of as-prepared g-C3N4, H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4 were studied by FTIR spectroscopy. As shown in Fig. 2d, for the BCQDs, the broad peak in the range of 3683–3045 cm−1 is assigned to the stretching vibration of surface –OH, the peak at 2950 cm−1 is assigned to the CH2 units, and the peaks in the range of 1826–962 cm−1 are assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O, C–N, and C–C stretching vibration. For g-C3N4, the broad peak in the range of 3650–2850 cm−1 originated from the stretching vibration of primary (–NH2) and secondary (
C, C–O, C–N, and C–C stretching vibration. For g-C3N4, the broad peak in the range of 3650–2850 cm−1 originated from the stretching vibration of primary (–NH2) and secondary (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH) amines. A series of peaks found in the range of 1800–1000 cm−1 can be attributed to the stretching vibration modes like C–N and C
NH) amines. A series of peaks found in the range of 1800–1000 cm−1 can be attributed to the stretching vibration modes like C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the CN heterocycles. The sharp peak at 815 cm−1 is the typical bending vibration of s-triazine units. The similar FTIR characteristic absorption peaks of as-prepared g-C3N4, H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4 indicate that the chemical structure of g-C3N4 remains intact after surface modification. The FTIR signal of BCQDs is covered by that of g-C3N4, so the FTIR peaks regarding BCQDs cannot be observed in the BCQDs/g-C3N4 and H+/BCQDs/g-C3N4.
N in the CN heterocycles. The sharp peak at 815 cm−1 is the typical bending vibration of s-triazine units. The similar FTIR characteristic absorption peaks of as-prepared g-C3N4, H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4 indicate that the chemical structure of g-C3N4 remains intact after surface modification. The FTIR signal of BCQDs is covered by that of g-C3N4, so the FTIR peaks regarding BCQDs cannot be observed in the BCQDs/g-C3N4 and H+/BCQDs/g-C3N4.
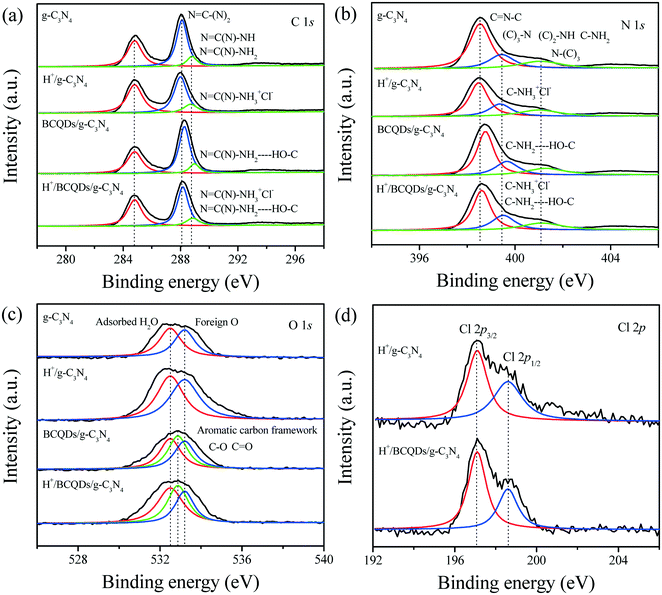
The interactions of surface-modified H+ or BCQDs with g-C3N4 can be studied by high-resolution XPS. As shown in Fig. 3a, the XPS peak of C 1s centered at 284.8 eV is typically assigned to the adventitious reference C element. For the g-C3N4, the XPS peak of C 1s centered at 288.1 eV can be attributed to the sp2 C bonded to N in an aromatic ring (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–(N)2), whereas the XPS peak of C 1s centered at 288.9 eV is assigned to sp2 C in the aromatic ring attached to the primary (N
C–(N)2), whereas the XPS peak of C 1s centered at 288.9 eV is assigned to sp2 C in the aromatic ring attached to the primary (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(N)–NH2) and secondary (N
C(N)–NH2) and secondary (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(N)–NH) amines. For H+/g-C3N4, the shift of sp2 C XPS peaks relative to g-C3N4 indicates that the N
C(N)–NH) amines. For H+/g-C3N4, the shift of sp2 C XPS peaks relative to g-C3N4 indicates that the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(N)–NH3+Cl− species were formed by the acid–base interaction between the surface –NH2 of g-C3N4 and HCl (Scheme 1), and the decreased binding energy of sp2 C can be attributed to the electron-withdrawing effect of H+. For the BCQDs/g-C3N4, the shift of sp2 C XPS peaks relative to g-C3N4 indicates that the N
C(N)–NH3+Cl− species were formed by the acid–base interaction between the surface –NH2 of g-C3N4 and HCl (Scheme 1), and the decreased binding energy of sp2 C can be attributed to the electron-withdrawing effect of H+. For the BCQDs/g-C3N4, the shift of sp2 C XPS peaks relative to g-C3N4 indicates that the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(N)–NH2–HO–C species were formed by the hydrogen bond interaction between the surface –NH2 of g-C3N4 and the surface –OH of BCQDs (Scheme 1), and the increased binding energy of sp2 C can be attributed to the electron-donating effect of BCQDs. As shown in Fig. S1a,† the C 1s XPS peaks of BCQDs centered at 284.8 and 288.9 eV overlaps with that of g-C3N4. Therefore, the characteristic C 1s XPS peaks of BCQDs cannot be observed in BCQDs/g-C3N4. The formation of N
C(N)–NH2–HO–C species were formed by the hydrogen bond interaction between the surface –NH2 of g-C3N4 and the surface –OH of BCQDs (Scheme 1), and the increased binding energy of sp2 C can be attributed to the electron-donating effect of BCQDs. As shown in Fig. S1a,† the C 1s XPS peaks of BCQDs centered at 284.8 and 288.9 eV overlaps with that of g-C3N4. Therefore, the characteristic C 1s XPS peaks of BCQDs cannot be observed in BCQDs/g-C3N4. The formation of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(N)–NH3+Cl− and N
C(N)–NH3+Cl− and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(N)–NH2–HO–C species in the H+/BCQDs/g-C3N4 eventually caused the XPS peaks of sp2 C to move slightly toward the high binding energy direction relative to g-C3N4. The XPS results of N 1s are consistent with that of C 1s. For the N 1s XPS peaks of g-C3N4 shown in Fig. 3b, the peak centered at 398.5 eV is assigned to sp2 hybridized aromatic N atoms bonded to C atoms (C
C(N)–NH2–HO–C species in the H+/BCQDs/g-C3N4 eventually caused the XPS peaks of sp2 C to move slightly toward the high binding energy direction relative to g-C3N4. The XPS results of N 1s are consistent with that of C 1s. For the N 1s XPS peaks of g-C3N4 shown in Fig. 3b, the peak centered at 398.5 eV is assigned to sp2 hybridized aromatic N atoms bonded to C atoms (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C), the peak centered at 399.4 eV is related to either tertiary nitrogen (C)3–N groups linking the structural motif (C6N7) or amino groups carrying hydrogen ((C)2–NH, C–NH2) to structural defects and incomplete condensation, and a weak peak centered at 401.1 eV corresponds to N atoms bonded to three C atoms in the aromatic cycles. The N 1s XPS peaks of H+/g-C3N4 shift to the low binding energy direction relative to g-C3N4 due to the formation of C–NH3+Cl− species by the acid–base interaction between the surface –NH2 of g-C3N4 and HCl. The N 1s XPS peaks of BCQDs/g-C3N4 shift to the high binding energy direction relative to g-C3N4 due to the formation of C–NH2–HO–C species by the hydrogen bond interaction between the surface –NH2 of g-C3N4 and the surface –OH of BCQDs. The characteristic N 1s XPS peaks of BCQDs centered at 400.0 eV cannot be observed in BCQDs/g-C3N4, because the atomic concentration of the N element in BCQDs is very low (Fig. S1b and Table S1†). Compared with g-C3N4, the formation of C–NH3+Cl− and C–NH2–HO–C species in H+/BCQDs/g-C3N4 makes the XPS peaks of N 1s move toward the high binding energy direction slightly. For the O 1s XPS peaks of g-C3N4 and H+/g-C3N4 shown in Fig. 3c, the XPS peak of O 1s centered at 532.5 eV can be attributed to the adsorbed H2O, and the XPS peak of O 1s centered at 533.2 eV is derived from a foreign O element during calcination in air. Compared with g-C3N4 and H+/g-C3N4, the additional XPS peak of O 1s centered at 532.8 eV for the BCQDs/g-C3N4 and H+/BCQDs/g-C3N4 can be attributed to the O element of the aromatic carbon framework in the BCQDs (Fig. 3c and S1c†). For the Cl 2p XPS peaks of H+/g-C3N4 and H+/BCQDs/g-C3N4 shown in Fig. 3d, the XPS peaks of Cl 2p centered at 197.1 and 198.6 eV can be attributed to the Cl 2p3/2 and Cl 2p1/2 electron orbits. The XPS results of Cl 2p further confirmed the successful introduction of H+ on the surface of g-C3N4 and BCQDs/g-C3N4 by the acidification with HCl at room temperature (Scheme 1). As shown in Fig. S1d,† the BCQD precursor prepared by using WCOSs mainly contains C and O elements, and the atomic concentrations of the C and O elements are 74.36% and 24.67%, respectively. In addition, the atomic concentrations of C, N, and O in g-C3N4 did not change obviously after surface modification because surface-modified BCQDs and/or HCl accounted for a small proportion in the as-prepared composites (Table S1†).
N–C), the peak centered at 399.4 eV is related to either tertiary nitrogen (C)3–N groups linking the structural motif (C6N7) or amino groups carrying hydrogen ((C)2–NH, C–NH2) to structural defects and incomplete condensation, and a weak peak centered at 401.1 eV corresponds to N atoms bonded to three C atoms in the aromatic cycles. The N 1s XPS peaks of H+/g-C3N4 shift to the low binding energy direction relative to g-C3N4 due to the formation of C–NH3+Cl− species by the acid–base interaction between the surface –NH2 of g-C3N4 and HCl. The N 1s XPS peaks of BCQDs/g-C3N4 shift to the high binding energy direction relative to g-C3N4 due to the formation of C–NH2–HO–C species by the hydrogen bond interaction between the surface –NH2 of g-C3N4 and the surface –OH of BCQDs. The characteristic N 1s XPS peaks of BCQDs centered at 400.0 eV cannot be observed in BCQDs/g-C3N4, because the atomic concentration of the N element in BCQDs is very low (Fig. S1b and Table S1†). Compared with g-C3N4, the formation of C–NH3+Cl− and C–NH2–HO–C species in H+/BCQDs/g-C3N4 makes the XPS peaks of N 1s move toward the high binding energy direction slightly. For the O 1s XPS peaks of g-C3N4 and H+/g-C3N4 shown in Fig. 3c, the XPS peak of O 1s centered at 532.5 eV can be attributed to the adsorbed H2O, and the XPS peak of O 1s centered at 533.2 eV is derived from a foreign O element during calcination in air. Compared with g-C3N4 and H+/g-C3N4, the additional XPS peak of O 1s centered at 532.8 eV for the BCQDs/g-C3N4 and H+/BCQDs/g-C3N4 can be attributed to the O element of the aromatic carbon framework in the BCQDs (Fig. 3c and S1c†). For the Cl 2p XPS peaks of H+/g-C3N4 and H+/BCQDs/g-C3N4 shown in Fig. 3d, the XPS peaks of Cl 2p centered at 197.1 and 198.6 eV can be attributed to the Cl 2p3/2 and Cl 2p1/2 electron orbits. The XPS results of Cl 2p further confirmed the successful introduction of H+ on the surface of g-C3N4 and BCQDs/g-C3N4 by the acidification with HCl at room temperature (Scheme 1). As shown in Fig. S1d,† the BCQD precursor prepared by using WCOSs mainly contains C and O elements, and the atomic concentrations of the C and O elements are 74.36% and 24.67%, respectively. In addition, the atomic concentrations of C, N, and O in g-C3N4 did not change obviously after surface modification because surface-modified BCQDs and/or HCl accounted for a small proportion in the as-prepared composites (Table S1†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + B2
exp(−t/τ1) + B2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2)], and two decay components, namely, τ1 and τ2, are derived. The short-lifetime component (τ1) usually originates from nonradiative relaxation related to material defects, whereas the long-lifetime component (τ2) can be attributed to radiation produced by the recombination of photo-generated e−–h+ pairs. τ2 of BCQDs/g-C3N4 is shorter than that of g-C3N4, indicating that the recombination number of photo-generated e−–h+ pairs is less in BCQDs/g-C3N4 than in g-C3N4 in the same time zone, suggesting that the electron-donating effect of BCQDs slow down the recombination of photo-generated e−–h+ pairs. Compared with g-C3N4 and BCQDs/g-C3N4, the slightly longer lifetime τ2 of H+/g-C3N4 and H+/BCQDs/g-C3N4 can be attributed to the increased number of photo-generated e−–h+ pairs by the electron-withdrawing effect of H+, resulting in an increased recombination number of photo-generated e−–h+ pairs in the same time zone. As a representative of the overall decay behavior, the average PL lifetimes of as-prepared g-C3N4, H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4 are 4.504, 4.516, 3.224, and 3.230 ns, respectively (Table 1).
exp(−t/τ2)], and two decay components, namely, τ1 and τ2, are derived. The short-lifetime component (τ1) usually originates from nonradiative relaxation related to material defects, whereas the long-lifetime component (τ2) can be attributed to radiation produced by the recombination of photo-generated e−–h+ pairs. τ2 of BCQDs/g-C3N4 is shorter than that of g-C3N4, indicating that the recombination number of photo-generated e−–h+ pairs is less in BCQDs/g-C3N4 than in g-C3N4 in the same time zone, suggesting that the electron-donating effect of BCQDs slow down the recombination of photo-generated e−–h+ pairs. Compared with g-C3N4 and BCQDs/g-C3N4, the slightly longer lifetime τ2 of H+/g-C3N4 and H+/BCQDs/g-C3N4 can be attributed to the increased number of photo-generated e−–h+ pairs by the electron-withdrawing effect of H+, resulting in an increased recombination number of photo-generated e−–h+ pairs in the same time zone. As a representative of the overall decay behavior, the average PL lifetimes of as-prepared g-C3N4, H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4 are 4.504, 4.516, 3.224, and 3.230 ns, respectively (Table 1).
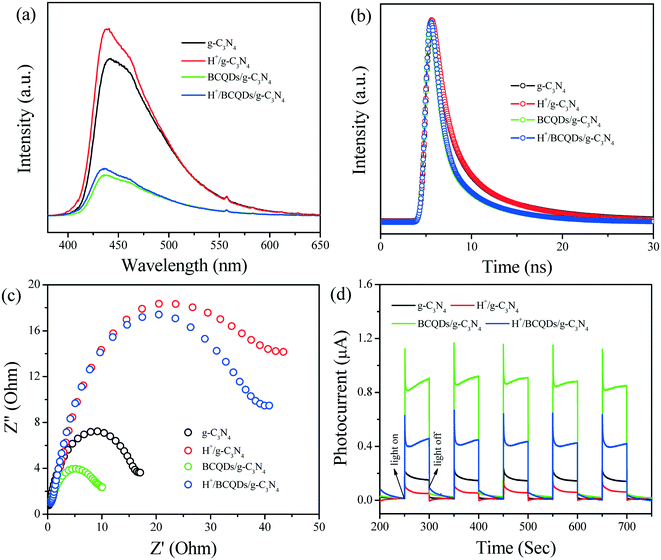 | ||
| Fig. 4 PL spectra (a), TR-PL spectra (b), EIS (c), and transient photocurrent (d) of as-prepared g-C3N4, H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4. | ||
| Photocatalysts | τ 1 (ns) | τ 2 (ns) | Ave. τ (ns) |
|---|---|---|---|
| g-C3N4 | 1.495 | 6.622 | 4.504 |
| H+/g-C3N4 | 1.526 | 6.898 | 4.516 |
| BCQDs/g-C3N4 | 0.965 | 5.105 | 3.224 |
| H+/BCQDs/g-C3N4 | 1.007 | 5.144 | 3.230 |
The transmission of photo-generated charges in the as-prepared g-C3N4, H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4 is investigated by EIS and transient photocurrent. As shown in Fig. 4c, the EIS of BCQDs/g-C3N4 displays a smaller semi-cycle radius than that of g-C3N4, indicating that the charge directional transfer is smoother in BCQDs/g-C3N4 than in g-C3N4 due to the electron-donating effect of BCQDs. Compared with g-C3N4 and BCQDs/g-C3N4, the EIS of H+/g-C3N4 and H+/BCQDs/g-C3N4 display a larger semi-cycle radius, which can be attributed to the enhanced charge directional transfer resistance by the electron-withdrawing effect of surface H+. As shown in Fig. 4d, the prompt increase in transient photocurrent from the light-off to the light-on state is ascribed to the quick directional transfer of photo-generated charges on the surfaces of working electrodes. Compared with g-C3N4, the higher transient photocurrent of BCQDs/g-C3N4 can be attributed to the smoother charge directional transfer due to the electron-donating effect of BCQDs. Compared with g-C3N4 and BCQDs/g-C3N4, the lower transient photocurrent of H+/g-C3N4 and H+/BCQDs/g-C3N4 can be attributed to the enhanced charge directional transfer resistance by the electron-withdrawing effect of surface H+.
The relative amount of surface charges of as-prepared g-C3N4, H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4 at different pH values was characterized via zeta potential measurements. As shown in Fig. 5a, the as-prepared g-C3N4 is positively charged at pH <5 due to the surface adsorption of H+ and negatively charged at pH >5 due to the surface adsorption of hydroxide ions (OH−). Compared with g-C3N4, the increased surface negative charges of BCQDs/g-C3N4 can be attributed to the electron-donating effect of surface-modified BCQDs. Compared with g-C3N4 and BCQDs/g-C3N4, the increased surface positive charges of H+/g-C3N4 and H+/BCQDs/g-C3N4 are derived from the surface-modified H+. Compared with H+/g-C3N4, the decreased surface positive charges of H+/BCQDs/g-C3N4 can be attributed to the cancellation of surface positive charges derived from H+ and surface negative charges derived from BCQDs.
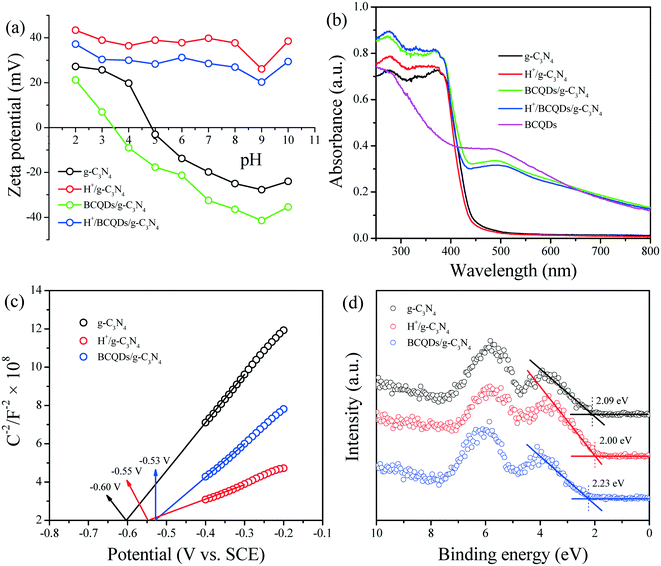 | ||
| Fig. 5 Zeta potential (a), UV-vis/DRS (b), Mott–Schottky plots (c), and XPS VB spectra (d) of as-prepared g-C3N4, H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4. | ||
The light absorption properties of as-prepared g-C3N4, H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4 were studied by UV-vis/DRS. As shown in Fig. 5b, g-C3N4 shows a typical semiconductor absorption within the region of 250–450 nm, which originates from the electron transition from the VB populated by the N 2p electron orbit to the CB formed by the C 2p electron orbit. For the BCQDs, the light absorption in both UV- and visible-light regions can be attributed to the electronic transitions within the BCQDs. Compared with g-C3N4, the increased light absorption for the H+/g-C3N4 and BCQDs/g-C3N4 in the region of 250–450 nm can be attributed to the improved electron transition efficiency by the electron-withdrawing effect of surface H+ and the electron-donating effect of surface BCQDs. The combined effect of surface H+ and BCQDs further improves the light absorption capacity of H+/BCQDs/g-C3N4 in the region of 250–450 nm. With reference to the light absorption of BCQDs in the visible-light region, we can judge that the visible-light absorption of BCQDs/g-C3N4 and H+/BCQDs/g-C3N4 in the region of 450–800 nm originates from BCQDs.
The CB potentials (ECB) of as-prepared g-C3N4, H+/g-C3N4, and BCQDs/g-C3N4 are roughly determined by their corresponding Mott–Schottky plots. As shown in Fig. 5c, the flat band potentials (Vfb) obtained by extrapolation of the Mott–Schottky plots are −0.60, −0.55, and −0.53 V for g-C3N4, H+/g-C3N4, and BCQDs/g-C3N4versus SCE at pH 7, respectively. After correction by adding +0.24 V, the ECB values of g-C3N4, H+/g-C3N4, and BCQDs/g-C3N4 are about −0.36, −0.31, and −0.29 V, respectively. The VB potentials (EVB) of as-prepared g-C3N4, H+/g-C3N4, and BCQDs/g-C3N4 are roughly determined by the XPS VB spectra. As shown in Fig. 5d, the EVB values of g-C3N4, H+/g-C3N4, and BCQDs/g-C3N4 are 2.09, 2.00, and 2.23 eV, respectively. The above Mott–Schottky plots and XPS VB spectra results indicate that the surface-modified H+ and BCQDs act as co-catalysts to change the ECB and EVB of g-C3N4, while surface-modified H+ slightly reduces the bandgap energy of g-C3N4 and the bandgap energy of g-C3N4 is slightly increased by BCQD surface modification.
3.2. Photocatalytic tests
In the field of photocatalytic wastewater treatment, traditional research models focus too much on the micro-kinetics of photocatalytic reactions, such as the degradation routes of target pollutants and hydroxyl radicals generated by photocatalysts, but ignore the macro-thermodynamics of photocatalytic reactions, which is not conducive to the practical application of photocatalytic technology for wastewater treatment. In this article, we establish a macro-thermodynamic model to discuss the synergistic photocatalytic effects of various components in photocatalysts or multi-component pollution systems. Compared with the complex micro-kinetic model, this simple macro-thermodynamic model is more conducive to the industrial development of photocatalytic wastewater treatment. Specifically, in the macro-thermodynamic model, all the elementary reactions will be ignored, there is a redox tendency between the substrates, and the excited photocatalyst looks like an electronic transmitter that switches on the electron transport from the reducing agent (RA) to the oxidizing agent (OA) (Scheme 1). Based on the macro-thermodynamic model, it is not difficult to understand that if one substrate is oxidized or reduced, the other reduced or oxidized substrate must be found in both single- and multi-component pollution systems. In the field of environmental photocatalysis, the photocatalytic reduction of Cr(VI) is essentially that of the electron transport effect of photocatalyst switches on the electron transport from O2− to Cr6+ in Cr2O72− under acidic conditions, and the photocatalytic degradation of organic pollutants is essentially that of the electron transport effect of photocatalyst switches on the electron transport from organic pollutants to dissolved O2. Based on the macro-thermodynamic model, from the perspective of photocatalytic substrates, we have confirmed in our previous studies that the synergistic photocatalytic effect in the 4-FP/Cr(VI) multi-component pollution system can be attributed to the electron transport from 4-FP to Cr(VI) being switched on by the electron transport effect of the photocatalyst.33,34 Therefore, compared with the 4-FP or Cr(VI) single-component pollution system, the degradation and reduction rates of 4-FP and Cr(VI) are simultaneously improved in the 4-FP/Cr(VI) multi-component pollution system. However, based on the macro-thermodynamic model, from the perspective of the photocatalyst, one of the key scientific issues needs to be clarified; that is, what is the difference of the synergistic photocatalytic effect for the photocatalysts with different photocatalytic performance? In order to answer the above question, we fabricated three types of surface-modified g-C3N4 and systematically investigated the photocatalytic performance of as-prepared materials under normal conditions in Cr(VI) reduction, 4-FP degradation, and simultaneous reduction of Cr(VI) with 4-FP degradation photocatalytic systems. The photocatalytic test results can be perfectly explained by the constructed macro-thermodynamic model. In addition, in order to clarify the role of dissolved O2, all photocatalytic tests were repeated under anoxic conditions.The kinetics of aqueous Cr(VI) reduction was studied quantitatively by applying the Langmuir–Hinshelwood first-order model as expressed by the following equation:
| ln(Ct/C0) = −Kt |
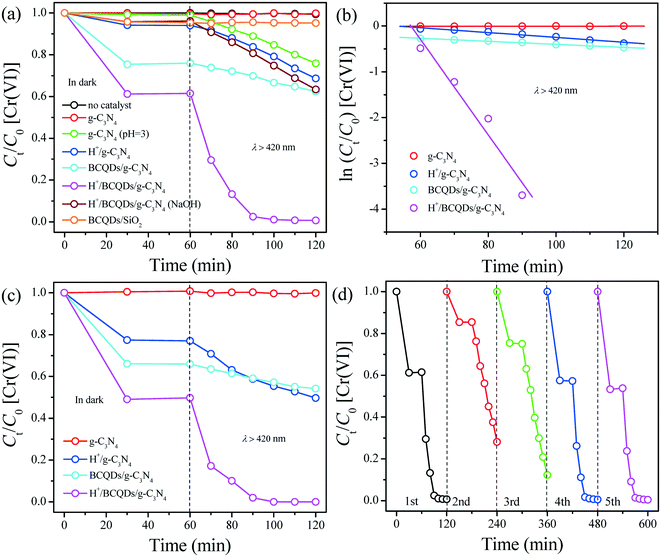
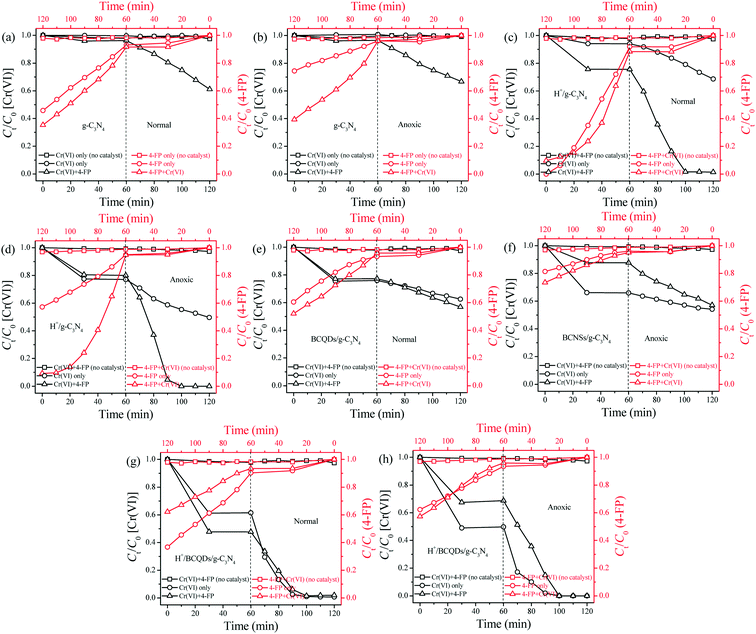
As shown in Fig. 6c, under anaerobic conditions, the photocatalytic activities of as-prepared g-C3N4, H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4 for the aqueous Cr(VI) reduction are basically the same as those under normal conditions, indicating that dissolved O2 in water did not participate in the photocatalytic reduction of aqueous Cr(VI). Compared with normal conditions, the as-prepared H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4 have enhanced adsorption capacity toward Cr(VI) under anaerobic conditions, indicating that the surface modifiers compete for adsorption of Cr(VI) and dissolved O2.
We further clarified the role of surface-modified H+ and BCQDs during aqueous Cr(VI) reduction through the recycling experiments of as-prepared H+/BCQDs/g-C3N4. As shown in Fig. 6d, the photocatalytic activity of H+/BCQDs/g-C3N4 decreased significantly after the first cycle. We regenerated the first cycled H+/BCQDs/g-C3N4 through H+ surface modification, but the activity of the first cycled H+/BCQDs/g-C3N4 did not return to its initial state. Then, we regenerated the second cycled H+/BCQDs/g-C3N4 by H+ and BCQDs surface modification and found that the activity of the second cycled H+/BCQDs/g-C3N4 returned to its original state. We can get the same result by regenerating the third cycled H+/BCQDs/g-C3N4 by H+ and BCQDs surface modification. The above results show that H+ and BCQDs on the surface of g-C3N4 are consumed simultaneously during aqueous Cr(VI) reduction. Specifically, H+ reduces the electron cloud density of O2− in Cr2O72− and makes Cr(VI) have a strong electron-withdrawing ability, BCQDs provide electrons like sacrificial agents, and the electron transport effect of g-C3N4 switch on the electron transport from BCQDs to Cr(VI) (equation C in Scheme 1). Therefore, the as-prepared H+/BCQDs/g-C3N4 has the highest photocatalytic activity for the aqueous Cr(VI) reduction in all the tested materials. The full-range XPS and atomic concentrations results of H+/BCQDs/g-C3N4 before and after photocatalytic reduction of aqueous Cr(VI) are shown in Fig. S6 and Table S3.† The reduced atomic concentrations of C, O, and Cl in the H+/BCQDs/g-C3N4 after the photocatalytic reduction of aqueous Cr(VI) further proves that the surface-modified H+ and BCQDs are involved in the photocatalytic reduction of aqueous Cr(VI). Compared with the fresh H+/BCQDs/g-C3N4, the adsorption capacity of the first cycled H+/BCQDs/g-C3N4 toward Cr(VI) is obviously weakened. After regenerating the first cycled H+/BCQDs/g-C3N4 by H+ surface modification, the adsorption capacity of the first cycled H+/BCQDs/g-C3N4 toward Cr(VI) is restored somewhat. After regenerating the second cycled H+/BCQDs/g-C3N4 by H+ and BCQDs surface modification, the adsorption capacity of the second cycled H+/BCQDs/g-C3N4 toward Cr(VI) is restored completely. The above results indicate that both surface-modified H+ and BCQDs have an attractive effect on Cr(VI).
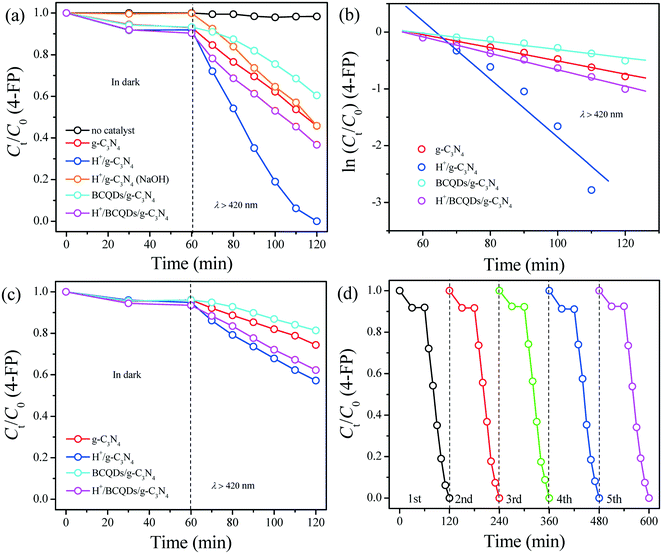
The kinetics of aqueous 4-FP degradation was also studied quantitatively by applying the Langmuir–Hinshelwood first-order model. The relationships between ln(Ct/C0) and reaction time for the aqueous 4-FP degradation over as-prepared photocatalysts are shown in Fig. 7b and the kinetic parameters in the current reaction system are summarized in Table S4.† The reduction rate of H+/g-C3N4 was the fastest among all the tested photocatalysts for aqueous 4-FP degradation under visible-light irradiation.
As shown in Fig. 7c, the photocatalytic activities of as-prepared g-C3N4, H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4 decreased significantly under anoxic conditions because the electron transport from 4-FP to dissolved O2 is impeded.
As shown in Fig. 7d, the as-prepared H+/g-C3N4 basically maintained the original photocatalytic activity toward aqueous 4-FP degradation after five recycles, indicating that the surface-modified H+ did not participate in the photocatalytic degradation of aqueous 4-FP. The as-prepared H+/g-C3N4 shows the highest photocatalytic activity toward aqueous 4-FP degradation in all the tested materials due to the internal synergy between g-C3N4 and H+ in the photocatalyst.
In addition, we added the same number of moles of HCl to the photocatalytic reaction system based on the atomic concentration of Cl element in the H+/BCQDs/g-C3N4. As shown in Fig. S8,† compared with H+/BCQDs/g-C3N4, both adsorption and photocatalytic activity of BCQDs/g-C3N4 (aq HCl) are slightly reduced. This result shows that the modified H+ on the surface of photocatalyst is more conducive to the capture and removal of target pollutants compared with H+ in the reaction solution.
As shown in Fig. 8c, for the H+/g-C3N4, in the simultaneous reduction of Cr(VI) with 4-FP degradation photocatalytic system, the electron transport from O2− to Cr(VI) in Cr2O72− is switched on by the electron transport effect of g-C3N4 because the electron cloud density of O2− in Cr2O72− is decreased by the surface-modified H+, the electron transport from 4-FP to dissolved O2 is accelerated because surface-modified H+ ions induce g-C3N4 to produce more photo-generated e−–h+ pairs, and the electron transport from 4-FP to Cr(VI) is accelerated by the surface-modified H+ because the electron cloud density of O2− in Cr2O72− is decreased (equations A, D, and G in Scheme 1). Therefore, relative to single-component pollution systems, the external synergy between Cr(VI) and 4-FP in the reaction solution and the internal synergy between g-C3N4 and H+ in the photocatalyst significantly increase the reduction and degradation rates of Cr(VI) and 4-FP in the multi-component pollution system. In addition, the degradation rate of 4-FP decreased slightly after 40 min of photocatalytic reaction due to consumption of Cr(VI) and H+. As shown in Fig. 8d, in the simultaneous reduction of Cr(VI) with 4-FP degradation photocatalytic system, the Cr(VI) reduction and 4-FP degradation rates under anoxic condition are similar to those of normal condition due to the external synergy between Cr(VI) and 4-FP in the reaction solution and the internal synergy between g-C3N4 and H+ in the photocatalyst (equations A and G in Scheme 1).
As shown in Fig. 8e, for the BCQDs/g-C3N4, the electron transport from BCQDs to Cr(VI), from 4-FP to dissolved O2, from BCQDs to dissolved O2, and from 4-FP to Cr(VI) are switched on by the electron transport effect of g-C3N4 in the simultaneous reduction of Cr(VI) with 4-FP degradation photocatalytic system (equations B, D, E, and F in Scheme 1). Therefore, relative to single-component pollution systems, the Cr(VI) reduction and 4-FP degradation are simultaneously accelerated in the multi-component pollution system due to the external synergy between Cr(VI) and 4-FP in the reaction solution. As shown in Fig. 8f, under anoxic conditions, the electron transport from BCQDs to Cr(VI) and from 4-FP to Cr(VI) are switched on by the electron transport effect of g-C3N4 in the simultaneous reduction of Cr(VI) with 4-FP degradation photocatalytic system (equations B and F in Scheme 1). Relative to single-component pollution systems, both Cr(VI) reduction and 4-FP degradation can also be accelerated in the multi-component pollution system due to the external synergy between Cr(VI) and 4-FP in the reaction solution.
As shown in Fig. 8g, for the H+/BCQDs/g-C3N4, the electron transport from O2− to Cr(VI) in Cr2O72− is switched on by g-C3N4 and the electron transport from BCQDs to Cr(VI), from 4-FP to dissolved O2, from BCQDs to dissolved O2, and from 4-FP to Cr(VI) are accelerated by surface-modified H+ (equations A, C, D, E, and G in Scheme 1). As shown in Fig. 8h, under anoxic conditions, the electron transport from O2− to Cr(VI) in Cr2O72− is switched on and the electron transport from BCQDs to Cr(VI) and from 4-FP to Cr(VI) are accelerated (equations A, C, and G in Scheme 1). The reduction rate of Cr(VI) is fast in the Cr(VI) reduction photocatalytic system due to the coexistence of electron transport from O2− and BCQDs to Cr(VI), and the degradation rate of 4-FP is slow in the 4-FP degradation photocatalytic system due to the coexistence of electron transport from 4-FP and BCQDs to dissolved O2. Relative to single-component pollution systems, the accelerated Cr(VI) reduction and 4-FP degradation efficiencies can no longer be observed in the multi-component pollution system both under normal and anoxic conditions due to the competition between too many types of electron transport toward the photo-generated carriers both in the photocatalyst and in the reaction solution, resulting in a severe shortage of total supplied photo-generated carriers.
4. Conclusions
H+/g-C3N4, BCQDs/g-C3N4, and H+/BCQDs/g-C3N4 were successfully fabricated by simple and low-cost strategies. The as-prepared materials showed unique photocatalytic performance in the Cr(VI) reduction, 4-FP degradation, and simultaneous reduction of Cr(VI) with 4-FP degradation photocatalytic systems under normal and anoxic conditions. Based on the macro-thermodynamic model, the highest photocatalytic activity of H+/BCQDs/g-C3N4 toward Cr(VI) reduction among all the tested materials can be attributed to the internal synergy between H+, BCQDs, and g-C3N4 in H+/BCQDs/g-C3N4. The highest photocatalytic activity of H+/g-C3N4 toward 4-FP degradation among all the tested materials is due to the internal synergy between H+ and g-C3N4 in H+/g-C3N4. Compared with the single-component pollution system, the Cr(VI) reduction and 4-FP degradation by g-C3N4, H+/g-C3N4, and BCQDs/g-C3N4 are simultaneously accelerated in the multi-component pollution system due to the existence of external synergy between Cr(VI) and 4-FP in the reaction solution and internal synergy between g-C3N4 and H+ in the photocatalyst. However, the synergistic photocatalytic effects in the multi-component pollution system can no longer be observed by using H+/BCQDs/g-C3N4 as a photocatalyst because too many types of redox reactions in the photocatalyst and the reaction solution compete for the photo-generated carriers.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Major International (Regional) Joint Research Program of China (51720105001), the National Natural Science Foundation of China (51568049) and the Natural Science Foundation of Jiangxi Province, China (20171ACB21035).References
- Y. Zou, X. Wang, A. Khan, P. Wang, Y. Liu, A. Alsaedi, T. Hayat and X. Wang, Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review, Environ. Sci. Technol., 2016, 50, 7290–7304 CrossRef CAS PubMed.
- S. Murgolo, S. Franz, H. Arab, M. Bestetti, E. Falletta and G. Mascolo, Degradation of emerging organic pollutants in wastewater effluents by electrochemical photocatalysis on nanostructured TiO2 meshes, Water Res., 2019, 164, 114920 CrossRef CAS PubMed.
- H. Yoneyama, Y. Yamashita and H. Tamura, Heterogeneous photocatalytic reduction of dichromate on n-type semiconductor catalysts, Nature, 1979, 282, 817–818 CrossRef CAS.
- Z. Yi, J. Ye, N. Kikugawa, T. Kako, S. Ouyang, H. Stuart-Williams, H. Yang, J. Cao, W. Luo, Z. Li, Y. Liu and R. L. Withers, An orthophosphate semiconductor with photooxidation properties under visible-light irradiation, Nat. Mater., 2010, 9, 559–564 CrossRef CAS PubMed.
- Y. Zhong, X. Qiu, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, Flexible electrospun carbon nanofiber/tin(IV) sulfide core/sheath membranes for photocatalytically treating chromium(VI)-containing wastewater, ACS Appl. Mater. Interfaces, 2016, 8, 28671–28677 CrossRef CAS PubMed.
- X. Q. Qiao, Z. W. Zhang, Q. H. Li, D. Hou, Q. Zhang, J. Zhang, D. S. Li, P. Feng and X. Bu, In situ synthesis of n–n Bi2MoO6 & Bi2S3 heterojunctions for highly efficient photocatalytic removal of Cr(VI), J. Mater. Chem. A, 2018, 6, 22580–22589 RSC.
- H. Li, F. Deng, Y. Zheng, L. Hua, C. Qu and X. Luo, Visible-light-driven Z-scheme rGO/Bi2S3–BiOBr heterojunctions with tunable exposed BiOBr (102) facets for efficient synchronous photocatalytic degradation of 2-nitrophenol and Cr(VI) reduction, Environ. Sci.: Nano, 2019, 6, 3670–3683 RSC.
- S. Bagheri, A. T. Yousefi and T. O. Do, Photocatalytic pathway toward degradation of environmental pharmaceutical pollutants: structure, kinetics and mechanism approach, Catal. Sci. Technol., 2017, 7, 4548–4569 RSC.
- C. C. Wang, J. R. Li, X. L. Lv, Y. Q. Zhang and G. Guo, Photocatalytic organic pollutants degradation in metal–organic frameworks, Energy Environ. Sci., 2014, 7, 2831–2867 RSC.
- L. Wang, D. W. Bahnemann, L. Bian, G. Dong, J. Zhao and C. Wang, Two-dimensional layered zinc silicate nanosheets with excellent photocatalytic performance for organic pollutant degradation and CO2 conversion, Angew. Chem., Int. Ed., 2019, 58, 8103–8108 CrossRef CAS PubMed.
- W. J. Ong, L. L. Tan, Y. H. Ng, S. T. Yong and S. P. Chai, Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability?, Chem. Rev., 2016, 116, 7159–7329 CrossRef CAS PubMed.
- Q. Wang and K. Domen, Particulate photocatalystsfor light-driven water splitting: mechanisms, challenges, and design strategies, Chem. Rev., 2020, 120, 919–985 CrossRef CAS PubMed.
- X. Li, J. Yu, M. Jaroniec and X. Chen, Cocatalysts for selective photoreduction of CO2 into solar fuels, Chem. Rev., 2019, 119, 3962–4179 CrossRef CAS PubMed.
- H. Yamashita, K. Mori, Y. Kuwahara, T. Kamegawa, M. Wen, P. Verma and M. Che, Single-site and nano-confined photocatalysts designed in porous materials for environmental uses and solar fuels, Chem. Soc. Rev., 2018, 47, 8072–8096 RSC.
- W. Wang, M. O. Tadé and Z. Shao, Research progress of perovskite materials in photocatalysis- and photovoltaics-related energy conversion and environmental treatment, Chem. Soc. Rev., 2015, 44, 5371–5408 RSC.
- M. Humayun, Y. Qu, F. Raziq, R. Yan, Z. Li, X. Zhang and L. Jing, Exceptional visible-light activities of TiO2-coupled N-doped porous perovskite LaFeO3 for 2,4-dichlorophenol decomposition and CO2 conversion, Environ. Sci. Technol., 2016, 50, 13600–13610 CrossRef CAS PubMed.
- M. Nolan, A. Iwaszuk, A. K. Lucid, J. J. Carey and M. Fronzi, Design of novel visible light active photocatalyst materials: surface modified TiO2, Adv. Mater., 2016, 28, 5425–5446 CrossRef CAS PubMed.
- A. Mukherji, R. Marschall, A. Tanksale, C. Sun, S. C. Smith, G. Q. Lu and L. Wang, N-doped CsTaWO6 as a new photocatalyst for hydrogen production from water splitting under solar irradiation, Adv. Funct. Mater., 2011, 21, 126–132 CrossRef CAS.
- A. Iwaszuk and M. Nolan, SnO-nanocluster modified anatase TiO2 photocatalyst: exploiting the Sn(II) lone pair for a new photocatalyst material with visible light absorption and charge carrier separation, J. Mater. Chem. A, 2013, 1, 6670–6677 RSC.
- Z. Wang, C. Li and K. Domen, Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting, Chem. Soc. Rev., 2019, 48, 2109–2125 RSC.
- Q. Wang, P. Wang, P. Xu, Y. Li, J. Duan, G. Zhang, L. Hu, X. Wang and W. Zhang, Visible-light-driven photo-Fenton reactions using Zn1-1.5xFexS/g-C3N4 photocatalyst: Degradation kinetics and mechanisms analysis, Appl. Catal., B, 2020, 266, 118653 CrossRef.
- S. W. Lv, J. M. Liu, N. Zhao, C. Y. Li, Z. H. Wang and S. Wang, Benzothiadiazole functionalized Co-doped MIL-53-NH2 with electron deficient units for enhanced photocatalytic degradation of bisphenol A and ofloxacin under visible light, J. Hazard. Mater., 2020, 387, 122011 CrossRef CAS PubMed.
- M. F. Atitar, A. Bouziani, R. Dillert, M. E. Azzouzi and D. W. Bahnemann, Photocatalytic degradation of the herbicide imazapyr: do the initial degradation rates correlate with the adsorption kinetics and isotherms?, Catal. Sci. Technol., 2018, 8, 985–995 RSC.
- F. Chen, Q. Yang, J. Sun, F. Yao, S. Wang, Y. Wang, X. Wang, X. Li, C. Niu, D. Wang and G. Zeng, Enhanced photocatalytic degradation of tetracycline by AgI/BiVO4 heterojunction under visible-light irradiation: mineralization efficiency and mechanism, ACS Appl. Mater. Interfaces, 2016, 8, 32887–32900 CrossRef CAS PubMed.
- J. Ding, J. Ming, D. Lu, W. Wu, M. Liu, X. Zhao, C. Li, M. Yang and P. Fang, Study of the enhanced visible-light-sensitive photocatalytic activity of Cr2O3-loaded titanate nanosheets for Cr(VI) degradation and H2 generation, Catal. Sci. Technol., 2017, 7, 2283–2297 RSC.
- W. Hao, X. Li, L. Qin, S. Han and S. Z. Kang, Facile preparation of Ti3+ self-doped TiO2 nanoparticles and their dramatic visible photocatalytic activity for the fast treatment of highly concentrated Cr(VI) effluent, Catal. Sci. Technol., 2019, 9, 2523–2531 RSC.
- D. K. Padhi, T. K. Panigrahi, K. Parida, S. K. Singh and P. M. Mishra, Green synthesis of Fe3O4/RGO nanocomposite with enhanced photocatalytic performance for Cr(VI) reduction, phenol degradation, and antibacterial activity, ACS Sustainable Chem. Eng., 2017, 5, 10551–10562 CrossRef CAS.
- H. Zhao, Q. Xia, H. Xing, D. Chen and H. Wang, Construction of pillared-layer MOF as efficient visible-light photocatalysts for aqueous Cr(VI) reduction and dye degradation, ACS Sustainable Chem. Eng., 2017, 5, 4449–4456 CrossRef CAS.
- Q. Li, X. Zhao, J. Yang, C. J. Jia, Z. Jin and W. Fan, Exploring the effects of nanocrystal facet orientations in g-C3N4/BiOCl heterostructures on photocatalytic performance, Nanoscale, 2015, 7, 18971–18983 RSC.
- Q. Zhang, S. Hu, Z. Fan, D. Liu, Y. Zhao, H. Ma and F. Li, Preparation of g-C3N4/ZnMoCdS hybrid heterojunction catalyst with outstanding nitrogen photofixation performance under visible light via hydrothermal post-treatment, Dalton Trans., 2016, 45, 3497–3505 RSC.
- L. Han, Y. Zhong, K. Lei, D. Mao, Y. Dong, G. Hong, Y. Zhou and D. Fang, Carbon dot–SnS2 heterojunction photocatalyst for photoreduction of Cr(VI) under visible light: A combined experimental and first-principles DFT study, J. Phys. Chem. C, 2019, 123, 2398–2409 CrossRef CAS.
- W. Wang, J. C. Yu, D. Xia, P. K. Wong and Y. Li, Graphene and g-C3N4 nanosheets cowrapped elemental α-sulfur as a novel metal-free heterojunction photocatalyst for bacterial inactivation under visible-light, Environ. Sci. Technol., 2013, 47, 8724–8732 CrossRef CAS PubMed.
- K. Li, Z. Huang, S. Zhu, S. Luo, L. Yan, Y. Dai, Y. Guo and Y. Yang, Removal of Cr(VI) from water by a biochar-coupled g-C3N4 nanosheets composite and performance of a recycled photocatalyst in single and combined pollution systems, Appl. Catal., B, 2019, 243, 386–396 CrossRef CAS.
- K. Wei, K. Li, L. Yan, S. Luo, H. Guo, Y. Dai and X. Luo, One-step fabrication of g-C3N4 nanosheets/TiO2 hollow microspheres heterojunctions with atomic level hybridization and their application in the multi-component synergistic photocatalytic systems, Appl. Catal., B, 2018, 222, 88–98 CrossRef CAS.
- C. Yu, D. Zeng, Q. Fan, K. Yang, J. Zeng, L. Wei, J. Yi and H. Ji, The distinct role of boron doping in Sn3O4 microspheres for synergistic removal of phenols and Cr(VI) in simulated wastewater, Environ. Sci.: Nano, 2020, 7, 286–303 RSC.
- A. Jin, X. Liu, M. Li, Y. Jia, C. Chen and X. Chen, One-pot ionothermal synthesized carbon nitride heterojunction nanorods for simultaneous photocatalytic reduction and oxidation reactions: synergistic effect and mechanism insight, ACS Sustainable Chem. Eng., 2019, 7, 5122–5133 CrossRef CAS.
- Y. Yang, G. Wang, Q. Deng, D. H. L. Ng and H. Zhao, Microwave-assisted fabrication of nanoparticulate TiO2 microspheres for synergistic photocatalytic removal of Cr(VI) and methyl orange, ACS Appl. Mater. Interfaces, 2014, 6, 3008–3015 CrossRef CAS PubMed.
- C. Dong, J. Ji, B. Shen, M. Xing and J. Zhang, Enhancement of H2O2 decomposition by the co-catalytic effect of WS2 on the Fenton reaction for the synchronous reduction of Cr(VI) and remediation of phenol, Environ. Sci. Technol., 2018, 52, 11297–11308 CrossRef CAS PubMed.
- M. Li, S. Song, C. Su, L. Li, Z. Yan and X. Cao, MOF-templated in situ fabrication of surface-modified Ni/graphitic carbon nitride with enhanced photocatalytic hydrogen evolution, Catal. Sci. Technol., 2019, 9, 3828–3835 RSC.
- S. S. Yi, J. M. Yan and Q. Jiang, Carbon quantum dot sensitized integrated Fe2O3@g-C3N4 core–shell nanoarray photoanode towards highly efficient water oxidation, J. Mater. Chem. A, 2018, 6, 9839–9845 RSC.
- Y. Wang, F. Wang, Y. Feng, Z. Xie, Q. Zhang, X. Jin, H. Liu, Y. Liu, W. Lv and G. Liu, Facile synthesis of carbon quantum dots loaded with mesoporous g-C3N4 for synergistic absorption and visible light photodegradation of fluoroquinolone antibiotics, Dalton Trans., 2018, 47, 1284–1293 RSC.
- G. Dong, L. Yang, F. Wang, L. Zang and C. Wang, Removal of nitric oxide through visible light photocatalysis by g-C3N4 modified with perylene imides, ACS Catal., 2016, 6, 6511–6519 CrossRef CAS.
- Z. Li, X. Meng and Z. Zhang, Fabrication of surface hydroxyl modified g-C3N4 with enhanced photocatalytic oxidation activity, Catal. Sci. Technol., 2019, 9, 3979–3993 RSC.
- F. Wang, Y. Wang, Y. Wu, D. Wei, L. Li, Q. Zhang, H. Liu, Y. Liu, W. Lv and G. Liu, Template-free synthesis of oxygen-containing ultrathin porous carbon quantum dots/g-C3N4 with superior photocatalytic activity for PPCPs remediation, Environ. Sci.: Nano, 2019, 6, 2565–2576 RSC.
- W. Ma, K. Li, H. Guo, L. Yan, Y. Dai, X. Luo and Y. Yao, Fabrication of porous carbon microspheres with numerous spherical microstructures directly from waste Camellia oleifera shells and their application in sustained-release of 5-fluorouracil, Microporous Mesoporous Mater., 2017, 250, 195–202 CrossRef CAS.
- S. Wang, L. Li, Z. Zhu, M. Zhao, L. Zhang, N. Zhang, Q. Wu, X. Wang and G. Li, Remarkable improvement in photocatalytic performance for tannery wastewater processing via SnS2 modified with N-doped carbon quantum dots: Synthesis, characterization, and 4-nitrophenol-aided Cr(VI) photoreduction, Small, 2019, 15, 1804515 CrossRef PubMed.
- X. Deng, Y. Chen, J. Wen, Y. Xu, J. Zhu and Z. Bian, Polyaniline-TiO2 composite photocatalysts for light-driven hexavalent chromium ions reduction, Sci. Bull., 2020, 65, 105–112 CrossRef CAS.
- J. Wu, W. Wang, Y. Tian, C. Song, H. Qiu and H. Xue, Piezotronic effect boosted photocatalytic performance of heterostructured BaTiO3/TiO2 nanofibers for degradation of organic pollutants, Nano Energy, 2020, 77, 105122 CrossRef CAS.
- L. Bergamonti, C. Bergonzi, C. Graiff, P. P. Lottici, R. Bettini and L. Elviri, 3D printed chitosan scaffolds: A new TiO2 support for the photocatalytic degradation of amoxicillin in water, Water Res., 2019, 163, 114841 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0en00759e |
| This journal is © The Royal Society of Chemistry 2021 |