Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nuclear spin relaxation as a probe of zeolite acidity: a combined NMR and TPD investigation of pyridine in HZSM-5†
Neil
Robinson
 a,
Pierre
Bräuer
b,
Andrew P. E.
York
c and
Carmine
D'Agostino
a,
Pierre
Bräuer
b,
Andrew P. E.
York
c and
Carmine
D'Agostino
 *d
*d
aDepartment of Chemical Engineering, University of Western Australia, 35 Stirling Highway, Perth, WA 6009, Australia
bDepartment of Chemical Engineering and Biotechnology, University of Cambridge, Philippa Fawcett Drive, Cambridge, CB3 0AS, UK
cJohnson Matthey Technology Centre, Blount's Court, Sonning Common, Reading, RG4 9NH, UK
dDepartment of Chemical Engineering and Analytical Science, University of Manchester, The Mill, Sackville Street, Manchester, M13 9PL, UK. E-mail: carmine.dagostino@manchester.ac.uk
First published on 18th June 2021
Abstract
The relative surface affinities of pyridine within microporous HZSM-5 zeolites are explored using two-dimensional 1H nuclear magnetic resonance (NMR) relaxation time measurements. The dimensionless ratio of longitudinal-to-transverse nuclear spin relaxation times T1/T2 is shown to exhibit strong sensitivity to the silica/alumina ratio (SAR) of these zeolites, which is indicative of material acidity. This trend is interpreted in terms of increased pyridine surface affinity with decreasing SAR. Temperature programmed desorption (TPD) analysis corroborates this observation, revealing a distinct increase in the heat of desorption associated with adsorbed pyridine as a function of decreasing SAR. A direct correlation between NMR and TPD data suggests NMR relaxation time analysis can be a valuable tool for the non-invasive characterisation of adsorption phenomena in microporous solids.
Introduction
Microporous solids (exhibiting pore diameters <2 nm) such as zeolites and metal organic frameworks have potential applications across a variety of processes including chemical conversion, storage, sensing and separations.1,2 In the field of heterogeneous catalysis zeolites are regularly applied to facilitate a range of reactions such as cracking,3,4 alkylation5 and dehydration.6–8 A key feature regarding the activity of such materials is that of surface acidity, characterised by the presence of Brønsted (proton donating) and/or Lewis (electron accepting) acid sites within the micropore network, and across the external material surface. As both the accessibility and acidity of these sites dictate the potential catalytic activity of zeolitic materials, extensive research efforts have been directed towards their characterisation.9,10Established techniques used to investigate the surface acidity of zeolites include infrared (IR) spectroscopy, temperature programmed desorption (TPD) and nuclear magnetic resonance (NMR) spectroscopy. The use of IR spectroscopy with pyridine as a probe molecule, for example, is particularly powerful since the assignment of vibrational modes associated with pyridinium ions at Brønsted sites and the coordination of complexes at Lewis sites are well-established.11–13 Quantitative analysis in terms of adsorbate density is also possible if molar extinction coefficient values are known.14 TPD analysis – again utilising basic probe molecules such as ammonia and pyridine – is also widely applied.15–19 Typical TPD spectra report the desorption rate of the chosen probe molecule as a function of temperature; the area beneath such a curve is proportional to the amount of adsorbate present, providing quantification of acid site density, while the position of desorption peaks provides information on acid site strength. Magic angle spinning (MAS) solid state NMR spectroscopy measurements of zeolitic materials are extensively reported; such measurements provide a direct and quantitative probe of Brønsted acid site density via1H (proton) analysis and utilise the observed 1H chemical shift values to both characterise site acidity20 and differentiate between bridging (Si–OH–Al) and terminal (Al–OH or Si–OH) groups.21 The measurement of 29Si and 27Al spectra also allows quantification of the material silica/alumina ratio (SiO2/Al2O3), which is considered an analogue of zeolite acidity.22 Indirect measurements of acid site characteristics are again possible via the use of probe molecules and facilitate the investigation of site accessibility. While 1H chemical shift features may be exploited to detail probe molecule interactions, a wide range of heteronuclear MAS NMR experiments (including 13C, 15N and 31P) have also been used to resolve adsorbate resonances.23–27
In the present work we detail an alternative magnetic resonance technique for the comparison and characterisation of zeolitic acidity based on analysis of the 1H nuclear spin relaxation characteristics of a liquid-phase basic probe molecule. The past decade has seen a rapid evolution in the application and interpretation of nuclear spin relaxation phenomena as a probe of surface affinity and adsorbate behaviour within catalytically active porous media.28,29 These measurements exploit relevant NMR pulse sequences to determine the rates longitudinal and/or transverse nuclear spin relaxation processes, which are characterised by the time constants T1 and T2, respectively. Within the unrestricted bulk liquid phase these time constants are known to conform to well-established relationships with molecular rotational and translational dynamics.30 For liquids imbibed within porous solids, however, the correspondence between time constants and molecular dynamics is influenced by the pore structure and surface chemistry properties of the confining material, providing a potential route for the non-destructive characterisation of adsorption phenomena and confinement effects.
For fluids confined to catalytically active porous media the evaluation and interpretation of dimensionless relaxation time ratios is often of particular utility.31,32 The ratio of longitudinal-to-transverse nuclear spin relaxation time constants T1/T2 is now established as a non-invasive probe of surface affinity,33 and is a regularly sought metric to aid in the evaluation mesoporous catalyst materials.34–38 Most notably, this ratio has been shown to correlate with the desorption energetics of liquids imbibed within mesoporous oxide materials as evaluated via both experimental32 (TPD) and theoretical39 (density functional theory) methods, and has been demonstrated as a useful probe of competitive adsorption processes in liquid-phase catalytic systems.40–42 It is of interest to note, however, that this approach is yet to be applied to the evaluation of liquid-saturated microporous materials, with previous relaxation studies instead focussing on the investigation of gas admission and storage phenomena,43–46 surface area screening protocols47,48 and the study of confinement effects.49–52 To this end, we detail here the measurement and interpretation of T1/T2 ratios exhibited by pyridine confined to the microporous zeolite HZSM-5 with varying silica/alumina ratios (SAR, a measure of zeolite acidity). Through a direct comparison with TPD analysis our results demonstrate for the first time a clear correlation between nuclear spin relaxation characteristics, SAR and pyridine desorption energetics.
Relaxation theory
For fluids confined to porous media the observed rates of nuclear spin relaxation Ti−1 (with i ∈ {1,2}) may be expressed as a linear combination of unrestricted bulk, surface, and topological contributions,53 | (1) |
Additional terms may also be required to fully describe transverse relaxation (i = 2) rates due to the influence of magnetic susceptibility differences between the confining solid and imbibed fluid.54,55 Here Ti,bulk−1 and D are the relaxation rates and self-diffusion coefficient of the unrestricted bulk fluid, respectively, α is a shape parameter that takes values of 1, 2 or 3 for planar, cylindrical or spherical pores, respectively, and dp is the pore diameter. The surface relativities ρi = δTi,surf−1 are defined by the relaxation rates of species at the pore surface Ti,surf−1 weighted by the length-scale of the adsorbed surface layer δ.56 Enhanced rates of relaxation occur at the solid–liquid interface due to the reduction in rotational and translational molecular mobility upon adsorption35,57 and through interactions with any paramagnetic species imbedded within the solid matrix,58,59 such that Ti,surf−1 ≫ Ti,bulk−1. As Ti,surf exhibits sensitivity to the surface chemistry of the porous medium under investigation,60–62 this parameter is central to the characterisation of surface interactions using nuclear spin relaxation measurements.63
There exists two limiting cases for eqn (1), which may be defined according to the dimensionless parameter59
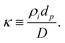 | (2) |
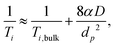 | (3) |
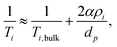 | (4) |
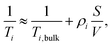 | (5) |
In the case of microporous zeolites careful consideration of an appropriate form of such expressions is required. In the present case, that of pyridine relaxation within HZSM-5 with various SAR, we note that the micropore diameter (dp = 5.1–5.6 Å)67 and molecular kinetic diameter (dk ≈ 5.3 Å)68 are essentially identical, such that there will be no contribution to the observed relaxation rates from bulk liquid away from the pore walls. An appropriate relaxation expression is therefore
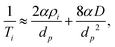 | (6) |
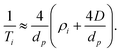 | (7) |
The corresponding ratio of observed relaxation time constants then becomes
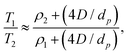 | (8) |
Experimental
Materials and sample preparation
Pyridine (≥99%) was obtained from Alfa Aesar and used as supplied. ZSM-5 zeolite powders exhibiting a range of SAR values (23, 30, 50, 80 and 300) were obtained from Alfa Aesar in NH4+ form. The solid powders were calcined in synthetic air (Air Liquide, 100 mL min−1) at 773 K for 4 hours to obtain the protonated form, HZSM-5. We note these materials have been characterised elsewhere via argon sorption measurements, infrared spectroscopy, elemental analysis, solid state 27Al MAS NMR spectroscopy and tapered element oscillating microbalance experiments.70–72Samples for TPD and NMR analysis were first prepared by pressing each zeolite powder into tablets using a manual hydraulic press. A 2 tonne compressive force was applied to approximately 250 mg of powder in each case, forming cylindrical tables measuring around 13 mm in dimeter and 1 mm in thickness. The tablets were then broken into approximately 10 mg pieces so as to fit within the active regions of the TPD and NMR equipment. Each material was dried in N2 (Air Liquide, 100 mL min−1) at 673 K for 1 hour to remove any adsorbed water, and soaked in excess pyridine under ambient conditions for at least 24 hours.
NMR relaxation measurements
1H NMR relaxation measurements were performed using a Bruker DMX 300 spectrometer equipped with a 7.1 T superconducting magnet, corresponding to a 1H frequency of 300.13 MHz. Experiments were performed under ambient pressure and at 298 ± 1 K as controlled by a Bruker Variable Temperature (BVT 3000) unit.Pyridine-saturated zeolite materials were first placed onto a pre-soaked filter paper to remove any excess liquid on the external surface, then transferred to sealed 5 mm NMR tubes. To minimise experimental uncertainties associated with the evaporation of pyridine from the zeolite structures during NMR analysis, the atmosphere within each NMR tube was saturated by placing a pyridine-soaked plug of filter paper beneath the cap. Each sample was left within the magnet bore for at least 15 minutes prior to analysis to attain thermal equilibrium.
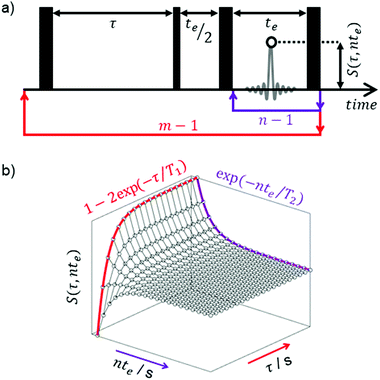
T 1–T2 correlation data was acquired by applying the two-dimensional (2D) NMR pulse sequence in Fig. 1, which comprises an inversion recovery component followed by a CPMG echo train.73 The indirect (T1) dimension was encoded using m = 16τ recovery times between 1 ms and 10 s, while data in the direct (T2) dimension was acquired by taking the magnitude of n = 512 spin echoes separated by an echo time of te = 0.5 ms. Echo magnitudes S(τ,nte) were acquired as a single data point (white data point in Fig. 1) generating an m × n data matrix with no spectral resolution. Each experiment took approximately 30 minutes to complete and included 16 repeat scans separated by a recycle delay of 5T1.
The acquired 2D NMR relaxation data may be described by a Fredholm integral equation of the first kind,74
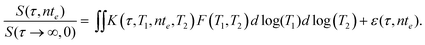 | (9) |
Here S(τ,nte)/S(τ → ∞,0) is the normalised spin echo magnitude and ε(τ,nte) represents the experimental noise, assumed Gaussian with zero mean. The kernel function K(τ,T1,nte,T2) describes the predicted forms of T1 and T2 relaxation, and for the NMR pulse sequence in Fig. 1 takes the form75
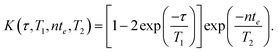 | (10) |
Finally, F(T1,T2) represents the desired 2D distributions of T1 and T2 relaxation time constants; distributions were obtained by applying a numerical inversion of the acquired 2D relaxation data according to the above expressions. As this is an ill-posed problem,76 stability of the inverted distributions in the presence of experimental noise was achieved through the use of Tikhonov regularisation,77 with the magnitude of the smoothing parameter chosen according to the Generalised Cross-validation method.78 Inverted distributions were bound within the range {10−3, 102} s and corrected for the influence of magnetic susceptibility contrast effects54 using the approach of Mitchell et al.79
TPD measurements
TPD measurements were performed using a Hidden Analytical CATLAB-PCS comprising a microreactor module and integrated mass spectrometer. Zeolite samples imbibed with pyridine were placed within a glass microreactor under a constant 40 mL min−1 flow of high-purity helium and left for 2 hours at 432 K; after this time the mass spectrometer signal was observed to have returned to its baseline, indicating removal of all physiosorbed and excess pyridine. TPD curves were then acquired across the temperature range 423–1273 K with heating rates of β = 2, 5, 10, 15 and 20 K min−1. Data from the mass fragments m/z = 52 and m/z = 79 were recorded, with each experiment repeated twice to ensure reproducibility; the acquisition of each TPD curve took between 4 and 10 hours.Results and discussion
NMR relaxation
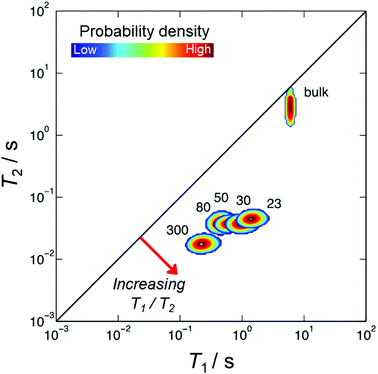
Fig. 2 summaries the T1–T2 correlation data obtained from our range of HZSM-5 zeolites. Correlation plots of this form facilitate a straightforward visual comparison of the nuclear spin relaxation characteristics exhibited by bulk (unrestricted) pyridine and pyridine adsorbed within zeolite structures of varying SAR. The diagonal line within this figure indicates T1/T2 = 1, characteristic of non-viscous bulk liquids.80 The correlation peak obtained from bulk pyridine can been seen close to this diagonal, consistent with the expectation that T1 = T2 in the absence of surface interactions or confinement effects. Correlation peaks away from this diagonal are characterised by T1/T2 > 1; as suggested by eqn (8), the position of these peaks is expected to be dictated by the relative surface affinities of pyridine within these structures. The T1/T2 values obtained from the logarithmic mean of these correlation peaks are summarised in Table 1 and discussed further below.Temperature programmed desorption
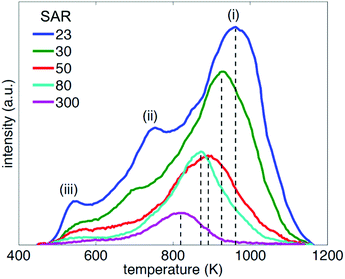
Example TPD spectra for pyridine with the range of HZSM-5 zeolites studied are shown in Fig. 3. For HZSM-5 with SAR = 23 three desorption rate maxima are evident, labelled (i), (ii) and (iii), suggesting pyridine desorbs from three distinct sites within this material. While peaks (ii) and (iii) are also evident at SAR = 30, only a single spectral desorption peak (peak (i)) is observed for the remaining materials, characterising the temperatures associated with the maximum pyridine desorption rates across these zeolites, Tp. Given the NMR relaxation time ratio T1/T2 is conjectured to be sensitive to the strongest adsorption sites present across a surface,32 we focus here on the consideration of this maximum desorption rate temperature across the five materials investigated, and a comparison of the associated desorption energetics with our acquired NMR relaxation data.Analysis of our TPD data was performed using the variable heating rate method of Cvetanović and Amenomiya,81,82 which has been applied to a variety of acidic zeolitic systems elsewhere.83–87 The relationship between desorption peak temperature Tp, heating rates β and the probe molecule heat of desorption ΔHdes may be written
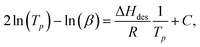 | (11) |
Fig. 4 summarises our acquired TPD data, obtained using a range of heating rates between β = 2 K min−1 and β = 20 K min−1. Solid lines indicate a fit to eqn (11) in each case, yielding values of ΔHdes for each SAR. These values, together with the T1/T2 ratios extracted from the data within Fig. 2, are summarised in Table 1.
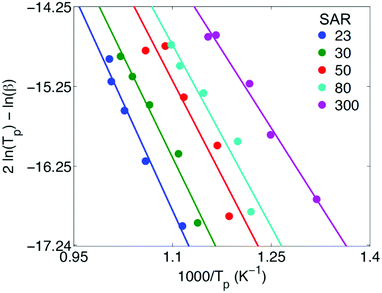 | ||
| Fig. 4 TPD data acquired for pyridine desorption from HZSM-5 zeolites with varying silica/alumina ratios (SAR). Data points indicate values of the maximum desorption rate temperature Tp obtained across multiple heating rates between β = 2 K min−1 and β = 20 K min−1. Solid lines indicate a fit to eqn (11) in each case, which yields values of the pyridine heat of desorption ΔHdes; the acquired values of ΔHdes are detailed in Table 1. | ||
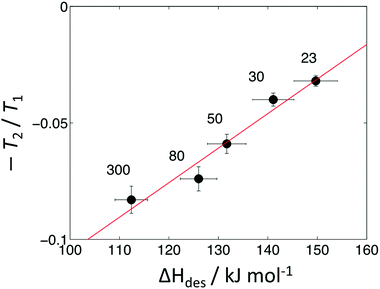
| SAR | T 1/T2 | ΔHdes/kJ mol−1 |
|---|---|---|
| 23 | 32 | 150 |
| 30 | 25 | 141 |
| 50 | 17 | 132 |
| 80 | 14 | 126 |
| 300 | 12 | 110 |
Correlating NMR relaxation with desorption energetics
We now provide a comparison of our acquired NMR relaxation data with the heats of desorption obtained from TPD analysis. The aim of this comparison is to validate the use of nuclear spin relaxation measurements for the comparison of zeolitic materials exhibiting different acidities, and more generally to extend the potential of such measurements – applied previously as a non-destructive probe of surface affinities in mesoporous systems – to microporous media.The data within Table 1 reveals clear and notable correlations between SAR, T1/T2 ratios and ΔHdes values. In particular, an increase in ΔHdes, which correlates with decreasing SAR due to an increase in the number of Brønsted acid sites,71 can be seen to correlate with an increase in T1/T2 ratio; this observation indicates the measurement of nuclear spin relaxation phenomena associated with basic probe molecules imbibed within such systems provides a useful method for the evaluation and comparison of zeolitic materials in terms of their acidity. Following our derivation of eqn (8) we attribute this observation to an increase in the ratio T1,surf/T2,surf with enhanced ΔHdes.
In previous work an empirical theory was developed to formally relate the ratio T1,surf/T2,surf with probe molecule desorption energetics.32 It was found that a linear correlation is expected to exist between desorption energetics and the inverse relaxation time ratio −T2/T1. This relationship has been verified for a range of water32 and short-chain hydrocarbons39 imbibed within mesoporous catalyst support materials. To explore whether this relationship also holds within microporous structures we provide in Fig. 5 a comparison of this inverse ratio, obtained from our NMR data in Table 1 as −T2/T1 = −1/(T1/T2), with our ΔHdes values. An extremely strong correlation is observed between these metrics, providing evidence that NMR relaxation data obtained from liquid-saturated microporous materials can provide a quantitative indication of surface interaction phenomena associated with the strongest adsorption sites present.
Conclusions
We have detailed an investigation into the application of nuclear spin relaxation measurements as a probe of sorption energetics within microporous HZSM-5 zeolites of varying SAR. Through a direct comparison with TPD analysis our results indicate that the dimensionless ratio of relaxation time constants T1/T2, obtained here through the analysis of 2D 1H T1–T2 correlation data, provides a non-invasive probe of surface affinity in microporous solids. For the specific case explored here, clear sensitivity of this relaxation time ratio to zeolite acidity has been demonstrated. Overall, our analysis method is of interest as it is rapid, non-destructive and simple to implement, and may be readily translated to portable and low-field benchtop NMR systems employed for materials screening and quality control. Measurements take on the order of tens of minutes to perform, reducing significantly the required experimental time required for such analysis compared to typical TPD analysis protocols, which may take >100 hours. Relaxation measurements may therefore be employed in standalone form to provide a rapid, qualitative indication of increasing surface interaction strength across a given material series, or performed in combination with at least two TPD calibration measurements to yield quantitative measures of surface interact strength, significantly reducing the required experimental time for such analysis. These factors suggest such relaxation time measurements represent a valuable tool for the characterisation of microporous materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Prof. Dame Lynn F. Gladden, University of Cambridge, for access to the experimental facilities. Carmine D’Agostino would also like to acknowledge the EPSRC, grant no. EP/S019138/1.Notes and references
- M. Shamzhy, M. Opanasenko, P. Concepción and A. Martínez, New trends in tailoring active sites in zeolite-based catalysts, Chem. Soc. Rev., 2019, 48, 1095–1149 RSC.
- S. Mintova, M. Jaber and V. Valtchev, Nanosized microporous crystals: emerging applications, Chem. Soc. Rev., 2015, 44, 7207–7233 RSC.
- N. Rahimi and R. Karimzadeh, Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: a review, Appl. Catal., A, 2011, 398, 1–17 CrossRef CAS.
- B. Xu, C. Sievers, S. B. Hong, R. Prins and J. A. van Bokhoven, Catalytic activity of Brønsted acid sites in zeolites: intrinsic activity, rate-limiting step, and influence of the local structure of the acid sites, J. Catal., 2006, 244, 163–168 CrossRef CAS.
- S. Al-Khattaf, C. D’Agostino, M. N. Akhtar, N. Al-Yassir, N. Y. Tan and L. F. Gladden, The effect of coke deposition on the activity and selectivity of the HZSM-5 zeolite during ethylbenzene alkylation reaction in the presence of ethanol, Catal. Sci. Technol., 2014, 4, 1017 RSC.
- D. E. Bryant and W. L. Kranich, Dehydration of alcohols over zeolite catalysts, J. Catal., 1967, 8, 8–13 CrossRef CAS.
- Y. T. Kim, K.-D. Jung and E. D. Park, A comparative study for gas-phase dehydration of glycerol over H-zeolites, Appl. Catal., A, 2011, 393, 275–287 CrossRef CAS.
- H. P. Decolatti, B. O. Dalla Costa and C. A. Querini, Dehydration of glycerol to acrolein using H-ZSM5 zeolite modified by alkali treatment with NaOH, Microporous Mesoporous Mater., 2015, 204, 180–189 CrossRef CAS.
- G. Busca, Acidity and basicity of zeolites: a fundamental approach, Microporous Mesoporous Mater., 2017, 254, 3–16 CrossRef CAS.
- E. G. Derouane, J. C. Védrine, R. R. Pinto, P. M. Borges, L. Costa, M. A. N. D. A. Lemos, F. Lemos and F. R. Ribeiro, The Acidity of Zeolites: Concepts, Measurements and Relation to Catalysis: A Review on Experimental and Theoretical Methods for the Study of Zeolite Acidity, Catal. Rev., 2013, 55, 454–515 CrossRef CAS.
- Y. Akacem, E. Kassab and E. Kassab, Vibrational Analysis of Pyridine Adsorption on the Brønsted Acid Sites of Zeolites Based on Density Functional Cluster Calculations, J. Phys. Chem. C, 2008, 112, 19045–19054 CrossRef.
- R. Buzzoni, S. Bordiga, G. Ricchiardi, C. Lamberti, A. Zecchina and G. Bellussi, Interaction of Pyridine with Acidic (H-ZSM5, H-β, H-MORD Zeolites) and Superacidic (H-Nafion Membrane) Systems: An IR Investigation, Langmuir, 1996, 12, 930–940 CrossRef CAS.
- J. N. Kondo, R. Nishitani, E. Yoda, T. Yokoi, T. Tatsumi and K. Domen, A comparative IR characterization of acidic sites on HY zeolite by pyridine and CO probes with silica–alumina and γ-alumina references, Phys. Chem. Chem. Phys., 2010, 12, 11576 RSC.
- C. A. Emeis, Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts, J. Catal., 1993, 141, 347–354 CrossRef CAS.
- L. Rodríguez-González, F. Hermes, M. Bertmer, E. Rodríguez-Castellón, A. Jiménez-López and U. Simon, The acid properties of H-ZSM-5 as studied by NH3-TPD and 27Al-MAS-NMR spectroscopy, Appl. Catal., A, 2007, 328, 174–182 CrossRef.
- F. Jin and Y. Li, A FTIR and TPD examination of the distributive properties of acid sites on ZSM-5 zeolite with pyridine as a probe molecule, Catal. Today, 2009, 145, 101–107 CrossRef CAS.
- A. Camiloti, S. Jahn, N. Velasco, L. Moura and D. Cardoso, Acidity of Beta zeolite determined by TPD of ammonia and ethylbenzene disproportionation, Appl. Catal., A, 1999, 182, 107–113 CrossRef CAS.
- B. Hunger, J. Hoffmann, O. Heitzsch and M. Hunger, Temperature-programmed desorption (TPD) of ammonia from HZSM-5 zeolites, J. Therm. Anal., 1990, 36, 1379–1391 CrossRef CAS.
- M. Niwa, K. Suzuki, N. Katada, T. Kanougi and T. Atoguchi, Ammonia IRMS-TPD study on the distribution of acid sites in mordenite, J. Phys. Chem. B, 2005, 109, 18749–18757 CrossRef CAS PubMed.
- J. Kanellopoulos, C. Gottert, D. Schneider, B. Knorr, D. Prager, H. Ernst and D. Freude, NMR investigation of proton mobility in zeolites, J. Catal., 2008, 255, 68–78 CrossRef CAS.
- A. Zheng, S. Li and F. Deng, Solid-State NMR Characterization of Acidity of Solid Catalysts, in Modern Magnetic Resonance, ed. G. Webb, Springer, Cham, 2018 Search PubMed.
- D. Barthomeuf, Zeolite acidity dependence on structure and chemical environment. Correlations with catalysis, Mater. Chem. Phys., 1987, 17, 49–71 CrossRef CAS.
- H. M. Kao, P. C. Chang, Y. W. Liao, L. P. Lee and C. H. Chien, Solid-state NMR characterization of the acid sites in cubic mesoporous Al-MCM-48 materials using trimethylphosphine oxide as a 31P NMR probe, Microporous Mesoporous Mater., 2008, 114, 352–364 CrossRef CAS.
- L. Peng, P. J. Chupas and C. P. Grey, Measuring Brønsted acid densites in zeolite HY with diphosphine molecules and solid state NMR spectroscopy, J. Am. Chem. Soc., 2004, 126, 12254–12255 CrossRef CAS PubMed.
- S. Li, S. J. Huang, W. Shen, H. Zhang, H. Fang, A. Zheng, S. Bin Liu and F. Deng, Probing the spatial proximities among acid sites in dealuminated H–Y zeolite by solid-state NMR spectroscopy, J. Phys. Chem. C, 2008, 112, 14486–14494 CrossRef CAS.
- W. R. Gunther, V. K. Michaelis, R. G. Griffin and Y. Román-Leshkov, Interrogating the Lewis Acidity of Metal Sites in Beta Zeolites with 15 N Pyridine Adsorption Coupled with MAS NMR Spectroscopy, J. Phys. Chem. C, 2016, 120, 28533–28544 CrossRef CAS PubMed.
- H. M. Kao and C. P. Grey, Probing the Brønsted and Lewis acidity of zeolite HY: A 1H/27Al and 15N/27Al TRAPDOR NMR study of monomethylamine adsorbed on HY, J. Phys. Chem., 1996, 100, 5105–5117 CrossRef CAS.
- L. F. Gladden and J. Mitchell, Measuring adsorption, diffusion and flow in chemical engineering: applications of magnetic resonance to porous media, New J. Phys., 2011, 13, 035001 CrossRef.
- L. F. Gladden, Magnetic resonance in reaction engineering: beyond spectroscopy, Curr. Opin. Chem. Eng., 2013, 2, 331–337 CrossRef.
- J. Kowalewski and L. Mäler, Nuclear spin relaxation in liquids: theory, experiments, and applications, CRC Press, 2nd edn, 2017 Search PubMed.
- P. A. Vecino, Z. Huang, J. Mitchell, J. McGregor, H. Daly, C. Hardacre, J. M. Thomson and L. F. Gladden, Determining adsorbate configuration on alumina surfaces with 13C nuclear magnetic resonance relaxation time analysis, Phys. Chem. Chem. Phys., 2015, 17, 20830–20839 RSC.
- C. D’Agostino, J. Mitchell, M. D. Mantle and L. F. Gladden, Interpretation of NMR Relaxation as a Tool for Characterising the Adsorption Strength of Liquids inside Porous Materials, Chem. – Eur. J., 2014, 20, 13009–13015 CrossRef PubMed.
- R. Pini and L. Joss, See the unseen: applications of imaging techniques to study adsorption in microporous materials, Curr. Opin. Chem. Eng., 2019, 24, 37–44 CrossRef.
- D. Weber, J. Mitchell, J. Mcgregor and L. F. Gladden, Comparing strengths of surface interactions for reactants and solvents in porous catalysts using Two-dimensional NMR relaxation correlations, J. Phys. Chem. C, 2009, 113, 6610–6615 CrossRef CAS.
- J. Mitchell, L. M. Broche, T. C. Chandrasekera, D. J. Lurie and L. F. Gladden, Exploring Surface Interactions in Catalysts Using Low-Field Nuclear Magnetic Resonance, J. Phys. Chem. C, 2013, 117, 17699–17706 CrossRef CAS.
- A. T. Krzyżak and I. Habina, Low field 1H NMR characterization of mesoporous silica MCM-41 and SBA-15 filled with different amount of water, Microporous Mesoporous Mater., 2016, 231, 230–239 CrossRef.
- K. Ralphs, C. D’Agostino, R. Burch, S. Chansai, L. F. Gladden, C. Hardacre, S. L. James, J. Mitchell and S. F. R. Taylor, Assessing the surface modifications following the mechanochemical preparation of a Ag/Al2O3 selective catalytic reduction catalyst, Catal. Sci. Technol., 2014, 4, 531–539 RSC.
- D. Espinat, F. Gaulier, F. Norrant, J. Barbier, B. Guichard, M. Rivallan and P. Levitz, Characterization of Asphaltenes in Solution and Inside the Pores of Catalysts by 1H NMR Relaxometry, Energy Fuels, 2017, 31, 7382–7395 CrossRef CAS.
- N. Robinson, C. Robertson, L. F. Gladden, S. J. Jenkins and C. D’Agostino, Direct correlation between adsorption energetics and nuclear spin relaxation in a liquid-saturated catalyst material, ChemPhysChem, 2018, 19, 2472–2479 CrossRef CAS PubMed.
- C. D’Agostino, M. R. Feaviour, G. L. Brett, J. Mitchell, A. P. E. York, G. J. Hutchings, M. D. Mantle and L. F. Gladden, Solvent inhibition in the liquid-phase catalytic oxidation of 1,4-butanediol: understanding the catalyst behaviour from NMR relaxation time measurements, Catal. Sci. Technol., 2016, 6, 7896–7901 RSC.
- C. D’Agostino, R. D. Armstrong, G. J. Hutchings and L. F. Gladden, Product Inhibition in Glycerol Oxidation over Au/TiO2 Catalysts Quantified by NMR Relaxation, ACS Catal., 2018, 8, 7334–7339 CrossRef.
- J. J. Varghese, L. Cao, C. Robertson, Y. Yang, L. F. Gladden, A. A. Lapkin and S. H. Mushrif, Synergistic Contribution of the Acidic Metal Oxide-Metal Couple and Solvent Environment in the Selective Hydrogenolysis of Glycerol: A Combined Experimental and Computational Study Using ReOx–Ir as the Catalyst, ACS Catal., 2019, 9, 485–503 CrossRef CAS.
- M. Wehring, J. Gascon, D. Dubbeldam, F. Kapteijn, R. Q. Snurr and F. Stallmach, Self-diffusion studies in CuBTC by PFG NMR and MD simulations, J. Phys. Chem. C, 2010, 114, 10527–10534 CrossRef CAS.
- N. Robinson, G. Xiao, P. R. J. Connolly, N. N. A. Ling, E. O. Fridjonsson, E. F. May and M. L. Johns, Low-field NMR relaxation-exchange measurements for the study of gas admission in microporous solids, Phys. Chem. Chem. Phys., 2020, 22, 13689–13697 RSC.
- V. J. Witherspoon, R. Mercado, E. Braun, A. Mace, J. Bachman, J. R. Long, B. Blümich, B. Smit and J. A. Reimer, Combined Nuclear Magnetic Resonance and Molecular Dynamics Study of Methane Adsorption in M2(dobdc) Metal–Organic Frameworks, J. Phys. Chem. C, 2019, 123, 12286–12295 CrossRef CAS.
- F. Stallmach, A. K. Pusch, T. Splith, C. Horch and S. Merker, NMR relaxation and diffusion studies of methane and carbon dioxide in nanoporous ZIF-8 and ZSM-58, Microporous Mesoporous Mater., 2015, 205, 36–39 CrossRef CAS.
- J. J. Chen, X. Kong, K. Sumida, M. A. Manumpil, J. R. Long and J. A. Reimer, Ex Situ NMR relaxometry of metal–organic frameworks for rapid surface-area screening, Angew. Chem., Int. Ed., 2013, 52, 12043–12046 CrossRef CAS PubMed.
- J. J. Chen, J. A. Mason, E. D. Bloch, D. Gygi, J. R. Long and J. A. Reimer, NMR relaxation and exchange in metal–organic frameworks for surface area screening, Microporous Mesoporous Mater., 2015, 205, 65–69 CrossRef CAS.
- T. Ueda, K. Kurokawa, Y. Kawamura, K. Miyakubo and T. Eguchi, 1H NMR study of molecular motion of benzene and n-decane confined in the nanocavities of metal–organic frameworks, J. Phys. Chem. C, 2012, 116, 1012–1019 CrossRef CAS.
- V. J. Witherspoon, L. M. Yu, S. Jawahery, E. Braun, S. M. Moosavi, S. K. Schnell, B. Smit and J. A. Reimer, Translational and Rotational Motion of C8 Aromatics Adsorbed in Isotropic Porous Media (MOF-5): NMR Studies and MD Simulations, J. Phys. Chem. C, 2017, 121, 15456–15462 CrossRef CAS.
- J.-P. Korb, S. Xu and J. Jonas, Confinement effects on dipolar relaxation by translational dynamics of liquids in porous silica glasses, J. Chem. Phys., 1993, 98, 2411–2422 CrossRef CAS.
- J.-P. Korb, A. Delville, S. Xu, G. Demeulenaere, P. Costa and J. Jonas, Relative role of surface interactions and topological effects in nuclear magnetic resonance of confined liquids, J. Chem. Phys., 1994, 101, 7074–7081 CrossRef.
- J.-P. Korb, Nuclear magnetic relaxation of liquids in porous media, New J. Phys., 2011, 13, 035016 CrossRef.
- J. Mitchell, T. C. Chandrasekera, M. L. Johns, L. F. Gladden and E. J. Fordham, Nuclear magnetic resonance relaxation and diffusion in the presence of internal gradients: the effect of magnetic field strength, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2010, 81, 026101 CrossRef CAS PubMed.
- J. Mitchell and T. C. Chandrasekera, Understanding generalized inversions of nuclear magnetic resonance transverse relaxation time in porous media, J. Chem. Phys., 2014, 141, 224201 CrossRef CAS PubMed.
- Y. Q. Song, Magnetic resonance of porous media (MRPM): a perspective, J. Magn. Reson., 2013, 229, 12–24 CrossRef CAS PubMed.
- G. Liu, Y. Li and J. Jonas, Confined geometry effects on reorientational dynamics of molecular liquids in porous silica glasses, J. Chem. Phys., 1991, 95, 6892–6901 CrossRef CAS.
- R. L. Kleinberg, W. E. Kenyon and P. P. Mitra, Mechanism of NMR Relaxation of Fluids in Rock, J. Magn. Reson., Ser. A, 1994, 108, 206–214 CrossRef CAS.
- S. Godefroy, J.-P. Korb, M. Fleury and R. G. Bryant, Surface nuclear magnetic relaxation and dynamics of water and oil in macroporous media, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2001, 64, 021605 CrossRef CAS PubMed.
- N. Robinson, L. F. Gladden and C. D’Agostino, Exploring catalyst passivation with NMR relaxation, Faraday Discuss., 2017, 204, 439–452 RSC.
- C. D’Agostino and P. Bräuer, Exploiting enhanced paramagnetic NMR relaxation for monitoring catalyst preparation using T1–T2 NMR correlation maps, React. Chem. Eng., 2019, 4, 268–272 RSC.
- C. D’Agostino, G. Brett, G. Divitini, C. Ducati, G. J. Hutchings, M. D. Mantle and L. F. Gladden, Increased Affinity of Small Gold Particles for Glycerol Oxidation over Au/TiO2 Probed by NMR Relaxation Methods, ACS Catal., 2017, 7, 4235–4241 CrossRef.
- B. E. Kinn, T. R. Myers and A. M. Allgeier, Surface enhanced nuclear magnetic resonance relaxation mechanisms and their significance in chemical engineering applications, Curr. Opin. Chem. Eng., 2019, 24, 115–121 CrossRef.
- J. P. Korb, S. Godefroy and M. Fleury, Surface nuclear magnetic relaxation and dynamics of water and oil in granular packings and rocks, Magn. Reson. Imaging, 2003, 21, 193–199 CrossRef CAS PubMed.
- W. F. J. Slijkerman and J. P. Hofman, Determination of surface relaxivity from NMR diffusion measurements, Magn. Reson. Imaging, 1998, 16, 541–544 CrossRef CAS PubMed.
- J. Mitchell and E. J. Fordham, Contributed Review: nuclear magnetic resonance core analysis at 0.3 T, Rev. Sci. Instrum., 2014, 85, 111502 CrossRef PubMed.
- D. Ohayon, R. Le Van Mao, D. Ciaravino, H. Hazel, A. Cochennec and N. Rolland, Methods for pore size engineering in ZSM-5 zeolite, Appl. Catal., A, 2001, 217, 241–251 CrossRef CAS.
- M. Che and J. C. Védrine, Characterization of solid materials and heterogeneous catalysts: from structure to surface reactivity, Wiley-VCH, 2012 Search PubMed.
- A. S. Al-Dughaither and H. De Lasa, HZSM-5 zeolites with different SiO2/Al2O3 ratios. Characterization and NH3 desorption kinetics, Ind. Eng. Chem. Res., 2014, 53, 15303–15316 CrossRef CAS.
- P. Braüer and C. D’Agostino, Base adsorption mechanism over zeolite catalysts at different Al contents probed by the tapered element oscillating microbalance (TEOM), Phys. Chem. Chem. Phys., 2018, 20, 25357–25364 RSC.
- P. Bräuer, O. Situmorang, P. L. Ng and C. D’Agostino, Effect of Al content on the strength of terminal silanol species in ZSM-5 zeolite catalysts: a quantitative DRIFTS study without the use of molar extinction coefficients, Phys. Chem. Chem. Phys., 2018, 20, 4250–4262 RSC.
- P. Bräuer, P. L. Ng, O. Situmorang, I. Hitchcock and C. D’Agostino, Effect of Al content on number and location of hydroxyl acid species in zeolites: a DRIFTS quantitative protocol without the need for molar extinction coefficients, RSC Adv., 2017, 7, 52604–52613 RSC.
- Y.-Q. Song, L. Venkataramanan, M. D. Hürlimann, M. Flaum, P. Frulla and C. Straley, T 1–T2 Correlation Spectra Obtained Using a Fast Two-Dimensional Laplace Inversion, J. Magn. Reson., 2002, 154, 261–268 CrossRef CAS PubMed.
- L. Venkataramanan, Y.-Q. Song and M. D. Hurlimann, Solving Fredholm integrals of the first kind with tensor product structure in 2 and 2.5 dimensions, IEEE Trans. Signal Process, 2002, 50, 1017–1026 CrossRef.
- J. Mitchell, L. F. Gladden, T. C. Chandrasekera and E. J. Fordham, Low-field permanent magnets for industrial process and quality control, Prog. Nucl. Magn. Reson. Spectrosc., 2014, 76, 1–60 CrossRef CAS PubMed.
- P. C. Hansen, Numerical tools for analysis and solution of Fredholm integral equations of the first kind, Inverse Probl., 1992, 8, 849–872 CrossRef.
- A. N. Tikhonov and V. I. A. Arsenin, Solutions of ill-posed problems, SIAM, 1977 Search PubMed.
- J. Mitchell, T. C. Chandrasekera and L. F. Gladden, Numerical estimation of relaxation and diffusion distributions in two dimensions, Prog. Nucl. Magn. Reson. Spectrosc., 2012, 62, 34–50 CrossRef CAS PubMed.
- J. Mitchell, T. C. Chandrasekera and L. F. Gladden, Measurement of the true transverse nuclear magnetic resonance relaxation in the presence of field gradients, J. Chem. Phys., 2013, 139, 074205 CrossRef CAS PubMed.
- N. Bloembergen, E. M. Purcell and R. V. Pound, Relaxation effects in nuclear magnetic resonance absorption, Phys. Rev., 1948, 73, 679–712 CrossRef CAS.
- R. J. Cvetanović and Y. Amenomiya, Application of a Temperature-Programmed Desorption Technique to Catalyst Studies, Adv. Catal., 1967, 17, 103–149 Search PubMed.
- R. J. Cvetanoviĉ and Y. Amenomiya, A Temperature Programmed Desorption Technique for Investigation of Practical Catalysts, Catal. Rev., 1972, 6, 21–48 CrossRef.
- S. B. Sharma, B. L. Meyers, D. T. Chen, J. Miller and J. A. Dumesic, Characterization of catalyst acidity by microcalorimetry and temperature-programmed desorption, Appl. Catal., A, 1993, 102, 253–265 CrossRef CAS.
- N. Y. Topsøe, K. Pedersen and E. G. Derouane, Infrared and temperature-programmed desorption study of the acidic properties of ZSM-5-type zeolites, J. Catal., 1981, 70, 41–52 CrossRef.
- G. I. Kapustin, T. R. Brueva, A. L. Klyachko, S. Beran and B. Wichterlova, Determination of the number and acid strength of acid sites in zeolites by ammonia adsorption. Comparison of calorimetry and temperature-programmed desorption of ammonia, Appl. Catal., 1988, 42, 239–246 CrossRef CAS.
- Y. Du, B. Wooler, M. Nines, P. Kortunov, C. S. Paur, J. Zengel, S. C. Weston and P. I. Ravikovitch, New High- and Low-Temperature Phase Changes of ZIF-7: Elucidation and Prediction of the Thermodynamics of Transitions, J. Am. Chem. Soc., 2015, 137, 13603–13611 CrossRef CAS PubMed.
- C. V. Hidalgo, H. Itoh, T. Hattori, M. Niwa and Y. Murakami, Measurement of the acidity of various zeolites by temperature-programmed desorption of ammonia, J. Catal., 1984, 85, 362–369 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Supplementary TPD discussion. See DOI: 10.1039/d1cp01515j |
| ‡ Here we recall that the surface-to-volume ratio (S/V) of a sphere of diameter dsphere may be expressed as S/V = 6/dsphere. |
| This journal is © the Owner Societies 2021 |